Significance
The majority of mammal species are nocturnal, but many are diurnal (active during the day), crepuscular (active mostly during twilight), or cathemeral (active during hours of daylight and darkness). These different strategies for regulating activity over a 24-h cycle are associated with suites of adaptations to light or semidarkness. The biogeography of these time partitioning strategies is, however, poorly understood. We show that global patterns in mammal diversity with different diel activity patterns are constrained by the duration of time that is both (i) illuminated by daylight, moonlight, and/or twilight and (ii) between thermal limits suitable for mammal activity.
Keywords: cathemeral, night
Abstract
Many animals regulate their activity over a 24-h sleep–wake cycle, concentrating their peak periods of activity to coincide with the hours of daylight, darkness, or twilight, or using different periods of light and darkness in more complex ways. These behavioral differences, which are in themselves functional traits, are associated with suites of physiological and morphological adaptations with implications for the ecological roles of species. The biogeography of diel time partitioning is, however, poorly understood. Here, we document basic biogeographic patterns of time partitioning by mammals and ecologically relevant large-scale patterns of natural variation in “illuminated activity time” constrained by temperature, and we determine how well the first of these are predicted by the second. Although the majority of mammals are nocturnal, the distributions of diurnal and crepuscular species richness are strongly associated with the availability of biologically useful daylight and twilight, respectively. Cathemerality is associated with relatively long hours of daylight and twilight in the northern Holarctic region, whereas the proportion of nocturnal species is highest in arid regions and lowest at extreme high altitudes. Although thermal constraints on activity have been identified as key to the distributions of organisms, constraints due to functional adaptation to the light environment are less well studied. Global patterns in diversity are constrained by the availability of the temporal niche; disruption of these constraints by the spread of artificial lighting and anthropogenic climate change, and the potential effects on time partitioning, are likely to be critical influences on species’ future distributions.
Natural cycles of light and darkness structure the environment of the majority of eukaryotic organisms. The rotation of the Earth partitions time into regular cycles of day and night, and although all points on the Earth’s surface receive roughly equal durations of light and darkness over the course of a year, at mid to high latitudes seasonal variation in day length imposes an uneven distribution throughout the annual cycle. During the hours when the sun is below the horizon, there is seasonal and latitudinal variation in the duration of “biologically useful semidarkness” in the form of twilight and moonlight (1), modified by both the lunar cycle and variable cloud cover, providing changing opportunities for animals able to use visual cues for key behaviors including foraging, predator avoidance, and reproduction (2–6). Activity during both daylight and semidarkness may be further constrained by covariance between the natural cycles of light and temperature; the metabolic costs of thermoregulation place constraints on the time available for activity (7). Thermal constraints may limit nocturnal activity when nighttime temperatures are low, and diurnal activity when temperatures are high. Furthermore, energetic constraints may force some species to be active throughout hours of both light and darkness (8). Where energetic and thermal costs are not prohibitive, temporal niche partitioning may occur as species specialize and avoid competition by concentrating their activity within a particular section along the light gradient (9, 10). Behavioral traits are associated with a range of specialized adaptations, particularly in visual systems and eye morphology (11) and energetics and resource use (6, 12). Thus, some species are apparently obligately diurnal in their peak activity patterns, some obligately nocturnal, obligately crepuscular (active primarily during twilight), or obligately cathemeral (significant activity both during daylight and night), and others make facultative use of both daylight and night (13), or show seasonal and/or geographical variation in their strategy. Strict nocturnality and diurnality are hence two ends of a continuum of possible strategies for partitioning time over the 24-h cycle. As properties of organisms that strongly influence performance within a particular environment, these strategies can be considered functional traits in themselves (14), but are also associated with suites of adaptations, with implications for the ecological roles of species and individuals. Crepuscular and cathemeral species may have intermediate adaptations (15), and behavior may be flexible to vary within species and among individuals according to factors such as time of year, habitat structure, food availability, age, temperature, and the presence or absence of predators (16–18).
The ecology of diel time partitioning by organisms remains rather poorly understood (19, 20). Studies have considered the adaptive mechanisms behind strategies within a single ecosystem, including predator avoidance, energetic constraints, diet quality, and interspecific competition (9, 21). Meanwhile, although mapping functional traits has become a core technique in functional biogeography (22, 23), surprisingly little is known about the biogeography of diel activity patterns, and the extent to which they are determined by geographic gradients in light and climate. Addressing such issues has become more pressing with growth in the evidence for a wide range of ecological impacts of both anthropogenic climatic change and nighttime light pollution (24–28). Natural cycles of light have remained consistent for extremely long geological periods, providing a rather invariant context, and a very reliable set of potential environmental cues. The continued spread of electric lighting has caused substantial disruption to how these cycles are experienced by many organisms, exerting a novel environmental pressure (29). Direct illumination of the environment has quite localized effects, but sky glow—the amplified night sky brightness that is produced by upwardly emitted and reflected electric light being scattered by water, dust, and gas molecules in the atmosphere—can alter light regimes over extensive areas. Indeed, under cloudy conditions in urban areas, sky glow has been shown to be of an equivalent or greater magnitude than high-elevation summer moonlight (30). Understanding the biogeography of time partitioning by organisms provides a first step toward determining where such changes are likely to have the greatest impact.
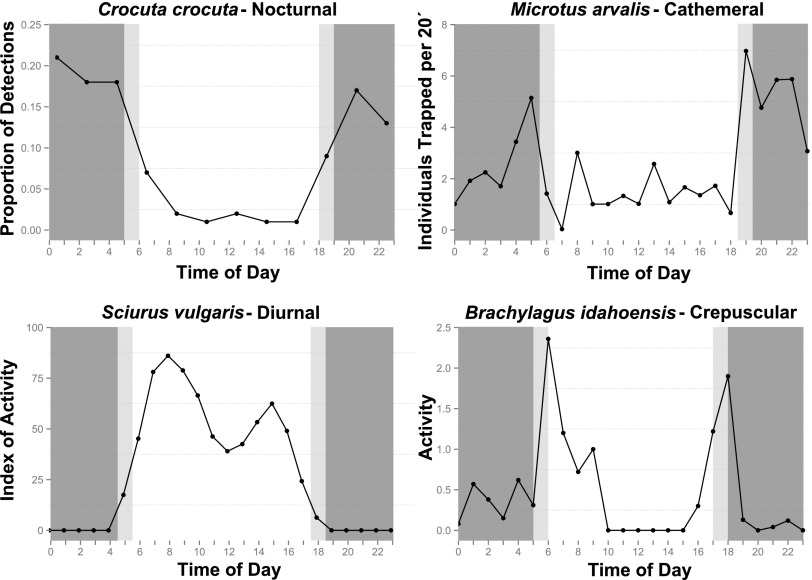
In this paper, we (i) document basic biogeographic patterns of time partitioning by organisms, using terrestrial mammals as a case study; (ii) document ecologically relevant large-scale patterns of natural variation in “biologically useful” natural light, constrained by temperature; and (iii) determine how well the first of these are predicted by the second. Mammals provide an interesting study group, being globally distributed, occupying a broad range of environments, and exhibiting a wide diversity of time-partitioning behavior. Much concern has also been expressed as to the potential impacts of nighttime light pollution on the group, and there are many studies documenting significant influences (31, 32). Due to the global nature of this study, and the paucity of detailed information on time partitioning reported for many species, our focus is on a high-level categorization, allocating species to one of four temporal niches: nocturnal, diurnal, cathemeral, and crepuscular (Fig. 1), albeit with the acknowledgment that in many species behavior occurs along a continuum of possible strategies that may be more flexible and complex.
Fig. 1.
Examples of recorded diel activity patterns illustrating the four main time-partitioning strategies used to classify terrestrial mammals in this study (64–67).
Results and Discussion
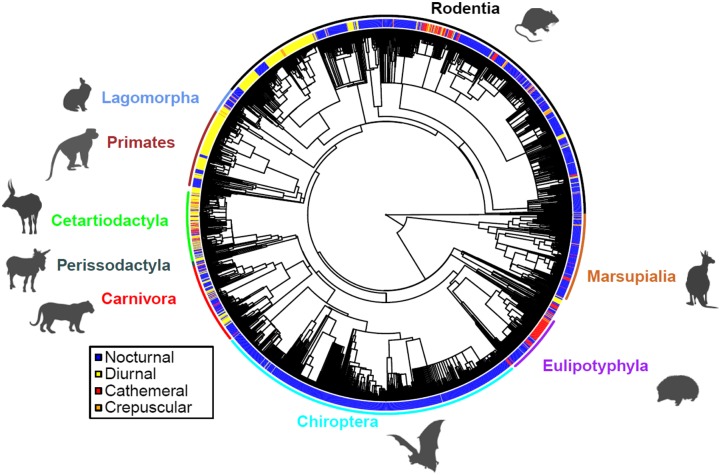
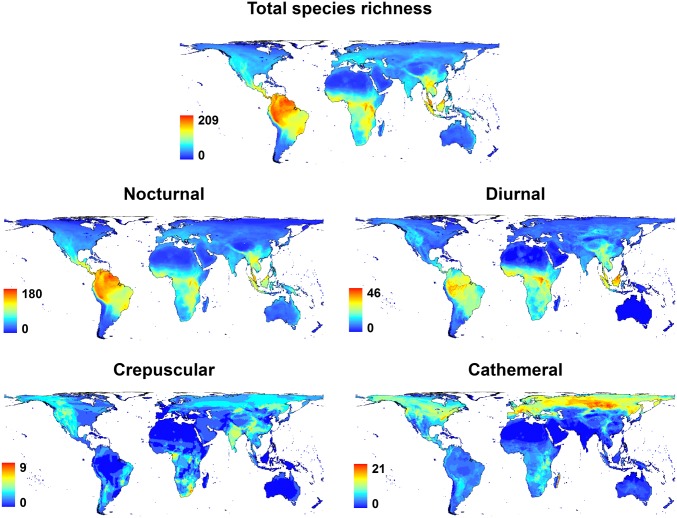
Globally, the majority of mammal species are nocturnal (69% in our dataset; 20% diurnal, 8.5% cathemeral, and 2.5% crepuscular), and it has been argued that nocturnality is an ancestral characteristic of the group (33). We found significant phylogenetic conservation in time-partitioning strategy within mammals as a whole (Fig. 2; Pagel’s λ = 0.947, P < 0.001). As a comparison with other mammalian traits, estimates of λ for solitary and group living are 0.93 and 0.86, respectively (34), 0.97 for life span (35), and 0.84 for dispersal distance (36). The global distributions of species richness within temporal niches, however, vary strikingly (Fig. 3). Although the species richness of both nocturnal and diurnal mammals is greatest in the humid tropics, broadly following well-known gradients in total species richness in this group and others (37), key differences occur between the two temporal niches; for example, in tropical South America diurnality is high along the river corridors of the Amazon basin, due to high richness of diurnal primates, whereas further north in seasonally dry forest and shrubland in Venezuela, high mammal diversity consists largely of bats and rodents, both groups in which diurnality is uncommon. In contrast, cathemeral species richness is highest at mid to high latitudes in the Northern Hemisphere, particularly central Siberia and midlatitude mountain ranges including the Alps, Pyrenees, and Appalachians, with relatively high cathemeral species richness also locally recorded in the forests of eastern Madagascar. Although strictly crepuscular activity is relatively rare globally, aggregations of crepuscular species occur in southern Africa, India, and China. When shown as a proportion of total species richness (Fig. 4), biogeographic patterns are particularly marked; although nocturnality is the global norm in mammals, diurnal activity patterns dominate at high altitude in the Tibetan Plateau and Andes, where nighttime temperatures are low and the energetic costs of nocturnality are prohibitively high; and crepuscular and cathemeral activity patterns dominate in the Arctic regions, characterized by long hours of twilight and high seasonal variation in the hours of daylight.
Fig. 2.
Phylogenetic tree showing 3,510 mammal species allocated to one of four time-partitioning strategies. The colored radial bars represent the dominant time-partitioning strategy for the species from an extensive literature search; the internal radial tree shows a species-level mammal phylogeny (55, 56). Selected major clades are shown.
Fig. 3.
Global distribution of mammal species richness, broken down by temporal niche.
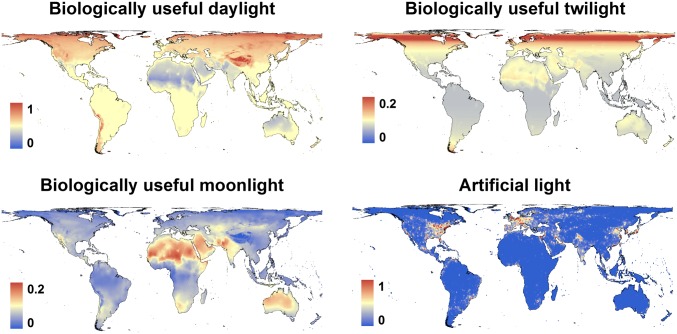
Fig. 4.
Global annual totals of biologically useful daylight, twilight, and moonlight, and global distribution of artificial light used in this analysis. Biologically useful light is expressed as the ratio of the illuminated duration when the estimated temperature is between 0 and 35 °C, and the total duration between these thermal limits (daylight, twilight, moonlight, and darkness). Twilight is defined as the duration when the sun is less than 12° below the horizon; moonlight is the duration when the lunar disk is more than 75% illuminated, unobscured by cloud cover and the sun is more than 12° below the horizon. Artificial light derived from DMSP satellite data for the year 2009 from National Oceanic and Atmospheric Administration National Geophysical Data Center.
There is considerable geographic variation in the relative proportions of time within thermal limits suitable for activity in mammals (“thermal activity time”—here defined as between 0 and 35 °C) illuminated by daylight, moonlight, and twilight, respectively (“illuminated activity time”). It is assumed that, outside these limits, activity is either greatly reduced or imposes high physiological or adaptive costs. The proportion of thermal activity time illuminated by daylight approximates 50% throughout the humid tropics, but is constrained by high daytime temperatures in the Sahara, Arabian Peninsula, Indian subcontinent, and Australia (Fig. 4). In parts of the high Andes, Rocky Mountains, and Tibetan Plateau, virtually all thermal activity time falls during the hours of daylight, due to low nighttime temperatures; at high latitudes in the Northern Hemisphere, a high proportion of thermal activity time falls within long days during summertime. The distribution of activity time illuminated by moonlight broadly follows global gradients in cloud cover, whereas relatively long hours of twilight occur at high latitudes where the sun remains close to the horizon for long periods of the year.
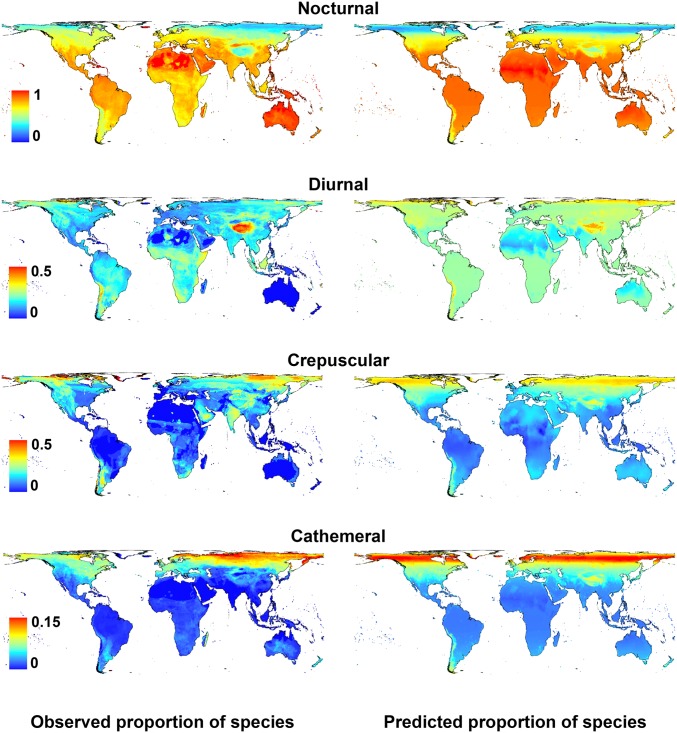
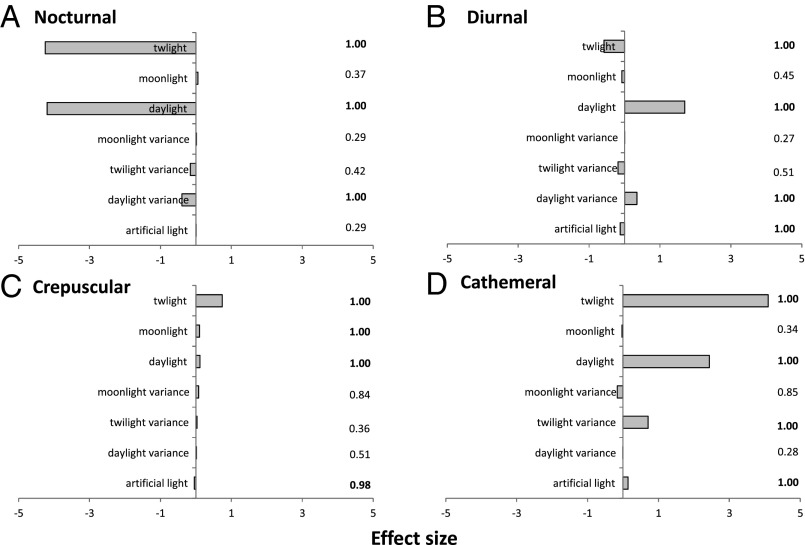
We predicted the proportion of species richness within each temporal niche as a function of spatial gradients in light using simultaneous autocorrelation models to allow for the effects of spatial autocorrelation. The results strongly indicate that global patterns in time partitioning are coincident with patterns in illuminated activity time, the availability of biologically useful daylight, twilight, and moonlight constrained by temperature (Figs. 5 and 6 and Table S1). R2 values for model fit predicting the proportion of total species richness attributed to each strategy are 0.701 for nocturnal species, 0.213 for diurnal, 0.668 for crepuscular, and 0.736 for cathemeral. Nocturnality is more frequent in regions with low ratios of both daylight and twilight, and low variance in daylight. Diurnality is more frequent where daylight hours are high and more variable, and twilight is limited; crepuscularity is most strongly influenced by the availability of twilight, and to a lesser extent both moonlight and daylight, whereas cathemerality is influenced by twilight, daylight, and twilight variance. The relatively low R2 values for diurnality reflect strong regional patterns that are not predicted by the modeled global light gradients, notably higher than expected rates of diurnality in localized parts of Africa, Borneo, and southern South America. These second-order patterns require further explanation.
Fig. 5.
Global distribution of observed (Left) and modeled (Right) proportions of mammal species with different time-partitioning strategies. Pseudo-R2 values, calculated following ref. 54 as the squared Pearson correlation coefficient between observed and predicted values, are as follows: nocturnal R2 = 0.701; diurnal R2 = 0.213; crepuscular R2 = 0.668; cathemeral R2 = 0.736.
Fig. 6.
Effect sizes and relative importance of predictor variables in spatial error models. The bars represent the effect size, calculated as the magnitude and direction of the Akaike-weighted coefficients of terms in the set of models within 5 AIC units of the best model. Figures to the Right of bars represent the relative importance, defined as the proportion of models within the set in which the term is included.
In addition, the distribution of artificial light is associated with the occurrence of diurnal, crepuscular, and cathemeral species. These effects are relatively small compared with those of global natural gradients of light, but are nevertheless robust enough to occur in the vast majority of models selected on the basis of Akaike information criterion (AIC) values. It is unlikely that at this spatial resolution this is a direct effect of artificial light on temporal niche use; the global distribution of artificial light is strongly correlated with human population density and may be acting as a proxy for a range of human impacts. These results provide tentative evidence that temporal niche is a component of extinction risk due to human impacts. The distribution of artificial light is associated with a decline in the proportion of diurnal and crepuscular species, and an increase in the proportion of cathemeral species, with the proportion of nocturnal species remaining unaffected. This pattern is echoed when the proportion of species attributed to International Union for Conservation of Nature (IUCN) red-list categories, representing their global extinction risk, is considered with respect to their time-partitioning strategies. Seventy percent of the 2,966 nonthreatened species in this study are nocturnal, 19% diurnal, 9% cathemeral, and 2% crepuscular; for the 926 species considered threatened (critically endangered, endangered, and vulnerable), only 59% are nocturnal and 7.5% cathemeral, but 30% are diurnal and 3.5% crepuscular (difference between groups significant at P < 0.001, χ2 = 105.71, df = 6). However, a significantly higher proportion of those species with insufficient data to classify their status are nocturnal (583 species classified as data deficient; 81% nocturnal, 11% diurnal, 6.5% cathemeral, 1% crepuscular). Cathemeral activity in mammals may increase following habitat fragmentation (38), and in other groups diurnality has been identified as a barrier to movement between habitat patches in artificially fragmented landscapes (39); however, more research is needed to elucidate any links between anthropogenic land use change and time-partitioning strategy. A nocturnal habit is likely to minimize contact with humans, and hence adaptation to facultative or obligate nocturnality may be a key determinant of extinction risk.
Just as temperature constraints on activity may limit the distribution of species (7, 40) and functional traits (41), it has been argued that light plays a key, but often neglected, role in organizing distributions and biological responses to climate (42, 43). The simple surfaces of activity time using different forms of light that are presented here give first-order predictions of the diversity of time-partitioning strategies along geographical gradients. Functional adaptations to activity during different sections of the diurnal cycle clearly place restrictions on the geographic distribution of species. Light and temperature combine to define available niches and constrain community structure. Global patterns in the duration of biologically useful light define a resource gradient, which is the context for niche partitioning and functional adaptation. Much remains to be discovered about the functional biogeography of the temporal niche. It is likely that patterns in time partitioning will emerge for other groups, which reflect the different phylogenetic constraints on adaptation to both light and temperature. Phylogenetic constraints have previously been shown to play a role in determining time-partitioning strategies in rodents (41, 44), and our analysis shows a high level of phylogenetic conservatism across mammals as a whole. Environmental constraints on the availability of temporal niches may therefore have implications for the distribution of taxonomic groups, and conversely the evolutionary history of regions may influence the diversity of strategies available. For example, a cathemeral habit is recorded for many lemurids, but is rare in other primates (13, 15, 21), and nocturnality is recorded for the majority of marsupials (11), and these patterns are not fully explained by our estimates of illuminated activity time for Madagascar and Australasia (Fig. 5). At resolutions finer than the broad-scale patterns identified by this study, other factors are likely to play a role in structuring temporal partitioning strategies. These include habitat structure (8, 38), interspecific or intraspecific competition (9, 10, 44), human disturbance, and predation pressure (4, 45, 46).
Our findings demonstrate that biogeographical patterns of global mammalian diversity are structured in part by the availability of the temporal niche, which is itself constrained by natural cycles of both light and temperature. In general, the nocturnal proportion of species diversity is highest in arid regions, whereas the diurnal proportion is higher at high altitudes, reflecting temperature constraints on activity during day and night, respectively. Crepuscular and cathemeral species provide a higher proportion of total species diversity at high latitudes, where hours of biologically useful twilight and annual variability in the light regime are greatest. Human modification of both the nighttime light environment (through the spread of artificial lighting) and global temperature patterns (through anthropogenic climate change) means that these constraints are changing at a probably unprecedented rate. We show that human impacts change the relative proportions of species diversity within each time-partitioning group, and that diurnal and crepuscular species are more likely to be classified as threatened than nocturnal and cathemeral. Understanding the links between the behavioral and physiological adaptations to circadian patterns of activity, the links between temporal niche and population declines, and the biogeography of time partitioning is a critical step toward predicting the implications of these changes for biodiversity.
Materials and Methods
Mammal Data.
The most recently available (October 2010) global species range maps for terrestrial mammals were downloaded from the IUCN (www.iucnredlist.org/technical-documents/spatial-data) in December 2011. Analysis was conducted in ArcGIS 9.3 and 10 (ESRI, 2012), except where otherwise stated. Range maps for each of 5,276 species were extracted and transformed to the Behrmann equal-area projection.
Each species was classified as using one of four temporal niches—nocturnal, diurnal, crepuscular, or cathemeral. An extensive literature search of books, peer-reviewed journal articles and their supplementary information, online databases and resources, gray literature, and consultation with experts was conducted to gather information on the daily activity patterns of the terrestrial mammals. One hundred eighty-four mammal species were described as highly/fully fossorial in the literature (47–49) and were therefore removed from the analysis. Information was successfully obtained for 2,893 species with a further 355 interpolated at the genus/family/order level where at least 75% of species for which data were available showed a common behavior or where the literature described a general behavior at a higher taxonomic level than species. Species for which data were interpolated at a higher taxonomic level were omitted from the phylogenetic analysis. In addition, 1,139 bat species were considered, and with the exception of Megachiroptera, the Old World fruit bats, all were assumed to be nocturnal unless described otherwise in the published literature. Species that were no longer formally described were also omitted. It is likely that the cathemeral and crepuscular habits are somewhat underrecorded in the literature, and this is likely to be the case in our dataset. In total, 4,477 species were used in this analysis, with 612 nonfossorial species omitted due to lack of data on their temporal niche. Knowledge gaps in particular exist within the mammals of China, Central Africa, and New Guinea; however, a sensitivity analysis suggests that neither these omissions, nor the interpolation of the 355 species from genus or family-level descriptions on the dataset are likely to have a strong effect on the spatial pattern of diversity within each time-partitioning strategy (SI Materials and Methods and Fig. S1). The classification for all 4,477 species used in the analysis is included in Table S2.
Following the classification of each species, vector maps of species ranges were converted into raster datasets of species richness attributed to the four temporal niches. This process was performed in the statistical and programming environment R (50), using the packages raster (51), rgdal (52), and sp (53). A pixel resolution of 56.2 km, equal to ∼0.5° at the equator, was used. To prevent loss of small polygons during the rasterization process, a polygon to point rasterization technique was used, where polygon edges were converted to points, and then these points were rasterized along with the polygons themselves, ensuring the inclusion of all range maps in the analysis. Duplication was avoided by taking the maximum value for a given pixel, ensuring only a 1 or a 0 was recorded per species in the running total. This procedure was repeated for each of the four groups of species resulting in a single species richness raster for each.
Global Light Data.
Global maps of illuminated activity time, in terms of biologically useful daylight, twilight, and moonlight constrained by temperature between 0 and 35 °C were generated by combining 0.5° resolution baseline monthly average climate data for 1961–1991 from the CRU 2.1 dataset (54) with calculated seasonal duration of light along latitudinal gradients. For each 0.5° latitude grid square, we calculated the daily duration of daylight (considered here to be the duration of time for which the midpoint of the solar disk is above the horizon), twilight (the duration for which the center of the solar disk is between 0° and 12° below the horizon; incorporating the conventional definition of both “civil” and “nautical” twilight), and full moonlight (defined here as the duration during which the illuminated fraction of the moon is greater than 75%, the center of the solar disk is more than 12° below the horizon, and the center of the lunar disk is above the horizon). The timing of sunset, sunrise, and positions of the sun and moon were derived from astronomical algorithms (55).
Global maps of illuminated activity time were created by adjusting the duration of each component of the daily light cycle using climate data to reflect both the effects of cloud cover on moonlight and seasonal temperature restrictions on the activity of mammals. Moonlight duration was adjusted to account for cloud cover obscuring the face of the moon [adjusted moonlight duration = moonlight duration × (1 – monthly cloud cover)]. Daily maximum, mean, and minimum temperatures were linearly interpolated from the monthly CRU data. A nominal lower temperature limit of 0 °C and upper temperature limit of 35 °C for mammal activity were imposed, on the assumption that, outside these limits, activity is either greatly reduced or imposes high physiological or adaptive costs. Monthly sums of illuminated activity time within these temperature limits were estimated using the following rules: daily hours of daylight were added to the monthly total only if the maximum daily temperature was below 35 °C and mean temperature above 0 °C; daily hours of moonlight were added if the minimum daily temperature was above 0 °C and below 35 °C; daily hours of presunrise twilight were included only if daily minimum temperatures were above 0 °C and below 35 °C; and daily hours of postsunset twilight were added only if daily mean temperatures were between 0 °C and 35 °C. The latter two restrictions are based on the assumption that minimum temperatures typically occur shortly before sunrise, making presunrise twilight temperatures close to the daily minimum temperature; whereas temperatures at sunset are typically closer to the daily mean temperature. Monthly totals were added to create an annual total duration for each natural light category, and the variance between months was calculated to create an index of seasonal variation in natural light. The script used for calculating illuminated activity time is included in R code file.
The distribution of artificial nighttime light was derived from satellite data for 2009 from the Defense Meteorological Satellite Program, Version 4, DMSP/OLS Nighttime Lights Time Series (56). All gridded raster data were converted to equal-area Behrmann projection with the same extent and resolution as the species richness maps.
Spatial Modeling.
We used autoregressive spatial linear models, or SLMs (57), using the “spdep” package (58) in the R statistical package (47) to predict species richness within each temporal niche. Niche species richness was modeled as a linear function of total species richness, interactions between total species richness and explanatory light variables, and a spatial error term. The model took the following form:
where Sniche is niche species richness, Stotal is total species richness, E1… En are a list of n standardized explanatory variables, β1… βn are the slopes associated with explanatory variables, γWu is the spatial structure (γW) in the spatially dependent error term u, and e is the spatially independent error. A neighborhood of 400 km was used to calculate the spatial structure weights matrix W, allowing all points within this distance to influence the values at a point. Residuals from all models were tested for significant spatial autocorrelation using Moran’s I; in all cases, no significant spatial autocorrelation remained when using this neighborhood distance. To ensure computation times were tractable, SLMs were run on a randomly selected 10% of the full dataset (4,494 points; the same selection of points was used in all analyses). Seven explanatory variables were used in the analysis—the annual ratios of biologically useful twilight, moonlight, and daylight, the variance in monthly values of each of these ratios, and a satellite-derived index of artificial light, which largely follows global patterns of human settlement (Fig. 4). All explanatory variables were rescaled to mean of zero and unit SD, and were included in the analysis as interactions with total mammalian species richness. SLMs were run for each temporal niche using all possible combinations of explanatory variables, and a model averaging procedure (59) was used to identify key explanatory variables. A “best” set of all models with AIC values within 5 units of the minimum AIC value were selected, and effect sizes were calculated from this model set as the Akaike-weighted slopes of the standardized variables. The relative importance of explanatory variables was calculated as the proportion of the “best” set of models containing each explanatory variable.
Because species richness within each temporal niche is correlated with total species richness, which was included as an interaction with all explanatory terms in the SLMs, to test model performance we divided predicted niche richness by total species richness to produce the predicted proportion of species within each niche as a function of explanatory terms (Fig. 5). Because there is no established method for calculating goodness of fit values from SLMs (60), we calculated pseudo-R2 values as the squared Pearson correlation coefficient between the predicted and observed proportions for the entire dataset.
Phylogenetic Analysis.
We cross-referenced our dataset with a published species-level mammal phylogeny (61, 62). To avoid circular reasoning, we omitted species from the phylogenetic analysis for which we had interpolated activity pattern on the basis of family, order, or genus without species-specific information (with the exception of bats). Species were also omitted if they could not be attributed to the phylogeny due to unresolved differences in taxonomy or nomenclature between the two datasets. A total of 3,510 species from our dataset could be allocated to species included in the phylogeny (Fig. 2). Pagel’s λ, a measure of phylogenetic signal in time-partitioning strategy, was calculated using the “geiger” package (63) in the R statistical package (50), and the significance was tested with a likelihood ratio test comparing this value of λ against a tree without phylogenetic signal (λ = 0).
Supplementary Material
Acknowledgments
We thank T. H. Clutton-Brock, M. Genoud, J. Gliwicz, K. Gourlay, D. J. Hosken, K. E. Jones, F. Matthews, R. A. McDonald, P. Racey, and A. T. Smith (and the IUCN Lagomorph Specialist Group) for help with compiling mammalian behavior data; S. Casalegno for technical advice; and R. A. McDonald and J. L. Osborne for comments and discussion. The research leading to this paper has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007–2013)/European Research Council Grant Agreement 268504 (to K.J.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.V. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216063110/-/DCSupplemental.
References
- 1.Mills AM. Latitudinal gradients of biologically useful semi-darkness. Ecography. 2008;31:578–582. [Google Scholar]
- 2.Baker GC, Dekker RWRJ. Lunar synchrony in the reproduction of the Moluccan megapode Megapodius wallacei. Ibis. 2000;142:382–388. [Google Scholar]
- 3.Mougeot F, Bretagnolle V. Predation risk and moonlight avoidance in nocturnal seabirds. J Avian Biol. 2000;31:376–386. [Google Scholar]
- 4.Hill RA. Why be diurnal? Or, why not be cathemeral? Folia Primatol (Basel) 2006;77(1-2):72–86. doi: 10.1159/000089696. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Duque E, de la Iglesia H, Erkert HG. Moonstruck primates: Owl monkeys (Aotus) need moonlight for nocturnal activity in their natural environment. PLoS One. 2010;5(9):e12572. doi: 10.1371/journal.pone.0012572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smit B, Boyles JG, Brigham RM, McKechnie AE. Torpor in dark times: Patterns of heterothermy are associated with the lunar cycle in a nocturnal bird. J Biol Rhythms. 2011;26(3):241–248. doi: 10.1177/0748730411402632. [DOI] [PubMed] [Google Scholar]
- 7.Angilletta MJ. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford: Oxford Univ Press; 2009. [Google Scholar]
- 8.Van Schaik CP, Griffiths M. Activity patterns in Indonesian rain forest mammals. Biotropica. 1996;28:105–112. [Google Scholar]
- 9.Di Bitetti MS, Di Blanco YE, Pereira JA, Paviolo A, Pérez IJ. Time partitioning favors the coexistence of sympatric crab-eating foxes (Cerdocyon thous) and pampas foxes (Lycalopex gymnocercus) J Mammal. 2009;90:479–490. [Google Scholar]
- 10.Gutman R, Dayan T. Temporal partitioning: An experiment with two species of spiny mice. Ecology. 2005;86:164–173. [Google Scholar]
- 11.Kirk EC. Comparative morphology of the eyes of primates. Anat Rec. 2004;281A:1095–1103. doi: 10.1002/ar.a.20115. [DOI] [PubMed] [Google Scholar]
- 12.Voigt CC, Lewanzik D. Trapped in the darkness of the night: Thermal and energetic constraints of daylight flight in bats. Proc Biol Sci. 2011;278(1716):2311–2317. doi: 10.1098/rspb.2010.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tattersall I. Cathemeral activity in primates: A definition. Folia Primatol (Basel) 1987;49:200–202. [Google Scholar]
- 14.McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends Ecol Evol. 2006;21(4):178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Kirk EC. Eye morphology in cathemeral lemurids and other mammals. Folia Primatol (Basel) 2006;77(1-2):27–49. doi: 10.1159/000089694. [DOI] [PubMed] [Google Scholar]
- 16.Hoogenboom I, Daan S, Dallings JH, Schoenmakers M. Seasonal change in the daily timing of behavior of the common vole Microtus arvalis. Oecologia. 1984;61:18–31. doi: 10.1007/BF00379084. [DOI] [PubMed] [Google Scholar]
- 17.Russo D, Maglio G, Rainho A, Meyer CFJ, Palmeirim JM. Out of the dark: Diurnal activity in the bat Hipposideros ruber on São Tomé Island (West Africa) Mamm Biol. 2011;76:701–708. [Google Scholar]
- 18.Mech LD, Cluff HD. Movements of wolves at the northern extreme of the species’ range, including during four months of darkness. PLoS One. 2011;6(10):e25328. doi: 10.1371/journal.pone.0025328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carothers JH, Jaksić FM. Time as a niche difference: The role of interference competition. Oikos. 1984;42:403–406. [Google Scholar]
- 20.Kronfeld-Schor N, Dayan T. Partitioning of time as an ecological resource. Annu Rev Ecol Evol Syst. 2003;34:153–181. [Google Scholar]
- 21.Tattersall I. Avoiding commitment: Cathemerality among primates. Biol Rhythm Res. 2008;39:213–228. [Google Scholar]
- 22.Newbold T, Butchart SHM, Sekercioğlu CH, Purves DW, Scharlemann JPW. Mapping functional traits: Comparing abundance and presence-absence estimates at large spatial scales. PLoS One. 2012;7(8):e44019. doi: 10.1371/journal.pone.0044019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swenson NG, et al. The biogeography and filtering of woody plant functional biodiversity in North and South America. Glob Ecol Biogeogr. 2012;21:798–808. [Google Scholar]
- 24.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333(6045):1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 25.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421(6918):37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 26.Longcore T, Rich C. Ecological light pollution. Front Ecol Environ. 2004;2:191–198. [Google Scholar]
- 27.Rich C, Longcore T, editors. Ecological Consequences of Artificial Night Lighting. Washington, DC: Island Press; 2006. [Google Scholar]
- 28.Perkin EK, et al. The influence of artificial light on stream and riparian ecosystems: Questions, challenges and perspectives. Ecosphere. 2011;2(11):122. [Google Scholar]
- 29.Hölker F, Wolter C, Perkin EK, Tockner K. Light pollution as a biodiversity threat. Trends Ecol Evol. 2010;25(12):681–682. doi: 10.1016/j.tree.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Kyba CCM, Ruhtz T, Fischer J, Hölker F. Cloud coverage acts as an amplifier for ecological light pollution in urban ecosystems. PLoS One. 2011;6(3):e17307. doi: 10.1371/journal.pone.0017307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beier P. In: Ecological Consequences of Artificial Night Lighting. Rich C, Longcore T, editors. Washington, DC: Island Press; 2006. pp. 19–42. [Google Scholar]
- 32.Rotics S, Dayan T, Kronfeld-Schor N. Effect of artificial night lighting on temporally partitioned spiny mice. J Mammal. 2011;92:159–168. [Google Scholar]
- 33.Heesy CP, Hall MI. The nocturnal bottleneck and the evolution of mammalian vision. Brain Behav Evol. 2010;75(3):195–203. doi: 10.1159/000314278. [DOI] [PubMed] [Google Scholar]
- 34.Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341(6145):526–530. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- 35.González-Lagos C, Sol D, Reader SM. Large-brained mammals live longer. J Evol Biol. 2010;23(5):1064–1074. doi: 10.1111/j.1420-9101.2010.01976.x. [DOI] [PubMed] [Google Scholar]
- 36.Whitmee S, Orme CD. Predicting dispersal distance in mammals: A trait-based approach. J Anim Ecol. 2012;82(1):211–221. doi: 10.1111/j.1365-2656.2012.02030.x. [DOI] [PubMed] [Google Scholar]
- 37.Grenyer R, et al. Global distribution and conservation of rare and threatened vertebrates. Nature. 2006;444(7115):93–96. doi: 10.1038/nature05237. [DOI] [PubMed] [Google Scholar]
- 38.Norris D, Fernanda M, Peres CA. Habitat patch size modulates terrestrial mammal activity patterns in Amazonian forest fragments. J Mammal. 2010;91:551–560. [Google Scholar]
- 39.Daily GC, Ehrlich PR. Nocturnality and species survival. Proc Natl Acad Sci USA. 1996;93(21):11709–11712. doi: 10.1073/pnas.93.21.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckley LB, et al. Can mechanism inform species’ distribution models? Ecol Lett. 2010;13(8):1041–1054. doi: 10.1111/j.1461-0248.2010.01479.x. [DOI] [PubMed] [Google Scholar]
- 41.Buckley LB, Hurlbert AH, Jetz W. Broad-scale ecological implications of ectothermy and endothermy in changing environments. Glob Ecol Biogeogr. 2012;21:873–885. [Google Scholar]
- 42.Dunbar RIM, Korstjens AH, Lehmann J. British Academy Centenary Research Project Time as an ecological constraint. Biol Rev Camb Philos Soc. 2009;84(3):413–429. doi: 10.1111/j.1469-185X.2009.00080.x. [DOI] [PubMed] [Google Scholar]
- 43.Bradshaw WE, Holzapfel CM. Light, time, and the physiology of biotic response to rapid climate change in animals. Annu Rev Physiol. 2010;72:147–166. doi: 10.1146/annurev-physiol-021909-135837. [DOI] [PubMed] [Google Scholar]
- 44.Roll U, Dayan T, Kronfeld-Schor N. On the role of phylogeny in determining activity patterns of rodents. Evol Ecol. 2006;20:479–490. [Google Scholar]
- 45.Rydell J, Speakman JR. Evolution of nocturnality in bats: Potential competitors and predators during their early history. Biol J Linn Soc Lond. 1995;54:183–191. [Google Scholar]
- 46.Berger D, Gotthard K. Time stress, predation risk and diurnal-nocturnal foraging trade-offs in larval prey. Behav Ecol Sociobiol. 2008;62:1655–1663. [Google Scholar]
- 47.Nowak RM. Walker's Mammals of the World. 6th Ed. Baltimore: Johns Hopkins Univ Press; 1999. [Google Scholar]
- 48.Vaughan TA, Ryan JM, Czaplewski NJ. Mammalogy. 5th Ed. Sudbury, MA: Jones and Bartlett; 2010. [Google Scholar]
- 49.Encyclopedia of Life Available at www.eol.org. Accessed July 10, 2012.
- 50.R Core Development Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2012. Available at www.R-project.org. Accessed June 1, 2012. [Google Scholar]
- 51.Hijmans RJ, van Etten J. The R Core Development Team 2012. raster: Geographic Analysis and Modelling with Raster Data. R package, version 1.9-70 (R Foundation for Statistical Computing, Vienna)
- 52.Keitt TH, Bivand R, Pebesma E, Rowlingson B. The R Core Development Team 2012. rgdal: Bindings for the Geospatial Data Abstraction Library. R package, version 0.7-8 (R Foundation for Statistical Computing, Vienna)
- 53.Pebesma E, Bivand R. The R Core Development Team 2012. sp: Classes and Methods for Spatial Data. R package, version 0.9-88 (R Foundation for Statistical Computing, Vienna)
- 54.Mitchell TD, Jones PD. An improved method of constructing a database of monthly climate observations and associated high-resolution grids. Int J Climatol. 2005;25:693–712. [Google Scholar]
- 55.Meeus J. Astronomical Formulae for Calculators. 4th Ed. Richmond, VA: Wilmann-Bell; 1988. [Google Scholar]
- 56.Elvidge CD, et al. Nighttime lights of the world: 1994-95. ISPRS J Photogramm Remote Sens. 2001;56:81–99. [Google Scholar]
- 57.Fortin M-J, Dale MRT. Spatial Analysis—A Guide for Ecologists. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 58.Bivand R. 2006. Spdep: Spatial Dependence: Weighting Schemes, Statistics and Models. R package, version 0.3-31 (R Foundation for Statistical Computing, Vienna)
- 59.Johnson JB, Omland KS. Model selection in ecology and evolution. Trends Ecol Evol. 2004;19(2):101–108. doi: 10.1016/j.tree.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 60.Kissling WD, Carl G. Spatial autocorrelation and the selection of simultaneous autoregressive models. Glob Ecol Biogeogr. 2007;17:59–71. [Google Scholar]
- 61.Bininda-Emonds OR, et al. The delayed rise of present-day mammals. Nature. 2007;446(7135):507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- 62.Bininda-Edmonds ORP, et al. Corrigendum: The delayed rise of present-day mammals. Nature. 2008;446:507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- 63.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. GEIGER: Investigating evolutionary radiations. Bioinformatics. 2008;24(1):129–131. doi: 10.1093/bioinformatics/btm538. [DOI] [PubMed] [Google Scholar]
- 64.Schuette P, Wagner AP, Wagner ME, Creel S. Occupancy patterns and niche partitioning within a diverse carnivore community exposed to anthropogenic pressures. Biol Conserv. 2013;158:301–312. [Google Scholar]
- 65.Tonkin JM. Activity patterns of the red squirrel (Sciurus vulgaris) Mammal Rev. 1983;13:99–111. [Google Scholar]
- 66.Daan S, Slopsema S. Short-term rhythms in foraging behaviour fo the common vole, Microtus arvalis. J Comp Physiol. 1978;127:215–227. [Google Scholar]
- 67.Larrucea ES, Brussard PF. Diel and seasonal activity patterns of pygmy rabbits (Brachylagus idahoensis) J Mammal. 2009;90:1176–1183. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.