Significance
In this study we sequenced bacterial communities present on tree leaves in a neotropical forest in Panama, to quantify the poorly understood relationships between bacterial biodiversity on leaves (the phyllosphere) vs. host tree attributes. Bacterial community structure on leaves was highly correlated with host evolutionary relatedness and suites of plant functional traits related to host ecological strategies for resource uptake and growth/mortality tradeoffs. The abundance of several bacterial taxa was correlated with host growth, mortality, and function. Our study quantifies the drivers of variation in plant-associated microbial biodiversity; our results suggest that incorporating information on plant-associated microbes will improve our understanding of the functional biogeography of plants and plant–microbe interactions.
Keywords: tropical forests, host–microbe associations, plant microbiome, microbial ecology
Abstract
The phyllosphere—the aerial surfaces of plants, including leaves—is a ubiquitous global habitat that harbors diverse bacterial communities. Phyllosphere bacterial communities have the potential to influence plant biogeography and ecosystem function through their influence on the fitness and function of their hosts, but the host attributes that drive community assembly in the phyllosphere are poorly understood. In this study we used high-throughput sequencing to quantify bacterial community structure on the leaves of 57 tree species in a neotropical forest in Panama. We tested for relationships between bacterial communities on tree leaves and the functional traits, taxonomy, and phylogeny of their plant hosts. Bacterial communities on tropical tree leaves were diverse; leaves from individual trees were host to more than 400 bacterial taxa. Bacterial communities in the phyllosphere were dominated by a core microbiome of taxa including Actinobacteria, Alpha-, Beta-, and Gammaproteobacteria, and Sphingobacteria. Host attributes including plant taxonomic identity, phylogeny, growth and mortality rates, wood density, leaf mass per area, and leaf nitrogen and phosphorous concentrations were correlated with bacterial community structure on leaves. The relative abundances of several bacterial taxa were correlated with suites of host plant traits related to major axes of plant trait variation, including the leaf economics spectrum and the wood density–growth/mortality tradeoff. These correlations between phyllosphere bacterial diversity and host growth, mortality, and function suggest that incorporating information on plant–microbe associations will improve our ability to understand plant functional biogeography and the drivers of variation in plant and ecosystem function.
The phyllosphere—the aerial surfaces of plants—is an important and ubiquitous habitat for bacteria (1). It is estimated that on a global scale, the phyllosphere spans more than 108 km2 and is home to up to 1026 bacterial cells (2). Bacteria are also important to their plant hosts. Leaf-associated bacteria represent a widespread and ancient symbiosis (3, 4) that can influence host growth and function in many ways, including the production of growth-promoting nutrients and hormones (5, 6) and protection of hosts against pathogen infection (7, 8). Phyllosphere bacteria have the potential to influence plant biogeography and ecosystem function through their influence on plant performance under different environmental conditions (9–11), but the drivers of variation in leaf-associated bacterial biodiversity among host plants are not well understood.
The ability to quantify microbial community structure in depth with environmental sequencing technologies has led to an increasing focus not only on the ecology of individual microbial taxa but on the entire genomic content of communities of microbes in different habitats, or “microbiomes” (12). Numerous studies of host-associated microbiomes have shown that microbial biodiversity is a trait (13) that forms part of the extended phenotype of the host organism (4, 14, 15) with important effects on the health and fitness (16–18) and evolution (19–21) of the host. Because of the importance of the microbiome for host fitness and function, there is a growing desire to model and manage host–microbiome interactions (22, 23), and understanding the drivers of host-associated microbial community assembly has thus become a cornerstone of microbiome research (24).
In animals, the assembly of host-associated microbiomes is known to be driven by ecologically important host attributes, such as diet, that covary with host evolutionary history (20, 25, 26). A similar understanding of the drivers of plant microbiome assembly is lacking. Most of our knowledge of plant–bacterial associations on leaves has been based on studies of individual bacterial strains and individual host species. Different plant species possess characteristic bacterial phyllosphere communities (27, 28), and there are several examples of variation in bacterial biodiversity on leaves among plant genotypes (29–31) as well as among species and higher taxonomic ranks (32). Although these patterns are presumably due to phylogenetic variation in ecologically important plant functional traits (33) among host populations and species, the influence of host functional traits on variation in phyllosphere community structure across host species has not been directly quantified. As a result, we have very little understanding of the potential of plant–microbe interaction networks to influence the distribution and functional biogeography of their hosts at large scales in the face of global change (34).
A first step toward integrating phyllosphere microbial communities into the study of plant biogeography will require establishing correlations between microbial community structure on leaves and the functional traits of plant hosts. To address this goal, we used high-throughput sequencing to characterize the structure of the bacterial phyllosphere microbiome on the leaves of multiple host tree species in a diverse neotropical forest in Panama. We combined phyllosphere microbiome data with a rich dataset on the attributes of plant hosts, including functional traits and evolutionary relationships, to (i) quantify the magnitude of leaf-associated bacterial biodiversity in a diverse natural forest community, (ii) identify the host plant attributes that influence microbiome community assembly on leaves, and (iii) understand relationships between bacterial biodiversity and suites of host plant traits and functions and discuss their implications for our understanding of plant functional biogeography.
Results
Microbial Phyllosphere Diversity and the Core Phyllosphere Microbiome.
We used high-throughput Illumina sequencing of the bacterial 16S rRNA gene (35) to quantify the composition of bacterial communities on the leaf surfaces of trees growing in a tropical lowland rainforest on Barro Colorado Island, Panama. We identified 7,293 bacterial operational taxonomic units (OTUs, sequences binned at a 97% similarity cutoff) on the leaves of 137 trees belonging to 57 species, an average of 418 ± 4 OTUs (mean ± SE) per sampled tree. Many of these bacterial taxa were rare, with 28% of bacterial OTUs occurring on a single tree. A collector’s curve of the number of OTUs per host species continued to reveal additional bacterial taxa with every additional host plant species sampled (Fig. 1). The total size of the phyllosphere bacterial OTU pool was estimated to be 11,615 ± 227 OTUs [mean ± SE of Chao2 estimator of total OTU pool richness (36)].
Fig. 1.
Collector’s curve (mean ± 95% confidence interval) of bacterial phyllosphere OTU (97% sequence similarity cutoff) richness vs. number of plant host species sampled on Barro Colorado Island, Panama.
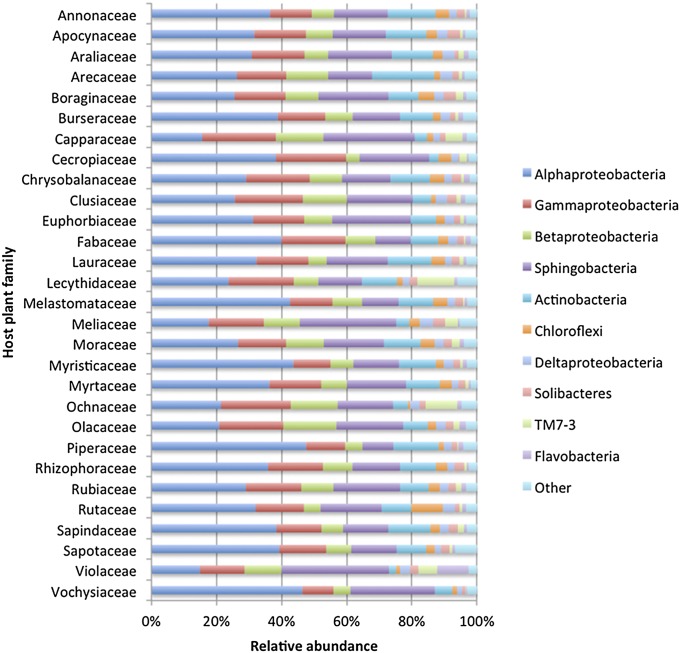
Studies of microbes in various habitats have sought to identify the “core microbiome” (37)—the potentially ecologically important microbial taxa shared among multiple communities sampled from the same habitat. We detected a “core phyllosphere microbiome” of common and abundant bacterial phyllosphere taxa present on nearly all trees sampled in this forest (Fig. 2). There were 104 bacterial OTUs belonging to eight phyla and 34 families that were present on 95% or more of all trees sampled, representing 1.4% of the bacterial taxonomic diversity but more than 73% of sequences. The five most dominant phyla represented in the core microbiome were Alphaproteobacteria [Beijerinckiaceae (7.0% of all sequences), Bradyrhizobiaceae (4.9%)], Sphingobacteria [Flexibacteriaceae (6.7%), Sphingobacteriaceae (4.2%)], Gammaproteobacteria [Pseudomonadaceae (3.3%), Xanthomonadaceae (8.7%)], Betaproteobacteria (8.1%), and Actinobacteria (5.5%). The most abundant individual OTUs were identified as Beijerinckia (6.5%), Leptothrix (3.9%), Stenotrophomonas (3.4%), Niastella (3.3%), and Spirosoma (2.9%).
Fig. 2.
Taxonomic composition of bacterial phyllosphere communities on different host plant families on Barro Colorado Island, Panama.
Drivers of Variation in Phyllosphere Bacterial Community Composition.
Variation in bacterial community structure on leaves was related to two groups of correlated plant host traits (Fig. 3). The first group of plant traits significantly (P < 0.05) correlated with variation in bacterial community structure (bacterial community ordination axis 1) included wood density and growth and mortality rates. The second group of plant traits significantly (P < 0.05) correlated with bacterial community structure (bacterial community ordination axis 2) included leaf mass per area, leaf thickness, and leaf nitrogen and phosphorous concentrations. Bacterial communities on leaves were phylogenetically clustered, containing OTUs more closely related than expected from a null model of random assembly from the pool of OTUs observed on all trees (mean ± SE, SESMPD = −3.0 ± 0.1; P < 0.05). The magnitude of phylogenetic clustering was significantly correlated with axis 1 of the bacterial community ordination (Fig. 3) and with the host traits associated with that axis (mortality rate, growth rate, and wood density).
Fig. 3.
Nonmetric multidimensional scaling (NMDS) ordination of variation in bacterial community structure across 137 samples from tropical tree phyllospheres on Barro Colorado Island, Panama. The ordination was based on weighted UniFrac dissimilarity among samples; the ordination axes explain 97% of the variance in the dissimilarities. Samples (points) are shaded according to taxonomic order of hosts; ellipses indicate 2 SD confidence intervals around samples from selected host taxonomic orders. Arrows outside plot margins indicate host plant traits with significant (P < 0.05) correlations with sample scores on each ordination axis.
The majority (51%) of the variation in bacterial community structure on leaves could be explained by host traits and taxonomy, with host taxonomy alone explaining 26% of the variation, host traits alone explaining 13% of the variation, and host traits and taxonomy jointly explaining 10% of the variation (variance partitioning of weighted UniFrac distances). A nested analysis of the variance in microbial community structure explained by different host plant taxonomic levels indicated that host plant order (26.0%), family (9.2%), and genus (8.1%) all explained significant amounts of variance [P < 0.05; nested permutational multivariate analysis of variance (ANOVA) vs. weighted UniFrac distances], and that the majority (51%) of variance in microbial community structure was among host species (P < 0.01; based on species with at least two individuals sampled).
We quantified the strength of evolutionary associations between host species and bacterial OTUs by testing both for an overall evolutionary association and identifying individual host–microbe associations that were stronger than expected by chance using the “host–parasite association test,” which uses a randomization test to evaluate the strength of associations between organisms from different phylogenetic groups (38). There was a significant overall evolutionary association between host species and bacterial OTUs (P < 0.001). We also identified numerous associations between host and bacterial clades that were stronger than expected (P < 0.05) (Fig. 4).
Fig. 4.
Cophylogeny of host plants (Left) and phyllosphere bacteria (Right) on Barro Colorado Island, Panama. Lines connecting tips on the phylogenies indicate plant–bacterial associations that were stronger than expected according to a host–parasite coevolution test [P < 0.05 (38)].
Correlations Between Plant Functional Trait Strategies and Microbial Diversity.
To understand how microbial communities on leaves are related to major dimensions of plant functional trait variation and plant ecological strategies, we summarized correlations among plant functional traits for 217 woody plant species from Barro Colorado Island (description in Methods) using a principal components analysis (PCA) of trait values. We then tested for relationships between plant trait strategies and the relative abundance of sequences assigned to different bacterial taxonomic classes for the 57 host species for which both plant trait and microbial community data were available, using a permutation test that determined whether relationships between microbial community composition variables vs. plant trait correlation axes were stronger than expected by chance.
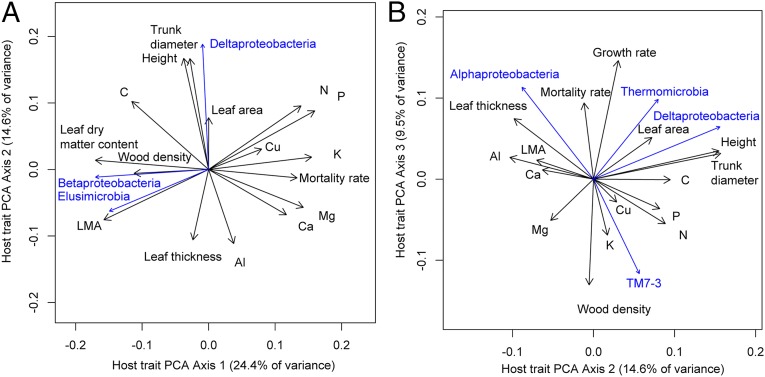
Analysis of correlations among plant traits (Fig. 5) revealed three major axes of plant trait variation among species that have been previously described for tropical trees (39–41). The first axis of correlated traits (PCA axis 1) includes traits related to the “leaf economics spectrum” of plant resource uptake strategies (42–44), such as leaf nutrient concentrations, leaf dry matter content, and leaf mass per area. The second axis of correlated traits (PCA axis 2) includes traits related to plant stature at maturity, including maximum height and diameter. The third axis of correlated traits (PCA axis 3) includes wood density, relative growth rate, and mortality rate. These traits are related to a wood density–growth/mortality tradeoff (39, 41).
Fig. 5.
Relationships between microbial community structure and suites of correlated plant host traits on Barro Colorado Island, Panama. (A and B) Axis 1 vs. 2 (A) and axis 2 vs. 3 (B) from a principal components analysis (PCA) of host plant traits across 217 plant species. These axes explain 49% of the variation in the data. Arrow direction indicates the correlation among traits, arrow length indicates the strength of the correlation. Black arrows indicate correlations among plant traits, blue arrows indicate significant correlations (P < 0.05) between PCA axes vs. the relative abundances of sequences from different microbial classes measured on a subset of 57 host species. LMA, leaf mass per area.
Microbial community structure and abundance were correlated with the major axes of plant trait variation (Fig. 5). The relative abundances of Betaproteobacteria and Elusimicrobia were significantly correlated (P < 0.05) with the cluster of traits related to the leaf economics spectrum (PCA axis 1). These bacterial classes were more abundant on the leaves of tree species with high leaf dry matter content and leaf mass per area and low leaf nitrogen and phosphorous concentrations. The relative abundances of Deltaproteobacteria were significantly correlated (P < 0.05) with the cluster of traits related to plant stature (PCA axis 2), whereas several clades, including the Alphaproteobacteria, Thermomicrobia, and TM7-3, were significantly correlated (P < 0.05) with the cluster of traits related to the wood density–growth/mortality tradeoff (PCA axis 3).
Discussion
Tropical tree leaves harbor diverse bacterial communities. More than 450 tree species have been recorded on Barro Colorado Island (45). Just 50–100 g of leaf tissue from an individual tree is home to nearly as many bacterial taxa as there are tree species on the entire 16-km2 island. Even at a sampling depth of hundreds of thousands of sequences there are still numerous undiscovered bacterial taxa living on tropical tree leaves. Most bacterial taxa on leaves in this forest were rare. However, in contrast to a previous study of temperate phyllosphere bacterial diversity that found few bacterial OTUs present across multiple temperate tree species (32), we detected a core microbiome of common bacterial taxa present on nearly all leaves.
Many of the dominant bacterial taxa in the phyllosphere belong to clades known to associate closely with plant hosts, including diazotrophic (nitrogen-fixing) and methylotrophic (methanol- and other one-carbon compound-consuming) taxa, such as Beijerinckia and Methylobacterium. We also detected many bacterial taxa from groups that are commonly encountered in other environments, including soil and water. Because of a lack of detailed knowledge about the ecological niches of bacteria (46), it is difficult to identify the sources of the bacterial populations on tropical tree leaves. It is likely that the communities we sampled represent a mixture of resident taxa living permanently on leaves in biofilms and on leaf surface substrates (2, 47), as well as transient taxa introduced from the atmosphere, rainwater, and contact with animal and plant dispersal vectors. Future studies comparing bacterial community composition across different potential source communities, including soil, air, water, and animals, will be required to identify the metacommunities (48) contributing to microbial diversity on leaf surfaces.
The plant attributes that explained variation in bacterial community structure on leaves included traits related to the leaf economics spectrum (42, 44) and the wood density–growth/mortality tradeoff (39, 41). The leaf economics spectrum is a suite of correlated traits related to the resource uptake and retention strategies of plants. This spectrum separates taxa with an “acquisitive” resource strategy and traits including relatively high resource uptake rates, short-lived leaves, and high concentrations of nutrients, including nitrogen and phosphorous, from taxa with a “retentive” resource strategy and the opposite set of traits (42, 44). The evidence that bacterial community structure is related to the leaf economics spectrum is likely a result of the profound impact of leaf resource uptake strategies on leaf morphology and physiology. Traits that could impact the resources available to bacteria living on the leaf surface include the availability of leaf nutrients and carbon compounds, the rate of production of volatile organic compounds, including methanol, and the amount of structural and antimicrobial compounds produced by leaves (49).
The other major axis of plant trait and functional covariation that was related to bacterial biodiversity on leaves was the wood density–growth/mortality tradeoff spectrum (39, 40). This spectrum separates fast-growing species with high mortality rates and low wood density (low structural investment) adapted to rapid growth in favorable environments, such as canopy gaps, from species with the opposite set of traits. Although wood density and growth/mortality rate would not be expected to directly influence bacterial populations on leaves, these traits are related to the overall growth and life-history strategy of plants. Taken together, our results suggest that the ecological strategies and functional traits of plants have a profound effect on phyllosphere bacterial communities and that incorporating information on plant-associated bacteria has the potential to improve our understanding of the mechanisms shaping plant trait correlations at biogeographic scales (50).
Using ecological null models to assess the degree of phylogenetic clustering in bacterial communities, we detected evidence of nonneutral community assembly on nearly all sampled leaves, in the form of widespread phylogenetic clustering. The correlation between bacterial phylogenetic clustering vs. host traits such as mortality and growth rate suggests that conditions on the leaves of fast-growing trees act as a stronger ecological filter on potential colonists of the phyllosphere, resulting in more phylogenetically clustered communities, or that microbial succession on long-lived leaves eventually leads to less strongly phylogenetically clustered communities. Given the long lifespans of some tropical leaves, future studies of succession on leaves of different ages (29) will be required to fully distinguish between these possibilities.
We found evidence for evolutionary associations between plants and phyllosphere bacteria (Fig. 4). For example, Alphaproteobacteria were highly abundant on plants from the Ochnaceae and Vochysiaceae but much less abundant on plants from the Violaceae, which were commonly colonized by Sphingobacteria (Fig. 2). These evolutionary associations are likely to be related to phylogenetic variation in host traits (51), given the interaction between microbial community variance explained by host traits and taxonomy. Several traits not measured by this study, such as volatile organic compound production, cuticle chemistry, and antibiotic production, are also likely to play a role. Future studies of additional functional traits and detailed investigations of the effects of host traits on the growth of microbial populations will be required to fully understand the processes responsible for these evolutionary associations.
The associations between bacterial phyllosphere community structure and host traits that we observed raise the question of which partner exerts greater control over interactions in the phyllosphere. For example, is the association between the relative abundance of certain bacterial taxa and host plant growth rates due to bacteria directly influencing the growth of their hosts, or do the traits of fast-growing host species filter for a certain bacterial community composition by promoting or inhibiting the growth of different bacterial clades? The adaptation of bacterial taxa to variation in leaf chemistry and microenvironment suggests that leaf chemistry has an important effect on the growth and survival of bacteria on leaves (1), and this is supported by our finding that leaf chemistry was strongly correlated with bacterial community structure. However, microbial populations have also been found to have direct effects on plant growth through resource exchange and protection from pathogen damage (7, 52). Fully disentangling the relative importance of plants vs. microbes to explain the associations we observed will require experimental manipulations of plant–microbe associations (7, 53).
Functional traits are defined as any trait that can potentially influence fitness through correlations with growth, reproduction, or survival (54). We have demonstrated that the biodiversity of bacterial communities on tree leaves is correlated with host growth and mortality rates, suggesting the possibility that the structure of the plant microbiome could be considered a plant functional trait. Many widely measured plant functional traits, such as association with nitrogen-fixing bacteria or mycorrhizal fungi, are simple measures of the potential influence of microbial communities on plant and ecosystem function. The ability to quantify plant-associated microbial biodiversity in depth offers the possibility to move beyond binary measures of plant–microbial associations (e.g., mycorrhizal status) toward a more detailed understanding of how microbial biodiversity is related to host function, both directly by considering microbial biodiversity as a potential functional trait (33) and indirectly through understanding how microbes mediate other plant functional traits (10). Expanding our notions of the functional biogeography of plants beyond phenotypic traits to include biotic interactions and species interaction networks (55, 56) is a research priority for biogeography and macroecology (57). Our knowledge of plant–microbe associations at biogeographic scales are currently very limited (58); our study provides a step toward incorporating information on plant–microbe associations to better understand the functional biogeography of plants and forecast species and ecosystem responses to global change (34, 59).
Methods
Bacterial Community Sampling and Sequencing.
We collected bacteria from the leaf surfaces of woody plants in a tropical lowland rainforest on Barro Colorado Island, Panama in December 2010. We sampled between one and five individual trees from each of 57 species. Each sample consisted of shade leaves collected from the subcanopy (2–10 m above ground) by clipping 50–100 g fresh mass of leaves from an individual plant into sterile roll bags with surface-sterilized shears. Microbial cells were collected from leaf surfaces by agitating leaves for 5 min in 100 mL of 1:50 diluted wash solution [1 M Tris·HCl, 0.5 M Na EDTA, and 1.2% CTAB (60)] and pelleting via centrifugation at 4,000 × g for 20 min. The supernatant was removed by pipetting, cells were resuspended in 500 µL MoBio PowerSoil bead solution, and DNA was extracted using the MoBio PowerSoil kit.
Amplicon libraries were prepared for Illumina sequencing using a two-stage PCR protocol. We first targeted the V5–V6 region of the bacterial 16S rRNA gene using cyanobacteria-excluding primers [16S primers 799F-1115R (32, 61)] to exclude chloroplast DNA. Primers were modified with a 5′ tail that added a 6-bp barcode and partial Illumina adaptor sequence to 16S fragments during PCR (modified 799F: 5′-CGGTCTCGGCATTCCTGCTGAACCGCTCTTCCGATCT xxxxxx AACMGGATTAGATACCCKG; modified 1115R: 5′-ACACTCTTTCCCTACACGACGCTCTTCCGATCT xxxxxx AGGGTTGCGCTCGTTG, where “x” represents barcode nucleotides). Twenty-five-microliter PCR reactions consisted of 5 µL 5× HF buffer (Thermo Scientific), 0.5 µL dNTPs (10 µM each), 0.25 µL Phusion Hot Start II polymerase (Thermo Scientific), 0.5 µL each primer (10 µM), 2–4 µL of genomic DNA, and 16.25–18.25 µL molecular-grade water. Reactions were performed in triplicate for each sample with the following conditions: 30 s initial denaturation at 98 °C, followed by 20 cycles of 10 s at 98 °C, 30 s at 64 °C, and 30 s at 72 °C, with a final 10-min elongation at 72 °C. Triplicate reactions were pooled, cleaned using MoBio UltraClean PCR cleanup kit, and resuspended in 50 µL of solution C6. Using cleaned PCR product as a template, a second PCR was performed with custom HPLC-cleaned primers to further amplify 16S products and complete the Illumina sequencing construct (PCRII_for: 5′-AAGCAGAAGACGGCATACGAGATCGGTCTCGGCATTCCTGC; PCRII_rev: 5′-ATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACG). Single 25-µL reactions were performed for each sample, with reagents and conditions as described above, but reactions were run for 15 rather than 20 cycles. A ∼445-bp fragment was isolated by electrophoresis in a 2% agarose gel, and DNA was recovered with the MoBio GelSpin kit. Multiplexed 16S libraries were prepared by mixing equimolar concentrations of DNA from each sample. The resulting DNA library was sequenced using Illumina HiSeq 150-bp paired-end sequencing at the University of Oregon Genomics Core Facility.
We processed raw sequence data with the fastx_toolkit and QIIME (62) software pipelines to trim and concatenate paired-end sequences to a single sequence of length of 212 bp (106 bp from each paired end), eliminate low-quality sequences by removing trimmed sequences with a mean quality score less than 30 or with any base pair with a quality score less than 25, and de-multiplex sequences into samples using barcode sequences. A combinatorial barcoding approach was used to allow bioinformatic identification of each sample via the unique combination of forward and reverse barcodes attached to each sequence (63). We binned sequences into OTUs (a species equivalent) at a 97% sequence similarity cutoff using uclust (64) and eliminated putative chimeric OTUs and estimated the taxonomic identity of each OTU using the BLAST algorithm as implemented in QIIME (62). After quality filtering and rarefaction of each sample to 4,000 sequences, 548,000 sequences from 137 samples representing 57 tree species remained and were included in all subsequent analyses. We inferred phylogenetic relationships among all bacterial OTUs using a maximum likelihood GTR+Gamma phylogenetic model in FastTree (65).
Plant Host Traits, Fitness, Function, and Phylogeny.
Data on host plant functional traits, including growth form and adult stature [average diameter at breast height (DBH) and average height of six largest individuals by DBH in a fully enumerated 50-ha forest plot], leaf elemental chemistry (concentration of aluminum, calcium, copper, potassium, magnesium, manganese, phosphorous, zinc, nitrogen, and carbon), leaf morphology (leaf mass per area, leaf dry matter content, leaf thickness, leaf area), wood density, sapling growth rate, and sapling mortality rate, were obtained for all species according to data previously collected from Barro Colorado Island (39). Phylogenetic relationships among plant hosts were estimated according to a maximum-likelihood phylogeny for Barro Colorado Island woody plant species (66).
Data Analyses.
Data analyses and visualization were performed using the ape (67), ggplot2 (68), picante (69), and vegan (70) packages for R (71). We quantified variation in bacterial community structure among samples using the weighted UniFrac index, an abundance-weighted measure of the phylogenetic differentiation among bacterial communities (72). We summarized major gradients in bacterial community structure among samples using a nonmetric multidimensional scaling ordination of weighted UniFrac distances. We identified relationships between bacterial community structure and host plant traits by calculating correlations between host plant traits and the scores of samples on the axes of the bacterial community ordination. We partitioned the variance in phyllosphere bacterial community structure explained by host traits and host taxonomy using variance partitioning (73) and permutational multivariate ANOVA analysis (74) of the variance in weighted UniFrac distances explained by different traits and taxonomic ranks. We quantified the evidence for nonneutral community assembly of microbial communities on leaves using null model testing of phylogenetic diversity (75). We estimated the abundance-weighted mean pairwise distance (MPD) among sequences in each sample and calculated a standardized effect size (SESMPD) by comparing the observed values to the value expected if communities were assembled at random from the pool of all OTUs observed on all leaves (75).
Acknowledgments
We thank Rufino Gonzalez and Omar Hernandez for their assistance in the field. This study was supported by a Center for Tropical Forest Science Research Grant, the Smithsonian Tropical Research Institute, and the University of Oregon. S.W.K. was supported by the Canada Research Chairs program and the Natural Sciences and Engineering Research Council of Canada. The Barro Colorado Island forest dynamics research project was made possible by the National Science Foundation, support from the Center for Tropical Forest Science, the Smithsonian Tropical Research Institute, the John D. and Catherine T. MacArthur Foundation, the Mellon Foundation, the Small World Institute Fund, and numerous private individuals, and through the hard work of more than 100 people from 10 countries over the past two decades.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.V. is a guest editor invited by the Editorial Board.
Data deposition: The sequences reported in this paper have been deposited in the figshare data repository, http://dx.doi.org/10.6084/m9.figshare.928573.
References
- 1.Vorholt JA. Microbial life in the phyllosphere. Nat Rev Microbiol. 2012;10(12):828–840. doi: 10.1038/nrmicro2910. [DOI] [PubMed] [Google Scholar]
- 2.Lindow SE, Brandl MT. Microbiology of the phyllosphere. Appl Environ Microbiol. 2003;69(4):1875–1883. doi: 10.1128/AEM.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fedorov DN, Doronina NV, Trotsenko YA. Phytosymbiosis of aerobic methylobacteria: New facts and views. Microbiology. 2011;80(4):443–454. [PubMed] [Google Scholar]
- 4.Partida-Martínez LP, Heil M. The microbe-free plant: Fact or artifact? Front Plant Sci. 2011;2:100. doi: 10.3389/fpls.2011.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gourion B, Rossignol M, Vorholt JA. A proteomic study of Methylobacterium extorquens reveals a response regulator essential for epiphytic growth. Proc Natl Acad Sci USA. 2006;103(35):13186–13191. doi: 10.1073/pnas.0603530103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed SC, Townsend AR, Cleveland CC, Nemergut DR. Microbial community shifts influence patterns in tropical forest nitrogen fixation. Oecologia. 2010;164(2):521–531. doi: 10.1007/s00442-010-1649-6. [DOI] [PubMed] [Google Scholar]
- 7.Innerebner G, Knief C, Vorholt JA. Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl Environ Microbiol. 2011;77(10):3202–3210. doi: 10.1128/AEM.00133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balint-Kurti P, Simmons SJ, Blum JE, Ballaré CL, Stapleton AE. Maize leaf epiphytic bacteria diversity patterns are genetically correlated with resistance to fungal pathogen infection. Mol Plant Microbe Interact. 2010;23(4):473–484. doi: 10.1094/MPMI-23-4-0473. [DOI] [PubMed] [Google Scholar]
- 9.Fürnkranz M, et al. Nitrogen fixation by phyllosphere bacteria associated with higher plants and their colonizing epiphytes of a tropical lowland rainforest of Costa Rica. ISME J. 2008;2(5):561–570. doi: 10.1038/ismej.2008.14. [DOI] [PubMed] [Google Scholar]
- 10.Friesen ML, et al. Microbially mediated plant functional traits. Annu Rev Ecol Evol Syst. 2011;42:23–46. [Google Scholar]
- 11.Meyer KM, Leveau JHJ. Microbiology of the phyllosphere: A playground for testing ecological concepts. Oecologia. 2012;168(3):621–629. doi: 10.1007/s00442-011-2138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbaugh PJ, et al. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benson AK, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci USA. 2010;107(44):18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitham TG, et al. Community and ecosystem genetics: A consequence of the extended phenotype. Ecology. 2003;84:559–573. [Google Scholar]
- 15.Li M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci USA. 2008;105(6):2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17(8):478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Cho I, Blaser MJ. The human microbiome: At the interface of health and disease. Nat Rev Genet. 2012;13(4):260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol Rev. 2008;32(5):723–735. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- 20.Ley RE, et al. Evolution of mammals and their gut microbes. Science. 2008;320(5883):1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449(7164):811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Project THM. Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336(6086):1255–1262. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson CJ, Bohannan BJM, Young VB. From structure to function: The ecology of host-associated microbial communities. Microbiol Mol Biol Rev. 2010;74(3):453–476. doi: 10.1128/MMBR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muegge BD, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332(6032):970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9(4):279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 27.Knief C, Ramette A, Frances L, Alonso-Blanco C, Vorholt JA. Site and plant species are important determinants of the Methylobacterium community composition in the plant phyllosphere. ISME J. 2010;4(6):719–728. doi: 10.1038/ismej.2010.9. [DOI] [PubMed] [Google Scholar]
- 28.Kim M, et al. Distinctive phyllosphere bacterial communities in tropical trees. Microb Ecol. 2012;63(3):674–681. doi: 10.1007/s00248-011-9953-1. [DOI] [PubMed] [Google Scholar]
- 29.Redford AJ, Fierer N. Bacterial succession on the leaf surface: A novel system for studying successional dynamics. Microb Ecol. 2009;58(1):189–198. doi: 10.1007/s00248-009-9495-y. [DOI] [PubMed] [Google Scholar]
- 30.Finkel OM, et al. Distance-decay relationships partially determine diversity patterns of phyllosphere bacteria on Tamarix trees across the Sonoran Desert [corrected] Appl Environ Microbiol. 2012;78(17):6187–6193. doi: 10.1128/AEM.00888-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peiffer JA, et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci USA. 2013;110(16):6548–6553. doi: 10.1073/pnas.1302837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N. The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ Microbiol. 2010;12(11):2885–2893. doi: 10.1111/j.1462-2920.2010.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends Ecol Evol. 2006;21(4):178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Compant S, van der Heijden MGA, Sessitsch A. Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiol Ecol. 2010;73(2):197–214. doi: 10.1111/j.1574-6941.2010.00900.x. [DOI] [PubMed] [Google Scholar]
- 35.Claesson MJ, et al. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res. 2010;38(22):e200. doi: 10.1093/nar/gkq873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chao A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics. 1987;43(4):783–791. [PubMed] [Google Scholar]
- 37.Shade A, Handelsman J. Beyond the Venn diagram: The hunt for a core microbiome. Environ Microbiol. 2012;14(1):4–12. doi: 10.1111/j.1462-2920.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 38.Legendre P, Desdevises Y, Bazin E. A statistical test for host-parasite coevolution. Syst Biol. 2002;51(2):217–234. doi: 10.1080/10635150252899734. [DOI] [PubMed] [Google Scholar]
- 39.Wright SJ, et al. Functional traits and the growth-mortality trade-off in tropical trees. Ecology. 2010;91(12):3664–3674. doi: 10.1890/09-2335.1. [DOI] [PubMed] [Google Scholar]
- 40.Wright IJ, et al. Relationships among ecologically important dimensions of plant trait variation in seven neotropical forests. Ann Bot. 2007;99(5):1003–1015. doi: 10.1093/aob/mcl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poorter L, et al. Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology. 2008;89(7):1908–1920. doi: 10.1890/07-0207.1. [DOI] [PubMed] [Google Scholar]
- 42.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428(6985):821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 43.Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: Global convergence in plant functioning. Proc Natl Acad Sci USA. 1997;94(25):13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osnas JLD, Lichstein JW, Reich PB, Pacala SW. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science. 2013;340(6133):741–744. doi: 10.1126/science.1231574. [DOI] [PubMed] [Google Scholar]
- 45.Croat T. Flora of Barro Colorado Island. Redwood City, CA: Stanford University Press; 1978. [Google Scholar]
- 46.Philippot L, et al. The ecological coherence of high bacterial taxonomic ranks. Nat Rev Microbiol. 2010;8(7):523–529. doi: 10.1038/nrmicro2367. [DOI] [PubMed] [Google Scholar]
- 47.Wilson M, Lindow SE. Coexistence among epiphytic bacterial populations mediated through nutritional resource partitioning. Appl Environ Microbiol. 1994;60(12):4468–4477. doi: 10.1128/aem.60.12.4468-4477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martiny JBH, et al. Microbial biogeography: Putting microorganisms on the map. Nat Rev Microbiol. 2006;4(2):102–112. doi: 10.1038/nrmicro1341. [DOI] [PubMed] [Google Scholar]
- 49.Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: Some leading dimensions of variation between species. Annu Rev Ecol Syst. 2002;33:125–159. [Google Scholar]
- 50.Westoby M, Wright IJ. Land-plant ecology on the basis of functional traits. Trends Ecol Evol. 2006;21(5):261–268. doi: 10.1016/j.tree.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Kraft NJB, Ackerly DD. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecol Monogr. 2010;80(3):401–422. [Google Scholar]
- 52.Arnold AE, et al. Fungal endophytes limit pathogen damage in a tropical tree. Proc Natl Acad Sci USA. 2003;100(26):15649–15654. doi: 10.1073/pnas.2533483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundberg DS, et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012;488(7409):86–90. doi: 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Violle C, et al. Let the concept of trait be functional! Oikos. 2007;116:882–892. [Google Scholar]
- 55.Thébault E, Loreau M. Food-web constraints on biodiversity-ecosystem functioning relationships. Proc Natl Acad Sci USA. 2003;100(25):14949–14954. doi: 10.1073/pnas.2434847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poulin R, Krasnov BR, Mouillot D. Host specificity in phylogenetic and geographic space. Trends Parasitol. 2011;27(8):355–361. doi: 10.1016/j.pt.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Beck J, et al. What’s on the horizon for macroecology? Ecography. 2012;35:673–683. [Google Scholar]
- 58.Andrews JH, Harris RF. The ecology and biogeography of microorganisms on plant surfaces. Annu Rev Phytopathol. 2000;38:145–180. doi: 10.1146/annurev.phyto.38.1.145. [DOI] [PubMed] [Google Scholar]
- 59.Sarmento H, Montoya JM, Vázquez-Domínguez E, Vaqué D, Gasol JM. Warming effects on marine microbial food web processes: How far can we go when it comes to predictions? Philos Trans R Soc Lond B Biol Sci. 2010;365(1549):2137–2149. doi: 10.1098/rstb.2010.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadivar H, Stapleton AE. Ultraviolet radiation alters maize phyllosphere bacterial diversity. Microb Ecol. 2003;45(4):353–361. doi: 10.1007/s00248-002-1065-5. [DOI] [PubMed] [Google Scholar]
- 61.Chelius MK, Triplett EW. The diversity of archaea and bacteria in association with the roots of Zea mays L. Microb Ecol. 2001;41(3):252–263. doi: 10.1007/s002480000087. [DOI] [PubMed] [Google Scholar]
- 62.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods. 2008;5(3):235–237. doi: 10.1038/nmeth.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 65.Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kress WJ, et al. Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc Natl Acad Sci USA. 2009;106(44):18621–18626. doi: 10.1073/pnas.0909820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paradis E, Claude J, Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20(2):289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 68.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2009. [Google Scholar]
- 69.Kembel SW, et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26(11):1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- 70.Oksanen J, et al. Vegan: Community Ecology Package. R Package Version 1.17. 2007. Available at http://cran.r-project.org/web/packages/vegan/. Accessed August 18, 2011. [Google Scholar]
- 71.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 72.Lozupone C, Hamady M, Knight R. UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics. 2006;7:371. doi: 10.1186/1471-2105-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borcard D, Legendre P, Drapeau P. Partialling out the Spatial Component of Ecological Variation. Ecology. 1992;73(3):1045. [Google Scholar]
- 74.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]
- 75.Kembel SW. Disentangling niche and neutral influences on community assembly: Assessing the performance of community phylogenetic structure tests. Ecol Lett. 2009;12(9):949–960. doi: 10.1111/j.1461-0248.2009.01354.x. [DOI] [PubMed] [Google Scholar]