Significance
Multipotent cells, such as mesenchymal stromal cells (MSCs), have the capacity to differentiate into cartilage-forming cells. Chondrocytes derived from MSCs obtain an epiphyseal cartilage-like phenotype, which turns into bone upon implantation via endochondral ossification. Here, we report that the chondrogenic fate of MSCs can be metabolically programmed by low oxygen tension to acquire an articular chondrocyte-like phenotype via mechanisms that resemble natural development. Our study identifies metabolic programming of stem cells by oxygen tension as a powerful tool to control cell fate, which may have broad applications for the way in which stem cells are now prepared for clinical use.
Keywords: tissue engineering, chondral defects, skeletogenesis, cell therapy, regenerative medicine
Abstract
Actively steering the chondrogenic differentiation of mesenchymal stromal cells (MSCs) into either permanent cartilage or hypertrophic cartilage destined to be replaced by bone has not yet been possible. During limb development, the developing long bone is exposed to a concentration gradient of oxygen, with lower oxygen tension in the region destined to become articular cartilage and higher oxygen tension in transient hypertrophic cartilage. Here, we prove that metabolic programming of MSCs by oxygen tension directs chondrogenesis into either permanent or transient hyaline cartilage. Human MSCs chondrogenically differentiated in vitro under hypoxia (2.5% O2) produced more hyaline cartilage, which expressed typical articular cartilage biomarkers, including established inhibitors of hypertrophic differentiation. In contrast, normoxia (21% O2) prevented the expression of these inhibitors and was associated with increased hypertrophic differentiation. Interestingly, gene network analysis revealed that oxygen tension resulted in metabolic programming of the MSCs directing chondrogenesis into articular- or epiphyseal cartilage-like tissue. This differentiation program resembled the embryological development of these distinct types of hyaline cartilage. Remarkably, the distinct cartilage phenotypes were preserved upon implantation in mice. Hypoxia-preconditioned implants remained cartilaginous, whereas normoxia-preconditioned implants readily underwent calcification, vascular invasion, and subsequent endochondral ossification. In conclusion, metabolic programming of MSCs by oxygen tension provides a simple yet effective mechanism by which to direct the chondrogenic differentiation program into either permanent articular-like cartilage or hypertrophic cartilage that is destined to become endochondral bone.
The limited regenerative capacity of articular cartilage, combined with its susceptibility to damage from high-energy impacts, repetitive shear, and torsional forces, has led to a growing need for new therapeutic strategies. The use of multipotent cells, such as mesenchymal stromal cells (MSCs), to form de novo hyaline cartilage remains a promising strategy (1). Importantly, unlike articular chondrocytes, MSCs can be isolated in high numbers from various sources (2, 3) without the creation of a secondary defect in the diseased joint (2, 4).
During the past decades, substantial progress has been made in gaining control over the derivation of chondrocytes from progenitor cells (5). Unfortunately, it is not yet possible to steer the differentiation of MSCs into the formation of permanent hyaline cartilage. Instead, the present protocols for chondrogenically differentiating MSCs result in the production of neocartilage that is characterized by hypertrophic differentiation (6–9). Consequently, the newly formed cartilage undergoes endochondral ossification upon implantation (8, 10–12). In fact, it has been reported that, currently, the most efficient way to engineer new bone from multipotent progenitor cells is via implantation of in vitro-generated neocartilage (6). Accumulating evidence indeed suggests that the current chondrogenic differentiation protocols for MSCs result in hypertrophic hyaline cartilage that more closely resembles growth plate-like cartilage than articular cartilage (7, 13). In line with these observations, the current protocols do not induce the transcription of genes encoding regulators of articular cartilage homeostasis that effectively inhibit hypertrophic differentiation (7, 13).
During embryonic development, the permanent articular cartilage and the transient hypertrophic cartilage both arise from the same cartilaginous anlage. However, specific sets of stimuli drive these two hyaline cartilages into distinct differentiation programs. Attempts to identify the required stimuli to drive the formation of permanent articular cartilage have been largely unsuccessful to date. Recent studies suggested that oxygen levels might play a role in driving hypertrophic differentiation (14, 15). Interestingly, in the cartilage anlage, permanent articular cartilage is formed under hypoxic conditions, whereas hypertrophic differentiation of cartilage and subsequent endochondral ossification are associated with vascular invasion and, consequently, much higher levels of oxygen. Remarkably, standard differentiation protocols for MSCs occur in normoxic conditions. Based on these observations, we hypothesized that the proper choice of oxygen tension might be a powerful mechanism with which to steer chondrogenic differentiation of MSCs.
Here, we report that oxygen levels control the chondrogenic differentiation program of MSCs to become either articular- or epiphyseal-like cartilage through metabolic programming.
Results
Hypoxia Stimulates Chondrogenic Differentiation of MSCs.
Micromasses of MSCs were differentiated into the chondrogenic lineage for up to 35 d in the presence of TGF-β3 in either normoxic or hypoxic conditions. Histological analysis demonstrated little to no positive glycosaminoglycan staining after 7 d of culture in either normoxic or hypoxic conditions (Fig. 1A). In contrast, at 21 d of chondrogenic differentiation, intense glycosaminoglycan staining throughout the pellet was observed in pellets cultured in hypoxia compared with modest glycosaminoglycan staining in pellets cultured in normoxia. Particularly in the periphery of the hypoxic cultured pellets, chondron formation was visible at lower density. After 35 d, glycosaminoglycan staining intensified in the pellets cultured in hypoxia. Interestingly, in micromasses cultured in normoxia glycosaminoglycan, staining remained lower and positive staining for glycosaminoglycans was predominantly found in the center of the pellet. Strikingly, these MSC-derived chondrocytes were characterized by a hypertrophic phenotype due to their enlarged size and the presence of lacunae (Fig. 1B). This phenomenon was absent under hypoxic conditions. Moreover, the rim of pellets cultured in normoxia suggested the presence of a more stratified cartilaginous matrix, indicating fibrous tissue formation. Biochemical quantification corroborated enhanced glycosaminoglycan deposition in hypoxia compared with normoxia at 21 and 35 d of differentiation (Fig. 1C).
Fig. 1.
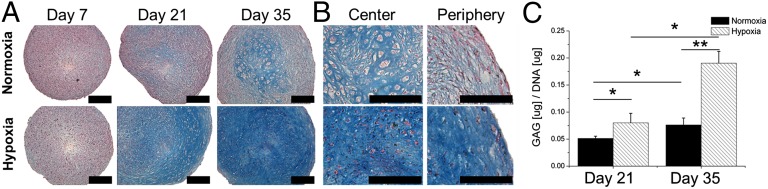
Hypoxia stimulates chondrogenic differentiation of MSCs. (A) Micromasses of MSCs were cultured for up to 35 d under either normoxic or hypoxic conditions. Histological analysis of midsagittal sections using Alcian blue and Nuclear Fast Red was used to visualize chondrogenic differentiation. (B) High-magnification microphotographs were taken of the center and periphery of the micromasses to visualize their cartilage phenotype. (C) Biochemical analysis of glycosaminoglycans (GAG) was used to quantify chondrogenesis. Data represent the mean of three donors, each measured in quadruplicate ± SD. *P < 0.05; **P < 0.01. (Scale bars: 100 μm.)
Effect of Oxygen on Gene Expression Profile of Chondrogenically Differentiating MSCs.
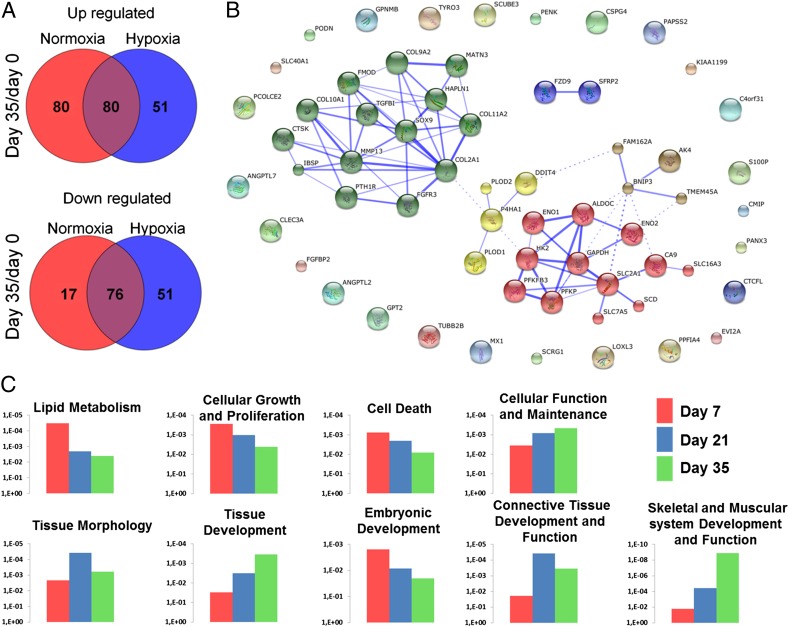
Whole-genome gene expression analysis was performed on MSCs that were chondrogenically differentiated for 7, 21, or 35 d under either hypoxic or normoxic culture conditions. These gene expression profiles were compared with the gene expression profiles of the initially undifferentiated MSCs. In total, 503 genes were significantly differentially expressed with a more than twofold change in at least one of the time points compared with the undifferentiated MSCs over the 35-d culture period. Hierarchical clustering of these 503 significantly differentially expressed genes revealed that during the first 21 d of culture, the time in culture accounted for larger changes in gene expression than those changes caused by differential oxygen exposure. However, after 35 d of chondrogenic differentiation, the effect of oxygen tension became more dominant. Cells cultured in normoxia for 35 d demonstrated more resemblance to cells cultured for 21 d in normoxia than to those cells cultured for 35 d in hypoxia. This observation suggested a potential difference in cell fate (Fig. S1A). Comparison of undifferentiated MSCs with chondrogenically differentiated pellets at day 35 revealed that more genes were up-regulated during normoxic culture compared with hypoxic culture (160 vs. 131 genes, respectively). In hypoxia, more genes were down-regulated compared with normoxic culture (93 vs. 127 genes, respectively) (Fig. 2A). Comparing overall differences in gene expression between hypoxia and normoxia demonstrated that 60 genes were up-regulated and nine genes were down-regulated more than twofold at day 35 (Fig. S1B).
Fig. 2.
Whole-genome gene expression analysis of chondrogenically differentiated MSCs in either normoxic or hypoxic conditions. (A) Venn diagrams depicting the gene transcripts with at least a twofold difference that were significantly differentially expressed between day 35 and day 0 under normoxic or hypoxic culture conditions. (B) Network analysis of predicted gene or protein interactions of gene transcripts differentially expressed in MSCs after 35 d of chondrogenic differentiation in either normoxic or hypoxic conditions. The red cluster predominantly contains genes involved in glycolysis, and the green cluster predominantly contains genes with a cartilage signature. Both red and green clusters are up-regulated under hypoxic conditions. In contrast, the yellow cluster containing COL10A1 and MMP13 is up-regulated in normoxic conditions. (C) We then visualized the significantly different biofunctions between normoxic and hypoxic culture conditions at day 7, day 21, and day 35 with their respective P values on the y axis. Each data point is based on the gene expression analysis of three donors.
Differentially expressed genes between hypoxia and normoxia at day 35 were used to analyze gene/protein network interactions. Using Markov clustering algorithms, two key nodes within the network were observed (Fig. 2B). Metabolism-related genes, predominantly involving regulation of glycolysis, characterized the smaller cluster. The larger node consisted predominantly of genes important for the formation and function of articular cartilage matrix, such as TGF-β1, collagen type II (COL2A1), and sex-determining region Y-box 9 (SOX9). Moreover, it included several genes that encoded proteins associated with inhibition of hypertrophic differentiation, such as FGF receptor 3 (FGFR3) and parathyroid hormone 1 receptor (PTH1R). The expression of these anabolic genes is up-regulated in hypoxia. In contrast, functional biomarkers of hypertrophic differentiation, such as collagen type X (COL10A1), matrix metalloproteinase 13 (MMP13), and cathepsin K (CTSK), were up-regulated in normoxia.
Pathway analysis revealed that the differences between hypoxia and normoxia at day 7 were dominated by biofunctions that are related to metabolism, proliferation, and cell death. Moreover, an important, significant, and progressive change in the classifiers’ cellular function and maintenance and tissue development was observed over time. In particular, this process was initiated at day 7 with a difference in “embryonic development,” followed by a transient increase in “connective tissue development and function” at day 21 and, finally, an increase of “skeletal and muscular system development and function” at day 35 (Fig. 2C). Taken together, these data suggested that continuous hypoxia steered the chondrogenic fate of MSCs in a manner that resembled natural embryological development.
Hypoxia Induces an Articular Cartilage-Like Profile in Chondrogenically Differentiated MSCs.
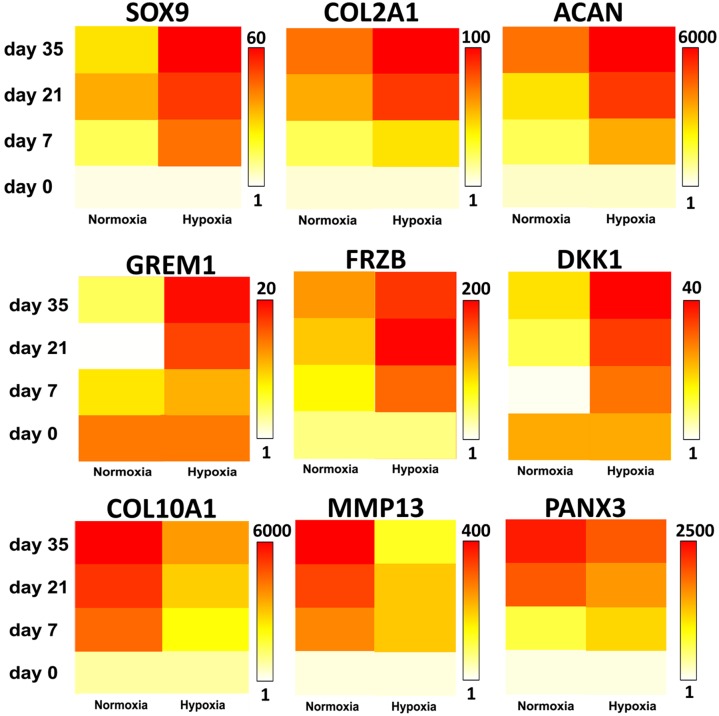
We validated that hypoxia enhanced the transcription of typical hyaline cartilage markers, such as SOX9, COL2A1, and aggrecan (ACAN) using quantitative PCR (qPCR) analysis (Fig. 3). Previously, we identified a panel of markers able to discriminate the two subtypes of hyaline cartilage: the permanent articular cartilage and the hypertrophic growth plate cartilage (7). The articular cartilage-enriched gene transcripts of gremlin 1 (GREM1), frizzled-related protein (FRZB), and Dickkopf WNT signaling pathway inhibitor 1 (DKK1), which are established inhibitors of hypertrophic differentiation (7, 16), were robustly increased under hypoxic conditions, whereas under normoxic conditions, these genes did not increase markedly (Fig. 3). The hypertrophic cartilage-enriched gene transcripts of COL10A1, MMP13, and pannexin 3 (PANX3) mRNA levels were strongly up-regulated under normoxic conditions compared with hypoxic conditions. The increased expression of the secreted antagonists GREM1, FRZB, and DKK1 at the mRNA level in hypoxia was corroborated by protein expression analysis. The protein levels of these three secreted proteins in culture medium were significantly higher after 35 d of chondrogenic differentiation under hypoxia compared with normoxia (Fig. 4). Together, these findings suggested that oxygen tension is selectively able to induce MSCs to express biomarkers that correlate with either permanent articular cartilage or transient hypertrophic cartilage.
Fig. 3.
Hypoxia stimulated the expression of gene transcripts toward an articular cartilage-like profile. Chondrogenically differentiating MSCs in either normoxic or hypoxic conditions were analyzed for gene expression of the hyaline cartilage markers SOX9, COL2A1, and ACAN; the hypertrophic cartilage markers COL10A1, MMP13, and PANX3, and the articular cartilage markers GREM1, FRZB, and DKK1 by qPCR. Data are illustrated in a linear heat map in which white represents the lowest gene expression and red represents the highest gene expression, of which the maximal value is given in fold change. Data represent the mean of three donors, each measured in triplicate.
Fig. 4.
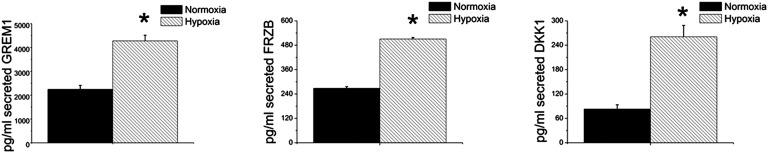
Normoxia increased secretion of GREM1, FRZB, and DKK1 in culture medium. The articular cartilage-enriched markers FRZB, GREM1, and DKK1 were analyzed using ELISA. Data represent the mean of three donors, each measured in triplicate ± SD. *P < 0.05.
Continued Hypoxia Is Needed to Retain Chondrogenic Stimulus.
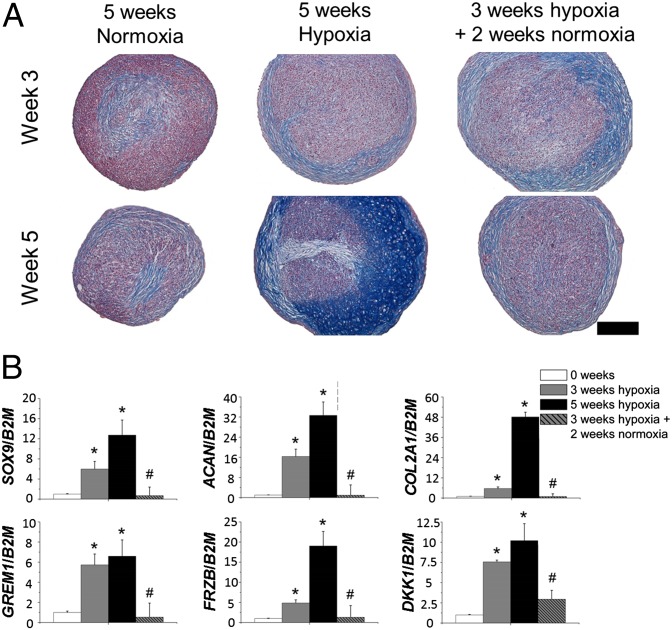
We next explored whether transient exposure to hypoxia was sufficient to steer the chondrogenesis of MSCs toward a permanent articular cartilage-like phenotype. MSCs were differentiated for 5 wk in normoxia, 5 wk in hypoxia, or 3 wk in hypoxia followed by 2 wk of normoxia, or 3 wk in normoxia followed by 2 wk in hypoxia. Hypoxia progressively enhanced glycosaminoglycan deposition and increased SOX9, ACAN, COL2A1, GREM1, FRZB, and DKK1 mRNA levels. However, when the hypoxic stress was alleviated after 3 wk, it reversed the chondrogenic benefit generated by the initial exposure to hypoxia, as witnessed by decreased glycosaminoglycan deposition (Fig. 5A). Moreover, when hypoxic preconditioned micromasses were transferred to normoxia after 21 d, the increased mRNA levels of chondrogenic genes dropped to levels found in undifferentiated MSCs (Fig. 5B). Inversely, mRNA expression levels of SOX9, ACAN, COL2A1, GREM1, FRZB, and DKK1 were not significantly different between MSCs that were allowed to differentiate chondrogenically for 2 wk in normoxia followed by 3 wk in hypoxia and MSCs that underwent 5 wk of continuous hypoxic differentiation (Fig. S2). This observation suggested that alleviation of hypoxia in hyaline cartilage, particularly after the onset of chondrogenic differentiation between day 14 and day 21, is detrimental to the expression of genes that are hallmarks of permanent articular cartilage homeostasis.
Fig. 5.
MSCs underwent chondrogenic differentiation for 5 wk in normoxia, 5 wk in hypoxia, or 3 wk in hypoxia followed by 2 wk in normoxia. (A) Histological analysis of glycosaminoglycans using Alcian blue and Nuclear Fast Red on midsagittal sections of MSC micromasses, which were chondrogenically differentiated for 3 or 5 wk. (Scale bar: 100 μm.) (B) Gene expression analysis of the articular cartilage markers SOX9, ACAN, and COL2A1 and the articular cartilage-enriched markers GREM1, FRZB, and DKK1. Data represent the mean of three donors, each measured in triplicate ± SD. *P < 0.05 for continuously hypoxic cultures compared with week 0; #P < 0.05 for 5-wk cultures that were noncontinuously hypoxic compared with 5-wk continuously hypoxic cultures.
Hypoxic Chondrogenic Differentiation of MSCs Strongly Reduces Calcification upon Implantation.
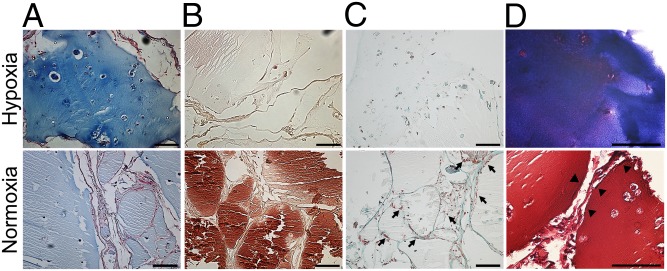
Lastly, we investigated whether predifferentiation of MSCs in vitro in either hypoxia or normoxia would affect the fate of the cartilaginous tissue upon s.c. implantation in a nude mouse model. To this end, MSCs were encapsulated in alginate hydrogels and chondrogenically differentiated for 5 wk in vitro under either hypoxia or normoxia before implantation. After 5 wk of implantation, hypoxia-preconditioned samples stained intensely for glycosaminoglycans, whereas normoxia-preconditioned samples only stained weakly (Fig. 6A). It is noteworthy that normoxic preconditioned samples demonstrated striking invasion of non–cartilage-forming cells that are presumably of the host. Furthermore, normoxic preconditioned samples stained strongly positive for calcium, indicating tissue calcification (Fig. 6B), and demonstrated the abundant presence of blood vessels (Fig. 6C). This phenotype is in great contrast to the hypoxic preconditioned samples that remained devoid of blood vessels and cartilaginous upon implantation for at least the investigated time frame, whereas the normoxic preconditioning resulted in replacement of the implanted cartilage with bone-like tissue (Fig. 6D). Together, these findings demonstrated that oxygen tension can steer the chondrogenic program of MSCs in tissue-engineering constructs toward an articular cartilage-like phenotype or epiphyseal cartilage-like phenotype that will undergo endochondral ossification upon implantation.
Fig. 6.
In vivo behavior of MSC-laden alginate implants that were preconditioned in either normoxia (21% oxygen) or hypoxia (2.5% oxygen) for 5 wk before implantation. Samples were explanted after 5 wk. Histological analysis of paraffin-embedded samples stained with Alcian blue with Nuclear Fast Red counterstaining for cartilage formation (A), Alizarin Red S for tissue calcification (B), Masson’s trichrome for vascular invasion (C), and methylene blue and basic fuchsin for transformation of cartilage into endochondral bone (D). Arrows indicate invading blood vessels containing erythrocytes. Arrowheads indicate lining cells. Data are representative for three samples. (Scale bars: 100 μm.)
Discussion
Cartilage tissue engineering has the potential to regenerate and restore the articular surface of diarthrodial joints. Consequently, it offers potential solutions for clinical issues ranging from trauma to osteoarthritis. Robust formation of permanent hyaline cartilage is essential to the successful development of such therapies. Because isolation of articular chondrocytes relies on inflicting additional damage to the injured joint, multipotent cells with chondrogenic potential have been intensively investigated and found to be a promising cell source for cartilage tissue engineering (1, 17). Although it is possible to induce chondrogenesis in multipotent cells, we currently lack the understanding to steer the chondrogenic differentiation program accurately. In fact, the current chondrogenic protocols efficiently create hypertrophic cartilage, which readily undergoes endochondral ossification upon implantation (6, 9, 12) resembling epiphyseal growth plate cartilage. However, reproducibly producing permanent articular cartilage from stem or progenitor cells has remained an unsolved challenge.
Understanding the natural environments of developmental processes can yield crucial information on the mechanisms of steering cell behavior. During development, skeletal formation in the cartilage anlage, from condensation to formation of articular cartilage and endochondral bone, coincides with a spatiotemporal controlled exposure to oxygen tension. Specifically, the development of articular cartilage in the cartilage anlage occurs under continuous hypoxic conditions, whereas terminal hypertrophic differentiation is associated with a physiological switch toward normoxia induced by orchestrated ingrowth of blood vessels. Mimicking this environmental factor in vitro has demonstrated similar effects. In particular, chondrogenic differentiation under hypoxic conditions results in enhanced cartilage formation and suppresses hypertrophic differentiation (15). In contrast, chondrogenic differentiation under normoxic conditions yields less cartilage, which has a clear hypertrophic signature (11, 18, 19). Here, we demonstrate that oxygen tension can, in fact, metabolically program the chondrogenic fate of MSCs into different subtypes of hyaline cartilage. More precisely, we revealed that continuous hypoxia induces chondrogenic MSCs to produce hyaline cartilage that is resistant to hypertrophic differentiation and subsequent endochondral ossification upon implantation in mice. This cartilage expressed typical biomarkers of articular cartilage and established inhibitors of hypertrophic differentiation, which have been implemented in the maintenance of articular cartilage homeostasis (7, 16). In contrast, chondrogenic differentiation of MSCs cultured under the conventionally used normoxic conditions resulted in hypertrophic hyaline cartilage that resembled epiphyseal cartilage. Interestingly, gene expression analysis indicated that the metabolic programming of the chondrogenic cell fate correlated with the natural development of distinct cartilage subtypes. This observation potentially implicates, or at least underlines, the powerful and pivotal role of environmental factors, such as oxygen tension, as cell fate programming agents.
The metabolic programming of MSCs, chondrogenic MSCs, or matured chondrocytes via oxygen tension has potentially important consequences. In vitro experimentation on mammalian cells is nearly exclusively performed under 21% oxygen conditions. This unphysiological environment influences the cell’s behavior, function, and fate, and is thus able to confound our fundamental understanding of naturally occurring differentiation processes, which may have implications for the development of novel therapies. For example, high oxygen tension metabolically programs MSCs and chondrocytes to induce the expression of genes involved in hypertrophic differentiation and matrix remodeling, which are also biomarkers for degenerative joint diseases, such as osteoarthritis. Thus, the standard use of supraphysiological oxygen levels during in vitro cultures can obscure our interpretation of in vitro studies on the behavior of cells from diseased tissue as well as interfere with the reliability of biomarkers that are used in drug development. Therefore, studying cells in environments that are as natural as possible, particularly with respect to oxygen tension, might prove both essential and inevitable.
Understanding the signaling mechanisms that underlie the metabolic programming of the chondrogenic cell fate might allow for the efficient production of either permanent articular cartilage or transient hypertrophic cartilage without the need for an extensive in vitro culture period. A possible candidate is the hypoxia-inducible factor signaling pathway, which is directly modulated by oxygen tension. Moreover, this pathway is associated with both chondrogenesis and the induction of hypertrophic differentiation in articular cartilage during the pathological development of osteoarthritis (20). Alternatively, it has recently been reported that the activity of the PI3K/AKT/FOXO pathway is influenced by oxygen tension and is able to enhance chondrogenesis, inhibit hypertrophic differentiation, and prevent endochondral ossification (21). Also, SMAD signaling induced by members of the TGF-β/BMP family of growth factors is influenced by oxygen tension (22). However, the determination of cell fate is typically a multistage decision involving multiple signaling pathways in a spatiotemporal manner. In addition to single candidates, it is likely that multiple signaling pathways are able to act in concert or even synergistically (21). The involvement of multiple signaling pathways upon exposure to distinct oxygen tension is also suggested by observations in our current study. Specifically, although programming of chondrogenic fates elicited a similar response among donors, differences in cell survival under low oxygen tensions were marked by interdonor variation. Moreover, hypoxic preconditioning resulted in implants that are characterized by relatively low cell densities. These implants proved sufficient for preservation of the chondrocyte phenotype in implantation studies for at least 5 wk. The long-term effects of metabolic preconditioning still remain to be elucidated. Our data indicate that a continuous hypoxic microenvironment is necessary to preserve the articular cartilage-like phenotype. In particular, hypoxia induced the expression of trophic factors involved in the inhibition of hypertrophic differentiation. This observation underlines the importance of the progenitor cell’s trophic role in tissue regeneration. Trophic factors can mediate tissue regeneration in both direct and indirect manners (23–25). Progressive insight indicates that current culture and treatment protocols allow progenitor cells to contribute mainly to regenerative effects via trophic roles rather than direct tissue formation (26). However, it is likely that the manner in which progenitor cells contribute to tissue regeneration is based on their preconditioning, manner of application, and in vivo microenvironment. Our study suggests the possibility of metabolic programming to prime the trophic role of the MSCs for a specific role in tissue repair.
The s.c. implantation of the distinctly preprogrammed chondrogenic constructs was characterized by remarkably dissimilar behaviors. Where the hypoxic pretreated implants resembled permanent mature articular-like cartilage devoid of signs of hypertrophic differentiation, the normoxic pretreated implants readily underwent hypertrophic differentiation and endochondral ossification. Most notably, the normoxic pretreated implants were strongly invaded by noncartilaginous tissue, which contained a high density of perfused small blood vessels. This phenomenon might be explained by the cartilaginous matrix degradation and expression of, for example, angiogenic factors that are associated with hypertrophic differentiation. Regardless, implanting pretreated implants exposes the tissue-engineered constructs to an environment that is dependent on diffusion of oxygen derived from the host for survival. Oxygen-generating or -scavenging biomaterials might prove to be an efficient way in which to control the chondrogenic differentiation program of progenitor cells in vivo.
Our observations might aid tissue-engineering approaches in important ways. For example, by inducing cartilage formation under hypoxic conditions, MSCs may generate permanent articular cartilage that could be used for better treatment of articular cartilage defects. In contrast, by inducing cartilage formation in normoxia, transient and hypertrophic cartilage is formed, which may be highly suited to endochondral healing of critical bone defects. Although our study is limited to the role of oxygen-mediated programming of MSCs in the chondrogenic lineages, it seems likely that oxygen-mediated metabolic programming may play a broader role in governing cellular differentiation processes into other tissue types. Control over oxygen tension and its manipulation appears to be a powerful tool with which to program the function and fate of MSCs, ultimately enabling control over their behavior in a clinical setting. Biomaterials that are designed either to release oxygen to stimulate cell survival or to mimic hypoxia to stimulate angiogenesis may, in fact, act as instructive materials for controlled differentiation of mesenchymal progenitor cells into cartilage and bone (27, 28).
Taken together, in the present study, we demonstrated that control over oxygen tension can actively steer the chondrogenic differentiation program and the fate of MSCs by metabolic programming. Importantly, this approach provides tissue-engineering strategies with a simple yet effective tool to create permanent articular cartilage or hypertrophic cartilage that will undergo endochondral ossification upon implantation.
Materials and Methods
Patient Material.
The use of patient material was approved by the local ethical committee of the Medisch Spectrum Twente, and informed written consent was obtained for all samples. Human MSCs of three donors were isolated from fresh bone marrow samples, cultured as described previously, and used individually in all presented experiments (29).
Chondrogenesis of MSCs.
Micromasses of MSCs were formed by gravitational seeding of 2.5 × 105 cells in 96 U-shaped, low-attachment well plates (Greiner Bio-One). Subsequently, chondrogenic differentiation of MSCs was chemically induced using chondrogenic medium containing 10 ng/mL TGF-β3. MSCs were allowed to differentiate up to 35 d under either normoxic (21% oxygen) or hypoxic (2.5% oxygen) conditions. Medium was refreshed every 3–4 d. For each individual donor, four pellets were pooled for RNA isolation and two were fixed in 10% (vol/vol) buffered formalin for histological analysis on days 7, 21 and 35.
Gene Expression Profiling.
MSC micromasses were lysed using TRIzol reagent (Invitrogen). Total RNA was isolated and purified using a Nucleospin RNA II kit (Bioke). Total RNA yields were measured using a Nanodrop2000 instrument (Isogen LifeScience). High quality of the RNA was verified using an Agilent 2100 Bioanalyzer (Agilent). For whole-genome gene expression analysis, amplified cDNA was synthesized with an Ovation PicoSL WTA System kit (NuGEN), biotinylated with an Encore BiotinIL module (NuGEN), and hybridized onto Illumina HumanHT-12 v4 Expression BeadChips. Genes were selected that had at least a twofold difference and were significantly differentially expressed according to a one-way ANOVA with a Benjamini–Hochberg false discovery rate correction and Tukey’s honestly significant difference post hoc test using a cutoff rate of P = 0.05. Changes in canonical pathways and biofunctions were visualized using Ingenuity Pathway Analysis software (Ingenuity Systems), and predicted gene/gene interaction networks were visualized using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING), version 9.0 (30). For single-gene expression analysis, cDNA was synthesized using iScript (BioRad), of which 20 ng was amplified in a real-time qPCR assay using Sensimix (Bioline) and a MyIQ detection system (BioRad). Gene expression was normalized using ACTB and B2M as housekeeping genes, which were unaffected by both chondrogenic differentiation and variation in oxygen tension. Data were visualized as a heat map generated using the software program R (R Project).
In Vivo Study.
Animal experimentation was performed in accordance with Dutch law and with the explicit approval of the local animal care and use committee of the University Medical Centre Utrecht (approval no. 104231-6). Implants were formed by encapsulating 1 million MSCs in 100 μL of 1.5% (wt/vol) sodium alginate (Sigma–Aldrich) using 100 mM CaCl2 (Sigma–Aldrich). Implants were preconditioned for 5 wk in vitro under either normoxic or hypoxic conditions in chondrogenic medium. Then, the cartilaginous implants were s.c. implanted in 8-wk-old nude male mice (NMRI-Nude; Harlan Laboratories). After 5 wk, the samples were explanted and histologically analyzed.
Histological Analysis.
Cell culture pellets and in vivo samples were washed and dehydrated in graded series of ethanol at room temperature. After overnight incubation in butanol at 4 °C, samples were embedded in paraffin and cut into 5-μm sections. Sections were deparaffinized in xylene and rehydrated using graded ethanol steps. Sections were stained for cartilage formation with 0.5% (wt/vol) Alcian blue (Sigma) and 0.1% (wt/vol) Nuclear Fast Red (Sigma), calcification with 2% (wt/vol) Alizarin Red S (Sigma), vascular invasion using Masson’s trichrome (Merck), or bone formation with 1% (wt/vol) methylene blue (Sigma) and basic fuchsin (Sigma) according to standard procedures. Histological sections were analyzed using a light microscope (E600; Nikon).
Quantitative Glycosaminoglycan and DNA Assay.
MSC micromasses were analyzed for glycosaminoglycan content as previously described (31). All values were normalized to their respective DNA amount and expressed as the glycosaminoglycan/DNA (μg/μg) ratio.
Quantification of GREM1, FRZB, and DKK1 Protein Levels in Conditioned Media.
After 32 d of chondrogenic differentiation, medium was conditioned for 3 d. Protein levels of GREM1 (USCN Life Science), FRZB (R&D Systems), and DKK1 (R&D Systems) secreted by the cells into the culture supernatant were measured by ELISA following the instructions of each manufacturer.
Statistical Analysis.
Statistical differences between two groups were analyzed using the Student t test or one-way ANOVA. Statistical significance was set to P < 0.05 and was indicated with an asterisk or hash (#) sign. Results are presented as mean ± SD.
Supplementary Material
Acknowledgments
We acknowledge the support of the translational excellence in regenerative medicine Smart Mix Program of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture, and Science. M.K. is supported by a long-term program subsidy of the Dutch Arthritis Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410977111/-/DCSupplemental.
References
- 1.Leijten JC, Georgi N, Wu L, van Blitterswijk CA, Karperien M. Cell sources for articular cartilage repair strategies: Shifting from monocultures to cocultures. Tissue Eng Part B Rev. 2013;19(1):31–40. doi: 10.1089/ten.TEB.2012.0273. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 5.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA. 2002;99(7):4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jukes JM, et al. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci USA. 2008;105(19):6840–6845. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leijten JC, et al. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012;64(10):3302–3312. doi: 10.1002/art.34535. [DOI] [PubMed] [Google Scholar]
- 8.Steck E, et al. Mesenchymal stem cell differentiation in an experimental cartilage defect: Restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009;18(7):969–978. doi: 10.1089/scd.2008.0213. [DOI] [PubMed] [Google Scholar]
- 9.Scotti C, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA. 2010;107(16):7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koga H, et al. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells. 2007;25(3):689–696. doi: 10.1634/stemcells.2006-0281. [DOI] [PubMed] [Google Scholar]
- 11.Pelttari K, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54(10):3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 12.Scotti C, et al. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci USA. 2013;110(10):3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Gool SA, et al. Fetal mesenchymal stromal cells differentiating towards chondrocytes acquire a gene expression profile resembling human growth plate cartilage. PLoS ONE. 2012;7(11):e44561. doi: 10.1371/journal.pone.0044561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morita K, et al. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med. 2007;204(7):1613–1623. doi: 10.1084/jem.20062525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leijten JC, Moreira Teixeira LS, Landman EB, van Blitterswijk CA, Karperien M. Hypoxia inhibits hypertrophic differentiation and endochondral ossification in explanted tibiae. PLoS ONE. 2012;7(11):e49896. doi: 10.1371/journal.pone.0049896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leijten JC, et al. GREM1, FRZB and DKK1 mRNA levels correlate with osteoarthritis and are regulated by osteoarthritis-associated factors. Arthritis Res Ther. 2013;15(5):R126. doi: 10.1186/ar4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreira-Teixeira LS, Georgi N, Leijten J, Wu L, Karperien M. Cartilage tissue engineering. Endocr Dev. 2011;21:102–115. doi: 10.1159/000328140. [DOI] [PubMed] [Google Scholar]
- 18.Khan WS, Adesida AB, Hardingham TE. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther. 2007;9(3):R55. doi: 10.1186/ar2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanichai M, Ferguson D, Prendergast PJ, Campbell VA. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: A role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol. 2008;216(3):708–715. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- 20.Husa M, Liu-Bryan R, Terkeltaub R. Shifting HIFs in osteoarthritis. Nat Med. 2010;16(6):641–644. doi: 10.1038/nm0610-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HH, et al. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Sci Rep. 2013;3:2683. doi: 10.1038/srep02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirao M, Tamai N, Tsumaki N, Yoshikawa H, Myoui A. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. J Biol Chem. 2006;281(41):31079–31092. doi: 10.1074/jbc.M602296200. [DOI] [PubMed] [Google Scholar]
- 23.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Leijten J, van Blitterswijk CA, Karperien M. Fibroblast growth factor-1 is a mesenchymal stromal cell-secreted factor stimulating proliferation of osteoarthritic chondrocytes in co-culture. Stem Cells Dev. 2013;22(17):2356–2367. doi: 10.1089/scd.2013.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L, et al. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17(9-10):1425–1436. doi: 10.1089/ten.TEA.2010.0517. [DOI] [PubMed] [Google Scholar]
- 26.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C, et al. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials. 2012;33(7):2076–2085. doi: 10.1016/j.biomaterials.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 28.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30(5):757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 29.Both SK, van der Muijsenberg AJ, van Blitterswijk CA, de Boer J, de Bruijn JD. A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng. 2007;13(1):3–9. doi: 10.1089/ten.2005.0513. [DOI] [PubMed] [Google Scholar]
- 30.Szklarczyk D, et al. The STRING database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011;39(Database issue):D561–D568. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin R, et al. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran-hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials. 2010;31(11):3103–3113. doi: 10.1016/j.biomaterials.2010.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.