Abstract
IMPORTANCE
Scalp electroencephalography (EEG) and intraoperative electrocorticography (ECoG) are routinely used in the evaluation of magnetic resonance imaging–negative temporal lobe epilepsy (TLE) undergoing standard anterior temporal lobectomy with amygdalohippocampectomy (ATL), but the utility of interictal epileptiform discharge (IED) identification and its role in outcome are poorly defined.
OBJECTIVES
To determine whether the following are associated with surgical outcomes in patients with magnetic resonance imaging–negative TLE who underwent standard ATL: (1) unilateral-only IEDs on preoperative scalp EEG; (2) complete resection of tissue generating IEDs on ECoG; (3) complete resection of opioid-induced IEDs recorded on ECoG; and (4) location of IEDs recorded on ECoG.
DESIGN, SETTING, AND PARTICIPANTS
Data were gathered through retrospective medical record review at a tertiary referral center. Adult and pediatric patients with TLE who underwent standard ATL between January 1, 1990, and October 15, 2010, were considered for inclusion. Inclusion criteria were magnetic resonance imaging–negative TLE, standard ECoG performed at the time of surgery, and a minimum follow-up of 12 months. Univariate analysis was performed using log-rank time-to-event analysis. Variables reaching significance with log-rank testing were further analyzed using Cox proportional hazards.
MAIN OUTCOMES AND MEASURES
Excellent or nonexcellent outcome at time of last follow-up. An excellent outcome was defined as Engel class I and a nonexcellent outcome as Engel classes II through IV.
RESULTS
Eighty-seven patients met inclusion criteria, with 48 (55%) achieving an excellent outcome following ATL. Unilateral IEDs on scalp EEG (P = .001) and complete resection of brain regions generating IEDs on baseline intraoperative ECoG (P = .02) were associated with excellent outcomes in univariate analysis. Both were associated with excellent outcomes when analyzed with Cox proportional hazards (unilateral-only IEDs, relative risk = 0.31 [95% CI, 0.16-0.64]; complete resection of IEDs on baseline ECoG, relative risk = 0.39 [95% CI, 0.20-0.76]). Overall, 25 of 35 patients (71%) with both unilateral-only IEDs and complete resection of baseline ECoG IEDs had an excellent outcome.
CONCLUSIONS AND RELEVANCE
Unilateral-only IEDs on preoperative scalp EEG and complete resection of IEDs on baseline ECoG are associated with better outcomes following standard ATL in magnetic resonance imaging–negative TLE. Prospective evaluation is needed to clarify the use of ECoG in tailoring temporal lobectomy.
The prognosis for surgery in magnetic resonance imaging (MRI)–negative temporal lobe epilepsy (TLE) is less favorable than lesional epilepsy. Previous studies demonstrate a favorable postoperative outcome in 36% to 76% of patients with MRI-negative TLE.1-8 Many factors have been associated with a favorable outcome in MRI-negative TLE,1,4,9-11 but there has been little improvement in postoperative outcomes.12,13 Both scalp and intracranial electroen-cephalography (EEG) play an important role in the selection of surgical candidates. While unilateral or localized ictal onset on scalp EEG has been associated with good outcomes,11,14 the role of interictal epileptiform discharges (IEDs) in outcomes is less clear.
Intraoperative electrocorticography (ECoG) is commonly performed prior to resection at comprehensive epilepsy centers. However, its prognostic value in TLE surgery is unclear. Some data suggest ECoG may be useful in tailoring the surgical approach15-17 or in selecting patients who may be able to bypass prolonged intracranial monitoring and proceed to surgery.15 Other studies have not supported its use in the evaluation or prognosis of surgical patients with TLE.18,19 The use of ECoG in epilepsy surgery specifically for MRI-negative TLE has not been extensively evaluated. Most studies have focused on ECoG in lesional epilepsy including mesial temporal sclerosis (MTS),17,20,21 contained heterogeneous patient groups,9,16-18,22 or involved relatively small numbers of patients.10,15
To clarify the role of interictal EEG and intraoperative ECoG in the surgical management of MRI-negative TLE, we performed a multivariate analysis of 4 EEG findings and surgical outcomes in this population, including unilateral-only or bilateral independent IEDs on preoperative scalp EEG, complete resection of baseline ECoG IEDs, complete resection of opioid-induced ECoG IEDs, and location of ECoG IEDs.
Methods
Patients
Approval for this study was obtained from the Mayo Clinic Institutional Review Board. Patients who underwent comprehensive epilepsy evaluation between January 1, 1990, and October 15, 2010, were identified from the Mayo Clinic Epilepsy Surgery Database (Figure 1). Informed consent was waived owing to the retrospective design of the study. Adult and pediatric patients were included if they had medically resistant TLE, normal MRI findings, no prior neurologic surgery, standardized ECoG at the time of surgery, and at least 12 months of post-operative follow-up. Follow-up included any office visit or direct correspondence from the patient (telephone call or written correspondence) documented in the medical record that outlined the patient’s seizure outcome. All patients underwent a comprehensive evaluation including a seizure-protocol MRI, prolonged scalp EEG monitoring, and preneuropsychological and postneuropsychological assessment. For most of the study period, patients with unilateral temporal lobe seizure onset on scalp EEG and semiology not suggestive of early eloquent cortical involvement proceeded directly to surgery without prolonged intracranial monitoring, regardless of laterality. Patients with poorly localized or indeterminate seizure onset or with semiology concerning for eloquent cortex involvement underwent prolonged intracranial EEG monitoring. Patients were excluded if they underwent surgery without standard intraoperative ECoG, regardless of other testing. All data were obtained through retrospective medical record review. Data collected included sex, age at epilepsy onset, age at surgery, history of febrile seizures in childhood, days monitored with scalp EEG, pursuit of intracranial monitoring, side of seizure focus and resection, cortical resection size, time of seizure recurrence, and time of last follow-up. Electrophysiological data included the presence of unilateral-only or bilateral independent IEDs on presurgical scalp EEG, active contacts during baseline ECoG, active contacts during opioid-induced ECoG, and location of ECoG IEDs (mesial only, mesiolateral, or lateral neocortex only).
Figure 1.
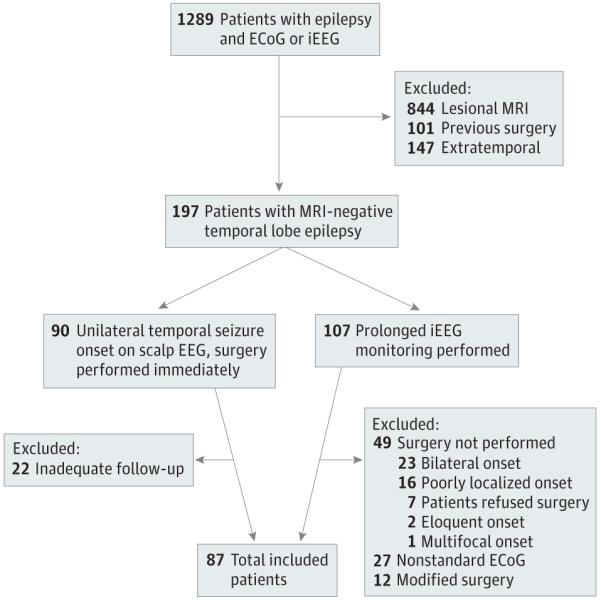
Patient Selection Process Through the Mayo Clinic Epilepsy Surgery Database
ECoG indicates electrocorticography; EEG, electroencephalography; iEEG, intraoperative electroencephalography; and MRI,magnetic resonance imaging.
Imaging
A standard epilepsy protocol was performed on all patients using 1.5-T or 3-T MRI scanners. These included 4-mm coronal fluid-attenuated inversion recovery and high-resolution 1.6-mm T1-weighted slices cut perpendicularly through the hippocampal axis and standard T2-weighted sequences. Quantitative hippocampal volumes were performed on patients with suspected subtle hippocampal atrophy.23 Imaging was reviewed at the time it was performed by board-certified neuroradiologists and subsequently at epilepsy conference by epileptologists, neurosurgeons, and epilepsy neuroradiologists. Patients were excluded if they demonstrated any hippocampal atrophy or signal change, tumor, encephalomalacia, cortical dysplasia, or vascular abnormalities other than noncortical developmental venous anomalies.
Scalp EEG
All patients underwent routine and prolonged scalp EEG using a 31-electrode modified 10-20 international system prior to surgery. Patients with any epileptiform abnormalities arising from the hemisphere contralateral to the seizure focus were classified as having bilateral independent IEDs. Epileptiform abnormalities included spikes, sharp waves, spike and wave discharges, and temporal intermittent rhythmic delta. All routine and prolonged scalp EEG studies performed prior to surgery were reviewed. All scalp EEG data were qualitative owing to retrospective medical record review. Data were available for all patients.
Anesthesia
All patients underwent standard anterior temporal lobectomy with amygdalohippocampectomy (ATL) under standardized general anesthesia. Anesthesia consisted of induction with intravenous thiopental sodium, 3 to 5 mg/kg, and intravenous fentanyl citrate, 2 μg/kg, followed by vecuronium neuromuscular blockade to maintain a single twitch with peripheral nerve stimulation. Anesthesia was maintained with 0.6% isoflurane inhaled with 50% nitrous oxide and intravenous fentanyl, 2 μg/kg/h. Prior to ECoG, isoflurane was held for at least 15 minutes and end-tidal concentration was monitored until it was less than 0.1%.24
Intraoperative ECoG
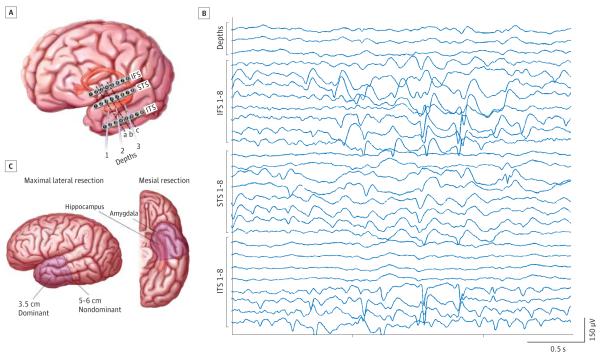
All patients underwent standardized intraoperative ECoG immediately prior to ATL (Figure 2). Anatomical landmarks were used to guide the placement of three 8-contact subdural strip electrodes (Adtech Inc) on the inferior frontal gyrus (IFS strip electrodes 1-8), superior temporal gyrus (STS strip electrodes 1-8), and inferior temporal gyrus (ITS strip electrodes 1-8). Three 1-contact depth electrodes (Adtech Inc) were placed laterally through the middle temporal gyrus targeting the amygdala, anterior hippocampus, and body of hippocampus. The ECoGs lasted 15 to 30 minutes and were read by an epileptologist at the time of recording using a standardized numbering system to indicate contact activity. After a baseline period of approximately 5 minutes, either alfentanil hydrochloride or remifentanil hydrochloride was used to induce IEDs in 63 patients.25 Baseline IEDs were assessed first, followed by opioid-induced IEDs.
Figure 2.
Intraoperative Electrocorticography and Anterior Temporal Lobectomy
A, Schematic of standardized electrode configuration for intraoperative electrocorticography in patients undergoing anterior temporal lobectomy. Dashed lines indicate cortical resections at 30 mm (a), 40 mm (b), and 50 mm (c). B, Intraoperative electrocorticography recording. IFS indicates the 8-contact strip electrode used to record from the inferior frontal gyrus; ITS, the 8-contact strip electrode used to record from the inferior temporal gyrus; and STS, the 8-contact strip electrode used to record from the superior temporal gyrus. C, Schematic of standard anterior temporal lobectomy performed in all patients. The extent of lateral neocortical resection for the dominant and nondominant temporal lobe is shown. The mesial temporal structures, amygdala, hippocampus, and parahippocampus are also resected.
Interictal epileptiform discharge resection was designated as complete or incomplete based on standard ECoG placement and resection size. Figure 2 illustrates the electrodes considered anterior to resections at 30, 40, and 50 mm. One contact each on the superior temporal gyrus and the inferior temporal gyrus was considered resected for every additional 10 mm. Interictal epileptiform discharge resection was also considered complete if the intraoperative or surgical report noted that all IEDs were within the resected tissue. All depth electrodes were within the hippocampal resection. All inferior frontal gyrus electrodes were outside the resection field. Outcomes were assessed separately for baseline ECoG IEDs and opioid-induced ECoG IEDs.
The location of the IED was assigned to be mesial only if the patient had IEDs as a result from the depth electrodes alone and to be mesiolateral or lateral only if the patient had IEDs from the neocortical contact strips, either with or without depth-electrode IEDs. The location of the IED was determined by baseline ECoG unless the ECoG was normal, in which case patients were assigned based on their opioid-induced IEDs.
Surgical Procedure and Postoperative Outcomes
Surgery consisted of a neocortical resection of 50 to 60 mm in nondominant temporal lobes or 35 to 40 mm in dominant temporal lobes, with amygdala and hippocampal resection to the trigone (Figure 2).
Patients were grouped into excellent or nonexcellent post-operative outcomes based on Engel classification. Excellent outcomes corresponded with Engel class I, and nonexcellent outcomes corresponded with Engel classes II through IV. All patients had outcome data at least 12 months from the time of surgery.
Statistical Analysis
Categorical statistics were presented as absolute numbers and corresponding percentages. Continuous statistics were presented as medians and interquartile ranges (IQRs) to prevent outlier bias. Analysis was carried out on electrophysiological variables first in a univariate model using a log-rank time-to-event analysis. Variables reaching statistical significance in univariate analysis, defined as P ≤ .05, were also analyzed using Cox proportional hazards. Patients with data missing for a variable were excluded from the analysis of that variable but included in the analysis of other variables. Patients were excluded from multivariate analysis if they had data missing for any variable included in the multivariate analysis. Results were considered statistically significant if P ≤ .05. Statistical analysis was performed using JMP statistical software, version 9.0.1 (SAS Institute, Inc).
Results
Patient Characteristics
Patient characteristics are outlined in Table 1. Of 1289 patients reviewed, 87 met criteria for inclusion (Figure 1). Eighty patients underwent 1.5-T MRI, and 7 underwent 3-T MRI. Surgical reports and ECoG were sufficient to describe the baseline ECoG IED field in 79 patients, including 3 patients based on intraoperative or surgical reports of complete IED resection. Eight patients had a normal baseline ECoG. Sixty-three patients had opioid-induced ECoG, with 60 having suitable data to describe the field of IED activation. Eighty-six patients had suitable data to group based on IED location, including 7 patients who were classified by opioid-induced ECoG. The remaining patient had a normal baseline ECoG and did not undergo opioid-induced ECoG.
Table 1.
Patient Characteristics
| Characteristic | No. (%) (N = 87) |
|---|---|
| Female | 56 (64) |
|
| |
| Age at onset, median (IQR), y | 16 (7-26) |
|
| |
| Age at surgery, median (IQR), y | 34 (25-43) |
|
| |
| Time to surgery, median (IQR), y | 14 (7-25) |
|
| |
| Focal seizures only | 33 (38) |
|
| |
| Right-sided seizure onset | 41 (47) |
|
| |
| Febrile seizures in childhood | 15 (17) |
|
| |
| Intracranial monitoring pursued | 19 (22) |
|
| |
| Scalp EEG | 87 |
|
| |
| Bilateral independent IEDs | 20 (23) |
|
| |
| Baseline ECoG | 79 |
|
| |
| Completely resected | 48 (61) |
|
| |
| Opioid-induced ECoG | 60 |
|
| |
| Completely resected | 35 (58) |
|
| |
| Baseline ECoG location | 86 |
|
| |
| Mesial only | 19 (22) |
|
| |
| Lateral only | 8 (9) |
|
| |
| Resection size, median (IQR), mm | |
|
| |
| Right | 40 (35-50) |
|
| |
| Left | 35 (33-40) |
|
| |
| Pathology | |
|
| |
| Gliosis | 63 (72) |
|
| |
| MTS | 21 (24) |
|
| |
| Tumor | 1 (1) |
|
| |
| Chronic encephalitis | 1 (1) |
|
| |
| Normal | 1 (1) |
|
| |
| Follow-up, median (IQR), mo | 41.8 (22.3-108.4) |
|
| |
| Excellent outcome | 48 (55) |
|
| |
| Right ATL | 22 (54) |
|
| |
| MTS, tumor, or encephalitis pathology | 13 (57) |
|
| |
| Gliosis or normal pathology | 35 (55) |
Abbreviations: ATL, anterior temporal lobectomy with amygdalohippocampectomy; ECoG, electrocorticography; EEG, electroencephalography; IEDs, interictal epileptiform discharges; IQR, interquartile range; MTS, mesial temporal sclerosis.
Fifty-six patients (64%) were female. The median time to surgery after epilepsy onset was 14 years (IQR, 7-25 years). Thirty-three patients (38%) had only focal seizures or only a single generalized tonic-clonic seizure throughout the course of their epilepsy. Presurgical scalp EEG monitoring was longer in patients with bilateral independent IEDs compared with those with unilateral-only IEDs (median, 6 days [IQR, 5-7 days] for bilateral, 4 days [IQR, 4-6 days] for unilateral; P < .01). Forty-one patients (47%) had a right temporal focus. The most common pathological finding was nonspecific gliosis, identified in 63 patients (72%). Pathology showed hippocampal neuronal loss and gliosis consistent with MTS in 21 patients (24%). One patient demonstrated chronic encephalitis and 1 patient had a small dysembryoplastic neuroepithelial tumor. Only 1 patient had no detectable abnormality.
Patient Outcomes
The median time of follow-up after surgery was 41.8 months (IQR, 22.3-108.4 months). The supplemental material provides data on follow-up and time to seizure recurrence (eTable in Supplement). Forty-eight patients (55%) had an excellent outcome following surgery. Excellent outcomes were similar with respect to laterality, with 22 patients (54%) with a right temporal focus achieving an excellent outcome compared with 26 patients (57%) with a left temporal focus. Twelve patients (57%) with MTS on histopathology had an excellent outcome.
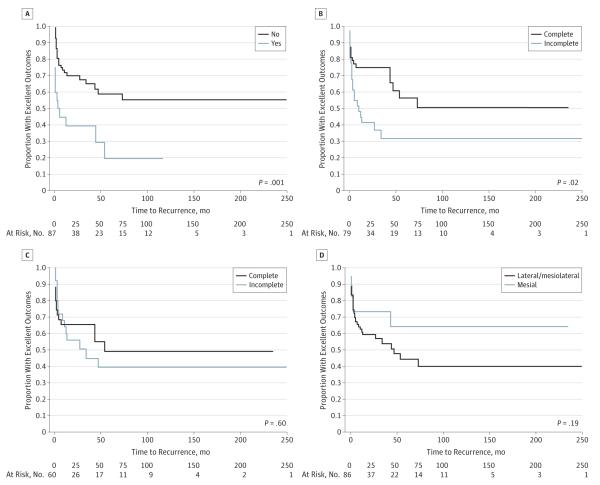
Unilateral-only IEDs on scalp EEG (P = .001) and complete resection of baseline ECoG IEDs (P = .02) were both statistically significant with regard to postoperative outcomes using log-rank analysis (Figure 3). Seventy-nine patients had data for both variables and were included in multivariate analysis. Both variables retained a significant association with outcome when assessed with Cox proportional hazards (Table 2).
Figure 3.
Log-Rank Kaplan-Meier Curves of Excellent Outcome Proportions
A, Bilateral independent interictal epileptiform discharges (IEDs) on scalp electroencephalography. B, Baseline electrocorticography (ECoG) IED resection, complete vs incomplete. C, Opioid-induced ECoG IED resection, complete vs incomplete. D, Location of ECoG IEDs, mesial only vs mesiolateral or lateral only.
Table 2.
Multivariate Analysis With Cox Proportional Hazards
| Feature | RR (95%CI) |
|---|---|
| Unilateral-only IEDs on scalp EEG | 0.31 (0.16-0.64) |
|
| |
| Complete baseline ECoG IED resection | 0.39 (0.20-0.76) |
Abbreviations: ECoG, electrocorticography; EEG, electroencephalograplny; IED, interictal epileptiform discharge; RR, relative risk.
Twenty patients with incomplete baseline ECoG resection had a nonexcellent outcome. Unresected IEDs were present in the inferior temporal gyrus alone in 14 patients (70%), inferior temporal gyrus and superior temporal gyrus in 2 patients (10%), inferior frontal gyrus alone in 2 patients (10%), inferior temporal gyrus and inferior frontal gyrus in 1 patient (5%), and all 3 neocortical strips in 1 patient (5%).
Twenty-five of 35 patients (71%) with both unilateral-only IEDs on scalp EEG and complete resection of baseline ECoG IEDs achieved an excellent outcome at last follow-up. Seventeen of 37 patients (46%) with either bilateral independent IEDs or incomplete resection of baseline ECoG IEDs had an excellent outcome at last follow-up. Seven patients had both bilateral independent IEDs and incomplete resection of base-line ECoG IEDs, and all had a nonexcellent outcome (Engel class II in 2 patients and Engel class IV in 5 patients) (eFigure in Supplement).
Discussion
Our study demonstrates that unilateral-only IEDs on scalp EEG and complete resection of baseline ECoG IEDs were associated with better outcomes in patients with MRI-negative TLE. These findings are useful in surgical outcome prognosis and, in the case of ECoG, could potentially be used to give an individual patient the best possible opportunity for seizure freedom.
Patients with unilateral-only IEDs on scalp EEG prior to surgery demonstrated better outcomes compared with patients with bilateral independent discharges. To our knowledge, our study is unique in this regard. Many studies have evaluated preoperative IEDs with mixed results. One small study of patients with MRI-negative TLE found that unilateral anterior temporal IEDs were associated with better outcomes,10 but other studies failed to demonstrate significance11 or demonstrated a trend with near significance.1,26 One study of consecutive patients with TLE did demonstrate an overall association, but the effect was driven by the lesional and hippocampal atrophy groups and was not seen in the MRI-negative group.7 Several other studies have failed to show any associated significance of scalp-recorded IEDs in surgical outcomes with predominantly lesional or MTS groups,27-29 in heterogeneous groups of patients,30 or in meta-analysis.13 There was a significant difference in the number of days monitored between unilateral-only and bilateral independent scalp EEG IED groups. While a longer monitoring time may lead to finding bilateral independent IEDs, this likely reflects that monitoring was longer in these patients to exclude independent bihemispheric seizures in the setting of bilateral independent scalp EEG IEDs. The statistical significance demonstrated in our study and suggested in other MRI-negative studies may indicate a more extensive epileptogenic network in MRI-negative patients with bilateral independent IEDs, making them less likely to achieve an excellent surgical outcome.
Intraoperative ECoG is often used to guide surgical resection of epileptogenic tissues.25 We found that patients with MRI-negative TLE were more likely to have better outcomes with complete resection of baseline ECoG IEDs. Thirty-one of 48 patients (65%) demonstrated an excellent outcome when all baseline ECoG IEDs were completely resected, compared with 11 of 31 patients (35%) with excellent outcomes when baseline ECoG IED resection was incomplete. When coupled with having unilateral-only scalp EEG IEDs, patients with complete resection had a high rate of excellent outcome at last follow-up. This likely reflects complete removal of the epileptogenic zone.
Previous ECoG studies have focused on groups composed primarily of patients with MTS. Cascino et al18 reported no association between postoperative outcomes and either the location of ECoG IEDs (neocortical vs depth) or the presence of postoperative ECoG IEDs in a cohort that included patients with MTS and evidence of hippocampal atrophy in 67% of patients. In another study of patients with MTS, neither presence of unresected ECoG IEDs nor IED location predicted surgical outcomes.19 Some studies have shown that ECoG-tailored resections in patients with mesial TLE, with or without MTS, may achieve outcomes comparable to standard surgical approaches.16,17 Improved long-term duration of favorable outcomes has also been linked to the use of ECoG.6 Our study differs from the aforementioned studies as we specifically excluded patients with radiographic evidence of MTS or hippocampal atrophy. Histopathology demonstrated MTS in 24% of patients, which was consistent with other MRI-negative TLE study populations, as was their excellent outcome rate of 57%.1,4,26 This excellent outcome rate was similar to the overall group, indicating that patients with MRI-negative TLE with positive pathology were not contributing unevenly to the overall proportion of excellent outcomes. Additionally, in contrast to the study by Cascino et al, which was also performed at the Mayo Clinic and included a 3-year overlap period with our study, we specifically evaluated IED resection rather than assessment of postoperative IEDs. Based on the results presented herein, baseline ECoG recordings do appear useful for prognosis in epilepsy surgery for patients with MRI-negative TLE. Tailored resection guided by ECoG may improve outcome and deserves further prospective evaluation.
In contrast to the significance found between outcome and completeness of baseline ECoG IED resection, we found no association with complete resection of opioid-induced ECoG IEDs. Our result is important as opioid-induced activity is thought to originate from the epileptogenic zone.24,31 Many studies have identified increased ECoG IED activation and seizures with administration of alfentanil or remifentanil.24,25,31-34 We have previously investigated this effect and reported that IED activation was most prominent in individuals having the fewest IEDs at baseline.24 These previous opioid-induced ECoG-related studies were performed in patients with MTS and did not investigate surgical outcome, prompting us to undertake the current study. Our data do not support that opioid-induced IED activation reflects the epileptogenic zone based on the lack of difference in clinical outcomes between resection groups. These opioid-induced IEDs may reflect propagation of discharges from the epileptogenic zone or independent activation of susceptible nonictogenic regions. A more thorough investigation including closer analysis of IED frequency changes, the presence of new independent IEDs, and associated tissue resection with patient outcomes would be helpful in the future.
There was no difference in outcomes between mesialonly vs mesiolateral and lateral-only IED location. A study by Luther et al15 suggests that carefully selected candidates may proceed to surgery on the basis of ECoG IED location rather than undergo prolonged intracranial monitoring. It should be noted that our ECoG was more limited in coverage than the ECoG used by Luther et al, which included more extensive coverage of the inferior and subtemporal cortex and the temporal pole but did not include hippocampal depth electrodes. All patients in the aforementioned study also underwent prolonged intracranial monitoring prior to resection regardless of ECoG findings, whereas our study did not assess the results of prolonged intracranial monitoring.
The major limitation of our study is its retrospective nature. Collection of EEG and ECoG information through unblinded medical record review rather than blinded review of raw EEG and ECoG data is suboptimal but required given the long study period. This limitation may also allow for incorporation of bias introduced at the time of ECoG and surgery. The evolution of imaging techniques over time may also introduce bias in lesion identification despite the use of a standardized imaging approach at our institution during the study period. Data were not available for all patients regarding all measures, most notably when assessing opioid-induced ECoG, as well as for 8 patients who had a normal baseline ECoG. Patients without data were not included in analysis for those specific measures. Patients with a normal baseline ECoG were also omitted from the multivariate analysis. A total of 22 patients were excluded owing to inadequate follow-up, with all of these patients having undergone ATL without prolonged intracranial monitoring. This may reflect a less severe disease state in patients who do not undergo intracranial monitoring, making them more likely to continue neurologic follow-up closer to home after surgery.
Conclusions
Ultimately, the use of a standardized ECoG protocol and consistent ATL surgical approach by the same 2 surgeons (W.R.M. and F.B.M.) in most patients throughout the study period provided compelling evidence that scalp EEG and intraoperative ECoG IEDs are potentially useful for postoperative prognosis, and the latter possibly for guiding surgical resection. A prospective study using a standardized ECoG approach would be useful to further clarify these issues.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by a generous gift from Mr and Mrs David Hawk, grant UL1TR000135 from the Mayo Clinic Center for Translational Science Activities, grant IGA MZCR NT/11536-5 from the Ministry of Health of the Czech Republic, Project FNUSA-ICRC (grant CZ.1.05/1.1.00/02.0123) from the European Regional Development Fund, and grant CZ.1.07/2.3.00/20.0117 from the European Social Fund Young Talent Incubator II. Dr Worrell is supported by grant R01-NS63039 from the National Institutes of Health.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Worrell consults for and has received research support from Medtronic Inc, Neuralynx Inc, and Neuropace Inc. No other disclosures were reported.
REFERENCES
- 1.Bell ML, Rao S, So EL, et al. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia. 2009;50(9):2053–2060. doi: 10.1111/j.1528-1167.2009.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkovic SF, McIntosh AM, Kalnins RM, et al. Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology. 1995;45(7):1358–1363. doi: 10.1212/wnl.45.7.1358. [DOI] [PubMed] [Google Scholar]
- 3.Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch Neurol. 2009;66(12):1491–1499. doi: 10.1001/archneurol.2009.283. [DOI] [PubMed] [Google Scholar]
- 4.Fong JS, Jehi L, Najm I, Prayson RA, Busch R, Bingaman W. Seizure outcome and its predictors after temporal lobe epilepsy surgery in patients with normal MRI. Epilepsia. 2011;52(8):1393–1401. doi: 10.1111/j.1528-1167.2011.03091.x. [DOI] [PubMed] [Google Scholar]
- 5.Immonen A, Jutila L, Muraja-Murro A, et al. Long-term epilepsy surgery outcomes in patients with MRI-negative temporal lobe epilepsy. Epilepsia. 2010;51(11):2260–2269. doi: 10.1111/j.1528-1167.2010.02720.x. [DOI] [PubMed] [Google Scholar]
- 6.Jeha LE, Najm IM, Bingaman WE, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66(12):1938–1940. doi: 10.1212/01.wnl.0000219810.71010.9b. [DOI] [PubMed] [Google Scholar]
- 7.Radhakrishnan K, So EL, Silbert PL, et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology. 1998;51(2):465–471. doi: 10.1212/wnl.51.2.465. [DOI] [PubMed] [Google Scholar]
- 8.Wieshmann UC, Larkin D, Varma T, Eldridge P. Predictors of outcome after temporal lobectomy for refractory temporal lobe epilepsy. Acta Neurol Scand. 2008;118(5):306–312. doi: 10.1111/j.1600-0404.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- 9.Jayakar P, Dunoyer C, Dean P, et al. Epilepsy surgery in patients with normal or nonfocal MRI scans: integrative strategies offer long-term seizure relief. Epilepsia. 2008;49(5):758–764. doi: 10.1111/j.1528-1167.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 10.Sylaja PN, Radhakrishnan K, Kesavadas C, Sarma PS. Seizure outcome after anterior temporal lobectomy and its predictors in patients with apparent temporal lobe epilepsy and normal MRI. Epilepsia. 2004;45(7):803–808. doi: 10.1111/j.0013-9580.2004.48503.x. [DOI] [PubMed] [Google Scholar]
- 11.Tatum WO IV, Benbadis SR, Hussain A, et al. Ictal EEG remains the prominent predictor of seizure-free outcome after temporal lobectomy in epileptic patients with normal brain MRI. Seizure. 2008;17(7):631–636. doi: 10.1016/j.seizure.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89(2-3):310–318. doi: 10.1016/j.eplepsyres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Tonini C, Beghi E, Berg AT, et al. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004;62(1):75–87. doi: 10.1016/j.eplepsyres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Steinhoff BJ, So NK, Lim S, Lüders HO. Ictal scalp EEG in temporal lobe epilepsy with unitemporal versus bitemporal interictal epileptiform discharges. Neurology. 1995;45(5):889–896. doi: 10.1212/wnl.45.5.889. [DOI] [PubMed] [Google Scholar]
- 15.Luther N, Rubens E, Sethi N, et al. The value of intraoperative electrocorticography in surgical decision making for temporal lobe epilepsy with normal MRI. Epilepsia. 2011;52(5):941–948. doi: 10.1111/j.1528-1167.2011.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKhann GM, II, Schoenfeld-McNeill J, Born DE, Haglund MM, Ojemann GA. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. J Neurosurg. 2000;93(1):44–52. doi: 10.3171/jns.2000.93.1.0044. [DOI] [PubMed] [Google Scholar]
- 17.San-juan D, Tapia CA, González-Aragón MF, Martínez Mayorga A, Staba RJ, Alonso-Vanegas M. The prognostic role of electrocorticography in tailored temporal lobe surgery. Seizure. 2011;20(7):564–569. doi: 10.1016/j.seizure.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Cascino GD, Trenerry MR, Jack CR, Jr, et al. Electrocorticography and temporal lobe epilepsy: relationship to quantitative MRI and operative outcome. Epilepsia. 1995;36(7):692–696. doi: 10.1111/j.1528-1157.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz TH, Bazil CW, Walczak TS, Chan S, Pedley TA, Goodman RR. The predictive value of intraoperative electrocorticography in resections for limbic epilepsy associated with mesial temporal sclerosis. Neurosurgery. 1997;40(2):302–309. doi: 10.1097/00006123-199702000-00014. discussion 309-311. [DOI] [PubMed] [Google Scholar]
- 20.Tripathi M, Garg A, Gaikwad S, et al. Intra-operative electrocorticography in lesional epilepsy. Epilepsy Res. 2010;89(1):133–141. doi: 10.1016/j.eplepsyres.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Van Gompel JJ, Rubio J, Cascino GD, Worrell GA, Meyer FB. Electrocorticography-guided resection of temporal cavernoma: is electrocorticography warranted and does it alter the surgical approach? J Neurosurg. 2009;110(6):1179–1185. doi: 10.3171/2008.10.JNS08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asano E, Juhász C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009;132:1038–1047. doi: 10.1093/brain/awp025. pt 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR., Jr Magnetic resonance imaging. Neuroimaging and anatomy. Neuroimaging Clin N Am. 1995;5(4):597–622. [PubMed] [Google Scholar]
- 24.Wass CT, Grady RE, Fessler AJ, et al. The effects of remifentanil on epileptiform discharges during intraoperative electrocorticography in patients undergoing epilepsy surgery. Epilepsia. 2001;42(10):1340–1344. doi: 10.1046/j.1528-1157.2001.05901.x. [DOI] [PubMed] [Google Scholar]
- 25.Cascino GD, So EL, Sharbrough FW, et al. Alfentanil-induced epileptiform activity in patients with partial epilepsy. J Clin Neurophysiol. 1993;10(4):520–525. doi: 10.1097/00004691-199310000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Vale FL, Effio E, Arredondo N, et al. Efficacy of temporal lobe surgery for epilepsy in patients with negative MRI for mesial temporal lobe sclerosis. J Clin Neurosci. 2012;19(1):101–106. doi: 10.1016/j.jocn.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Hardy SG, Miller JW, Holmes MD, et al. Factors predicting outcome of surgery for intractable epilepsy with pathologically verified mesial temporal sclerosis. Epilepsia. 2003;44(4):565–568. doi: 10.1046/j.1528-1157.2003.39202.x. [DOI] [PubMed] [Google Scholar]
- 28.Kilpatrick C, O’Brien T, Matkovic Z, Cook M, Kaye A. Preoperative evaluation for temporal lobe surgery. J Clin Neurosci. 2003;10(5):535–539. doi: 10.1016/s0967-5868(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 29.Krendl R, Lurger S, Baumgartner C. Absolute spike frequency predicts surgical outcome in TLE with unilateral hippocampal atrophy. Neurology. 2008;71(6):413–418. doi: 10.1212/01.wnl.0000310775.87331.90. [DOI] [PubMed] [Google Scholar]
- 30.Lee SK, Kim KK, Hong KS, Kim JY, Chung CK. The lateralizing and surgical prognostic value of a single 2-hour EEG in mesial TLE. Seizure. 2000;9(5):336–339. doi: 10.1053/seiz.2000.0414. [DOI] [PubMed] [Google Scholar]
- 31.Grønlykke L, Knudsen ML, Høgenhaven H, Moltke FB, Madsen FF, Kjaer TW. Remifentanil-induced spike activity as a diagnostic tool in epilepsy surgery. Acta Neurol Scand. 2008;117(2):90–93. doi: 10.1111/j.1600-0404.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 32.Manninen PH, Burke SJ, Wennberg R, Lozano AM, El Beheiry H. Intraoperative localization of an epileptogenic focus with alfentanil and fentanyl. Anesth Analg. 1999;88(5):1101–1106. doi: 10.1097/00000539-199905000-00025. [DOI] [PubMed] [Google Scholar]
- 33.McGuire G, El-Beheiry H, Manninen P, Lozano A, Wennberg R. Activation of electrocorticographic activity with remifentanil and alfentanil during neurosurgical excision of epileptogenic focus. Br J Anaesth. 2003;91(5):651–655. doi: 10.1093/bja/aeg241. [DOI] [PubMed] [Google Scholar]
- 34.Ross J, Kearse LA, Jr, Barlow MK, Houghton KJ, Cosgrove GR. Alfentanil-induced epileptiform activity: a simultaneous surface and depth electroencephalographic study in complex partial epilepsy. Epilepsia. 2001;42(2):220–225. doi: 10.1046/j.1528-1157.2001.18600.x. [DOI] [PubMed] [Google Scholar]
- 35.Zieglgänsberger W, French ED, Siggins GR, Bloom FE. Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science. 1979;205(4404):415–417. doi: 10.1126/science.451610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.