Significance
Glutamate is the principal excitatory neurotransmitter in the brain. Kainate receptors, a subfamily of the ionotropic glutamate receptors, mediate the pre- and postsynaptic actions of glutamate. Overactivation of postsynaptic kainate receptors plays an important role in neurodegeneration after ischemic stroke. Because Src kinases show increased activity after brain ischemia, we evaluated their roles in regulating kainate receptor function after brain ischemia. Our results demonstrate that Src kinases bind to and phosphorylate GluK2 at Y590 and facilitate kainate-evoked whole-cell currents and calcium influx. Furthermore, long-term kainate stimulation promotes apoptosis through the endocytosis of GluK2 subunits and activation of JNK3–c-Jun signaling. In summary, our results demonstrate that Src phosphorylation of GluK2 regulates kainate receptor activity and downstream excitatory signaling.
Abstract
Although kainate receptors play important roles in ischemic stroke, the molecular mechanisms underlying postischemic regulation of kainate receptors remain unclear. In this study we demonstrate that Src family kinases contribute to the potentiation of kainate receptor function. Brain ischemia and reperfusion induce rapid and sustained phosphorylation of the kainate receptor subunit GluK2 by Src in the rat hippocampus, implicating a critical role for Src-mediated GluK2 phosphorylation in ischemic brain injury. The NMDA and kainate receptors are involved in the tyrosine phosphorylation of GluK2. GluK2 binds to Src, and the tyrosine residue at position 590 (Y590) on GluK2 is a major site of phosphorylation by Src kinases. GluK2 phosphorylation at Y590 is responsible for increases in whole-cell currents and calcium influx in response to transient kainate stimulation. In addition, GluK2 phosphorylation at Y590 facilitates the endocytosis of GluK2 subunits, and the activation of JNK3 and its substrate c-Jun after long-term kainate treatment. Thus, Src phosphorylation of GluK2 plays an important role in the opening of kainate receptor channels and downstream proapoptosis signaling after brain ischemia. The present study reveals an additional mechanism for the regulation of GluK2-containing kainate receptors by Src family kinases, which may be of pathological significance in ischemic stroke.
Kainate receptors are widely expressed in the mammalian central nervous system, particularly in the hippocampus, where they are involved in synaptic transmission (1), neuronal plasticity (2), and excitotoxic lesions (3). Overactivation of postsynaptic kainate receptor-mediated responses is associated with neurological disorders resulting from ischemic stroke (4). However, the intracellular processes responsible for the postischemic up-regulation of kainate receptors, and its molecular consequences, have not yet been elucidated.
Reversible phosphorylation is one of the most common mechanisms regulating the function of receptor proteins. In particular, serine/threonine phosphorylation is important in the functional regulation of NMDA (5), AMPA (6), and kainate receptors (6–9), with tyrosine phosphorylation of NMDA receptors the most extensively studied (10, 11). There is accumulating evidence to show that tyrosine phosphorylation of NMDA receptors modulates their assembly at synapses after brain ischemia (12–15). However, less is known about the regulation of kainate receptor activity by tyrosine phosphorylation. Src is an important member of Src family kinases, the largest family of nonreceptor protein tyrosine kinases, and is highly expressed in the brain. Brain ischemia increases Src kinase activity in vulnerable brain regions, including the hippocampus (15–18), but it is not known whether Src phosphorylates kainate receptors.
Kainate receptors are tetrameric glutamate-gated ion channels consisting of GluK1–GluK5 subunits, formerly known as GluR5–GluR7, KA1, and KA2, respectively. Functional kainate receptors can be expressed as homomers and heteromers of GluK1–3 subunits, whereas GluK4 and GluK5 subunits combine with GluK1–3 to form functional channels. It has been reported that GluK2-deficient mice are resistant to kainic acid-induced neuronal degeneration and seizures (19), and GluK2 knockdown protects against postischemic neuronal loss in the rat hippocampal CA1 region (20). In addition to sodium and potassium ions, GluK2-containing kainate receptors are permeable to Ca2+ (21, 22). Glutamate-induced intracellular Ca2+ ([Ca2+]i) overload is a major mechanism underlying excitotoxicity and ischemic cell death. Furthermore, excessive activation of GluK2-containing kainate receptors triggers the proapoptotic JNK signal cascade, which contributes to ischemic brain damage (23, 24). These findings suggest that GluK2-containing kainate receptor-mediated responses are critical events in the induction of neuronal cell death after stroke.
In this study we found that Src family kinases are involved in kainate-evoked whole-cell currents. In the vulnerable hippocampal CA1 region, GluK2 is phosphorylated on tyrosine 590 (Y590) by Src family kinases after brain ischemia. Conversely, the mutation of the Y590 residue on GluK2 decreases whole-cell peak currents and [Ca2+]i increases elicited by kainate, and deficiency of GluK2 phosphorylation at Y590 attenuates the endocytosis of GluK2 subunits and JNK3–c-Jun activation in response to kainate. These data indicate that Src-mediated phosphorylation promotes the opening of GluK2-containing kainate receptor channels and facilitates GluK2–JNK3 signaling. Our results contribute to the elucidation of molecular mechanisms underlying brain ischemic excitotoxicity.
Results
Src Family Kinases Strengthen Kainate Receptor Activity in Hippocampal Neurons and GluK2-Overexpressing HEK293 Cells.
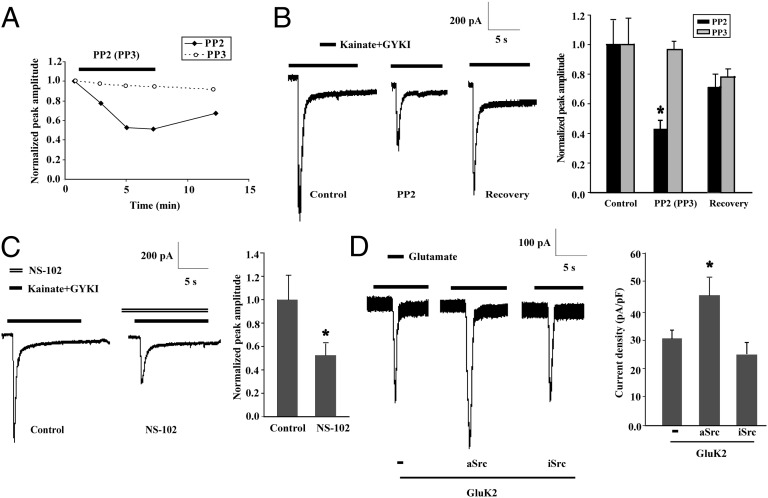
To determine whether Src family kinases are involved in the modulation of kainate receptor function, we evaluated the effect of PP2, a specific inhibitor of Src family kinases, on whole-cell currents evoked by kainate in cultured primary hippocampal neurons. Transient application of kainate (300 μmol/L) together with the AMPA receptor antagonist GYKI-52466 (100 μmol/L) was used to trigger kainate receptor-mediated whole-cell currents. Whole-cell patch-clamp recording showed that the kainate-triggered peak currents decreased gradually after the addition of PP2 (1 μmol/L) (Fig. 1A) and decreased to 42.7% ± 6.1% after 5 min (Fig. 1B). The current amplitude recovered to 71.6% ± 8.3% after PP2 washout. In contrast, treatment with PP3, an inactive PP2 analog, did not significantly change kainate receptor-mediated currents (Fig. 1 A and B). In addition, another Src family kinase inhibitor, SU6656 (1 μmol/L), significantly inhibited kainate-evoked currents (Fig. S1). These data indicate that Src family kinases enhance kainate receptor activity in hippocampal neurons.
Fig. 1.
Src family kinases strengthen kainate receptor activity. (A) Time course of kainate-stimulated peak currents from two representative hippocampal neurons treated with PP2 or PP3. After 6 min, the drugs were washed out. (B) Whole-cell patch-clamp recordings show an Src-dependent increase in kainate-evoked currents. The representative traces show the kainate receptor-mediated currents before (Control), during treatment (PP2 or PP3), and after treatment (recovery) in a single hippocampal neuron. The peak amplitudes are normalized to respective control and expressed as mean ± SEM (n = 8).*P < 0.05 vs. Control. (C) Kainate receptor currents were blocked by the GluK2 inhibitor NS-102. The peak amplitudes are normalized to control and expressed as mean ± SEM (n = 6). *P < 0.05 vs. control. (D) Constitutively active Src (aSrc), but not inactive Src (iSrc), increases GluK2(Q)-mediated currents induced by glutamate. The current density is expressed as current amplitude divided by capacitance (pA/pF). Results are expressed as mean ± SEM (n = 20–25). *P < 0.05 vs. GluK2.
A selective antagonist of GluK2-containing receptors, NS-102 (3 μmol/L), markedly decreased the peak whole-cell current after transient kainate application (Fig. 1C), suggesting a vital role for GluK2 subunits in kainate-induced currents in hippocampal neurons. To confirm the role of Src kinases in the functional regulation of GluK2-containing kainate receptors, full-length wild-type GluK2(Q) was overexpressed in HEK293 cells to form homologous functional kainate receptors that could be activated by glutamate or kainate. As shown in Fig. 1D, transient application of glutamate (1 mmol/L) elicited a rapid-onset inward current at a membrane voltage of −60 mV in transfected cells, which increased when wild-type GluK2(Q) was coexpressed with constitutively active Src (aSrc) but not with constitutively inactive form of Src (iSrc).
GluK2 subunits undergo editing at the Q/R site in the channel pore loop, which controls the channel properties of GluK2-containing receptors (25, 26). We found that coexpressing aSrc had no effect on glutamate-induced currents in HEK293 cells expressing homomeric channels composed of the edited form of GluK2(R) (Fig. S2). These results suggest that activated Src up-regulates the function of unedited GluK2(Q)-containing kainate receptors.
Brain Ischemia and Reperfusion Increase GluK2 Phosphorylation by Src Family Kinases in the Rat Hippocampal CA1 Region.
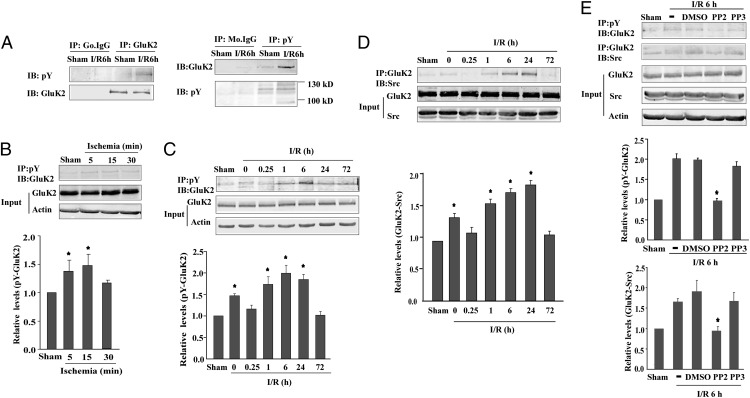
Src kinase, a central tyrosine kinase at the excitatory postsynaptic density in the adult central nervous system, is significantly activated after brain ischemia (15–18). Therefore, we investigated whether GluK2 subunits are phosphorylated by Src kinases during ischemia and reperfusion (I/R) in the vulnerable hippocampal CA1 subfield. Rats were subjected to global brain ischemia (15 min) followed by 6-h reperfusion. Hippocampal samples were then immunoprecipitated with an anti-phosphotyrosine antibody followed by immunoblotting with an anti-GluK2 antibody, or immunoprecipitated with an anti-GluK2 antibody followed by immunoblotting with an anti-phosphotyrosine antibody. The resulting 116-kDa protein band corresponding to tyrosine phosphorylated GluK2 was more intense in the hippocampus of rats that underwent I/R than in the sham control group (Fig. 2A). No bands were observed after immunoprecipitation with nonspecific IgG. Next, we found that tyrosine phosphorylation of GluK2 increased during 5-min and 15-min ischemia (Fig. 2B). After the 15-min brain ischemia, the level of tyrosine-phosphorylated GluK2 was increased until 24 h of reperfusion, peaking at 6 h (Fig. 2C), whereas the levels of total GluK2 were unchanged during ischemia and reperfusion.
Fig. 2.
Src family kinases are responsible for GluK2 tyrosine phosphorylation in rat hippocampal CA1 subfield after brain ischemia. Tyrosine phosphorylation of GluK2 and GluK2-Src binding were examined by immunoprecipitation (IP) followed by immunoblot (IB). Results are normalized to their respective sham controls and expressed as mean ± SD (n = 3). (A) GluK2 tyrosine phosphorylation after 6-h reperfusion (I/R6h) and in sham-operated rats. (B and C) Tyrosine phosphorylation of GluK2 after ischemia (5, 15, or 30 min) (B) and reperfusion after 15-min ischemia (C). *P < 0.05 vs. sham control. (D) GluK2-Src interaction during reperfusion after 15-min ischemia. *P < 0.05 vs. sham. (E) GluK2 tyrosine phosphorylation and GluK2-Src binding were prevented by Src family kinase inhibitor PP2. *P < 0.05 vs. I/R6h.
To investigate the role of Src kinases in tyrosine phosphorylation of GluK2, the interaction between Src and GluK2 was first examined by coimmunoprecipitation after ischemia (15 min). The binding between Src and GluK2 increased at 1 h, 6 h, and 24 h of reperfusion (Fig. 2D). In addition, pretreatment with PP2 (50 nmol per rat) or SU6656 (10 nmol per rat) attenuated the GluK2-Src binding and tyrosine phosphorylation of GluK2 (Fig. 2E and Fig. S3). These results suggest that brain ischemia and reperfusion promote Src kinase phosphorylation of GluK2 subunits in the hippocampus.
The overactivation of NMDA and kainate receptors is responsible for excitotoxic brain injury after ischemic stroke (27–29). Both kainate (100 μmol/L) and NMDA (100 μmol/L) increased the GluK2-Src binding and tyrosine phosphorylation of GluK2 in primary cultured cortical neurons, indicating that Src regulation of GluK2 may be associated with the postischemic excitotoxicity elicited by NMDA and kainate receptors (Fig. S4).
Src Kinase Binds Directly to and Phosphorylates GluK2 Primarily at Y590.
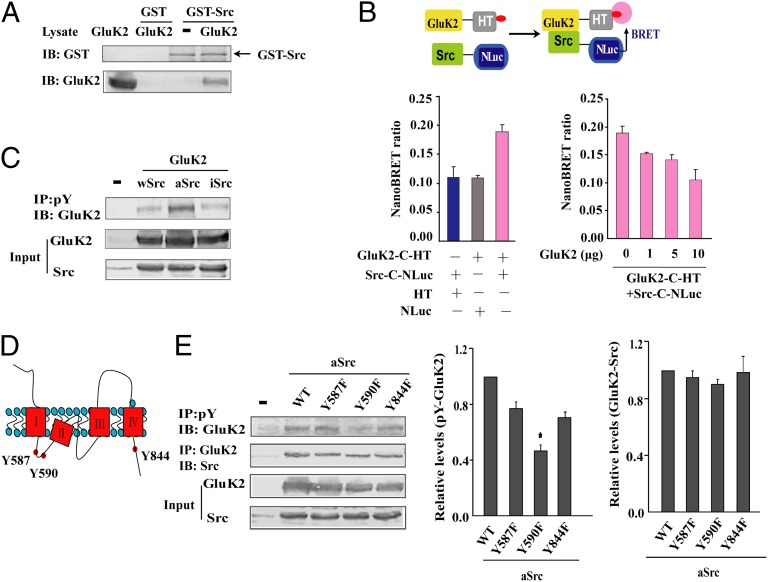
Results of the GST pull-down assay confirmed a direct interaction between Src and GluK2 in vitro (Fig. 3A). A bioluminescence resonance energy transfer (BRET) assay was then carried out to investigate the interaction of GluK2 and Src in live HEK293 cells. NanoLuc luciferase (NLuc) fused to the C terminus of Src (Src-C-NLuc) was the energy donor, and HaloTag-tagged wild-type GluK2(Q) (GluK2-C-HT) was the energy acceptor. As shown in Fig. 3B, coexpression of GluK2-C-HT and Src-C-NLuc resulted in a significant NanoBRET ratio. The increased expression of untagged-GluK2, a competitor for the interaction between GluK2-C-HT and Src-C-NLuc, gradually reduced the NanoBRET ratio. These results demonstrate that GluK2 interacts directly with Src in living cells.
Fig. 3.
GluK2(Q) Tyr590 is the principal phosphorylation site for active Src. (A) GST pull-down analysis of direct binding between Src and GluK2 in vitro. GluK2 in HEK293 cells lysate was pulled down by GST-Src but not GST. (B) Results of the BRET assay demonstrate GluK2-Src binding in live HEK293 cells. (Upper) Schematic diagram shows energy transfer upon binding of NanoLuc luciferase-tagged donor (Src-C-NLuc) and HaloTag-tagged acceptor (GluK2-C-HT) labeled with the fluorescent ligand in HEK293 cells. The NanoBRET ratio was highest in cells expressing GluK2-C-HT and Src-C-NLuc, decreasing after untagged-GluK2 expression. Results are expressed as mean ± SEM from a single experiment performed in triplicate and are representative of three independent experiments. (C) GluK2 tyrosine phosphorylation in cells coexpressing wild-type (wSrc), constitutively active (aSrc), or inactive Src (iSrc). (D) Three tyrosine residues on the cytoplasmic side of GluK2 are potential phosphorylation sites for intracellular Src kinases. (E) Tyrosine phosphorylation of wild-type GluK2 (WT) and the single tyrosine mutants Y587F, Y590F, and Y844F in HEK293 cells. GluK2 tyrosine phosphorylation and GluK2-Src binding results are normalized to that of WT group. Data are mean ± SD (n = 3); *P < 0.05 vs. WT.
In HEK293 cells, the construct for wild-type GluK2(Q) was cotransfected with a plasmid encoding wild-type Src (wSrc), aSrc, or iSrc. After 24 h, significant tyrosine phosphorylation of GluK2 was observed in cells carrying the aSrc construct (Fig. 3C), confirming that GluK2 is a substrate of activated Src. The 3D structures of the GluK2 subunits have been elucidated (30), and three tyrosine residues on the cytoplasmic side of GluK2 are potential phosphorylation sites for intracellular Src kinases (Fig. 3D). To identify the sites phosphorylated by Src kinases, wild-type GluK2(Q) and its single tyrosine mutants Y587F, Y590F, and Y844F were coexpressed with aSrc. Compared with cells expressing wild-type GluK2(Q), tyrosine phosphorylation was significantly lower in cells expressing the mutant Y590F. In contrast, single tyrosine mutations at Y587, Y590, or Y844 did not affect binding between Src and GluK2 (Fig. 3E). This result indicates that Y590 is the major phosphorylation site of GluK2 by Src kinases. The Y587/590/844F triple mutation nearly abolished GluK2 tyrosine phosphorylation (Fig. S5) but simultaneously compromised the binding of Src. This finding indicates that residues Y587 and Y844 may also contribute to GluK2 tyrosine phosphorylation.
GluK2 Phosphorylation at Y590 Enhances Kainate Receptor Responses.
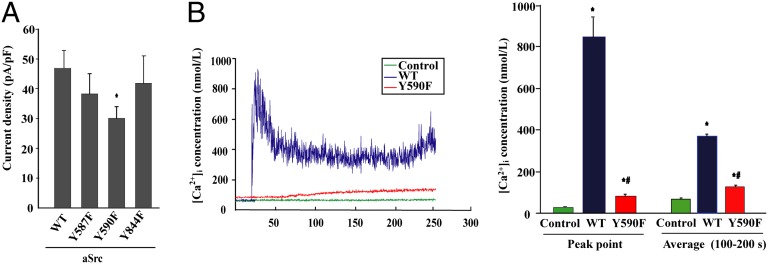
To further understand the role GluK2 phosphorylation at Y590 plays in the regulation of kainate receptors, whole-cell patch-clamp recording was used to detect glutamate-triggered responses in HEK293 cells cotransfected with constructs encoding aSrc with the nonphosphorylatable mutants Y587F, Y590F, and Y844F. Compared with cells expressing the wild-type GluK2 control, the normalized current amplitude was decreased in cells expressing Y590F but not in cells expressing Y587F or Y844F (Fig. 4A). No difference in the surface localization of GluK2 was detected in these cells expressing these constructs (Fig. S6).
Fig. 4.
Tyr590 phosphorylation on GluK2 enhances kainate receptor responses. (A) Whole-cell patch-clamp recordings of HEK293 cells expressing active Src (aSrc) with wild-type GluK2 (WT) and the single tyrosine mutants Y587F, Y590F, and Y844F. Current density is expressed as current amplitude divided by capacitance (pA/pF). Data are mean ± SEM (n = 12–26); *P < 0.05 vs. WT. (B) Kainate-induced increase in [Ca2+]i concentration in WT or Y590F-expressing HEK293 cells. Data are mean ± SEM (n = 12); *P < 0.05 vs. Control (empty vector); #P < 0.05 vs. WT.
Kainate receptors composed of GluK2(Q) are highly permeable to calcium (26), and exposure to kainate leads to intracellular Ca2+ overload. Immediately after kainate (300 μmol/L) stimulation, intracellular Ca2+ was significantly elevated in wild-type GluK2(Q)-expressing HEK293 cells (Fig. 4B). These data demonstrate that tyrosine phosphorylation at Y590 of GluK2-containing kainate receptors increases opening of the channel and calcium influx.
GluK2 Phosphorylation at Y590 Facilitates Kainate Receptor Endocytosis and JNK3–c-Jun Activation.
Excessive stimulation of GluK2-containing kainate receptors mediates the activation of proapoptotic JNK3 signaling, which is responsible for postischemic delayed neuronal loss (24). It has been found that sustained agonist treatment induces the endocytosis of GluK2-containing kainate receptors (31). Our previous study indicated that GluK2 endocytosis promotes MLK3-JNK3 activation in response to kainate incubation (32). In this study, we investigate the association of Src phosphorylation of GluK2 with the endocytosis of GluK2-containing receptors and intracellular activation of proapoptotic JNK3 signaling.
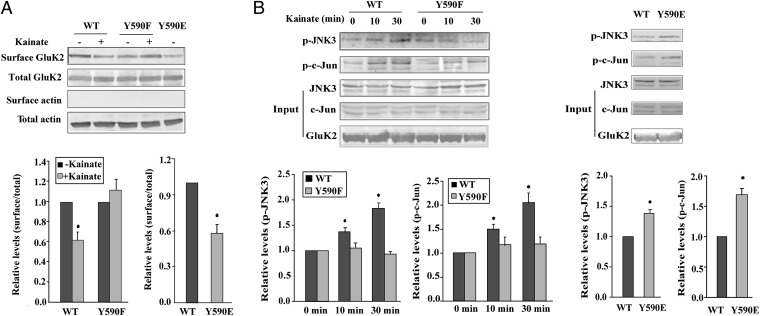
Long-term kainate incubation (300 μmol/L, 10 min) attenuated the membrane expression of wild-type GluK2(Q) in transfected HEK293 cells (Fig. 5A). In contrast, surface localization of the phosphorylation-deficient mutant GluK2Y590F was unchanged by kainate treatment. Membrane localization of the phosphomimetic mutant GluK2Y590E, in which a single tyrosine was mutated to glutamate, was low even in the absence of kainate. Under these conditions, total protein levels were unchanged. These results suggest that Src phosphorylation of GluK2 facilitates endocytosis after kainate stimulation.
Fig. 5.
Tyr590 phosphorylation facilitates GluK2 endocytosis and JNK3–c-Jun activation. (A) Surface biotinylation of HEK293 cells expressing wild-type GluK2 (WT), Y590F, or Y590E (with or without kainate). The surface and total GluK2 were immunoblotted with an anti-GluK2/3 antibody. The surface/total ratio is normalized to that of control (untreated cells expressing wild-type GluK2). Data are mean ± SD (n = 3); *P < 0.05. (B) Representative blot showing the JNK3 activation and phosphorylation of c-Jun in HEK293 cells overexpressing WT, Y590F (kainate stimulation for indicated times), or Y590E. Data are mean ± SD (n = 3). *P < 0.05 vs. 0 min or WT.
As expected, kainate incubation (300 μmol/L, 10 or 30 min) promoted the phosphorylation (activation) of JNK3 and its substrate c-Jun in HEK293 cells overexpressing wild-type GluK2(Q) but not in cells overexpressing GluK2Y590F (Fig. 5B). However, in GluK2Y590E-overexpressing cells, JNK3 and c-Jun activation was sustained in the absence of kainate (Fig. 5B). These results indicate that Src phosphorylation of GluK2 contributes to the activation of JNK3–c-Jun signaling.
Discussion
After an ischemic episode, overactivation of postsynaptic glutamate receptors, mainly NMDA and kainate receptors, triggers excessive intracellular signaling, eventually leading to neuronal cell death. This process, collectively known as excitotoxicity, represents a major mechanism underlying neurodegeneration after ischemic stroke. However, the clinical application of glutamate receptor antagonists is limited by severe side effects. A better understanding of the regulation of postsynaptic glutamate receptors and molecular consequences may reveal potential targets for ischemic stroke therapy. In this article, we provide the first evidence (to our knowledge) that the Src family of protein tyrosine kinases up-regulates GluK2-containing kainate receptor function in hippocampal neurons. Src phosphorylation of GluK2 facilitates the opening of the receptor channel, calcium overload, and proapoptotic JNK3–c-Jun signaling, which contributes to ischemic brain damage.
Both kainate and NMDA receptors are agonist-specific glutamate receptors. NMDA receptor activation increases Ca2+ influx and activates Ca2+-dependent Pyk2 (33) and its substrate Src (33, 34), which in turn phosphorylates GluK2 and up-regulates kainate receptor function. The tyrosine phosphorylation-induced opening of GluK2-containing channels may be due to a conformational change. On the other hand, kainate stimulates the opening of calcium-permeable kainate receptor channels and increases intracellular Ca2+ concentration, which may activate the Pyk2/Src pathway, thereby promoting tyrosine phosphorylation of GluN2 subunits and the functional enhancement of NMDA receptors. Our results suggest cross-talk between kainate and NMDA receptors based on Src-mediated tyrosine phosphorylation. Because the scaffold protein PSD-95 recruits more GluK2 and Src after ischemia (24, 35), we reason that PSD-95 promotes interaction between Src and GluK2, and GluK2 tyrosine phosphorylation mediated by Src kinases.
Our results show that long-term kainate stimulation induces GluK2 receptor endocytosis mediated by Src phosphorylation of GluK2. Endocytosis, along with recycling, degradation, and lateral diffusion, is responsible for changes in the surface expression and compartmentalization of membrane receptors. At low concentration (10 μmol/L), kainate induces the endocytic sorting of GluK2 to degradation pathways (36). However, in this study we found that at high concentrations (300 μmol/L) of kainate, GluK2 endocytosis resulted in downstream JNK3 signaling. We speculate that postischemic internalization of GluK2 leads to interactions between GluK2 subunits and intracellular proteins. The small ubiquitin-like modifier (SUMO) conjugation of the kainate receptor subunit GluK2 has been shown to enhance the endocytosis of kainate receptors (32). Here we demonstrated that tyrosine phosphorylation also regulates GluK2 surface expression. Thus, the relationship between tyrosine phosphorylation and SUMOylation of GluK2 requires further study.
Calcium signaling contributes greatly to cell death in excitotoxicity. Our results indicate that Src phosphorylation of GluK2(Q) promotes the opening of kainate receptor channels. The Q type of GluK2, but not the edited R type, increases Ca2+ permeability and single-channel conductance (26, 37). The extent of GluK2 Q/R editing is significantly decreased in ischemic vulnerable regions, including the hippocampus (38); therefore, calcium conductance through kainate receptors increases after brain ischemia, contributing to ischemic cell damage. Furthermore, a transient but substantial [Ca2+]i increase and the subsequent sustained lower [Ca2+]i increase are observed after kainate stimulation in GluK2(Q)-expressing cells. Immediate opening after kainate receptor activation causes the transient [Ca2+]i increase, which selectively activates Ca2+/calmodulin-dependent protein kinase II (CaMKII)/JNK, whereas the intracellular Ca2+ store release may contribute to the sustained Ca2+ increase, which activates calcineurin/nuclear factor of activated T cells (NFAT) (22, 39). CaMKII/JNK and calcineurin/NFAT-induced up-regulation of Fas/FasL is involved in cell apoptosis (40, 41). In addition, activated calcineurin may be involved in the endocytosis of kainate receptors through the dephosphorylation of endocytic proteins such as amphiphysin I and dynamin I (42, 43). In our experiments, kainate-evoked [Ca2+]i and reduced membrane expression of GluK2 were observed simultaneously; therefore, we speculate that Ca2+/calmodulin-activated calcineurin may induce kainate receptor endocytosis. Taken together, these findings indicate that tyrosine phosphorylation of GluK2(Q)-containing kainate receptors changes the intracellular Ca2+ concentration, activates Ca2+ signaling, initiates endocytosis, and plays critical roles in ischemic cell death.
In conclusion, this study revealed another target for GluK2 regulation. Our findings demonstrate that Src interacts with subunits of GluK2 and mediates its tyrosine phosphorylation. After ischemia/reperfusion, GluK2 phosphorylation, primarily on Y590, promotes opening of the channel, decreases receptor surface expression, and enhances JNK3 activation and c-Jun phosphorylation. These results reveal the molecular mechanisms underlying the regulation of GluK2 function and provide a potential target for the treatment for ischemic injury.
Materials and Methods
Brain ischemia was induced by the four-vessel occlusion method. Electrophysiology was detected by whole-cell patch-clamp recording. Detailed protocols regarding the brain ischemia experiments, neuron culture, surface biotinylation, immunoprecipitation, immunoblot, electrophysiology, GST pulldown assay, BRET assay, and calcium imaging can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China Grants 81173030 (to X.-Y.H.) and 81202610 (to T.L.), Major Basic Research Project of Jiangsu Higher Education Institutions Grant 11KJA310005 (to X.-Y.H.), a project funded by Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Jiangsu Qinlan Project for Innovative Team.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403493111/-/DCSupplemental.
References
- 1.Huettner JE. Kainate receptors and synaptic transmission. Prog Neurobiol. 2003;70(5):387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 2.Sihra TS, Flores G, Rodríguez-Moreno A. Kainate receptors: Multiple roles in neuronal plasticity. Neuroscientist. 2014;20(1):29–43. doi: 10.1177/1073858413478196. [DOI] [PubMed] [Google Scholar]
- 3.Vincent P, Mulle C. Kainate receptors in epilepsy and excitotoxicity. Neuroscience. 2009;158(1):309–323. doi: 10.1016/j.neuroscience.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 4.Mitani A, et al. Postischemic enhancements of N-methyl-D-aspartic acid (NMDA) and non-NMDA receptor-mediated responses in hippocampal CA1 pyramidal neurons. J Cereb Blood Flow Metab. 1998;18(10):1088–1098. doi: 10.1097/00004647-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Li BS, et al. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci USA. 2001;98(22):12742–12747. doi: 10.1073/pnas.211428098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang LY, Dudek EM, Browning MD, MacDonald JF. Modulation of AMPA/kainate receptors in cultured murine hippocampal neurones by protein kinase C. J Physiol. 1994;475(3):431–437. doi: 10.1113/jphysiol.1994.sp020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raymond LA, Blackstone CD, Huganir RL. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993;361(6413):637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- 8.Wang LY, Taverna FA, Huang XP, MacDonald JF, Hampson DR. Phosphorylation and modulation of a kainate receptor (GluR6) by cAMP-dependent protein kinase. Science. 1993;259(5098):1173–1175. doi: 10.1126/science.8382377. [DOI] [PubMed] [Google Scholar]
- 9.Nasu-Nishimura Y, Jaffe H, Isaac JT, Roche KW. Differential regulation of kainate receptor trafficking by phosphorylation of distinct sites on GluR6. J Biol Chem. 2010;285(4):2847–2856. doi: 10.1074/jbc.M109.081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11(3):336–342. doi: 10.1016/s0959-4388(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 11.Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 2012;279(1):12–19. doi: 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- 12.Hou XY, Zhang GY, Yan JZ, Chen M, Liu Y. Activation of NMDA receptors and L-type voltage-gated calcium channels mediates enhanced formation of Fyn-PSD95-NR2A complex after transient brain ischemia. Brain Res. 2002;955(1-2):123–132. doi: 10.1016/s0006-8993(02)03376-0. [DOI] [PubMed] [Google Scholar]
- 13.Besshoh S, Bawa D, Teves L, Wallace MC, Gurd JW. Increased phosphorylation and redistribution of NMDA receptors between synaptic lipid rafts and post-synaptic densities following transient global ischemia in the rat brain. J Neurochem. 2005;93(1):186–194. doi: 10.1111/j.1471-4159.2004.03009.x. [DOI] [PubMed] [Google Scholar]
- 14.Knox R, et al. Enhanced NMDA receptor tyrosine phosphorylation and increased brain injury following neonatal hypoxia-ischemia in mice with neuronal Fyn overexpression. Neurobiol Dis. 2013;51:113–119. doi: 10.1016/j.nbd.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang F, Guo A, Liu C, Comb M, Hu B. Phosphorylation and assembly of glutamate receptors after brain ischemia. Stroke. 2013;44(1):170–176. doi: 10.1161/STROKEAHA.112.667253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Hou XY, Zhang GY, Xu TL. L-type voltage-gated calcium channel attends regulation of tyrosine phosphorylation of NMDA receptor subunit 2A induced by transient brain ischemia. Brain Res. 2003;972(1-2):142–148. doi: 10.1016/s0006-8993(03)02519-8. [DOI] [PubMed] [Google Scholar]
- 17.Choi JS, et al. Activation of Src tyrosine kinase in microglia in the rat hippocampus following transient forebrain ischemia. Neurosci Lett. 2005;380(1-2):1–5. doi: 10.1016/j.neulet.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X, et al. Activated Src kinases interact with the N-methyl-D-aspartate receptor after neonatal brain ischemia. Ann Neurol. 2008;63(5):632–641. doi: 10.1002/ana.21365. [DOI] [PubMed] [Google Scholar]
- 19.Mulle C, et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392(6676):601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- 20.Pei DS, et al. Neuroprotective effects of GluR6 antisense oligodeoxynucleotides on transient brain ischemia/reperfusion-induced neuronal death in rat hippocampal CA1 region. J Neurosci Res. 2005;82(5):642–649. doi: 10.1002/jnr.20669. [DOI] [PubMed] [Google Scholar]
- 21.Silva AP, et al. Role of kainate receptor activation and desensitization on the [Ca(2+)](i) changes in cultured rat hippocampal neurons. J Neurosci Res. 2001;65(5):378–386. doi: 10.1002/jnr.1164. [DOI] [PubMed] [Google Scholar]
- 22.Ouardouz M, et al. Glutamate receptors on myelinated spinal cord axons: I. GluR6 kainate receptors. Ann Neurol. 2009;65(2):151–159. doi: 10.1002/ana.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savinainen A, Garcia EP, Dorow D, Marshall J, Liu YF. Kainate receptor activation induces mixed lineage kinase-mediated cellular signaling cascades via post-synaptic density protein 95. J Biol Chem. 2001;276(14):11382–11386. doi: 10.1074/jbc.M100190200. [DOI] [PubMed] [Google Scholar]
- 24.Pei DS, et al. Neuroprotection against ischaemic brain injury by a GluR6-9c peptide containing the TAT protein transduction sequence. Brain. 2006;129(Pt 2):465–479. doi: 10.1093/brain/awh700. [DOI] [PubMed] [Google Scholar]
- 25.Köhler M, Burnashev N, Sakmann B, Seeburg PH. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: Diversity by RNA editing. Neuron. 1993;10(3):491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- 26.Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. Effect of RNA editing and subunit co-assembly single-channel properties of recombinant kainate receptors. J Physiol. 1996;492(Pt 1):129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon RP, Swan JH, Griffiths T, Meldrum BS. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984;226(4676):850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- 28.Seo SY, et al. Complestatin is a noncompetitive peptide antagonist of N-methyl-D-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptors: Secure blockade of ischemic neuronal death. J Pharmacol Exp Ther. 2001;299(1):377–384. [PubMed] [Google Scholar]
- 29.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61(6):657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutcliffe MJ, Wo ZG, Oswald RE. Three-dimensional models of non-NMDA glutamate receptors. Biophys J. 1996;70(4):1575–1589. doi: 10.1016/S0006-3495(96)79724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin S, Nishimune A, Mellor JR, Henley JM. SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature. 2007;447(7142):321–325. doi: 10.1038/nature05736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu QJ, Xu Y, Du CP, Hou XY. SUMOylation of the kainate receptor subunit GluK2 contributes to the activation of the MLK3-JNK3 pathway following kainate stimulation. FEBS Lett. 2012;586(9):1259–1264. doi: 10.1016/j.febslet.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Guo J, et al. N-methyl-D-aspartate receptor and L-type voltage-gated Ca2+ channel activation mediate proline-rich tyrosine kinase 2 phosphorylation during cerebral ischemia in rats. Neurosci Lett. 2004;355(3):177–180. doi: 10.1016/j.neulet.2003.10.076. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Zhang G, Gao C, Hou X. NMDA receptor activation results in tyrosine phosphorylation of NMDA receptor subunit 2A(NR2A) and interaction of Pyk2 and Src with NR2A after transient cerebral ischemia and reperfusion. Brain Res. 2001;909(1-2):51–58. doi: 10.1016/s0006-8993(01)02619-1. [DOI] [PubMed] [Google Scholar]
- 35.Du CP, et al. Increased tyrosine phosphorylation of PSD-95 by Src family kinases after brain ischaemia. Biochem J. 2009;417(1):277–285. doi: 10.1042/BJ20080004. [DOI] [PubMed] [Google Scholar]
- 36.Martin S, Henley JM. Activity-dependent endocytic sorting of kainate receptors to recycling or degradation pathways. EMBO J. 2004;23(24):4749–4759. doi: 10.1038/sj.emboj.7600483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egebjerg J, Heinemann SF. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proc Natl Acad Sci USA. 1993;90(2):755–759. doi: 10.1073/pnas.90.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paschen W, Schmitt J, Uto A. RNA editing of glutamate receptor subunits GluR2, GluR5 and GluR6 in transient cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1996;16(4):548–556. doi: 10.1097/00004647-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386(6627):855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 40.Jayanthi S, et al. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci USA. 2005;102(3):868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timmins JM, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119(10):2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomizawa K, et al. Cophosphorylation of amphiphysin I and dynamin I by Cdk5 regulates clathrin-mediated endocytosis of synaptic vesicles. J Cell Biol. 2003;163(4):813–824. doi: 10.1083/jcb.200308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun T, et al. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J Neurosci. 2010;30(35):11838–11847. doi: 10.1523/JNEUROSCI.1481-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.