Abstract
Understanding the developmental mechanisms of T follicular helper (TFH) cells in humans is a highly relevant topic to clinic. However, factors that drive human CD4+ helper T (TH) cell differentiation program towards TFH cells remain largely undefined. Here we show that TGF-β provides critical additional signals for the transcription factors STAT3 and STAT4 to promote the initial TFH differentiation programs in humans. This mechanism does not appear to be shared with mouse TH cells. The developing human Bcl-6+ TFH cells also expressed RORγt, a transcription factor typically expressed by TH17 cells. Our study documents a mechanism by which TFH and TH17 cells co-emerge in inflammatory environments in humans, as often observed in many human autoimmune diseases.
T follicular helper (TFH) cells play a major role in the generation of antigen-specific antibody responses by providing help to B cells1. TFH cells are essential for the formation of germinal centers (GCs), where high-affinity B cells are selected and differentiate into long-lived memory B cells and plasma cells2. The chemokine receptor CXCR5 is expressed by TFH cells and guides their migration towards B cell follicles1. TFH cells highly express the inducible co-stimulatory molecule ICOS, which is critical for their development3, 4, migration into follicles5 and function6. TFH cells support the survival of GC B cells and their differentiation into memory cells and plasma cells through secretion of interleukin 21 (IL-21)7 and by providing signals through the TNF family receptor superfamily molecule CD401. While TFH cells are important for antibody responses against infectious agents, exaggerated TFH responses cause autoimmunity8. Therefore, defining the developmental mechanism of TFH cells in humans is a highly relevant topic to human pathophysiology, and would provide direct insights into designing novel vaccines for infectious diseases and developing novel therapeutic approaches for autoimmune diseases.
TFH precursors interact with B cells at the border of T cell zone and follicles. Prolonged and stable interactions with B cells are essential for their maturation into GC TFH cells1, 9. Nonetheless, dendritic cells (DCs) are important in the early stage of TFH cell generation. The programing of CD4+ helper T (TH) cell towards TFH cell differentiation occurs as early as the first few divisions following interaction with DCs4, 9, 10. DC-derived cytokines activating the transcription factors STAT311 and STAT412 induce the interacting Th cells to express Bcl-6, a transcriptional repressor essential for TFH maturation13, 14, 15. The function of Bcl-6 is inhibited by the transcriptional repressor Blimp-1, and accordingly Blimp-1 inhibits the generation of TFH cells13. ICOS ligand expressed by DCs also contributes to the expression of Bcl-6 in TH cells4. Therefore, encounter with DCs largely pre-determines whether TH cells differentiate into the TFH lineage9.
Similar to other TH subsets, cytokine signals are important for the early development of TFH cells. Previous studies suggest differences between humans and mice regarding the dominant cytokines involved in TFH cell development. In mice, IL-6, IL-21 and IL-27 (that activate STAT3) play dominant roles1, 16, while IL-12 (that mainly activates STAT4) can also participate in the early phase12. In contrast, IL-12 appears to be more important than IL-6, IL-21, and IL-27 for TFH cell generation in humans17, 18. IL-12 induces higher expression of IL-21, ICOS, CXCR5 and Bcl-6 on activated human naïve TH cells compared with the other cytokines18, 19. However, IL-12 is also implicated in the generation of TH1 cells, suggesting that additional factors may also contribute to the generation of human TFH cells. How STAT4 and STAT3 signaling contributes to the generation of human TFH cells also remains to be established.
Here we show that TGF-β is an important co-factor for the early differentiation of human TFH cells. TGF-β co-operated with IL-12 and IL-23 for the expression of multiple TFH molecules by human naïve TH cells including CXCR5, ICOS, IL-21, Bcl-6, BATF and c-Maf, and the downregulation of Blimp-1. This stimulatory effect of TGF-β for TFH development was not found in mice. In the presence of TGF-β, STAT4 and STAT3 shaped the human TH differentiation gene programs towards the TFH lineage in a largely redundant manner, and cooperated to induce the expression of TFH molecules. Furthermore, we found that human TH17 cells generated in vitro with the cytokine combination of IL-23+IL-6+IL-1β+TGF–β largely shared properties with TFH cells, suggesting that the early developmental path of TFH and TH17 cells is shared in humans. We also found TFH cells co-expressing Bcl-6 and RORγt in human tonsils, providing supportive evidence for co-development of TFH and TH17 cells in inflammatory environment in humans.
Results
TGF–β cooperates with IL-12 and IL-23 for TFH molecule expression
We took a systematic approach to determine the cytokine signals promoting the initial TFH differentiation programs in humans. We cultured adult blood naïve TH cells from 13 different donors with CD3-CD28 mAbs in the presence of different cytokine combinations for 2–4 days, and analyzed the expression of multiple molecules expressed by TFH cells, including CXCR5, Bcl-6, ICOS and IL-21. For this analysis we selected cytokines known to regulate T cell differentiation, many of which are secreted by DCs: IL-1β IL-6, IL-10, IL-12, IL-21, IL-23, type I interferons (IFN-α, β and ω), type III IFNs (IFN-λ1, λ2) and TGF-β To circumvent the direct inhibitory effect of TGF-β and IFNs on cell cycle, naïve TH cells were primed overnight with CD3-CD28 mAbs before addition of cytokines to the cultures. This procedure yielded comparable cell recovery and viability across different cytokine conditions (Supplementary Fig. 1a). The data obtained from naïve TH cells cultured with various cytokines were ranked and the cytokine conditions that increased the expression of each TFH molecule were determined. Of note, we excluded IL-4, a major driver for TH2 differentiation, from the analysis, because previous studies suggested that IL-4 by itself does not induce human naïve Th cells to express TFH molecules18, 19. We further found that IL-4 strongly inhibited the expression of ICOS and IL-21 in IL-12-stimulated naïve Th cells (Supplementary Fig. 1b,c) and as such IL-4 might negatively regulate the generation of human TFH cells.
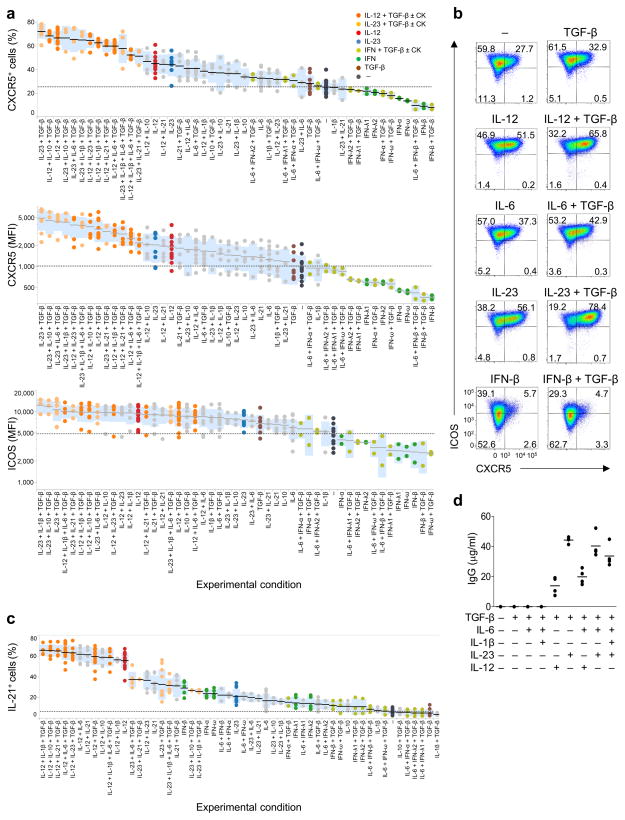
Analysis of the expression of CXCR5 by activated (FSChiSSChi) human naïve TH cells cultured with various cytokines for 3 days showed that the highest expression of CXCR5 was induced by the cytokine combination of TGF-β+IL-23 (Fig. 1a,b, and Supplementary Fig. 1d). The stimulation with TGF-β+IL-23 and TGF-β+IL-12 (also together with IL-1β, IL-6, IL-10, and IL-21) induced higher expression of CXCR5 than the stimulation with IL-23 or IL-12 alone. The cytokine conditions containing TGF-β+IL-23 and TGF-β+IL-12 also promoted the expression of ICOS and IL-21 (Fig. 1a–c). In contrast, type I and III IFNs yielded low expression of CXCR5 and ICOS, and few IL-21+ T cells (Fig. 1a–c). TH cells cultured with TGF–β+IL-12 and TGF–β+IL-23 efficiently induced B cells to produce IgG in vitro, while TH cells cultured with TGF–β or TGF–β+IL-6 did not (Fig. 1d). Thus, the combination of TGF-β+IL-12 and TGF-β+IL-23 promoted the expression of TFH markers and induced efficient B cell help.
Fig. 1. TGF–β+IL-12 and TGF–β+IL-23 induce TFH molecule expression by human naïve TH cells.
(a) Ranking of the differentiation conditions inducing the expression of CXCR5 and ICOS on activated (FSChiSSChi) human adult blood naïve TH cells following 4 d stimulation with CD3-CD28 mAbs in the presence of the indicated cytokines (CK) during the last 3 days. Each dot represents a result from 13 sets of 4 d-culture experiments. Black bars show the mean value and the blue boxes show the range of ± 1 s.d. (b) Expression of CXCR5 and ICOS on activated TH cells stimulated with the indicated cytokines as in (a). Results are from one representative of 13 sets of 4 d-culture experiments. (c) Ranking of the differentiation conditions inducing IL-21 expression by activated human naïve TH cells cultured as in (a). (d) IgG ELISA at day 14 in the supernatants of co-cultures of activated naïve TH cells (after 3 d culture with the indicated cytokines) and autologous memory B cells in the presence of the superantigen Staphylococcal enterotoxin B. Mean, n=4. A representative experiments out of 3 experiments.
TGF–β+ IL-12 and TGF–β+ IL-23 induce Bcl-6 in human naïve TH
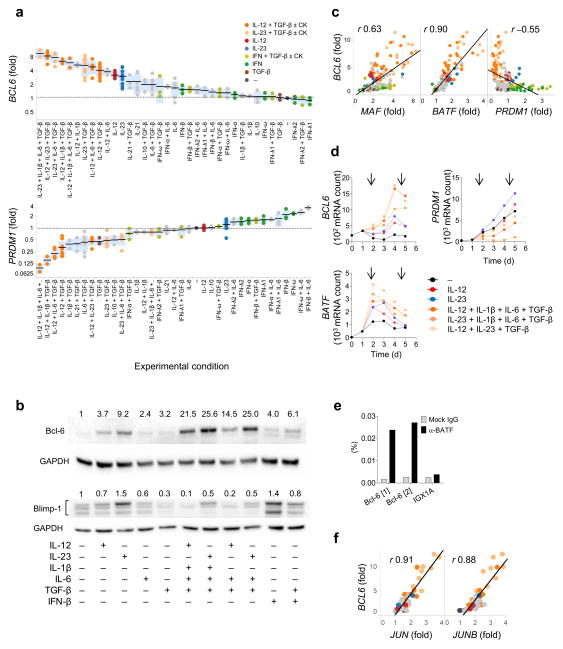
To assess the early TH differentiation programs at a transcriptional level, we analyzed the expression of transcription factor transcripts by NanoString nCounter® Analysis System, which allows direct measurement of mRNA transcript abundance. Similar to mouse TFH cells1, 16, we confirmed that human ex vivo tonsillar GC TFH cells and their precursors7 have high expression of BCL6, BATF and MAF, but low expression of PRDM1 (encoding Blimp-1; Supplementary Fig. 2a,b). We assessed the expression of these transcription factors in human adult blood naïve TH cells cultured for 3 days with different cytokines as described above. The data obtained were normalized to the values in the control culture (no cytokines) in each experiment, and the ranking of the cytokine conditions that determined. TGF-β+IL-12 and TGF-β+IL-23 induced higher BCL6 expression than IL-12 or IL-23 alone (Fig. 2a). Furthermore, addition of IL-1β and IL-6 to TGF-β+IL-12 and TGF-β+IL-23 further increased BCL6 expression. TGF-β+IL-12 and TGF-β+IL-23 also suppressed the expression of PRDM1. IL-1β and IL-6 further decreased PRDM1 expression when added to TGF-β+IL-12. Immunoblotting showed that protein expression correlate with the transcript data (Fig. 2b). Furthermore, overall PRDM1 abundance negatively correlated with BCL6 abundance (Fig. 2c), and accordingly, the combinations of TGF-β+IL-12+IL-1β+IL-6 yielded the highest ratio of BCL6 to PRDM1 (Supplementary Fig. 2c). Largely similar results were obtained with human cord blood-derived naïve TH cells (Supplementary Fig. 2d).
Fig. 2. TGF–β+IL-12 and TGF–β+IL-23 promote the induction of TFH transcriptional signature.
(a) Ranking of the differentiation conditions inducing BCL6 and suppressing PRDM1 by human adult blood naïve TH cells cultured as in Fig. 1a. The data were normalized to the values in the culture with no cytokines in each experiment. Each dot represents a result from 11 sets of 4 d culture experiments. Black bars show the mean value and blue boxes indicate ± 1 s.d. (b) Immunoblot of Bcl-6 and Blimp-1 with naïve TH cells cultured with the indicated cytokines for 3 days. The band density after normalization with GAPDH is shown in number. Note that human Blimp-1 has three isoforms. A representative out of 3 independent experiments. (c) Correlation between BCL6 and MAF, BATF, and PRDM1 expressed by human naïve TH cells cultured as in (a). Pearson R values are indicated. P values were all <0.0001. (d) Kinetics of BCL6, PRDM1, and BATF expression by naïve TH cells cultured with cytokines for 1–4 days as in (a). Arrows indicate days when cytokines (CK) were added. (e) The chromatin fraction of human naïve TH cells cultured with IL-23+IL-6+IL-1β+TGF–β for 3 days were immunoprecipitated with anti-BATF or Rabbit IgG, followed by quantitative PCR analysis of the two BATF binding sites within BCL6 promoter. Results were normalized to input. IGX1A region represents a negative control. A representative out of 5 independent experiments. (e) Correlation between BCL6 and JUN and JUNB expressed by cord blood naïve TH cells cultured as in (a). Pearson R values are indicated. P values were both <0.0001.
The stimulation of human naïve TH cells with the combinations of TGF-β+IL-12 and TGF-β+IL-23 together with IL-1β and IL-6 also resulted in high expression of BATF and MAF (Supplementary Fig. 3a). In particular, the abundance of BCL6 showed a strong positive correlation with the abundance of BATF (R=0.90 in adult blood naïve TH and R=0.84 in cord blood naïve TH; Fig. 2c and Supplementary Fig. 3b). Kinetic analysis showed that the cytokine combinations of TGF-β+IL-12+IL-1β+IL-6 and TGF-β+IL-23+IL-1β+IL-6 as well as TGF-β+IL-12+IL-23 increased the expression of BCL6 and BATF within 24 h (Fig. 2d). While BATF expression peaked at 24 h after cytokine stimulation, BCL6 expression peaked at 96–120 h, suggesting that BATF directly induces Bcl-6 expression in human TH cells, as shown in mice20, 21. Chromatin immunoprecipitation experiments indicated that BATF binds to promoter regions of the human BCL6 gene (Fig. 2e, Supplementary Fig. 3c). Furthermore, the combinations of TGF-β+IL-12 and TGF-β+IL-23 together with IL-1β and IL-6 enhanced the expression of JUN and JUNB, two transcription factors which form functional heterodimers with BATF22 (Supplementary Fig. 2d). The abundance of JUN and JUNB also showed a strong positive correlation with the abundance of BCL6 (R=0.91 and 0.88, respectively; Fig. 3a). These results suggest that TGF-β+IL-12 and TGF-β+IL-23 together with IL-1β and IL-6 promote Bcl-6 expression at least in part by upregulating both components of BATF-Jun heterodimers.
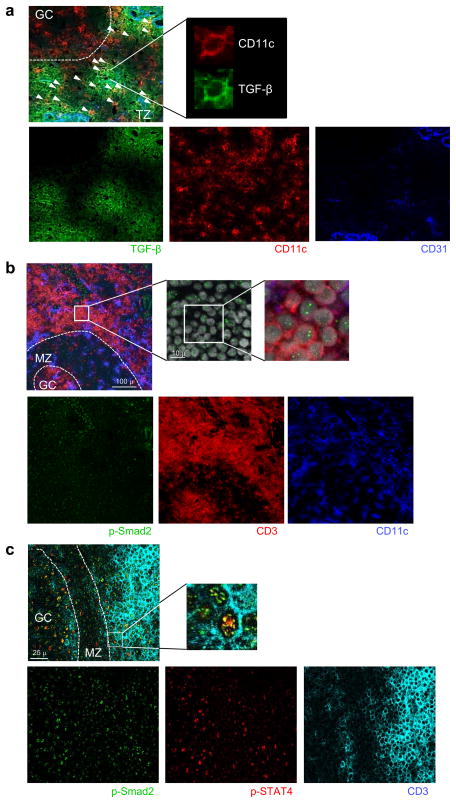
Fig. 3. TH cells receive TGF–β and IL-12 signals in the T cell zone.
(a) Tonsil sections were stained for the expression of TGF–β (indicated by green), CD11c (red), and CD31 (blue) and analyzed with fluorescent microscopy GC: germinal center. TZ: T cell zone. In the top left panel, small arrows indicate cells positive for both CD11c and TGF–β. A representative cell is shown in the top right panel. A representative out of 3 independent experiments. (b) Tonsil sections were stained for p-Smad2 (indicated by green), CD11c (blue), and CD3 (red) and analyzed with confocal microscopy. The top right panels show the localization of p-Smad2 in DAPI+ nuclei in T cells in the close proximity of GCs. A representative out of 3 independent experiments. (c) Tonsil sections were stained for p-Smad2 (indicated by green), p-STAT4 (red), and CD3 (light blue) and analyzed with confocal microscopy. The top right panels show T cells positive for both p-Smad2 and p-STAT4 in the close proximity of GCs. A representative out of 3 independent experiments.
To address whether TGF-β also promotes the expression of TFH markers in naïve TH cells primed under physiological conditions, human adult blood naïve TH cells were stimulated with allogeneic monocyte-derived DCs (activated either by heat-killed E. coli or CD40L stimulation) in the presence of titrated amounts of TGF-β. The expression of CXCR5, IL-21 and Bcl-6 was enhanced by a supplementation of 0.2–1 ng/ml of TGF-β (Supplementary Fig. 3d,e), consistent with the results obtained with anti-CD3-CD28-stimulated TH cells.
Collectively, TGF-β+IL-12 and TGF-β+IL-23 together with IL-1β and IL-6 promoted human naïve TH cells to express multiple TFH molecules including CXCR5, ICOS, IL-21, Bcl-6, BATF-Jun, and c-Maf, while downregulating Blimp-1.
TH cells at proximity of GCs receive TGF–β signaling
These results suggest that when naïve TH cells interact at T cell zone with activated DCs that produce inflammatory cytokines including IL-12 and IL-237, TGF-β promotes TH differentiation towards the TFH lineage. TH cells in the proximity of tonsillar GCs are known to express phosphorylated STAT4 (p-STAT4), suggesting that TH cells receive IL-12 signals at these sites17. We examined whether TGF–β is also expressed in the T cell zone of inflammatory pediatric tonsils. We observed abundant expression of TGF–β in the T cell zone near GCs, in particular in the proximity of CD31+ lymphatic and vascular endothelial cells (Fig. 3a). More than 50% of the CD11c+ DCs localized in the T cell zone were positive for TGF–β staining, indicating that TH cells have access to environmental TGF–β and/or TGF–β expressed by DCs.
TGF–β mediates its biological functions by binding to TGF–β type I and type II receptors, which phosphorylate the transcription factors Smad2 and Smad3. Activated Smad molecules translocate into the nucleus23. To determine whether TH cells receive TGF–β signals in the T cell zone, we analyzed the expression of phosphorylated Smad2 (p-Smad2) in human pediatric tonsils. p-Smad2 was expressed in many cells in the inflamed tonsils, including endothelial cells (Fig 3b), consistent with abundant expression of TGF–β. TH cells in the proximity of GCs (determined by DAPI staining pattern. not shown) showed p-Smad2 nuclear expression (Fig. 3b) and p-STAT4 expression (Fig. 3c) These observations indicate that the T cell zone in inflamed tonsils is enriched in TGF–β, and that TH cells in the proximity of GCs receive TGF–β and IL-12 signals.
TFH-promoting conditions induce TH17 transcriptional signatures
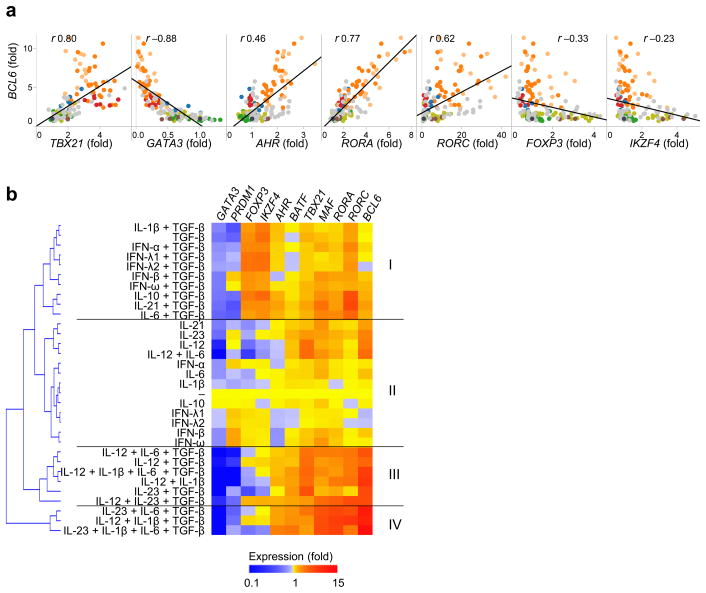
The observations that the IL-23+IL-1β+IL-6+TGF-β cytokine combination, which is commonly used for the generation of human Th17 cells in vitro24, was one of the most efficient combinations tested at inducing BCL6 expression in naïve TH cells (derived from both adult peripheral blood and cord blood samples) suggested that the initial differentiation process of human TH17 cells might be shared with TFH cells. In this context, the global abundance of BCL6 in the cultured adult blood naïve TH cells was found to positively correlate with the abundance of the TH17-associated transcription factors AHR, RORA and RORC (encoding RORγt) (Fig. 4A). In contrast, BCL6 abundance negatively correlated with the abundance of GATA3, FOXP3 and IKZF4 (encoding Eos). Largely similar results were obtained in experiments with cord-blood naïve TH cells, except TBX21 (encoding T-bet) which did not show any correlation with BCL6 (Supplementary Fig. 4). Thus, the conditions promoting the TFH differentiation also increased the expression of TH17-associated transcription factors, but decreased the expression of TH2- or Treg cell-associated transcription factors.
Fig. 4. TGF–β+IL-12 and TGF–β+IL-23 induce both TFH and TH17 transcriptional signatures.
(a) Correlation between BCL6 and the indicated transcription factors expressed by human adult blood naïve TH cells cultured for 3 days with different cytokines as in Fig. 2a. Pearson R values are indicated. P values were all <0.0001, except for IKZF4 (P=0.0003). (b) An unsupervised clustering of the culture conditions according to the expression of transcription factors by adult naïve TH cells cultured with various cytokines for 3 days as shown in Fig. 2a. For the analysis, the data were first normalized to the values in the culture with no cytokines in each experiment, and the mean values from 11 sets of experiments were determined.
An unsupervised clustering of the culture conditions according to the transcription factors expressed by adult naïve TH cells cultured for 3 days with various cytokines (indicated in Fig. 2a) revealed 4 clusters (hereafter called cluster I, II, III and IV; Fig. 4b). The pattern of the transcription factor expression in Clusters III and IV was largely similar, and represented a TFH transcriptional signature (such as upregulation of BCL6, BATF and MAF, and downregulation of PRDM1). Clusters III and IV also represented a TH17 transcriptional signature (characterized by the upregulation of AHR, RORA and RORC, while GATA3 expression was substantially diminished). The culture conditions that induced the transcriptional signatures characteristic to Clusters III and IV included TGF-β+IL-12 and TGF-β+IL-23 with or without IL-1β and/or IL-6. A Treg transcriptional signature (upregulation of FOXP3 and IKZF4) was dominant in Cluster I and found in conditions including TGF–β alone and combinations of TGF–β with each of the following: IFNs, IL-1β, IL-6, IL-10 and IL-21. This signature was absent in Clusters III and IV. Cluster II did not show strong transcriptional signatures of either TFH, TH17 or Tregs, and was characteristic of stimulatory conditions that lacked TGF–β. These results suggest that the culture conditions that promoted TFH transcriptional signatures also promoted TH17 transcriptional signatures, but not TH2 or Treg transcriptional signatures.
Bcl-6+ developing TFH cells co-express RORγt
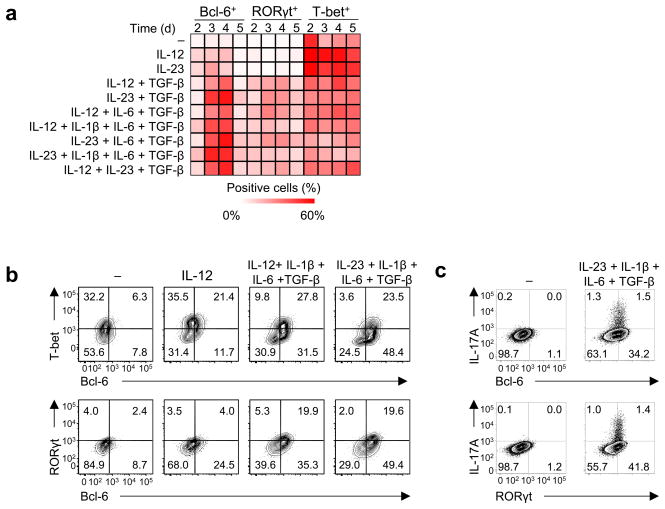
We next determined whether the developing TFH cells share properties with TH17 cells, or these two subsets emerge separately. Adult blood naïve TH cells stimulated with CD3-CD28 mAbs were cultured under no cytokines, IL-12, IL-23, TGF-β+IL-12 (with or without IL-1β and/or IL-6), and TGF-β+IL-23 (with or without IL-1β and/or IL-6, and thus including the TH17 condition IL-23+TGF–β+IL-6+IL-1β24), and analyzed for single cell expression of Bcl-6, T-bet, and RORγt by flow cytometry. Kinetics analysis showed that TGF–β+IL-12 and TGF–β+IL-23 increased the expression of Bcl-6 and RORγt, which peaked 2–3 days after the addition of cytokines (meaning at day 3–4 of culture; Fig. 5a,b). T-bet expression was decreased by TGF–β+IL-12 and TGF–β+IL-23 compared to IL-12 and IL-23 alone, respectively (Fig. 5b, Supplementary Fig. 5a).
Fig. 5. RORγt is expressed by CXCR5+ Bcl-6+ TH cells generated with TGF–β+IL-12 and TGF–β+IL-23.
(a) The expression of Bcl-6, RORγt, and T-bet by human adult blood naïve TH cells cultured with the indicated cytokines for 1–4 days (cytokines were added on day 1 of culture, so culture days 2–5) was analyzed by flow cytometry. Percentage of positive cells within the activated (FSChiSSChi) TH cells is indicated in a heatmap. A representative of 4 independent experiments. (b) The expression of Bcl-6, RORγt, and T-bet by CXCR5+ TH cells generated by culturing naïve TH cells with no cytokine, IL-12, IL-12+IL-1β+IL-6+TGF-β and IL-23+IL-1β+IL-6+TGF-β for 3 days as in (a). A representative of 4 independent experiments. (c) The expression of IL-17A, Bcl-6, and RORγt by activated (FSChiSSChi) cord blood naïve TH cells cultured for 3 days with no cytokines or IL-23+IL-1β+IL-6+TGF-β. A representative of 3 independent experiments.
We next determined the expression of Bcl-6, T-bet, and RORγt of CXCR5+ TH cells differentiated in various cytokine conditions. Whereas CXCR5+ TH cells differentiated with IL-12 or IL-23 alone expressed little Bcl-6, more than 60% of CXCR5+ TH cells differentiated with TGF–β+IL-12 and TGF–β+IL-23 expressed Bcl-6 (Fig. 5c, Supplementary Fig. 5b). Within the CXCR5+ TH cells differentiated with TGF–β+IL-12 and TGF–β+IL-23, more than 70% of RORγt+ cells co-expressed Bcl-6. The differentiation of CXCR5+Bcl-6+RORγt+ TH cells required IL-12 and IL-23, because TGF–β+IL-6 induced CXCR5+ TH cells expressing RORγt, but not Bcl-6 (Supplementary Fig. 5c). siRNA-mediated knock down of RORγt in TH cells cultured in the TH17 condition IL-23+TGF–β+IL-6+IL-1β increased the development of RORγt−Bcl-6+ cells, confirming that RORγt was not essential for expression of Bcl-6 (Supplementary Fig. 5d). Furthermore, IL-17A+ cells differentiated by culturing cord-blood naïve Th cells in the TH17 condition IL-23+TGF–β+IL-6+IL-1β expressed both RORγt and Bcl-6 (Fig. 5d), and approximately 40% of IL-17A+ TH cells differentiated in this condition co-expressed IL-21 (Supplementary Fig. 5e).
Collectively, these results show that TGF–β+IL-12 and TGF–β+IL-23 induced CXCR5+ TH cells co-expressing Bcl-6 and RORγt.
Both STAT3 and STAT4 cooperate with TGF–β in TFH differentiation
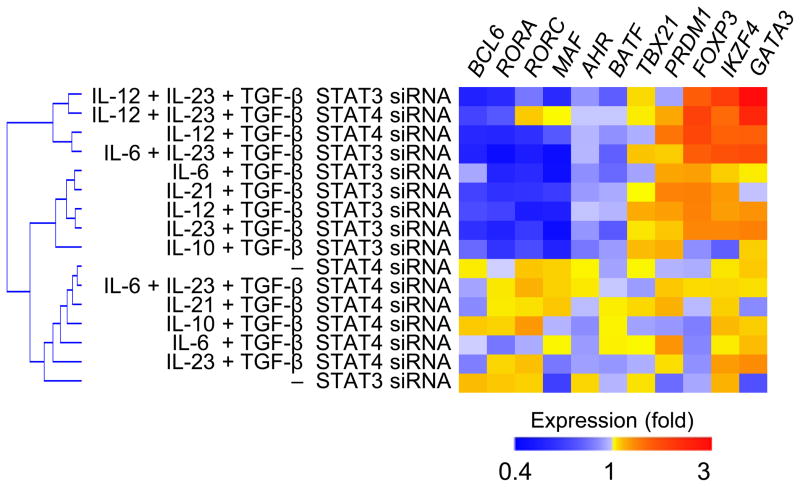
Whereas IL-12 and IL-23 activate both STAT4 and STAT3 in human TH cells, STAT4 in IL-12 signaling and STAT3 in IL-23 signaling deliver the major activation signals25. We next determined whether STAT4 and STAT3 play distinct or redundant roles in expression of TFH and TH17 transcriptional signatures in human adult blood naïve TH cells cultured with TGF–β+IL-12 and TGF–β+IL-23 (as shown in Cluster III and IV in Fig. 2b). Human adult blood naïve TH cells were first transfected with specific siRNA to downregulate the expression of STAT4 and STAT318, and cultured with TGF–β+IL-12 and TGF–β+IL-23. siRNA-transfected naïve TH cells were also cultured with combinations of TGF–β with each of STAT3-activating cytokines IL-6, IL-10 and IL-21. For the analysis, the abundance of each transcript analyzed in STAT3 and STAT4-siRNA transfected TH cells was normalized separately against data obtained from scrambled siRNA-transfected cells cultured in the same cytokine conditions. Knock down of STAT3 in TH cells cultured with combinations of TGF–β with each of IL-23, IL-21 and IL-6, and knock down of STAT4 in TH cells cultured with TGF–β+IL-12 resulted in a largely similar TF modulation pattern, in which the TFH transcriptional signature (characterized by high BCL6, BATF and MAF, and low PRDM1) as well as the TH17 transcriptional signature (high AHR, RORA, and RORC) were inhibited, while TH2 (GATA3) and Treg (FOXP3 and IKZF4) transcriptional signatures were enhanced. Of note, STAT3 knock down in TH cells cultured with IL-12+TGF-β also resulted in downregulation of TFH and TH17 signatures, and upregulation of TH2 and Treg signatures, supporting the importance of STAT3 in the TFH programing of IL-12-stimulated human TH cells26. Thus, in the presence of TGF–β signaling, STAT3 and STAT4 play redundant roles to drive human TH differentiation gene programs towards TFH and TH17 cells, and away from Treg and TH2 cells.
A fraction of tonsillar TFH cells express RORγt and T-bet
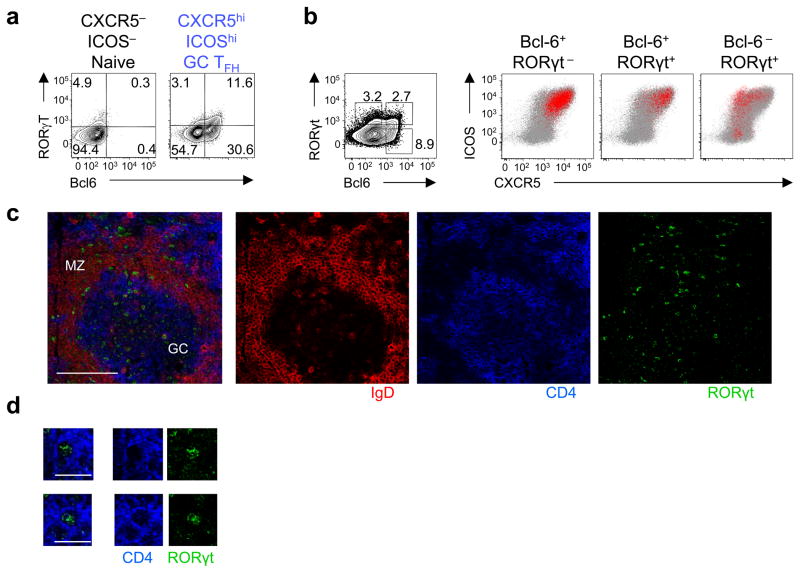
Our observations suggest the existence of human TFH cells that express RORγt. However, previous studies concluded that human tonsillar CXCR5hiICOShi GC TFH cells do not express RORγt, because RORC mRNA expression in GC TFH cells was substantially lower than in TH17 cells27. Analysis of mRNA transcripts showed that human ex vivo tonsillar CXCR5hiICOShi GC TFH cells expressed modest, yet more abundant RORC mRNA than CXCR5negICOSneg naïve TH cells (Supplementary Fig. 6a), suggesting that a subset of CXCR5hiICOShi GC TFH cells might express RORγt. To address this, we analyzed the expression of Bcl-6 and RORγt in single human tonsillar TFH cells by flow cytometry and confocal microscopy. Flow cytometry analysis showed that approximately 25% of CXCR5hiICOShi Bcl-6+ GC TFH cells expressed RORγt (Fig. 7a). As expected, backgating of Bcl-6+ cells showed that they were largely confined to the CXCR5hiICOShi GC TFH subset among tonsillar TH cells (Fig. 7b). Analysis by confocal microscopy confirmed that Bcl-6+ TH cells were largely limited within GC (Supplementary Fig. 6b,c). Importantly, backgating of Bcl-6+RORγt+ TH cells revealed that these cells were also confined to CXCR5hiICOShi GC TFH cells (Fig. 7b). In contrast, RORγt+ cells lacking the expression of Bcl-6 were largely confined to CXCR5loICOShi TH subset (Fig. 7b), which are inefficient B cell helpers7. Furthermore, by confocal microscopy, TH cells expressing RORγt were found both outside and within the GCs (Fig. 7c,d).
Fig. 7. A fraction of tonsillar TFH cells co-express RORγt.
(a) Bcl-6 and RORγt expression by ex vivo tonsillar GC TFH cells and naïve TH cells was analyzed by flow cytometry. A representative of 4 independent experiments. (b) Expression of CXCR5 and ICOS by ex vivo tonsillar Bcl-6+RORγt−,Bcl-6+RORγt+ and Bcl-6−RORγt+ TH cells (defined as shown in the left panel). The red dots indicate the expression of CXCR5 and ICOS by the cells with the indicated transcription factor expression pattern. The global tonsillar TH cells are indicated by gray dots. A representative out of 5 independent experiments. (c) Human tonsil sections were stained for IgD (indicated by red), CD4 (indicated by blue), and RORγt (indicated by green), and analyzed by confocal microscopy. GC: germinal center. MZ: mantle zone. Representative RORγt+ TH cells in GCs are shown in panel (d).
We also analyzed whether human tonsillar GC TFH cells contained subsets of cells expressing T-bet or GATA-3. GC TFH cells expressed more abundant TBX21 mRNA than naïve TH cells (Supplementary Fig. 6a). In contrast, GATA3 mRNA expression was similar between GC TFH cells and naïve TH cells. Flow cytometry analysis revealed that a fraction of Bcl-6+ GC TFH cells co-expressed T-bet, but not GATA3 (Supplementary Fig. 6d). Thus, human tonsillar Bcl-6+ GC TFH cells contain cells co-expressing RORγt and T-bet, but not GATA3. Given that the microenvironment of inflamed tonsils is enriched in TGF-β and inflammatory cytokines, including IL-12 (Fig. 3), these observations supports the observation that the combination of TGF–β and STAT3- and STAT4-activating cytokines induces naïve TH cell differentiation programs towards TFH and TH17 lineage, but away from TH2 cells.
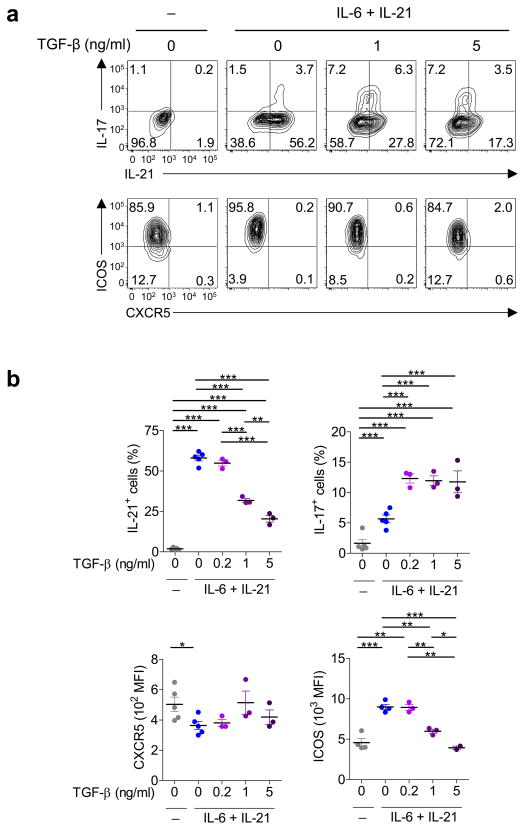
TGF-β suppresses ICOS and IL-21 expression by mouse TH cells
The significance of TGF-β for the generation of TFH cells in vivo can be potentially tested in mouse models. However, a previous study in mouse models showed that blocking TGF-β did not decrease the expression of CXCR5 on activated TH cells or the generation of GC B cells28. Furthermore, multiple studies with mouse TH cells show that TGF–β inhibits the expression of Bcl-614, 28 (partly via promoting the expression of miR-10a that suppresses Bcl-6 expression29) and IL-2130. These observations suggest that TGF-β might inhibit the generation and/or the function of TFH cells in mice. To directly address whether the effect of TGF-β on TFH development is different between humans and mice, mouse naïve TH cells were first primed overnight with CD3-CD28 mAbs (as with human naïve TH cells), and then cultured with combinations of titrated amounts of TGF-β with IL-6+IL-21 (commonly used to generate mouse TFH-like cells in vitro31), IL-12+ IL-6+IL-21, and IL-23+ IL-6+IL-21. Consistent with previous studies30, while promoting IL-17A expression, TGF-β dose-dependently inhibited IL-21 expression by mouse TH cells cultured under any tested cytokine combinations (Fig. 8a,b; Supplementary Fig. 7). Furthermore, TGF-β also dose-dependently suppressed the expression of ICOS. CXCR5 expression by TH cells was minimal under any culture conditions (consistent with previous studies32), and TGF-β did not substantially affect its expression. These results show that the effect of TGF-β on the expression of TFH molecules differs between mice and humans, and that the positive regulation by TGF-β for the generation of TFH cells appears to be limited to humans.
Fig. 8. TGF–β inhibits IL-21 and ICOS expression by mouse TH cells.
(a) Expression of CXCR5, ICOS, and IL-21 by activated (FSChiSSChi) mouse naïve TH cells following 4 d stimulation with CD3-CD28 mAbs in the presence of titrated amounts of TGF-β plus IL-6+IL-21 during the last 3 days. A representative of 4 independent experiments. Statistical analysis of results from 5 sets of experiments is shown in panel (b). Two-sided t-test: p-values * <0.05, ** <0.01, and *** <0.001.
Discussion
Previous studies suggested that IL-12 is important for TFH cell generation in humans17, 18. Individuals (particularly children) deficient for the IL-12 receptor β1 (IL-12Rβ1) chain have reduced TFH and GC responses, providing in vivo evidence that signals via IL-12 receptor are essential for the generation of TFH cells in humans17. However, IL-12 stimulation by itself seems insufficient, because Bcl-6 expression in IL-12-stimulated human naïve TH cells is much lower than in tonsillar TFH cells17. Furthermore, whereas IL-12Rβ1 is shared by receptors for IL-12 and IL-23, whether IL-23 also contributes to the generation of human TFH cells was unknown. Our present study demonstrates that both IL-12 and IL-23 contribute to human TFH generation, and TGF–β acts as a critical co-factor of IL-12 and IL-23 for the TFH cell differentiation in humans. The TFH transcriptional signature (that is upregulation of Bcl-6, c-Maf and BATF; downregulation of Blimp-1) was strongly induced when TGF–β was combined with IL-12 and IL-23. IL-23, but not by IL-12, promoted the expression of Blimp-1. However, TGF–β strongly diminished the capacity of IL-23 to induce Blimp-1 expression. In inflammatory lymphoid organs, TGF–β was abundantly expressed in the T cell zone, where naïve TH cells interact with activated DCs. The presence of TH cells co-expressing p-Smad2 and p-STAT4 adjacent to GCs provided evidence that developing TFH cells receive signals that activate both pathways. We propose that an integration of these signals promote human naïve TH cells to undergo TFH differentiation gene programs along with the upregulation of multiple TFH molecules. Such initial differentiation process likely promotes their migration towards follicles and the interactions with B cells to differentiate into mature TFH cells9.
Our study shows that the cooperation of TGF–β and STAT3-STAT4 cytokines occurs in at least two aspects of human TFH differentiation. First, while lacking this capacity by itself, TGF–β enhances the function of STAT3-STAT4 to induce human naïve TH cells to express TFH molecules, including CXCR5, ICOS, IL-21, Bcl-6, BATF-Jun and c-Maf. Importantly, this stimulatory effect of TGF-β for TFH development seems limited to human TH cells, and not shared with mouse TH cells. Enhanced Bcl-6 expression in human naïve TH cells cultured with TGF–β+IL-12 and TGF–β+IL-23 together with IL-1β and IL-6 was at least partly mediated by increased BATF-Jun expression. Second, TGF–β suppresses, and STAT3-STAT4 further downregulates (in the presence of TGF–β), Blimp-1 expression in human naive TH cells. Because TCR stimulation is sufficient to induce human TH cells to express Blimp-1, but not Bcl-633, inhibition of Blimp-1 expression likely represents an important mechanism to shift the Bcl-6 versus Blimp-1 balance towards Bcl-6 dominance, and thus to promote the TFH differentiation.
In contrast to TGF-β, type I and III IFNs were found to inhibit TFH cell differentiation by inhibiting the establishment of a TFH transcriptional signature, as well as the expression of TFH molecules. Type I IFN signals were recently shown to inhibit the development of TFH cells in mice in vivo34, suggesting that this mechanism is shared between mice and humans. These observations would suggest that exaggerated TFH response in type I IFN-mediated human autoimmune diseases, such as systematic lupus erythematosus (SLE)35, is not caused by the direct effect of type I IFNs on TH cells, but by an indirect effect on other immune cells, such as DCs. This hypothesis is further supported by mouse studies demonstrating that the adjuvant effect of type I IFNs for antibody response is solely mediated by IFN-responsive DCs36, 37.
The combination of TGF–β and STAT3-STAT4 induced in human naïve TH cells a differentiation program directed towards the TFH and TH17 lineages, and away from the TH2 and Treg lineages. In this context, STAT3 and STAT4 contributed in a largely similar fashion, and complemented each other. IL-23+TGF–β+IL-6+IL-1β, the most common cytokine cocktail for the generation of human TH17 cells in vitro, was one of the most efficient cytokine combinations to induce Bcl-6 expression. TH17 cells expressing RORγt and IL-17A generated with this cytokine cocktail co-expressed TFH molecules such as CXCR5, ICOS, IL-21, and Bcl-6, indicating that developing TH17 cells can share properties with TFH cells. This does not appear to be limited to developing cells, as human tonsillar Bcl-6+ GC TFH cells contained a subset that co-expressed RORγt. In addition, blood memory TFH cells also contain a subset sharing properties with TH17 cells38. This blood memory TFH subset can be defined by the co-expression of CXCR5 and CCR6, and is more efficient at providing help to B cells than CCR6− blood TFH subset38, 39. Importantly, the frequency of CCR6+ blood TFH subset was found to be increased in patients with various autoimmune diseases including juvenile dermatomyositis38, Sjogren’s syndrome40 and multiple sclerosis41. These observations suggest that TFH subset sharing properties with TH17 cells represents more efficient B cell helpers than other subsets, and an increase of these cells is associated with the development of human autoimmunity. Furthermore, both TFH and TH17 cells co-emerge in many human autoimmune diseases, including SLE42, rheumatoid arthritis8, Sjogren’s syndrome43, multiple sclerosis44 and juvenile dermatomyositis 38. The shared developmental mechanism for TFH and Th17 cells provides a strong mechanistic insight for their co-emergence in these diseases. Given that TGF–β is also abundantly expressed in inflammatory sites in human autoimmune diseases23, 45 where tertiary lymphoid organs are often formed, it is presumable that TGF–β-rich inflammatory sites also contribute to the generation of TFH and TH17 cells.
In conclusion, our study demonstrates that TGF–β promotes TFH response in inflammatory environment by collaborating with STAT3 and STAT4-activating cytokines. Our conclusion will provide insights in the pathogenesis of human autoimmune diseases. Establishing the global transcriptional network promoting the human TFH cell differentiation program and the expression of multiple TFH molecules, in a comprehensive approach as shown in mouse TH17 cells46, might identify novel therapeutic target molecules for their treatment.
Online Methods
Isolation of naïve TH cells
For human cells, the study was approved by the Institutional Review Board of Baylor Research Institute. PBMCs were purified from apheresis blood samples obtained from adult volunteers and cord blood samples obtained at Baylor Health Care System. Informed consent was obtained from all the donors. Naïve TH cells were first enriched by negative selection with purified CD8 (HIT8a), CD11b (LM1/2), CD11c (B-ly6), CD14 (M5E2), CD15 (W6D3), CD16 (3G8), CD19 (J4.119), CD45RO (UCHL1), CD56 (C218) and HLA-DR (B8.12.2) mAbs, and Dynabeads Pan Mouse IgG (Dynal). The mouse study was approved by Institutional Animal Care and Usage Committee of Baylor Research Institute. For isolation of mouse naïve TH cells, splenocytes were isolated by mechanical disruption of spleen harvested from C57BL/6 female mice (6 to 10 weeks old). Mouse TH cells were then enriched by negative selection with the TH cell isolation kit II (Miltenyi). Naive TH cells were further purified by sorting with FACSAria (BD Biosciences) as CD4+ CCR7+ CD45RA+ CD8− CD56− HLA-DR− for human and as CD4+ CD62Lhigh CD44low B220− CD8− CD11b− CD11c− CD25− for mouse. Cell purity was >99%.
Tonsillar cells
Tonsil samples were obtained from healthy subjects undergoing tonsillectomies, and single cells were collected by mechanical disruption. B cells were removed with CD19 MACS Microbeads (Miltenyi Biotech). The tonsillar TH subsets were sorted from CD8−CD19−CD56−CD4+ cells according to their different level of CXCR5 and ICOS expression7. Tonsillar GC TFH cells express CXCR5 and ICOS at high levels (CXCR5hiICOShi). TH cells expressing CXCR5 and ICOS at low levels (CXCR5loICOSlo) show phenotypic and functional similarities with GC TFH cells, yet localize outside GCs (Pre-TFH cells)7. Tonsillar CXCR5negICOSneg Th cells are largely constituted by naïve TH cells, while CXCR5loICOShi TH cells are enriched with IL-17A-secreting cells 7. Cell purity was >98%.
Stimulation of naïve TH cells with CD3-CD28 mAbs
After overnight stimulation of naïve TH cells with anti-human or mouse CD3-CD28 Dynabeads (Invitrogen) in RPMI complete medium supplemented with 10% FCS, cells were transferred to flat-bottomed 96 well plates coated with CD3 mAb (5 μg/ml, clone OKT3 for human and 145-2C11 for mouse) and supplemented with soluble CD28 mAb (1 μg/ml, clone CD28.2 for human and 37.51 for mouse) and the following human (h) or mouse (m) recombinant cytokines: hIL-1β (10 ng/ml), hIL-4 (10 ng/ml), hIL-6 (25 ng/ml), hIL-10 (10 ng/ml), hIL-12 (1 ng/ml), hIL-21 (25 ng/ml), hIL-23 (25 ng/ml), hTGF-β (5 ng/ml unless indicated), hIFN-α (50 IU/ml), hIFN-β(10 ng/ml), hIFN-λ1 (10 ng/ml), hIFN-λ2 (10 ng/ml), hIFN-ω (10 ng/ml), mIL-12p70 (10ng/ml), mIL-23 (20ng/ml), mIL-6 (20ng/ml) and mIL-21 (50ng/ml). Cell viability was determined by trypan blue staining (Vi-Cell Cell Viability Analyzer. Beckman Counter).
Stimulation of naïve TH cells with DCs
Monocyte-derived DCs were generated as described previously18. Briefly, Monocytes were isolated from adult PBMCs by negative selection using Monocyte Isolation Kit II (Miltenyi Biotec). DCs were generated by culturing monocytes with 50 ng/ml IL-4 (R&D) and 100 ng/ml GM-CSF (Leukine) in RPMI complete medium supplemented with 10% FCS in 6 well plates (2 × 106 cells/3 ml/well). Cytokines were added every 2 days. At day 6, DCs were stimulated with irradiated CD40L-transfected L-cells or heat killed Escherichia coli. After 5 hours stimulation, DCs were harvested and carefully washed. Allogeneic naïve TH cells (4 × 104 cells/well) were cultured for 4 d with activated DCs (8 × 103 cells/well) loaded with SEB (0.2 μg/ml) in the presence of titrated amounts of TGF-β in 96 well round bottom plates. The phenotype and the cytokine expression profiles were analyzed by flow cytometry.
Flow cytometry
Cultured human TH cells were stained with CXCR5 AF647 (RF8B2) and ICOS biotin (ISA-3) mAbs + Streptavidin PerCP. For intranuclear staining, cells were fixed with Cytofix buffer (BD) for 10 min at 37 °C and incubated with Bcl6 (K112-91), RORγt (AFKJS-9) and T-bet (O4-46) mAbs or isotype control in Perm/wash buffer I (BD) for 30 min at room temperature. For the analysis of intracytoplasmic IL-21, cultured TH cells or tonsillar TFH cells were stimulated with PMA (25 ng/ml) and ionomycin (1 μg/ml) for 6 h in the presence of GolgiStop (BD Biosciences) and Brefeldin (eBioscience) for the last 4 h. Human TH cells were stained with CXCR5 mAb and then for intracytoplasmic cytokines with IL-21 (3A3-N2) and IL-17A (BL168). Cultured mouse naïve TH cells were stained with CXCR5 (L138D7) and ICOS (C398.4A). IL-21 expression in mouse TH cells was detected as previously reported30. Briefly, permeabilized T cells were incubated sequentially with IL-21R/Fc, PE conjugated F(ab′)2 goat anti-human IgG antibody and then with IL-17A (TC11-18H10.1) antibodies. All the samples were incubated with LIVE/DEAD fixable Aqua to exclude dead cells from the analysis. Cells were acquired on a BD FACS Canto II or a BD LSRII. Expression of each molecule was assessed with FlowJo software (TreeStar) in each tonsillar TFH subsets or for cultured cells in activated (FSChighSSChigh) TH cells.
Nanostring
Freshly isolated or cultured TH cells were lysed in RLT buffer. Total RNA was purified using RNeasy Micro Kit (Qiagen). The NanoString reactions were done according to manufacturer’s instructions, and the data were normalized to multiple housekeeping genes included in the codeset. The analysis of ex vivo tonsillar TH subsets included probes for Bcl-6 cofactors CTBP1 (encoding CtBP), BCL6B (encoding BAZF), MTA3, NCOR2 (encoding SMRT), BCOR, ZBTB17 (encoding MIZ1), RUNX1T1 (encoding ETO), and ZBTB16 (encoding PLZF).
siRNA transfection
After overnight stimulation of naïve TH cells with CD3-CD28 Dynabeads, cells were transfected with siRNA using the Human T cell Nucleofector Kit and Nucleofector II device (Amaxa) according to manufacturer’s instructions. siRNA to target STAT3 (s743), STAT4 (s13531) and silencer select negative control #1 siRNA (Ambion) were used at 5 μM (0.5 nmol/5 × 106 cells/transfection)18. Six hours post-transfection, the cells were transferred to flat-bottomed 96 well plates coated with CD3 mAb in presence of soluble CD28 mAb. The indicated cytokines were added to the culture at 24 h post-transfection.
Immunoblotting
Whole cell extract were prepared using RIPA buffer. Equal amounts of total protein were electrophoresed on 4%–12% Bis-Tris gels (Invitrogen), transferred to PVDF membrane, and blotted with the following antibodies: anti-Bcl6 (clone D-8, Santa-Cruz), anti-Blimp1 (clone 6D3, Santa-Cruz) or Peroxidase-conjugated anti-GAPDH (clone GAPDH-71.1, Sigma). Peroxidase-conjugated Goat anti-mouse or Goat anti-rat antibodies (Jackson ImmunoResearch) were used for detection and specific bands were visualized with ChemiDoc™ MP System. Band intensity was quantified and normalized to the GAPDH loading controls.
Chromatin Immunoprecipitation
After 3 days culture of naïve TH cells with IL-23+IL-1β+IL-6+TGF-β, the cells were crosslinked with formaldehyde treatment and the chromatin was fragmented to 200 to 500 bp by sonication. Each ChIP experiment was performed on chromatin from 2 × 107 cells. The chromatin fraction was incubated overnight with 5 μg of anti-BATF (Brookwood Biomedical) or Rabbit IgG (Invitrogen) linked to protein A Dynabeads (Invitrogen). Immune complexes were washed and protein-DNA cross-links were reversed for 4 hours at 65°C. The samples were purified using ChIP DNA purification kit (Active Motif), resuspended in 50μl of Tris-EDTA and subsequently used for SYBR Green real-time PCR amplification. The primers were designed to cover two BATF binding sites predicted with MatInspector Professional (Genomatix) within the promoter region of BCL6 gene. ChIP-qPCR Human IGX1A Negative Control (Qiagen) was used for a negative control. The percentage of total genomic input was determined.
T-B coculture assay
B cells were enriched from apheresis PBMCs by positive selection using CD19 MicroBeads and LS column (Miltenyi Biotec). Memory B cells were sorted with FACSAria as CD27+ CD3− CD11c− CD14− cells, after staining with CD27 PE (L128), CD3 APC (SK7), CD11c APC (S-HCL-3), and CD14 APC (TüK4) mAbs. Cell purity was >98%.
Activated FSChigh TH cells after 3 d culture with the indicated cytokines were sorted and co-cultured with autologous memory B cells (2.5 ×103 T cells for 40 × 103 memory B cells per well) in 96-well U-bottom plates in Yssel medium/10% FBS in the presence of endotoxin-reduced SEB (0.25 ng/ml; Toxin technology, Inc.). The amounts of IgG produced in the cultures were quantified by ELISA at day 14.
Immunofluorescence
6 μm-frozen sections from tonsils fixed with cold acetone were stained with TGF-β (TB21, AbD Serotec), CD31 (Rabbit polyclonal, Abcam) and CD11c (S-HLC-3, BD) antibodies followed by anti–mouse IgG1 conjugated to A568, anti-Rabbit A488 and anti-mouse IgG2b A647, respectively. Finally, sections were counterstained for 2 min with 3 μM of the nuclear stain DAPI. Slides were mounted with Fluoromount G (Southern Biotec) and observed under Nikon Ti-E Inverted microscope with NIS Elements software and Coolsnap HQ2 camera using Planapo 4x/0.2 and Planapo 20/0.75 objectives.
Confocal microscopy
6 μm cryostat sections were fixed for 10 min in cold acetone and permeabilized with 0.1 % Triton X100 for 10 min. After treatment with Fc Receptor Blocker and Background Buster (Innovex Biosciences) for 30 min each, slides were incubated overnight with primary antibodies at 4°C. For the pSmad2, CD3, CD11c staining, fixed sections were stained with Rabbit polyclonal anti-pSmad2 (Cell Signaling), mouse IgG2a anti-CD3 (HIT3a, BD Bioscience) and mouse IgG2b anti-CD11c (S-HLC-3, BD) antibodies followed by anti-Rabbit A488, anti–mouse IgG2a A568, and anti–mouse IgG2b A647, respectively. For the pSmad2, pSTAT4, CD3 staining, the primary staining was done with rabbit polyclonal anti-pSmad2, mouse IgG1 anti-pSTAT4 (E-2, Santa Cruz) and mouse IgG2a anti-CD3 (HIT3a, BD) followed by a secondary staining with goat anti-rabbit A488, anti-mouse IgG1 A568 and anti-mouse IgG2a A647. For the RORγt, IgD, CD4 staining, the tissue section was first incubated with polyclonal rabbit anti RORγt (Abcam), mouse IgG2a anti-IgD (IA6-2, BD) and mouse IgG1 anti-CD4 (RPA-T4, BD) followed by incubation with anti-rabbit A568, anti-mouse IgG2a A488 and anti-mouse IgG1 A647. For the Bcl-6, IgD, CD4 staining, the tissue section was first incubated with mouse IgG1 anti-Bcl-6 (PG-B6P, Abcam), rabbit anti-IgD (Dako) and mouse IgG2b anti-CD4 (OKT4, Biolegend) then secondarily stained with anti-mouse IgG1 A568, anti-Rabbit A488 and anti-mouse IgG2b A647. Finally, sections were counterstained for 2 min with 3 μM of the nuclear stain DAPI, and mounted with prolong gold antifade reagent (Invitrogen). The slide images were observed under a Leica SP5 confocal microscopy with 203/0.7.403/1.25, and 633/1.4 Planapo Objectives.
Supplementary Material
Fig. 6. Both STAT3 and STAT4 cooperate with TGF–β in the induction of TFH and TH17 transcriptional signatures.
The expression of transcription factors by human naïve TH cells transfected with STAT3 or STAT4 siRNA followed by 2 d culture with combinations of TGF–β with each of the following: IL-12, IL-23, IL-6, IL-12+IL-23, IL-6+IL-23, IL-10 and IL-21. For the analysis, the transcript values in STAT siRNA-transfected TH cells were first normalized to those in scrambled siRNA-transfected TH cells cultured in the same cytokine conditions in each experiment, and the mean values from 3 experiments were determined.
Acknowledgments
We thank E. Kowalski, S. Coquery, N. Loof and K. Kayembe for cell sorting. We thank K. Palucka, V. Pascual, and Y-J Liu for discussions. We thank S. Clayton for confocal imaging. We thank C. Quinn for the development of the heatmap generation software. We thank Y. Kanno for the advice for ChIP. We thank Y. C. Lin for the discussion of ChIP data analysis. We thank M. Kathania for technical help for mouse experiments. This study was supported by research funding from NIH grants U19-AI057234, U19-AI082715, U19-AI089987, Alliance for Lupus Research, and Baylor Health Care System.
Footnotes
Author contribution:
N.S. conceived and executed the experiments, and analyzed the data. Y.L and L.B. performed the tonsillar tissue staining. S.E.B. contributed to the preparation of tonsillar Th cells. I.M. performed NanoString assay. K.V. contributed to the experiments with mouse cells. H.U. oversaw and conceived the entire project, and analyzed the data. N.S., J.B., and H.U. wrote the manuscript.
References
- 1.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 2.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 3.Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, et al. ICOS Deficiency Is Associated with a Severe Reduction of CXCR5+CD4 Germinal Center Th Cells. J Immunol. 2006;177(7):4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 4.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011;34(6):932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H, Li X, Liu D, Li J, Zhang X, Chen X, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496(7446):523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 6.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29(1):127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci U S A. 2011;108(33):E488–497. doi: 10.1073/pnas.1100898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8(6):337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35(5):671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Baumjohann D, Okada T, Ansel KM. Cutting Edge: Distinct Waves of BCL6 Expression during T Follicular Helper Cell Development. J Immunol. 2011;187(5):2089–2092. doi: 10.4049/jimmunol.1101393. [DOI] [PubMed] [Google Scholar]
- 11.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30(3):324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, et al. Early Th1 Cell Differentiation Is Marked by a Tfh Cell-like Transition. Immunity. 2011;35(6):919–931. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31(3):457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Sweet RA, Lee SK, Vinuesa CG. Developing connections amongst key cytokines and dysregulated germinal centers in autoimmunity. Curr Opin Immunol. 2012;24(6):658–664. doi: 10.1016/j.coi.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt N, Bustamante J, Bourdery L, Bentebibel SE, Boisson-Dupuis S, Hamlin F, et al. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 2013;121(17):3375–3385. doi: 10.1182/blood-2012-08-448902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31(1):158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87(8):590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 20.Schraml BU, Hildner K, Ise W, Lee W-L, Smith WAE, Solomon B, et al. The AP-1 transcription factor Batf controls TH17 differentiation. Nature. 2009;460(7253):405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat Immunol. 2011;12(6):536–543. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy TL, Tussiwand R, Murphy KM. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat Rev Immunol. 2013;13(7):499–509. doi: 10.1038/nri3470. [DOI] [PubMed] [Google Scholar]
- 23.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134(3):392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9(6):641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19(5):641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 26.Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119(17):3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173(1):68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 28.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29(1):138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciume G, Muljo SA, et al. TGF-beta and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol. 2012;13(6):587–595. doi: 10.1038/ni.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205(6):1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Kristina T, Kanno Y, Cannons Jennifer L, Handon R, Bible P, Elkahloun Abdel G, et al. Functional and Epigenetic Studies Reveal Multistep Differentiation and Plasticity of In Vitro-Generated and In Vivo-Derived Follicular T Helper Cells. Immunity. 2011;35(4):622–632. doi: 10.1016/j.immuni.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eto D, Lao C, Ditoro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 Are Critical for Different Aspects of B Cell Immunity and Redundantly Induce Optimal Follicular Helper CD4 T Cell (Tfh) Differentiation. PLoS One. 2011;6(3):e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallies A, Hawkins ED, Belz GT, Metcalf D, Hommel M, Corcoran LM, et al. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7(5):466–474. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- 34.Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription Factor STAT3 and Type I Interferons Are Corepressive Insulators for Differentiation of Follicular Helper and T Helper 1 Cells. Immunity. 2014;40(3):367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25(3):383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14(4):461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 37.Cucak H, Yrlid U, Reizis B, Kalinke U, Johansson-Lindbom B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity. 2009;31(3):491–501. doi: 10.1016/j.immuni.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boswell KL, Paris R, Boritz E, Ambrozak D, Yamamoto T, Darko S, et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 2014;10(1):e1003853. doi: 10.1371/journal.ppat.1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li XY, Wu ZB, Ding J, Zheng ZH, Li XY, Chen LN, et al. Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjogren’s syndrome. Biochem Biophys Res Commun. 2012;422(2):238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- 41.Romme Christensen J, Bornsen L, Ratzer R, Piehl F, Khademi M, Olsson T, et al. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One. 2013;8(3):e57820. doi: 10.1371/journal.pone.0057820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Coz C, Joublin A, Pasquali JL, Korganow AS, Dumortier H, Monneaux F. Circulating TFH Subset Distribution Is Strongly Affected in Lupus Patients with an Active Disease. PLoS One. 2013;8(9):e75319. doi: 10.1371/journal.pone.0075319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh N, Cohen PL. The T cell in Sjogren’s syndrome: force majeure, not spectateur. J Autoimmun. 2012;39(3):229–233. doi: 10.1016/j.jaut.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122(4):1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, et al. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165(6):3423–3429. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- 46.Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151(2):289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.