Abstract
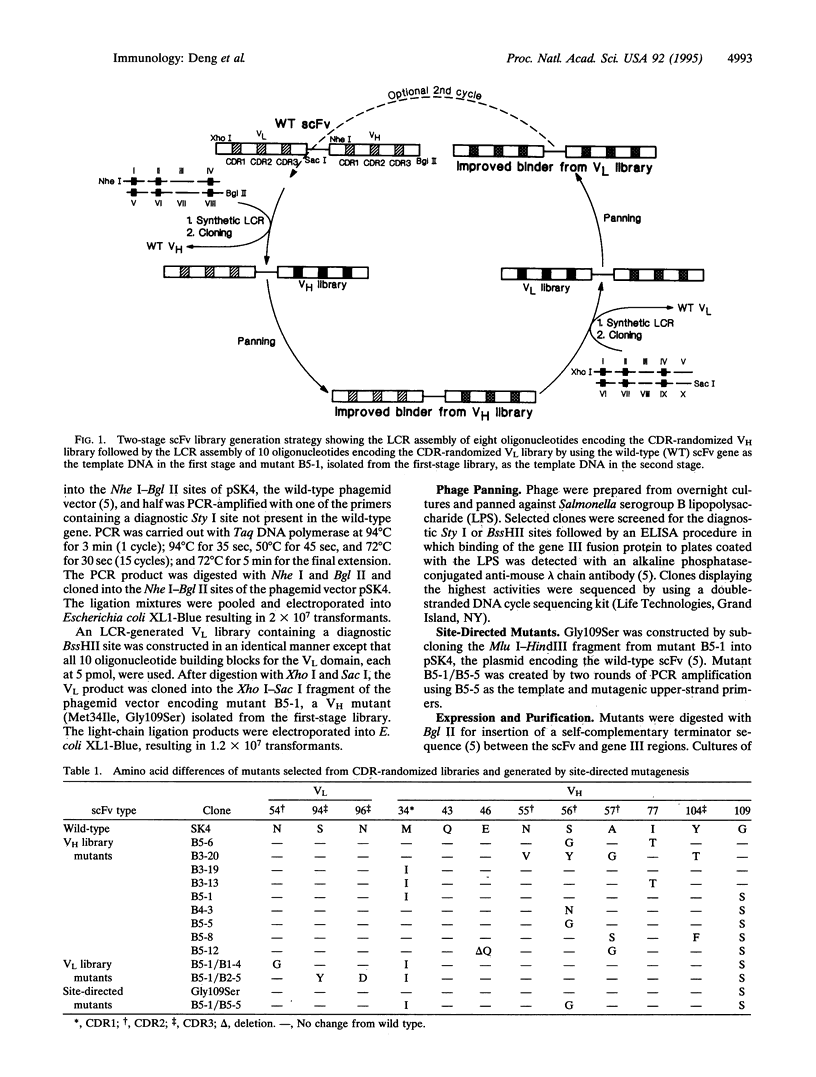
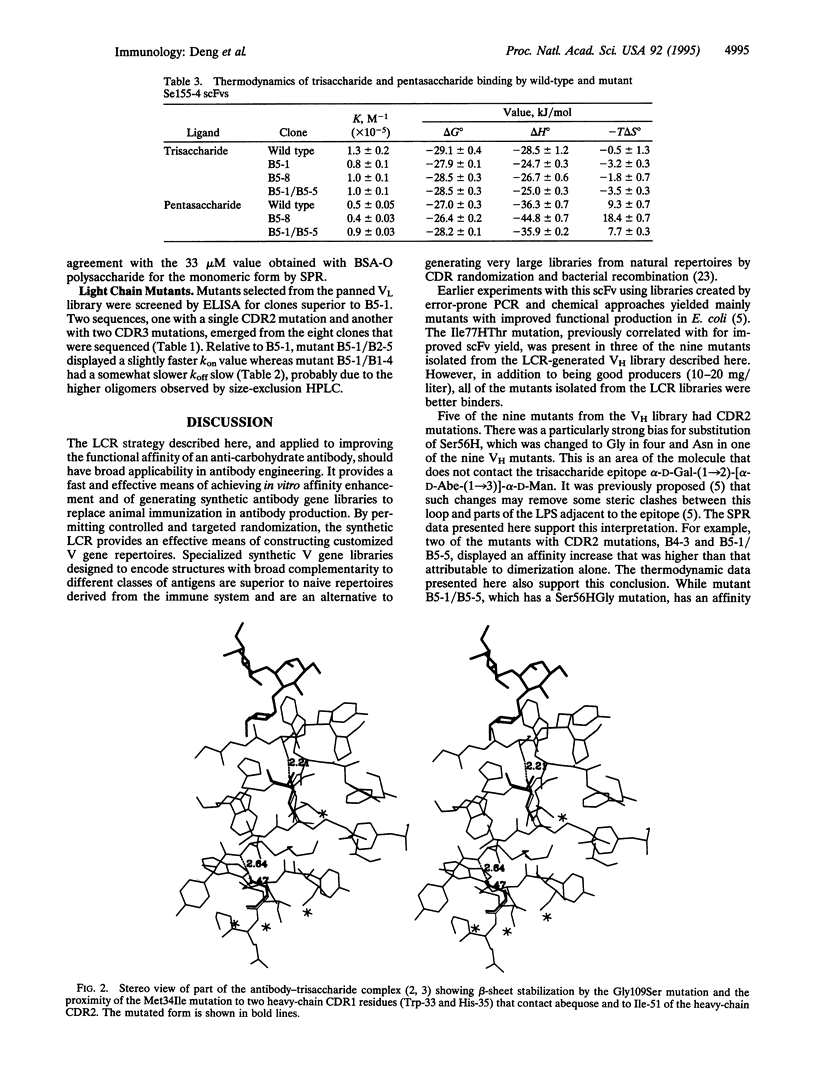
A technique is described for the simultaneous and controlled random mutation of all three heavy or light chain complementarity-determining regions (CDRs) in a single-chain Fv specific for the O polysaccharide of Salmonella serogroup B. Sense oligonucleotides were synthesized such that the central bases encoding a CDR were randomized by equimolar spiking with A, G, C, and T at a level of 10% while the antisense strands contained inosine in the spiked regions. Phage display of libraries assembled from the spiked oligonucleotides by a synthetic ligase chain reaction demonstrated a bias for selection of mutants that formed dimers and higher oligomers. Kinetic analyses showed that oligomerization increased association rates in addition to slowing dissociation rates. In combination with some contribution from reduced steric clashes with residues in heavy-chain CDR2, oligomerization resulted in functional affinities that were much higher than that of the monomeric form of the wild-type single-chain Fv.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbas C. F., 3rd, Bain J. D., Hoekstra D. M., Lerner R. A. Semisynthetic combinatorial antibody libraries: a chemical solution to the diversity problem. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4457–4461. doi: 10.1073/pnas.89.10.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Hu D., Dunlop N., Sawyer L., Cababa D., Hendry R. M., Nara P. L., Burton D. R. In vitro evolution of a neutralizing human antibody to human immunodeficiency virus type 1 to enhance affinity and broaden strain cross-reactivity. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3809–3813. doi: 10.1073/pnas.91.9.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C., Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987 Apr;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Brummell D. A., Sharma V. P., Anand N. N., Bilous D., Dubuc G., Michniewicz J., MacKenzie C. R., Sadowska J., Sigurskjold B. W., Sinnott B. Probing the combining site of an anti-carbohydrate antibody by saturation-mutagenesis: role of the heavy-chain CDR3 residues. Biochemistry. 1993 Feb 2;32(4):1180–1187. doi: 10.1021/bi00055a024. [DOI] [PubMed] [Google Scholar]
- Bundle D. R., Eichler E., Gidney M. A., Meldal M., Ragauskas A., Sigurskjold B. W., Sinnott B., Watson D. C., Yaguchi M., Young N. M. Molecular recognition of a Salmonella trisaccharide epitope by monoclonal antibody Se155-4. Biochemistry. 1994 May 3;33(17):5172–5182. doi: 10.1021/bi00183a022. [DOI] [PubMed] [Google Scholar]
- Chothia C., Novotný J., Bruccoleri R., Karplus M. Domain association in immunoglobulin molecules. The packing of variable domains. J Mol Biol. 1985 Dec 5;186(3):651–663. doi: 10.1016/0022-2836(85)90137-8. [DOI] [PubMed] [Google Scholar]
- Cooper L. J., Robertson D., Granzow R., Greenspan N. S. Variable domain-identical antibodies exhibit IgG subclass-related differences in affinity and kinetic constants as determined by surface plasmon resonance. Mol Immunol. 1994 Jun;31(8):577–584. doi: 10.1016/0161-5890(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Metzger H. The influence of polyvalency on the binding properties of antibodies. Immunochemistry. 1972 Mar;9(3):341–357. doi: 10.1016/0019-2791(72)90097-3. [DOI] [PubMed] [Google Scholar]
- Cygler M., Rose D. R., Bundle D. R. Recognition of a cell-surface oligosaccharide of pathogenic Salmonella by an antibody Fab fragment. Science. 1991 Jul 26;253(5018):442–445. doi: 10.1126/science.1713710. [DOI] [PubMed] [Google Scholar]
- Deng S. J., MacKenzie C. R., Narang S. A. Simultaneous randomization of antibody CDRs by a synthetic ligase chain reaction strategy. Nucleic Acids Res. 1993 Sep 11;21(18):4418–4419. doi: 10.1093/nar/21.18.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S. J., MacKenzie C. R., Sadowska J., Michniewicz J., Young N. M., Bundle D. R., Narang S. A. Selection of antibody single-chain variable fragments with improved carbohydrate binding by phage display. J Biol Chem. 1994 Apr 1;269(13):9533–9538. [PubMed] [Google Scholar]
- Desplancq D., King D. J., Lawson A. D., Mountain A. Multimerization behaviour of single chain Fv variants for the tumour-binding antibody B72.3. Protein Eng. 1994 Aug;7(8):1027–1033. doi: 10.1093/protein/7.8.1027. [DOI] [PubMed] [Google Scholar]
- Glaser S. M., Yelton D. E., Huse W. D. Antibody engineering by codon-based mutagenesis in a filamentous phage vector system. J Immunol. 1992 Dec 15;149(12):3903–3913. [PubMed] [Google Scholar]
- Gram H., Marconi L. A., Barbas C. F., 3rd, Collet T. A., Lerner R. A., Kang A. S. In vitro selection and affinity maturation of antibodies from a naive combinatorial immunoglobulin library. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3576–3580. doi: 10.1073/pnas.89.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths A. D., Malmqvist M., Marks J. D., Bye J. M., Embleton M. J., McCafferty J., Baier M., Holliger K. P., Gorick B. D., Hughes-Jones N. C. Human anti-self antibodies with high specificity from phage display libraries. EMBO J. 1993 Feb;12(2):725–734. doi: 10.1002/j.1460-2075.1993.tb05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths A. D., Williams S. C., Hartley O., Tomlinson I. M., Waterhouse P., Crosby W. L., Kontermann R. E., Jones P. T., Low N. M., Allison T. J. Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J. 1994 Jul 15;13(14):3245–3260. doi: 10.1002/j.1460-2075.1994.tb06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. E., Russell S. J., Winter G. Selection of phage antibodies by binding affinity. Mimicking affinity maturation. J Mol Biol. 1992 Aug 5;226(3):889–896. doi: 10.1016/0022-2836(92)90639-2. [DOI] [PubMed] [Google Scholar]
- Holliger P., Prospero T., Winter G. "Diabodies": small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom H. R., Marks J. D., Griffiths A. D., Winter G. Building antibodies from their genes. Immunol Rev. 1992 Dec;130:41–68. doi: 10.1111/j.1600-065x.1992.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Hoogenboom H. R., Winter G. By-passing immunisation. Human antibodies from synthetic repertoires of germline VH gene segments rearranged in vitro. J Mol Biol. 1992 Sep 20;227(2):381–388. doi: 10.1016/0022-2836(92)90894-p. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Nordeen S. K., Vogt K., Edgell M. H. A complete library of point substitution mutations in the glucocorticoid response element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1986 Feb;83(3):710–714. doi: 10.1073/pnas.83.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie C. R., Sharma V., Brummell D., Bilous D., Dubuc G., Sadowska J., Young N. M., Bundle D. R., Narang S. A. Effect of C lambda-C kappa domain switching on Fab activity and yield in Escherichia coli: synthesis and expression of genes encoding two anti-carbohydrate Fabs. Biotechnology (N Y) 1994 Apr;12(4):390–395. doi: 10.1038/nbt0494-390. [DOI] [PubMed] [Google Scholar]
- Padlan E. A. Anatomy of the antibody molecule. Mol Immunol. 1994 Feb;31(3):169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Riechmann L., Weill M. Phage display and selection of a site-directed randomized single-chain antibody Fv fragment for its affinity improvement. Biochemistry. 1993 Aug 31;32(34):8848–8855. doi: 10.1021/bi00085a016. [DOI] [PubMed] [Google Scholar]
- Sigurskjold B. W., Altman E., Bundle D. R. Sensitive titration microcalorimetric study of the binding of Salmonella O-antigenic oligosaccharides by a monoclonal antibody. Eur J Biochem. 1991 Apr 10;197(1):239–246. doi: 10.1111/j.1432-1033.1991.tb15904.x. [DOI] [PubMed] [Google Scholar]
- Sigurskjold B. W., Bundle D. R. Thermodynamics of oligosaccharide binding to a monoclonal antibody specific for a Salmonella O-antigen point to hydrophobic interactions in the binding site. J Biol Chem. 1992 Apr 25;267(12):8371–8376. [PubMed] [Google Scholar]
- Whitlow M., Bell B. A., Feng S. L., Filpula D., Hardman K. D., Hubert S. L., Rollence M. L., Wood J. F., Schott M. E., Milenic D. E. An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Eng. 1993 Nov;6(8):989–995. doi: 10.1093/protein/6.8.989. [DOI] [PubMed] [Google Scholar]
- Whitlow M., Filpula D., Rollence M. L., Feng S. L., Wood J. F. Multivalent Fvs: characterization of single-chain Fv oligomers and preparation of a bispecific Fv. Protein Eng. 1994 Aug;7(8):1017–1026. doi: 10.1093/protein/7.8.1017. [DOI] [PubMed] [Google Scholar]
- Zdanov A., Li Y., Bundle D. R., Deng S. J., MacKenzie C. R., Narang S. A., Young N. M., Cygler M. Structure of a single-chain antibody variable domain (Fv) fragment complexed with a carbohydrate antigen at 1.7-A resolution. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6423–6427. doi: 10.1073/pnas.91.14.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]


