Abstract
Experimentally naïve outbred rats display varying rates of locomotor reactivity in response to the mild stress of a novel environment. Namely, some display high rates (HR) whereas some display low rates (LR) of locomotor reactivity. Previous reports from our laboratory show that HRs, but not LRs, develop locomotor sensitization to a low dose nicotine challenge and exhibit increased social anxiety-like behavior following chronic intermittent nicotine training. Moreover, the hippocampus, specifically hippocampal Y2 receptor (Y2R)-mediated neuropeptide Y signaling is implicated in these nicotine-induced behavioral effects observed in HRs. The present study examines the structural substrates of the expression of locomotor sensitization to a low dose nicotine challenge and associated social anxiety-like behavior following chronic intermittent nicotine exposure during adolescence in the LRHR hippocampi. Our data showed that the expression of locomotor sensitization to the low dose nicotine challenge and the increase in social anxiety-like behavior were accompanied by an increase in mossy fiber terminal field size, as well as an increase in spinophilin mRNA levels in the hippocampus in nicotine pre-trained HRs compared to saline pre-trained controls. Furthermore, a novel, selective Y2R antagonist administered systemically during 1 wk of abstinence reversed the behavioral, molecular and neuromorphological effects observed in nicotine-exposed HRs. These results suggest that nicotine-induced neuroplasticity within the hippocampus may regulate abstinence-related negative affect in HRs, and implicate hippocampal Y2R in vulnerability to the behavioral and neuroplastic effects of nicotine in the novelty-seeking phenotype.
Keywords: Behavioral sensitization, social anxiety, mossy fiber, nicotine abstinence, hippocampus, neuroplasticity, Y2 receptor
1. Introduction
Individual differences in the degree to which humans voluntarily participate in activities associated with personal risk (Zuckerman, 1984) positively correlate with drug addiction and other psychopathologies (Zuckerman & Neeb, 1979). The novelty-seeking phenotype (Piazza et al., 1989; Hooks et al., 1991) was originally introduced as an animal model that overlaps with the human sensation-seeking trait (Zuckerman, 1984), and is identified in the laboratory rat by assessing locomotor reactivity to the mild stress of a novel environment. Namely, some rats display high locomotor reactivity in novel environments and are identified as high responders (HRs), whereas some display low locomotor behavior in novel environments and are identified as low responders (LRs). We have previously shown that a behaviorally-sensitizing nicotine regimen results in increased suprapyramidal mossy fiber (SP-MF) terminal field size in the HR but not LR rats (Bhatti et al., 2007). Moreover, temporarily inactivating the hippocampal hilus region results in augmented locomotor response to challenge nicotine in HRs, implicating MF plasticity in the behaviorally-sensitizing effects of nicotine (Bhatti et al., 2007).
The hippocampal MF comprise the axons of the dentate gyrus granule neurons that innervate the CA3 field (Gaarskjaer, 1986). Morpho-behavioral correlations in genetically-altered mice implicate hippocampal MF with exploratory behavior (Roullet & Lasalle, 1990; Ivanco & Greenough, 2002; Mineur et al., 2002). Moreover, data from inbred mice correlate heritable variance in MF size with different emotional behaviors, such as fear conditioning and anxiety-like behavior (Prior et al., 1997). Likewise, studies on outbred rats show a positive correlation between MF sprouting and anxiety-like behavior in the light-dark box test (de Oliveira et al., 2008), implicating MF plasticity in regulation of anxiety.
We have recently shown an upregulation in Y2 receptor (Y2R) and a downregulation in neuropeptide Y (NPY) mRNA levels in the hippocampal CA3 field in HRs following a behaviorally-sensitizing nicotine regimen (Aydin et al., 2011a, b). Y2R–specific mechanism of NPY binding is implicated in the MF system following prolonged seizures, where NPY reduces glutamate release by activating presynaptic Y2R in MF pathway (Nadler et al., 2007), suggesting a regulatory role for NPY via Y2R in the hippocampal MF system. Incidentally, NPY in the hippocampus has been reported to have anxiolytic properties (Thorsell et al., 2000; Lin et al., 2010; Cohen et al., 2011). Indeed, reversal of the deficit in hippocampal NPY mRNA levels in nicotine pre-trained HRs by systemic injections of a Y2R antagonist, JNJ-31020028 during 1 wk of abstinence accompanies reversal of the increased anxiety-like behavior in these animals (Aydin et al., 2011b), confirming the regulatory role of hippocampal NPY in anxiety-like responses. On the other hand, whether Y2R-mediated modulation of the MF system alters anxiety-like behavior remains to be answered.
The present study investigated the efficacy of a systemically administered Y2R antagonist on reversing nicotine-induced MF sprouting and the increase in social anxiety-like behavior observed in HRs following a behaviorally-sensitizing nicotine regimen. We also determined whether nicotine- induced MF sprouting observed in HRs was reflected on the CA3 dendritic receptive fields by assessing mRNA levels of a postsynaptic marker, spinophilin that is preferentially located in dendritic spines (Allen et al., 1997), and whether Y2R antagonist treatment altered these nicotine-induced neuroplastic effects.
2. Materials and methods
2.1. Drugs
Nicotine hydrogen tartrate was obtained from a commercial supplier (Sigma), dissolved in 0.9% NaCl and the pH was adjusted to 7.4. The Y2R antagonist, JNJ-31020028, was generously donated by Janssen Research & Development, L.L.C, and dissolved in 20% 2-hydroxypropyl-beta-cyclodextrin. The doses for nicotine and JNJ-31020028 were chosen based on effective doses used in the literature in several reports by others (Miller et al., 2001; Suto et al., 2001; Shoblock et al., 2010), and by us (Bhatti et al., 2007, 2009; Aydin et al., 2011a, 2011b, 2012a).
2.2. Animal housing and the LRHR phenotype screening
Animals were treated in accordance with the National Institute of Health guidelines on laboratory animal use and care. All efforts were made to minimize animal suffering and to reduce the number of animals used. A grand total of 72 male Sprague-Dawley rats (Charles River, Wilmington, MA) arrived at weaning (postnatal day, PN 22), were housed 3 per cage in 43 × 21.5 × 25 cm3 Plexiglas cages and were kept on a 12 h light/dark cycle (lights on at 7:00 A.M.). Food and water were available ad libitum. Animals were allowed to habituate to the housing conditions and were handled daily for 2 days. On PN 25, animals were screened for locomotor reactivity to the mild stress of a novel environment for 1 hr using commercially available locomotion chambers (San Diego Instruments, San Diego, CA). Briefly, locomotor reactivity to novelty was tested in 43 × 43 × 24.5-cm3 (high) clear Plexiglas cages with stainless steel grid flooring. Activity was monitored by means of photocells (a total of X = 16 by Y = 16 photocells) 2.5 cm above the grid floor and equally spaced along the sides of the box. Horizontal locomotion was monitored by this lower bank of photocells. Each locomotor count recorded a minimum of 3-cm traversing of the cage. Additional photocells were located 11.5 cm above the grid floor and 9 cm above the lower bank of photocells. Rearing (i.e., locomotion in the Z plane) was monitored by this upper bank of photocells. At the end of the screening session, total locomotor activity (i.e., X, Y, and Z locomotion) was pooled and the rats were ranked as HRs (i.e., rats that exhibited locomotor scores in the highest third of the sample; n=24) or LRs (i.e., rats that exhibited locomotor scores in the lowest third of the sample n=24). The intermediary responders were only used as residents rats in the social interaction test described below.
2.3. Behavioral sensitization to nicotine and therapeutics administration
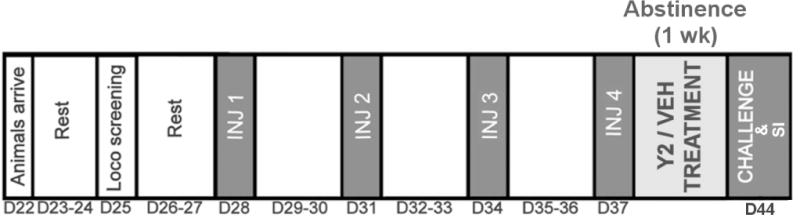
The behavioral sensitization to nicotine procedure is outlined in Table 1. Male Sprague-Dawley rats (PN 22) were allowed to rest until PN 28 after phenotype screening, and were assigned to saline (1 ml/kg; s.c.) or nicotine (0.35 mg/kg; s.c.) training injection groups. On injection days, rats were given 1 hr to habituate to the locomotor chambers before they received an injection of the assigned drug. Their locomotor response was recorded for 90 min. This procedure was repeated four times at a 3-d interval. Following the fourth training injection, rats were further assigned to vehicle (1 ml/kg, i.p.) or JNJ-31020028 (20 mg/kg, i.p.) therapeutic injection groups, and underwent 1 wk of abstinence where they received daily vehicle or JNJ-31020028 injections. At the end of the abstinence period, all LRHR rats were challenged with a low dose of nicotine (0.1 mg/kg, s.c.), and their locomotor response was monitored for 45 min. Upon completion of the challenge session, rats were tested on the social interaction test for assessing anxiety-like behavioral profile as described below.
Table 1. Behavioral sensitization to nicotine procedure.
Phenotype pre-screened LRHR rats received saline (1 ml/kg; s.c.) or nicotine (0.35 mg/kg; s.c.) injections four times at 3-day intervals (PN 28–37). Following the last saline or nicotine injection, all animals underwent 1 wk of abstinence during which they received daily, systemic Y2R antagonist (20 mg/kg; i.p) or vehicle (1 ml/kg; i.p) injections. At the end of the abstinence period (PN 45), all animals were challenged with a low dose of nicotine (0.1 mg/kg; s.c.).
|
2.4. Social interaction test (SI)
Testing took place in an open topped, rectangular, transparent social interaction box. The resident rat was placed in the box 8 min prior to placement of the experimental rat. The resident rat was of similar weight that was housed under identical conditions as the experimental rat, which had no previous contact with the experimental rat. The experimental rat was placed in the box and the amount of time the experimental rat spent initiating social interaction (i.e., grooming, sniffing, following, crawling over or under) with the resident was determined for 5 min. Upon completion of testing, animals were rapidly decapitated, brain tissues were harvested and processed for in situ hybridization histochemistry and Timm’s method for silver sulfide staining as described below.
2.5. In situ hybridization histochemistry
Brain tissues were collected and immediately frozen in isopentane cooled to −30°C. Brains were then sectioned on a cryostat and 20-μm-thick coronal sections were mounted on electrostatically charged slides. These slides were kept at −80°C until processed. On the day of hybridization, sections were fixed in 4% paraformaldehyde at room temperature for 1 hr, followed by three washes in 2× SSC (1× SSC is 150 mM sodium chloride, 15 mM sodium citrate). Sections were placed in a solution containing acetic anhydride (0.25%) in triethanolamine (0.1 M, pH 8) for 10 min, rinsed in distilled water, dehydrated through graded alcohols (50%, 75%, 85%, 95% and 100%) and air-dried. Rat spinophilin cDNA was cloned in our laboratory (accession #: NM_053474), antisense linearized, transcribed, and 35S labeled in reaction mixtures consisting of 1 ml of linearized plasmid, 1× transcription buffer, 125 mCi [35S ]UTP, 125 mCi [35S ]CTP, 150 mM each of ATP, and GTP, 12.5 mM dithiothreitol, 20 U RNAase inhibitor and 6 U polymerase. Reactions were incubated for 90 min at 37°C, and separated from unincorporated nucleotides over Micro Bio-Spin chromatography columns (Bio-Rad, CA). Probes were diluted in hybridization buffer (50% formamide, 10% dextran sulfate, 2× SSC, 50 mM sodium phosphate buffer, pH 7.4, 1× Denhardt’s solution, 0.1 mg/ml yeast tRNA and 10 mM dithiothreitol) to yield 106 dpm/70 ml. Sections were hybridized with probe mixture inside a humidified chamber over night at 55°C. Next day, sections were washed in 3× SSC for 5 min each, incubated for 1 hr in RNAase (20 mg/ml in Tris buffer containing 0.5 M NaCl, pH 8) at 37°C. Sections were washed with 2×, 1× and 0.5× SSC, and incubated for 1 hr in 0.1× SSC at 65°C. After rinsing in distilled water, sections were dehydrated, air dried and exposed to a Kodak XAR film (Eastman Kodak, NY). Section images were captured digitally from x-ray films with a CCD camera, and relative optical densities were determined. Only pixels with gray values exceeding 3.5× above background were included in the analyses. Optical density measurements were sampled from templates made for each brain region from both hemispheres and in the rostro-caudal axis using the Scion Image software. Levels of mRNA for spinophilin were quantified in the dorsal hippocampus. Optical density values were corrected for background and then averaged to produce one data point for each brain region for each animal. In addition, integrated density was calculated as optical density multiplied by area and included in the analyses. These data points were averaged per group and compared statistically.
2.6. Timm’s method for silver sulfide staining
Sections were collected at 200-μm intervals throughout the hippocampus, and were kept at −80°C until staining. On the day of staining, sections were air-dried and immersed in a phosphate-buffered (pH 7.4) 0.5% sodium sulfide solution for 2 min, briefly rinsed in two changes of phosphate buffer, fixed in 96% ethanol and rehydrated. Subsequently sections were stained as described previously (Danscher, 1981) by immersion in a citrate-buffered hydroquinone-silver lactate developer containing gum arabic as a protective colloid. Sections were rinsed vigorously with tap water following development and counterstained with cresyl violet stain and coverslipped. This protocol was successfully used on fresh frozen tissue before in previously published articles (Isgor et al., 2004b; Bhatti et al., 2007; Oztan et al., 2011).
2.7. Stereological estimation of mossy fiber terminal field volumes
The volumes of the two major components of the mossy fiber system (shown in Figure 1; i.e., the intra- and infra-pyramidal mossy fibers, IIP-MF; and supra-pyramidal mossy fibers, SP-MF were estimated using the Cavalieri estimator (Stereoinvestigator, Micro-BrightField, Colchester, VT). The tissue was viewed under brightfield illumination on a Zeiss Axiophot microscope interfaced with a CCD color video camera, and displayed on a high-resolution video monitor at a final magnification of 250×. Rostral and caudal extents of the hippocampus were determined following the convention of Paxinos and Watson (1982). A systematic, random sampling scheme was utilized such that estimates are based on every 10th section throughout the rostra-caudal extent of the hippocampus, yielding an average of 20 analyzed sections. The cross-sectional areas of the IIP-MF and SP-MF were estimated by an automated point-counting technique using a grid of test points displayed on the video monitor superimposed upon the structure of interest. Volumes of different MF terminal fields were estimated from the total number of points that fell within the respective field, the sampling interval and the nominal section thickness. Values were plotted for unilateral terminal field volume estimations for SP-MF, IIP-MF, and total (SP + IIP) mossy fiber systems.
Figure 1.
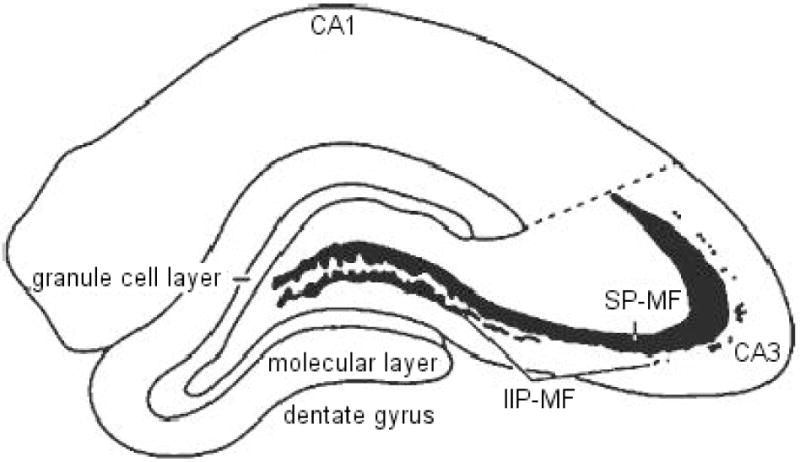
An illustration of a coronal hemisection of the dorsal hippocampus, depicting the two compartments of the mossy fiber system (IIP-MF, intra- and infra-pyramidal mossy fibers; SP-MF, supra-pyramidal mossy fibers) in relation to the major hippocampal subdivisions.
2.8. Statistical analysis
Locomotor reactivity to novelty data were analyzed by a two-tailed t-test. Behavioral data pertaining to repeated nicotine injections were analyzed by repeated-measures ANOVA: Phenotype (LR, HR) × Pre-training (SAL, NIC) × Injection Days (INJ 1, INJ 2, INJ 3, INJ 4). Locomotor reactivity to challenge nicotine, percent time spent engaged in social interaction, mRNA levels obtained from in situ hybridization histochemistry, and SP-MF, IIP-MF, and total MF terminal field volumes were analyzed by three-way ANOVAs: Phenotype (LR, HR), Pre-training (SAL, NIC) and Therapy (VEH, Y2). Significant main effects and interaction were followed by post-hoc comparisons using Fisher’s protected least significant difference tests, and significance was set at p ≤ 0.05.
3. Results
Prior to experimentation, rats were classified as LRs versus HRs based on their locomotor response to novelty, where LRs scored significantly lower compared to HRs (data not shown; t = 20.82, p = 0.0001). Figure 2 shows total locomotor reactivity to four intermittent nicotine or saline injections (A) and the subsequent low dose nicotine challenge following 1 wk of abstinence in LRs and HRs (B). Repeated measures ANOVA revealed significant interactions between Injection Days (INJ 1, INJ 2, INJ 3, INJ 4) and Pre-training [SAL, NIC; F= 3.04, p = 0.031], and between Phenotype (LR, HR) and Pre-training [SAL, NIC; F= 10.95, p = 0.002]. The same analysis also revealed significant main effects of Pre-training [SAL, NIC; F = 47.74, p = 0.0001] and Phenotype [LR, HR; F = 17.15, p = 0.0001]. Specific post-hoc comparisons showed that at all injection days, nicotine-injected HRs exhibited higher locomotor reactivity compared to saline-injected controls [ps ≤ 0.010], while in LRs such nicotine-induced elevations in locomotor reactivity were observed on INJ 3 [p = 0.043] and on INJ 4 [p = 0.033] compared to saline controls. Moreover, a three-way ANOVA showed significant interactions between Pre-training (SAL, NIC), Therapy (VEH, Y2) and Phenotype [LR, HR; F = 4.12, p = 0.048] and between Phenotype (LR, HR) and Pre-training [SAL, NIC; F = 4.47, p = 0.040] in locomotor response to the challenge nicotine. Furthermore, post-hoc comparisons showed that nicotine pre-trained HRs, but not LRs, exhibited increased locomotor reactivity to the low dose nicotine challenge compared to saline pre-trained controls following 1 wk of abstinence [p = 0.035]. This effect was reversed by daily Y2R antagonist administration during abstinence [p = 0.036] in nicotine pre-trained HRs back to vehicle control levels.
Figure 2.
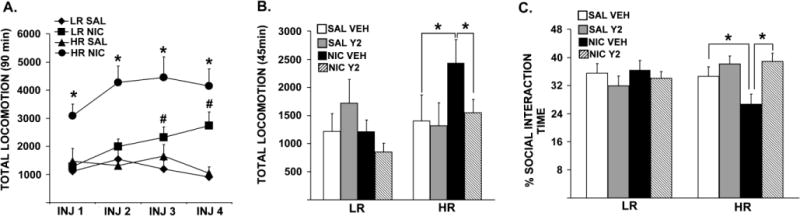
Total locomotor reactivity to four intermittent nicotine (0.35 mg/kg, s.c.) or saline (1.0 ml/kg, s.c.) training injections (A), total locomotor reactivity to a low dose nicotine (0.1 mg/kg, s.c.) challenge (B), and percent time spent interacting with the resident rat in the SI test (C) are shown. Group means ±SEMs are plotted in line (A) and bar (B and C) graphs. In panel A, (*) represents significant effects in total locomotor reactivity to nicotine in HRs compared to saline-injected controls, whereas (#) represents significant effects in nicotine-injected LRs compared to saline-injected controls. Significance is set at p ≤ 0.005.
Figure 2C shows percent time the experimental animals spent interacting with the resident rats in a social context following 1 wk of abstinence and a low dose nicotine challenge. A three-way ANOVA revealed significant interactions between Therapy (VEH, Y2) and Phenotype [LR, HR; F = 8.46, p = 0.006] in the SI test. Specific post-hoc comparisons showed that, nicotine pre-training and a low dose nicotine challenge resulted in decreased percentage of time spent in social interaction in HRs compared to saline controls [p = 0.045], and this nicotine-induced social anxiety-like behavior was fully reversed by the Y2R antagonist treatment in HRs back to levels observed with saline pre-trained controls [p = 0.004].
Figure 3 depicts dorsal hippocampal sections of Timm-stained hippocampal MF from representative HR animals. Three-way ANOVAs revealed significant interactions between Phenotype (LR, HR), Pre-training (SAL, NIC) and Therapy (VEH, Y2) on SP-MF [F = 9.98, p = 0.004] and the total MF [F = 4.97, p = 0.0354] terminal field volume. The same analysis also showed significant interactions between Phenotype (LR, HR) and Therapy (VEH, Y2) in SP-MF terminal field volume [F = 5.51, p = 0.027]. Specific post-hoc comparisons showed a significant increase in the total MF terminal field volume [p = 0.0465] and SP-MF volume [p = 0.027] in nicotine pre-trained HRs compared to saline pre-trained controls. Moreover, these effects were reversed by daily, systemic Y2R antagonist administration during abstinence [ps ≤ 0.029].
Figure 3.
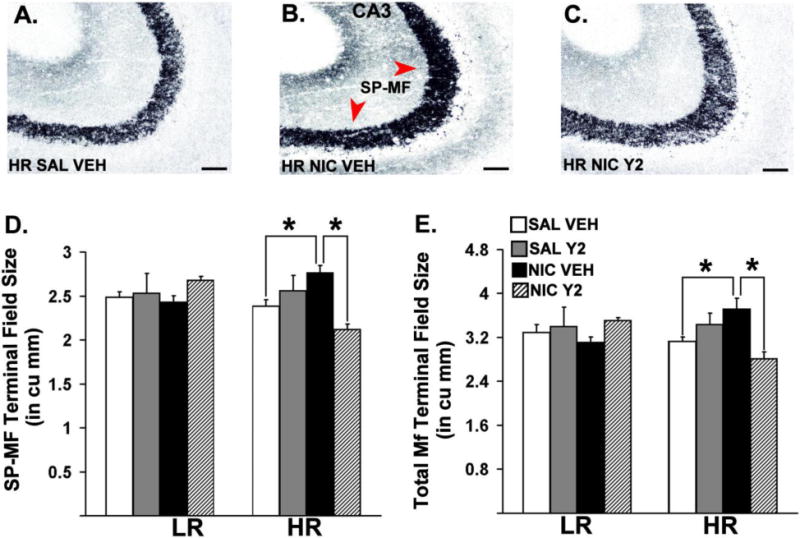
Coronal hemisections of Timm-stained hippocampal mossy fibers counterstained by cresyl violet from representative HR animals (A, B, C). Means of stereological estimates of the supra-pyramidal mossy fiber (SP-MF, D), and the total mossy fiber (E) terminal field volumes for LRHR animals are shown in bar graphs (*: p ≤ 0.05). Scale bar = 50 μm.
Figure 4 shows spinophilin mRNA expression in the hippocampus in representative HR rats. Three-way ANOVAs revealed significant interactions between Phenotype (LR, HR), Pre-training (SAL, NIC) and Therapy (VEH, Y2) in the CA1 [F= 4.38, p = 0.044] and the CA3 [F= 4.54, p = 0.0405] fields of the hippocampus. Significant interactions between Therapy (VEH, Y2) and Phenotype (LR, HR) were observed in the CA1 [F= 4.69, p = 0.0376], CA3 [F= 6.38, p = 0.016] and the DG [F = 5.57, p = 0.0244] of the hippocampus. The same analysis also revealed significant interactions between Phenotype (LR, HR) and Pre-training (SAL, NIC) in the CA1 [F= 5.05, p = 0.031], CA3 [F= 10.08, p = 0.003] and the DG [F= 5.51, p = 0.025] of the hippocampus. In addition, significant main effects of Therapy (VEH, Y2) were observed in the CA1 [F= 6.72, p = 0.014] and the CA3 [F= 6.93, p = 0.013], whereas a significant main effect of Pre-training (SAL, NIC) was observed only in the CA3 field of the hippocampus [F = 6.03, p = 0.019]. Specific post-hoc comparisons showed that a behaviorally-sensitizing nicotine regimen resulted in increased spinophilin mRNA levels in all hippocampal subfields in nicotine pre-trained HRs compared to saline pre-trained controls [ps ≤ 0.002]. Moreover, these effects were reversed by daily, systemic Y2R antagonist administration during abstinence [ps ≤ 0.0004].
Figure 4.
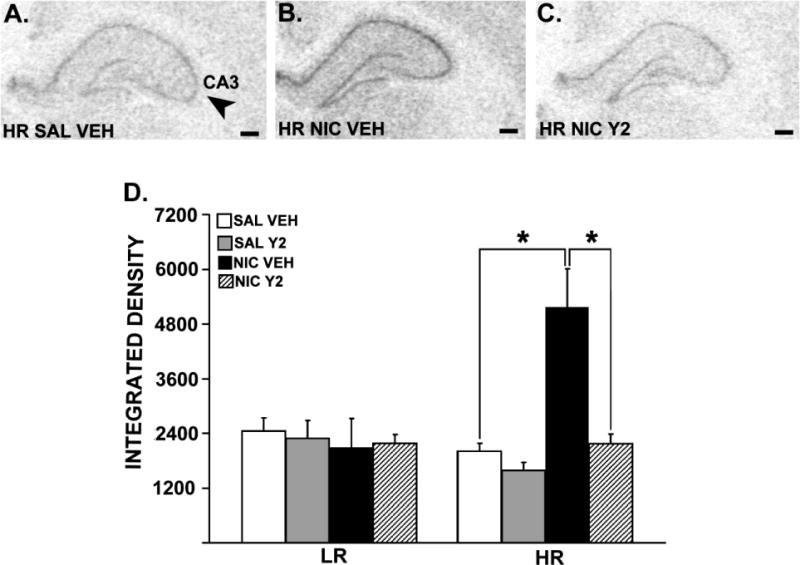
Spinophilin mRNA expression in the hippocampal CA3 field of a representative HR rat that is pre-trained with saline and treated with vehicle (A), HR rat that is pre-trained with nicotine and treated with vehicle (B) and HR rat that is pre-trained with nicotine, and treated with Y2R antagonist (C). Panels A, B and C show images of coronal hemisections of the hippocampus that were radioactively labeled with an antisense cRNA probe against spinophilin mRNA, and exposed on an x-ray film. Means of quantification results for integrated density ± SEMs are plotted with the bar graph (D; *: p ≤ 0.05). Scale bar = 250 μm.
4. Discussion
In confirmation of our previous reports, the present data show that a behaviorally-sensitizing nicotine regimen results in increased social anxiety-like behavior (Aydin et al., 2011a, b; 2012a, b), along with increased SP-MF and total MF terminal field size in the hippocampus (Bhatti et al., 2007). In addition, the current data show an increase in spinophilin mRNA levels in all subfields of the hippocampus in nicotine pre-trained HRs compared to saline controls. Importantly, the present findings demonstrate the efficacy of the novel, selective and brain penetrant Y2R antagonist, JNJ-31020028 in reversing nicotine-induced neuroplasticity in the HR hippocampus; in that daily, systemic Y2R antagonist treatment during abstinence normalizes the MF terminal field size and spinophilin mRNA levels in the hippocampus, in addition to reversing the behavioral effects of nicotine observed in nicotine pre-trained HRs.
Present data are in confirmation with our previously published report (Bhatti et al., 2007), in that the HR MF system structurally reorganizes following a behaviorally sensitizing nicotine regimen. This effect is seen despite the use of a lower training dose of nicotine in this experiment, indicating sensitivity of the HR MF to nicotine-induced plasticity. Moreover, the nicotine-induced MF sprouting observed in HRs correlates with increased spinophilin mRNA levels in all three fields of the hippocampus. Spinophilin mRNA has been used as a marker for hippocampal synaptic plasticity (Zhong et al., 2006; Schang et al., 2011) as well as an indicator of spinogenesis, as it is enriched in spines (Allen et al. 1997; Feng et al. 2000) and its expression correlates with changes in dendritic spine density (Zhou et al. 2010; Allen, 2004; Sarrouilhe et al., 2006; Prange-Kiel et al., 2009). Therefore, increased spinophilin mRNA levels, specifically in the CA3 field of the hippocampus, observed in nicotine pre-trained HRs may suggest that the increase in MF sprouting observed in these animals is reflected on the CA3 dendritic receptive fields. Moreover, the finding that this effect is observed in all fields of the hippocampus may indicate an overall remodeling of the hippocampal circuitry in nicotine pre-trained HRs, suggesting that a behaviorally-sensitizing nicotine regimen results in hyperactivity in the HR hippocampus compared to that of saline controls.
Our results also show that Y2R antagonist administration during abstinence reverses the nicotine-induced increase in SP-MF and total MF field volume as well as the increase in spinophilin mRNA levels in the hippocampus in nicotine pre-trained HRs. The role of Y2R in the hippocampal MF pathway has been well demonstrated in the case of epileptic seizures. Namely, during acute seizures NPY is released from the MF terminals to suppress concominant release of glutamate, by binding to Y2R (Gruber et al., 1994; Colmers et al., 1988; Klapstein & Colmers, 1993). Moreover, seizures induce MF sprouting (Tauck & Nadler, 1985), which is accompanied by synthesis and activation of the presynaptic Y2R, and reduced probability of glutamate release (Schwarzer et al., 1998; Tu et al., 2005, 2006), indicating a direct correlation between Y2R expression and MF volume, and suggesting inhibitory effects of Y2R activation on hippocampal excitation. In line with these reports, our current and previous data show that a behaviorally-sensitizing nicotine regimen results in increased MF terminal field size and increased Y2R mRNA levels (Aydin et al., 2011a, b) in the hippocampal CA3 field, respectively in HRs compared to saline controls. However, considering the role of Y2R activation in reducing excitatory neurotransmission in the hippocampus, reversal of MF sprouting observed in the present study in nicotine pre-trained HRs following Y2R antagonist treatment during abstinence may appear paradoxical, as this may indicate an inhibitory effect of the treatment on the circuitry. Nevertheless, Y2R-like immunoreactivity has also been found on MF that are immunoreactive for GABA (Stanic et al., 2011), suggesting that Y2R in the MF pathway can also regulate inhibitory neurotransmission. Moreover, the CA3 network dynamics under pathophysiological conditions, such as a behaviorally-sensitizing nicotine regimen, may depend on the net innervation ratio between the excitatory primary neurons and inhibitory interneurons (Mori et al., 2004). Furthermore, although MF are considered excitatory projections on to the CA3 field, it has been shown that the inhibitory interneurons receive approximately 10 times more synapses than do the principle neurons (Acsady et al., 1998) and generate a strong feedforward inhibition at physiological firing frequencies of dentate granule cells (Lawrence & McBain, 2003). It has also been shown that MF synapses onto CA3 can switch from inhibition to excitation depending on the stimulation frequency of the dentate granule neurons (Mori et al., 2004), and can be selectively muted (Losonczy et al., 2003), providing a state-dependent switch in the network. These reports suggest that the excitatory/inhibitory characteristics of the newly-formed MF synapses following a behaviorally-sensitizing nicotine regimen in HRs may be different than those formed under normal conditions. Moreover, due to the altered morphological makeup of the newly formed MF-CA3 circuitry, Y2R antagonist may reverse nicotine-induced MF plasticity instead of facilitating it further in HRs. Overall, these data suggest that Y2R treatment during 1 wk of abstinence that inhibits the expression of behavioral sensitization to nicotine and associated social anxiety-like behavior may also normalize the nicotine-induced structural changes in the hippocampus in the HR phenotype.
Hippocampus is an important component of neuronal circuitry controlling anxiety-related behaviors (Andrews et al., 1997; Gonzalez et al., 1998). Although the direct link between hippocampal MF plasticity and anxiety-like behavior has not been reported, studies have shown a positive correlation between SP-MF sprouting and increased anxiety-like behavior in inbred (Prior et al., 1997), as well as in outbred rats (de Oliveira et al., 2008; Oztan et al., 2011), suggesting that the MF system may contribute to modulation of anxiety-like responses. In support of this premise, our present data show that increased social anxiety-like behavior observed in HRs following a behaviorally sensitizing nicotine regimen is accompanied by increased SP-MF terminal field size, in comparison to saline pre-trained controls. Moreover, daily, systemic Y2R antagonist treatment during 1 wk of abstinence reverses increased social withdrawal and the SP-MF terminal field size in nicotine pre-trained HRs back to the levels observed in saline controls. These findings provide the first evidence of a causal relationship between anxiety-like behavior and hippocampal MF plasticity, and assign a role for Y2R in the MF-CA3 synapse in nicotine-induced negative affective state.
The present study focused on the behavioral as well as the morphological consequences of adolescent nicotine exposure in LRHR rats. Studies have shown that adolescence is a highly plastic and sensitive period of brain maturation, during which environmental and pharmacological influences strongly affect neural structure and function (Spear & Brake, 1983; Teicher et al., 1995). Specifically, the significance of this developmental period in development of nicotine addiction has been highlighted by several studies in the literature. For example, clinical data demonstrate that there is higher likelihood to shift from use to abuse, and a higher probability to develop dependence following early-onset drug use (Anthony & Petronis 1995; Robins & Przybeck 1985). Similarly, rodent studies have shown that repeated juvenile nicotine exposure results in increased self-administration of nicotine in adulthood, compared to equivalent exposure at adulthood (Adriani et al., 2003; Natividad et al., 2013), and nicotine-induced structural plasticity is considerably more pronounced during adolescence compared to adulthood (McDonald et al., 2007) in rats. These studies suggest enhanced vulnerability of the adolescent brain to the effects of nicotine. Moreover, this period in rats has been shown to be critical in hippocampal development when stressful experiences can result in delayed and enduring alterations in hippocampal morphology (Isgor et al., 2004a), emphasizing heightened vulnerability of the hippocampus during adolescence. Although the effects of the identical nicotine regimen in adulthood was not tested in the present study and hence the observed effects cannot be directly linked to adolescent nicotine exposure, based on the above-mentioned reports, it is plausible to suggest that the molecular and morphological effects in the HR hippocampi following nicotine may be related to introduction of nicotine during this critical period where hippocampal plasticity is peaking. Future studies will compare and contrast the effects of adolescence versus adulthood exposure to nicotine on hippocampal plasticity in the LRHR phenotype.
In summary, the present study demonstrates the efficacy of a novel, selective Y2R antagonist, JNJ-31020028; in reversing the nicotine-induced behavioral, as well as neuroplastic changes in the hippocampus, in the nicotine vulnerable HR rats. Namely, a behaviorally sensitizing nicotine regimen results in increased social anxiety-like behavior and induces MF sprouting, along with increased spinophilin mRNA levels in the HR hippocampus; all of which are reversed by a daily, systemic Y2R antagonist treatment during 1 wk of abstinence. Moreover, together with our previously published findings showing that the identical treatment regimen also reverses the neuropeptidergic adaptations in the hippocampus and the amygdala in nicotine pre-trained HRs (Aydin et al., 2011b); the present data provide further evidence for a link between a presynaptic Y2R-mediated pathway and the development of nicotine-induced behavioral effects. Furthermore, the present findings present an additional mechanism by which JNJ-31020028 reverses the nicotine-induced behavioral adaptations in the HR phenotype and confirm the efficacy of JNJ-31020028; a potent, selective, brain-penetrant Y2R antagonist, as a promising treatment option in combating nicotine relapse and associated negative affect.
Research Highlights.
Behavioral sensitization to nicotine is marked with increased social anxiety in HRs
A behaviorally-sensitizing nicotine regimen results in mossy fiber sprouting in HRs
Repeated nicotine induces increased spinophilin mRNA levels in the HR hippocampus
JNJ-31020028 reverses the behavioral and neuroplastic effects of nicotine in HRs
Acknowledgments
The Y2R antagonist, JNJ-31020028, is generously donated by the Janssen Research & Development, L.L.C, and we thank Dr. Pascal Bonaventure for his valuable technical assistance. This research is supported by the NIH grant DA023675 awarded to Dr. Isgor.
Abbreviations
- HR
high responder
- IIP
intra- and infra-pyramidal
- LR
low responder
- MF
mossy fiber
- NPY
neuropeptide Y
- SP
supra-pyramidal
- SI
social interaction
- Y2R
Y2 receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18(9):3386–403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23(11):4712–6. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci U S A. 1997;94(18):9956–61. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PB. Functional plasticity in the organization of signaling complexes in the striatum. Parkinsonism Relat Disord. 2004;10(5):287–92. doi: 10.1016/j.parkreldis.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Allen PB, Zachariou V, Svenningsson P, Lepore AC, Centonze D, Costa C, Rossi S, Bender G, Chen G, Feng J, Snyder GL, Bernardi G, Nestler EJ, Yan Z, Calabresi P, Greengard P. Distinct roles for spinophilin and neurabin in dopamine-mediated plasticity. Neuroscience. 2006;140(3):897–911. doi: 10.1016/j.neuroscience.2006.02.067. [DOI] [PubMed] [Google Scholar]
- Andrews N, File SE, Fernandes C, Gonzalez LE, Barnes NM. Evidence that the median raphé nucleus—dorsal hippocampal pathway mediates diazepam withdrawal-induced anxiety. Psychopharmacology (Berl) 1997;130:228–34. doi: 10.1007/s002130050233. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40(1):9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Aydin C, Oztan O, Isgor C. Vulnerability to nicotine abstinence-related social anxietylike behavior: molecular correlates in neuropeptide Y, Y2 receptor and corticotropin releasing factor. Neurosci Lett. 2011a;490(3):220–5. doi: 10.1016/j.neulet.2010.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin C, Oztan O, Isgor C. Effects of a selective Y2R antagonist, JNJ-31020028, on nicotine abstinence-related social anxiety-like behavior, neuropeptide Y and corticotropin releasing factor mRNA levels in the novelty-seeking phenotype. Behav Brain Res. 2011b;222(2):332–41. doi: 10.1016/j.bbr.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin C, Oztan O, Isgor C. Long-term effects of juvenile nicotine exposure on abstinence-related social anxiety-like behavior and amygdalar cannabinoid receptor 1 (CB1R) mRNA expression in the novelty-seeking phenotype. Behav Brain Res. 2012a;228(1):236–9. doi: 10.1016/j.bbr.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin C, Oztan O, Isgor C. Nicotine-induced anxiety-like behavior in a rat model of the novelty-seeking phenotype is associated with long-lasting neuropeptidergic and neuroplastic adaptations in the amygdala: effects of the cannabinoid receptor 1 antagonist AM251. Neuropharmacology. 2012b;63(8):1335–45. doi: 10.1016/j.neuropharm.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti AS, Hall P, Ma Z, Tao R, Isgor C. Hippocampus modulates the behaviorally-sensitizing effects of nicotine in a rat model of novelty-seeking: potential role for mossy fibers. Hippocampus. 2007;17:922–33. doi: 10.1002/hipo.20310. [DOI] [PubMed] [Google Scholar]
- Bhatti AS, Aydin C, Oztan O, Ma Z, Hall P, Tao R, Isgor C. Effects of a cannabinoid receptor (CB) 1 antagonist AM251 on behavioral sensitization to nicotine in a rat model of novelty-seeking behavior: correlation with hippocampal 5HT. Psychopharmacology (Berl) 2009;203:23–32. doi: 10.1007/s00213-008-1366-6. [DOI] [PubMed] [Google Scholar]
- Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathé AA. The Neuropeptide Y (NPY)-ergic System is Associated with Behavioral Resilience to Stress Exposure in an Animal Model of Post-Traumatic Stress Disorder. Neuropsychopharmacology. 2011 Oct 5; doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers WF, Lukowiak K, Pittman QJ. Neuropeptide Y action in the rat hippocampal slice: site and mechanism of presynaptic inhibition. J Neurosci. 1988;8(10):3827–37. doi: 10.1523/JNEUROSCI.08-10-03827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danscher G. Light and electron microscopic localization of silver in biological tissue. Histochemistry. 1981;71(2):177–86. doi: 10.1007/BF00507822. [DOI] [PubMed] [Google Scholar]
- de Oliveira DL, Fischer A, Jorge RS, da Silva MC, Leite M, Gonçalves CA, Quillfeldt JA, Souza DO, e Souza TM, Wofchuk S. Effects of early-life LiCl-pilocarpine-induced status epilepticus on memory and anxiety in adult rats are associated with mossy fiber sprouting and elevated CSF S100B protein. Epilepsia. 2008;49(5):842–52. doi: 10.1111/j.1528-1167.2007.01484.x. [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci U S A. 2000;97(16):9287–92. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaarskjaer FB. The organization and development of the hippocampal mossy fiber system. Brain Res. 1986;396(4):335–57. doi: 10.1016/0165-0173(86)90004-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Ouagazzal AM, File SE. Stimulation of benzodiazepine receptors in the dorsal hippocampus and median raphé reveals differential GABAergic control in two animal tests of anxiety. Eur J Neurosci. 1998;10:3673–80. doi: 10.1046/j.1460-9568.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- Gruber B, Greber S, Rupp E, Sperk G. Differential NPY mRNA expression in granule cells and interneurons of the rat dentate gyrus after kainic acid injection. Hippocampus. 1994;4(4):474–82. doi: 10.1002/hipo.450040409. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed Effects of Chronic Variable Stress During Peripubertal-Juvenile Period on Hippocampal Morphology and on Cognitive and Stress Axis Functions in Rats. 2004a;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Isgor C, Slomianka L, Watson SJ. Hippocampal mossy fibre terminal field size is differentially affected in a rat model of risk-taking behaviour. Behav Brain Res. 2004b;153(1):7–14. doi: 10.1016/j.bbr.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Ivanco TL, Greenough WT. Altered mossy fiber distributions in adult Fmr1 (FVB) knockout mice. Hippocampus. 2002;12(1):47–54. doi: 10.1002/hipo.10004. [DOI] [PubMed] [Google Scholar]
- Klapstein GJ, Colmers WF. 4-Aminopyridine and low Ca2+ differentiate presynaptic inhibition mediated by neuropeptide Y, baclofen and 2-chloroadenosine in rat hippocampal CA1 in vitro. Br J Pharmacol. 1992;105:470–4. doi: 10.1111/j.1476-5381.1992.tb14277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation—feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26(11):631–40. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Lin EJ, Lin S, Aljanova A, During MJ, Herzog H. Adult-onset hippocampal-specific neuropeptide Y overexpression confers mild anxiolytic effect in mice. Eur Neuropsychopharmacol. 2010;20:164–75. doi: 10.1016/j.euroneuro.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Somogyi P, Nusser Z. Reduction of excitatory postsynaptic responses by persistently active metabotropic glutamate receptors in the hippocampus. J Neurophysiol. 2003;89(4):1910–9. doi: 10.1152/jn.00842.2002. [DOI] [PubMed] [Google Scholar]
- McDonald CG, Eppolito AK, Brielmaier JM, Smith LN, Bergstrom HC, Lawhead MR, Smith RF. Evidence for elevated nicotine-induced structural plasticity in nucleus accumbens of adolescent rats. Brain Res. 2007;1151:211–8. doi: 10.1016/j.brainres.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Miller DK, Wilkins LH, Bardo MT, Crooks PA, Dwoskin LP. Once weekly administration of nicotine produces long-lasting locomotor sensitization in rats via a nicotinic receptor-mediated mechanism. Psychopharmacology (Berl) 2001;156:469–476. doi: 10.1007/s002130100747. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Sluyter F, de Wit S, Oostra BA, Crusio WE. Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus. 2002;12(1):39–46. doi: 10.1002/hipo.10005. [DOI] [PubMed] [Google Scholar]
- Mori M, Abegg MH, Gähwiler BH, Gerber U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature. 2004;431(7007):453–6. doi: 10.1038/nature02854. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Tu B, Timofeeva O, Jiao Y, Herzog H. Neuropeptide Y in the recurrent mossy fiber pathway. Peptides. 2007;28(2):357–64. doi: 10.1016/j.peptides.2006.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Torres OV, Friedman TC, O’Dell LE. Adolescence is a period of development characterized by short- and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug. Behav Brain Res. 2013;257:275–85. doi: 10.1016/j.bbr.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztan O, Aydin C, Isgor C. Chronic variable physical stress during the peripubertal-juvenile period causes differential depressive and anxiogenic effects in the novelty-seeking phenotype: functional implications for hippocampal and amygdalar brain-derived neurotrophic factor and the mossy fibre plasticity. Neuroscience. 2011;192:334–44. doi: 10.1016/j.neuroscience.2011.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1982. 1982. [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Fester L, Zhou L, Jarry H, Rune GM. Estrus cyclicity of spinogenesis: underlying mechanisms. J Neural Transm. 2009;116(11):1417–25. doi: 10.1007/s00702-009-0294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt HD, Greydanus DE. Adolescent violence: concepts for a new millennium. Adolesc Med. 2000;11(1):103–25. [PubMed] [Google Scholar]
- Prior H, Schwegler H, Dücker G. Dissociation of spatial reference memory, spatial working memory, and hippocampal mossy fiber distribution in two rat strains differing in emotionality. Behav Brain Res. 1997;87(2):183–94. doi: 10.1016/s0166-4328(97)02282-1. [DOI] [PubMed] [Google Scholar]
- Robins LN, Przybeck TR. Age of onset of drug use as a factor in drug and other disorders. NIDA Res Monogr. 1985;56:178–92. [PubMed] [Google Scholar]
- Qian J, Colmers WF, Saggau P. Inhibition of synaptic transmission by neuropeptide Y in rat hippocampal area CA1: modulation of presynaptic Ca2+ entry. J Neurosci. 1997;17:8169–77. doi: 10.1523/JNEUROSCI.17-21-08169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet P, Lassalle JM. Genetic variation, hippocampal mossy fibres distribution, novelty reactions and spatial representation in mice. Behav Brain Res. 1990;41(1):61–70. doi: 10.1016/0166-4328(90)90054-i. [DOI] [PubMed] [Google Scholar]
- Sarrouilhe D, di Tommaso A, Métayé T, Ladeveze V. Spinophilin: from partners to functions. Biochimie. 2006;88(9):1099–113. doi: 10.1016/j.biochi.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Schang AL, Ngô-Muller V, Bleux C, Granger A, Chenut MC, Loudes C, Magre S, Counis R, Cohen-Tannoudji J, Laverrière JN. GnRH receptor gene expression in the developing rat hippocampus: transcriptional regulation and potential roles in neuronal plasticity. Endocrinology. 2011;152(2):568–80. doi: 10.1210/en.2010-0840. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Kofler N, Sperk G. Up-regulation of neuropeptide Y-Y2 receptors in an animal model of temporal lobe epilepsy. Mol Pharmacol. 1998;53(1):6–13. doi: 10.1124/mol.53.1.6. [DOI] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24(2):115–23. [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Stanić D, Mulder J, Watanabe M, Hökfelt T. Characterization of NPY Y2 receptor protein expression in the mouse brain. II. Coexistence with NPY, the Y1 receptor, and other neurotransmitter-related molecules. J Comp Neurol. 2011;519(7):1219–57. doi: 10.1002/cne.22608. [DOI] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158(2):175–180. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Nepomuceno D, Lord B, Aluisio L, Fraser I, Motley ST, Sutton SW, Morton K, Galici R, Atack JR, Dvorak L, Swanson DM, Carruthers NI, Dvorak C, Lovenberg TW, Bonaventure P. In vitro and in vivo characterization of JNJ-31020028 (N-(4-{4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl}-3-fluorophenyl)-2-pyridin-3-ylbenzamide), a selective brain penetrant small molecule antagonist of the neuropeptide Y Y(2) receptor. Psychopharmacology (Berl) 2010;208(2):265–77. doi: 10.1007/s00213-009-1726-x. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5(4):1016–22. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, Mathé AA, Heilig M. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A. 2000;97:12852–7. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu B, Timofeeva O, Jiao Y, Nadler JV. Spontaneous release of neuropeptide Y tonically inhibits recurrent mossy fiber synaptic transmission in epileptic brain. J Neurosci. 2005;25:1718–29. doi: 10.1523/JNEUROSCI.4835-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu B, Jiao Y, Herzog H, Nadler JV. Neuropeptide Y regulates recurrent mossy fiber synaptic transmission less effectively in mice than in rats: Correlation with Y2 receptor plasticity. Neuroscience. 2006;143:1085–94. doi: 10.1016/j.neuroscience.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Zhong J, Zhang T, Bloch LM. Dendritic mRNAs encode diversified functionalities in hippocampal pyramidal neurons. BMC Neurosci. 2006;7:17. doi: 10.1186/1471-2202-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Wang S, Zhu X. Prenatal ethanol exposure attenuates GABAergic inhibition in basolateral amygdala leading to neuronal hyperexcitability and anxiety-like behavior of adult rat offspring. Neuroscience. 2010;170(3):749–57. doi: 10.1016/j.neuroscience.2010.07.055. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Neeb M. Sensation seeking and psychopathology. Psychiatry Res. 1979;1(3):255–64. doi: 10.1016/0165-1781(79)90007-6. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation-seeking: a comparative approach to a human trait. Behav Brain Sci. 1984;7:413–471. [Google Scholar]


