Significance
Our results indicate that, even in highly diverse systems like coral reefs, we can no longer assume that the erosion of species diversity can be discounted by the high probability of functional redundancy: i.e., that several species can support the same function. Indeed, we show that fish species tend to disproportionately pack into a few particular functions while leaving many functions highly vulnerable, i.e., they are supported by just one species. Even the Coral Triangle, which has a high concentration of tropical-reef fishes, may experience a loss of functional diversity following fisheries pressure and local species extirpation. Our results suggest that the promised benefits of functional insurance from high species diversity may not be as strong as we once hoped.
Keywords: fish ecology, coral reefs
Abstract
When tropical systems lose species, they are often assumed to be buffered against declines in functional diversity by the ability of the species-rich biota to display high functional redundancy: i.e., a high number of species performing similar functions. We tested this hypothesis using a ninefold richness gradient in global fish faunas on tropical reefs encompassing 6,316 species distributed among 646 functional entities (FEs): i.e., unique combinations of functional traits. We found that the highest functional redundancy is located in the Central Indo-Pacific with a mean of 7.9 species per FE. However, this overall level of redundancy is disproportionately packed into few FEs, a pattern termed functional over-redundancy (FOR). For instance, the most speciose FE in the Central Indo-Pacific contains 222 species (out of 3,689) whereas 38% of FEs (180 out of 468) have no functional insurance with only one species. Surprisingly, the level of FOR is consistent across the six fish faunas, meaning that, whatever the richness, over a third of the species may still be in overrepresented FEs whereas more than one third of the FEs are left without insurance, these levels all being significantly higher than expected by chance. Thus, our study shows that, even in high-diversity systems, such as tropical reefs, functional diversity remains highly vulnerable to species loss. Although further investigations are needed to specifically address the influence of redundant vs. vulnerable FEs on ecosystem functioning, our results suggest that the promised benefits from tropical biodiversity may not be as strong as previously thought.
The human-induced collapse of species has triggered a sixth mass extinction crisis worldwide (1). This ongoing biotic impoverishment may, in turn, markedly alter key ecosystem processes, such as productivity, nutrient cycling, and bioerosion, with undisputed consequences on ecosystem services that humanity needs to prosper (2–4).
Beyond the loss of species, the loss of particular functions appears to be the main threat imperiling ecosystem processes and services (4, 5). When several species perform similar functions, this functional redundancy may ensure against the loss of ecosystem functioning following declines in species diversity (6, 7). The critical issue is whether the extraordinary species diversity on Earth matters for ecosystem functioning or whether a smaller proportion of species is enough to perform most of the key functions (8).
This debate, at the core of ecological science (5), is even more vigorous in species-rich ecosystems where high functional redundancy among species is likely and where it is thus often assumed that ecosystem functioning is buffered against species loss. For instance, 75% of species could be lost before any functional group would disappear in an Argentinean plant community (6). Such high functional redundancy may ensure the level of functional diversity—i.e., the breadth of functions—against species loss following disturbance (9, 10) or explain why the relationship between species richness and ecosystem functioning may be weak (11) and asymptotic (12).
Some tropical ecosystems that hold many more species than their temperate counterparts do not show a higher functional diversity (13). This pattern suggests that functional redundancy may be comparatively higher in the tropics (14). In contrast, despite high levels of species richness, some tropical ecosystems show little functional redundancy among species (15, 16), thus revealing their functional vulnerability: i.e., a potential decrease of functional diversity following species loss (17).
Beyond aesthetic and moral arguments, the importance of conserving the whole of tropical biodiversity for maintaining the breadth of potential functions performed in species assemblages is still under scrutiny. Indeed, local or regional assessments are often based on a limited number of species and have offered contradictory results depending on ecosystems, taxa, and functional traits considered (9, 15, 18). We therefore still lack a global study, along a steep gradient of species richness, investigating how species are distributed among functional groups and, more particularly, the extent of functional redundancy and vulnerability.
In the marine realm, tropical reefs host a remarkable diversity of fishes that sustain essential ecosystem processes (e.g., trophic control, bioerosion, nutrient cycling) (18). Within the diverse array of species, some perform unique roles and appear to be irreplaceable (19). Moreover, tropical-reef fishes from the Indo-Pacific show a richness that declines with increasing distance from the Indo-Australian Archipelago (IAA) (20), and tropical-reef fish faunas from the Atlantic Ocean have a markedly lower diversity than their Indo-Pacific counterparts (21). Thus, tropical-reef fishes constitute an archetypal situation where we expect a gradient of functional richness, redundancy, and vulnerability, depending on the distribution of species among functional groups. Instead of functional groups, built a priori or after clustering species based on an arbitrary level of trait similarity, we identified 646 functional entities (FEs) based on unique combinations of six categorical functional traits to classify the 6,316 fish species of the global pool. We also built a functional space where FEs were placed according to their trait combinations (22). We then assessed the level of functional diversity, functional redundancy, and functional vulnerability for the tropical-reef fish faunas along a ninefold gradient of species richness. Because the identity, the number, and the categorization of functional traits may influence the results (16), we also performed a series of sensitivity analyses to test the robustness of the findings when decreasing the number of FEs by up to one order of magnitude.
Results and Discussion
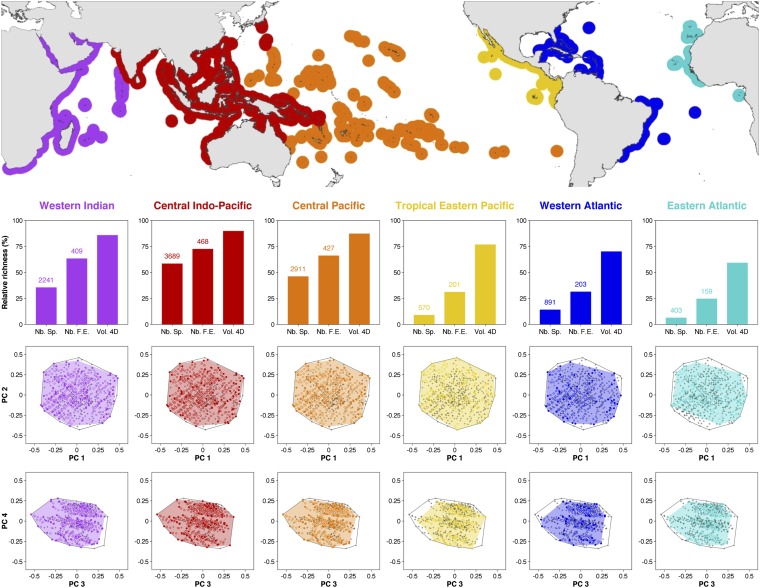
Across the six fish faunas of tropical reefs (23), species richness ranges between 403 species in the Eastern Atlantic to 3,689 in the Central Indo-Pacific, i.e., between 6.4% and 58.4% of the global pool respectively (Fig. 1). Despite hosting only a very small proportion of tropical-reef fishes, the three poorest faunas (Western and Eastern Atlantic and Tropical Eastern Pacific) have at least 25% of global FEs and fill at least 59% of the global functional space. Therefore, the ninefold gradient in fish richness across faunas translates into only a threefold gradient in terms of FE diversity. The most striking example is provided by the Central Pacific fish fauna, which fills nearly the same amount of functional space as the richest fauna of the Central Indo-Pacific (87.2% vs. 89.8%, respectively) despite hosting 21.1% fewer species (2,911 vs. 3,689, respectively). This relative stability in functional diversity is not due to a high taxonomic similarity among faunas because fish species composition is highly variable (global Jaccard dissimilarity index of 90.4%). These results support the idea that poorer faunas in the Tropical Eastern Pacific and in the Atlantic can maintain the range of ecological processes necessary for the growth and persistence of tropical reefs because they share most of the key functions with richer faunas (18, 24).
Fig. 1.
Species and functional diversity in six tropical-reef fish faunas. Histograms show fish richness, functional entity richness (i.e., unique trait combinations), and functional richness (i.e., volume filled by each fish fauna), expressed as a percentage relative to the global pool. Despite a ninefold change in species richness, the functional space richness remains largely unchanged. Number of species and number of functional entities are displayed above corresponding bars. Distribution of functional entities is shown in functional spaces where axes represent PC1–PC2 and PC3–PC4 from a Principal Coordinate Analysis on functional traits, respectively (Materials and Methods). The global convex hull, including the 6,316 species split into 646 functional entities, is in gray. The color-filled areas show the functional volume filled by each fauna. Colored circles represent functional entities present in the fauna, and filled points represent functional entities present in the fauna that are vertices of the convex hull: i.e., the ones that shape edges. Gray crosses are functional entities absent in the fauna.
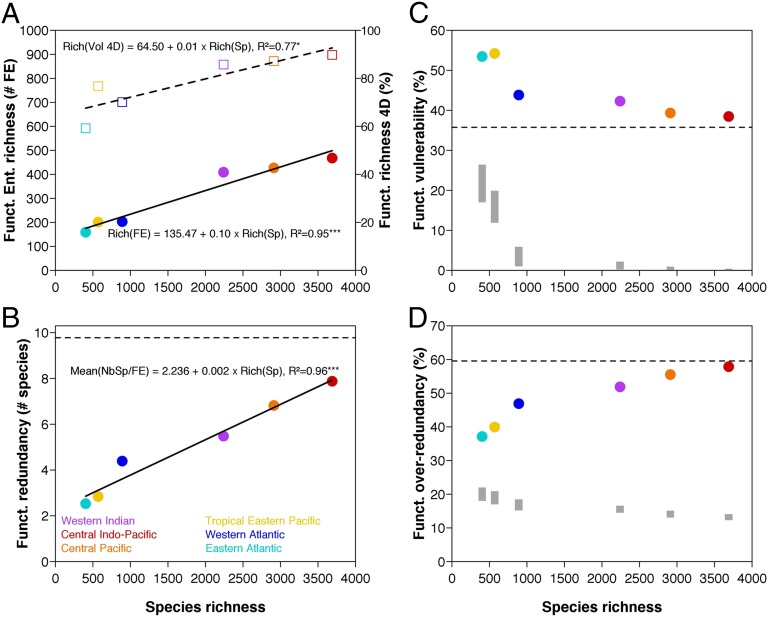
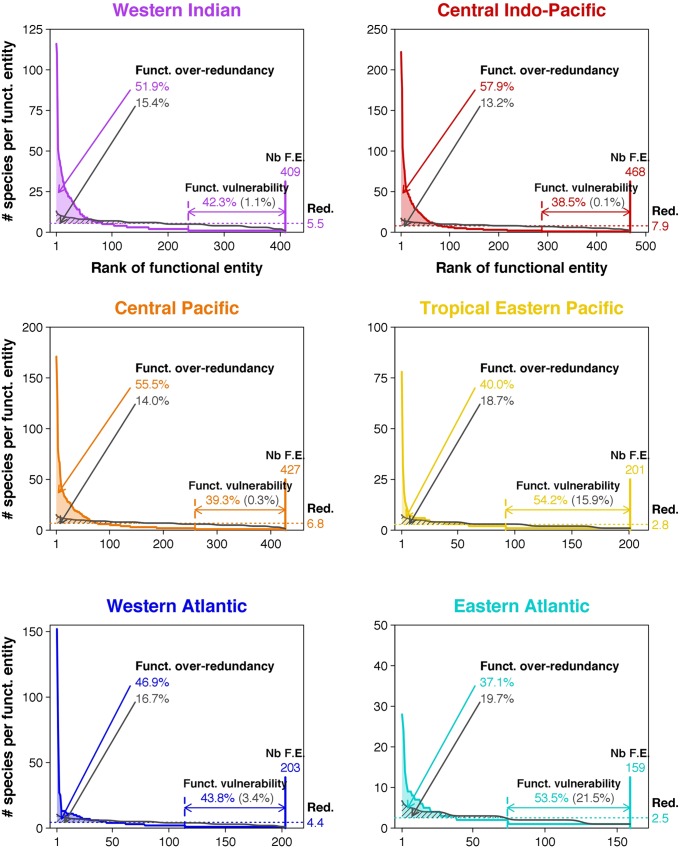
If the functional space is similarly filled by tropical fish faunas despite a strong richness gradient, it implies that species are more densely packed in that functional space for richer faunas. The number of FEs increases at a slower rate than species richness, with a slope for the linear regression of 0.10, meaning that 10 additional species, on average, are needed to have another FE across faunas (Fig. 2A). Consequently, the richest faunas have the highest functional redundancy, with, on average, 7.9 species per FE in the Central Indo-Pacific whereas there are only 2.8 species per FE in the Tropical Eastern Pacific (Fig. 2B). However, this increasing functional redundancy along the species-richness gradient does not result in the lowest functional vulnerability in the richest faunas: i.e., the lowest proportion of FEs with only one species. Indeed, because species are disproportionally packed into a small set of FEs (Fig. 3), the apparent insurance provided by the high functional redundancy level hides a large proportion of FEs vulnerable to species extinction. More precisely, functional vulnerability ranges between 38.5% and 54.2% of FEs across faunas (Fig. 2C), and observed values are all significantly higher than expected when species are randomly assigned to FEs (Materials and Methods) because the observed distribution of species richness among FEs is more right skewed than expected, with a long tail at one species (Fig. 3).
Fig. 2.
Relation between species richness and (A) functional richness, (B) functional redundancy, (C) functional vulnerability, and (D) functional over-redundancy across six tropical-reef fish faunas. Functional richness is expressed in terms of both the number of functional entities (circles) and the functional volume (squares) filled by fish faunas. Functional redundancy is the mean number of species per functional entity, functional vulnerability is the percentage of functional entities having only one species, with functional over-redundancy as the percentage of species in functional entities having more species than expected from functional redundancy. Horizontal dashed lines symbolize the index values measured on the global pool of species in B, C, and D. Linear regressions are plotted for functional richness (continuous line for entities and dashed line for volume) and functional redundancy whereas results from null models are provided for functional vulnerability and functional over-redundancy. Gray bars in C and D represent 95%-confidence interval of expected values under a null model simulating a random assignment of species into functional entities.
Fig. 3.
Functional over-redundancy and functional vulnerability in six tropical-reef fish faunas. The distribution of fish species into functional entities is displayed for each fauna. The number of functional entities (“Nb F.E.”) present in each fauna is shown at the bottom right of the distribution. Functional redundancy (“Red.”) (i.e., the mean number of species per functional entity) is illustrated by the horizontal dashed line, and the value is provided on the right margin of the panel. Functional vulnerability (i.e., percentage of functional entities having only one species) is illustrated by the horizontal colored line with arrows. Functional over-redundancy, the percentage of species in excess in functional entities having more species than expected from functional redundancy, is colored. The expected distribution (under a random assignment of species to functional entities) is represented by the gray line, with the corresponding values of functional over-redundancy and functional vulnerability in gray.
We coin the expression “Functional Over-Redundancy” (FOR) to describe the overrepresentation of some FEs in terms of species richness because the overall functional redundancy across FEs is in fact exacerbated in some FEs. For instance, species richness peaks at 222 for the most speciose FEs (6% of the fauna) in the Central Indo-Pacific whereas 180 FEs (out of 468) are represented by just one species (Fig. 3). FOR was calculated as the percentage of species that fill FEs above the mean level of functional redundancy (Materials and Methods). Surprisingly, the level of FOR remains in a narrow range (37−58%) across the six fish faunas (Fig. 2D), meaning that, whatever the level of species richness, roughly half of the species contribute to FOR. We also estimated the expected FOR under a null hypothesis, randomly assigning species to FEs to examine the sampling effect on FOR values. All of the observed FOR values are significantly higher than expected by chance (Fig. 2D), further showing that species are more densely packed into few FEs than one would expect based on simple random assignment.
To test the sensitivity of the results to the number and the identity of traits, we reran all analyses with all combinations of five traits out of six (Ecological Relevance of Fish Functional Traits). Whatever the combination, even the poorest faunas fill a large amount of the global functional space (Fig. S1C) whereas the levels of functional vulnerability (Fig. S1E) and FOR (Fig. S1F) are close to those observed with six traits. These findings suggest that (i) whereas the number of FEs was divided by up to 2.6, trivially increasing the mean functional redundancy (Fig. S1D), species are still packed into a small subset of FEs leaving many FEs (>21%) with no insurance, and (ii) no particular trait is driving the pattern because the variations among trait combinations remain small compared with the mean. To test the sensitivity of the results to the categorization of traits, we considered a crude, instead of a fine, categorization (Materials and Methods and Ecological Relevance of Fish Functional Traits), thereby decreasing the number of FEs by a 7.5-fold factor (86 instead of 646), and rerunning all analyses. Even in this case, the level of FOR is surprisingly very close to that observed with a much finer categorization (Fig. S1F). The level of functional vulnerability decreases with the crude categorization, but, despite having on average 25.4 species by FE across faunas, between 9% and 25% of FEs still have just one species because FOR is still strong. Indeed, even when considering a small number of FEs, the observed distribution of species richness among FEs is more right skewed than expected, with a long tail with few species (Fig. S2). Overall, our sensitivity analyses show the consistent over-redundancy of some FEs, whatever the number, the identity, and the categorization of traits.
Under the hypothesis of phylogenetic trait conservatism—i.e., the tendency of closely related species to share similar traits (25)—we may expect that FEs with many species are made of some particularly speciose families or genera whereas single species FEs are made of genera with one species only. At the global scale, 262 functional entities (i.e., 63% of FEs with at least two species) have species belonging to at least two families, which illustrates that, beyond phylogenetic trait conservatism, there is also functional convergence between families. On the other hand, among the 168 families of the global pool, only 44 (26%) have all their species within a single FE. Furthermore, the species in 38 families (23%) are distributed in at least 10 FEs, demonstrating functional divergence within families. Although we cannot use the number of species per genus or family in a given fauna as a simple surrogate for functional vulnerability and FOR, the observed patterns can be, at least partially, explained by evolutionary trajectories and historical contingencies within each oceanic basin. For instance, the proportion of monospecific genera was relatively similar among the six faunas, ranging from 44% to 59%, and the distribution of species within the main reef-fish families is surprisingly stable across the Indo-Pacific (20). Such consistent taxonomic patterns across fish faunas certainly contribute to the surprisingly stable level of FOR along the richness gradient, but the level of trait conservatism remains too low to infer FOR from taxonomic composition.
Our results indicate that, even in highly diverse systems, we can no longer assume that the erosion of species diversity can be discounted by the high probability of functional redundancy because species tend to disproportionately pack into a few particular FEs instead of spreading evenly. Although ensuring most FEs, many FEs are left with just one species. Thus, the exceptional biodiversity characterizing tropical biotas does not preclude functional vulnerability because even the loss of a few species may erode the breadth of functions performed in assemblages. For instance, the Indo-Australian Archipelago (Coral Triangle), which supports most tropical-reef fish species, may experience a loss of FEs following fisheries pressure and local species extirpation (26). When seeking particularly vulnerable functional trait categories across fish faunas, it appears that only few are over- or underrepresented into FEs (Fig. S3) compared with their proportions among the pool of species. For instance, FEs grouping mobile invertebrate and plankton feeders, as well as pelagic fishes, tend to be more vulnerable to species loss than expected, whereas FEs grouping sessile invertebrate feeders are less vulnerable, whatever the fauna (Fig. S3 E and F). In addition, FEs grouping large body-sized fishes tend to be more vulnerable than FEs with small body-sized fishes (Fig. S3A). This latter bias in vulnerability distribution among categories can increase the ecosystem impacts of overexploitation, which targets large species (27). However, at the large spatial scale, the level of insurance for FEs is given by the number of species, but realistic scenarios of FE erosion, based on local species depletion rates according to their traits and their sensitivity to various pressures, are needed to better anticipate the consequences of human-mediated disturbances on ecosystem functioning. Nevertheless, the potential erosion of the diversity of FEs at the macroecological scale cannot be directly translated into a potential decline of ecosystem functioning. Indeed, species–trait combinations do not map easily onto ecological functions. For instance, interactions between species are not taken into account nor are potential shifts in trait values following the loss of competitors or ontogenic shifts (28). Moreover, at a longer time scale, macroevolutionary processes may lead to niche filling and recovery of functions that would be lost following selective FE extinctions (29), thus potentially mitigating long-term impacts of functional vulnerability on ecosystem functioning.
Although high species diversity does offer a moderate reduction in potential functional vulnerability, the extent of vulnerability in high-diversity systems is striking. Equally striking is the extent of over-redundancy in all systems, whatever the combination and the categorization of traits (Fig. S1F). In high diversity systems, up to 58% of species may represent FOR: i.e., occupying FEs that are more redundant than average. However, even in more depauperate systems, over a third of the species may still be in overrepresented FEs. Even in these systems, where there are relatively few species to fill FEs and provide functional insurance, species still cluster in specific FEs, but with very little bias toward specific trait categories (Fig. S3). Our results call for caution when considering the protection of high-diversity ecosystems (26, 30). Diversity does not appear to provide the insurance, functional redundancy, or reduced vulnerability once hoped for. For reef-fish faunas, high diversity and low diversity systems appear to share a common fate: having a third of functional entities being vulnerable. This result seems contradictory to the recent investigations, carried out at the community scale (500 m2), suggesting that fishes from temperate reefs contribute proportionally more to ecological processes on average than fishes from tropical reefs (30). However, this claim is based on a functional diversity index (Rao’s quadratic entropy) that is calculated using the number of individuals as a measure of fish abundance within communities. Because small species tend to have more individuals than large ones and because small species tend to be overrepresented in tropical compared with temperate communities (31), the largest fishes that carry unique trait combinations are expected to have limited proportional abundances on tropical reefs, thus undercontributing to this functional-diversity index. This pattern does not imply that such rare but large fish species have low impacts on ecological processes in the tropics. Indeed, their high biomass, along with associated functional traits, enables them to play essential roles that drive ecosystem functioning (4, 18).
The conclusion that the promised benefits from massive levels of species richness may not be as strong as we have thought before deserves further investigation across ecosystems and taxa. Other studies provide comparable results although they have considered many fewer species per FE (1.3), e.g., in North American lotic insects (32), or use a multivariate index of functional dispersion that is independent from species richness, in temperate rainforests (10). Nevertheless, large-scale assessments aiming at identifying hotspots of functional vulnerability will be needed in both high- and low-diversity systems to guide ongoing conservation strategies. In a similar way that some indices combine species evolutionary distinctiveness and rarity to inform conservation at biogeographical scales (33), we may propose vulnerability indices based on species functional distinctiveness, abundance, rarity, dispersal, and colonizing capacity. For instance, the risk of losing an FE could be estimated through the cumulated vulnerability of species within this FE to particular threats (climate change, direct human impacts, and habitat degradation), including their geographic range sizes and their local abundances. For coral-reef fishes, species-based vulnerability values (27) could then scale up to FEs and assemblages.
Our framework, proposing the concept of functional over-redundancy, is not limited to fishes or tropical faunas but may also apply in many contexts where functional entities or roles can be confidently inferred from individual traits or other biological features. At the community scale, it would be relevant to replace the number of species by the biomass or the number of individuals to include the abundance distribution among FEs instead of the number of species. This approach would determine whether abundance is packed into particular functions and which functions are the most vulnerable to abundance loss. This kind of analysis was not performed in this study due to a lack of reliable abundance data at the global scale for coral-reef fishes. However, recent findings show that functionally unique species tend to be rarer in tropical than in temperate reef-fish communities (30), suggesting that, beyond the number of species, abundance also tends to pack into a restricted number of FEs, particularly in species-rich regions where functional vulnerability to local species extinction would be high. Species extinction is also a nonrandom process that closely depends on abundance and geographic range size that would need to be taken into account when dealing with the functional implications of ecological extinction. This kind of assessment may guide future conservation strategies toward critical functions and may call for a new generation of experiments that will specifically address the influence of functional over-redundancy and functional vulnerability in ecosystem functioning by, for example, testing the loss of species from either redundant or vulnerable FEs.
Materials and Methods
Tropical-Reef Fish Faunas.
We limited our study to tropical reefs, defined as areas including both coral and rocky reefs showing a minimum monthly sea-surface temperature (hereafter SST) of 17 °C. This limit of 17 °C was set because, although focusing on the tropics (SST limit usually set at 20 °C), we decided to include locations where species of tropical affinity are present (34). The inclusion of those areas between 17 °C and 20 °C broadens the range of variation in species richness and species composition, thereby further extending the range of functional traits.
We obtained information on reef-fish species composition at 169 locations worldwide by examining more than 300 references. We then considered six different faunas (Western Indian, Central-Indo Pacific, Central Pacific, Tropical Eastern Pacific, Western Atlantic, and Eastern Atlantic) according to several biogeographical works conducted on tropical-reef fishes (23). The distinction between the Central Indo-Pacific and the Central Pacific (Fig.1), previously considered as a single province (34), is largely supported by several biogeographical studies, conducted at the Indo-Pacific scale, that identified the Pacific Plate as a biogeographical barrier of major significance (35, 36).
Fish Functional Traits.
The functional strategy of each tropical-reef fish species was described using six categorical traits (8, 16) (SI Text). Fish size was coded using six ordered categories: 0–7 cm, 7.1–15 cm, 15.1–30 cm, 30.1–50 cm, 50.1–80 cm, and >80 cm. Mobility was coded using three ordered categories: sedentary (including territorial species), mobile within a reef, and mobile between reefs. The period of activity was coded using three ordered categories: diurnal, both diurnal and nocturnal, and nocturnal. Schooling was coded using five ordered categories: solitary, pairing, or living in small (3–20 individuals), medium (20–50 individuals), or large (>50 individuals) groups. Vertical position in the water column was coded using three ordered categories: benthic, bentho-pelagic, and pelagic. Diet was characterized based on main items consumed by each species, which led to seven trophic categories: herbivorous-detritivorous (i.e., fish feeding on turf or filamentous algae and/or undefined organic material), macroalgal herbivorous (i.e., fish eating large fleshy algae and/or seagrass), invertivorous targeting sessile invertebrates (i.e., corals, sponges, ascidians), invertivorous targeting mobile invertebrate (i.e., benthic species such as crustaceans), planktivorous (i.e., fish eating small organisms in the water column), piscivorous (including fish and cephalopods), and omnivorous (i.e., fish for which both vegetal and animal material are important in their diet). The traits have already been used to investigate community assembly rules (16) and to seek vulnerable fish functions (8); their relevance is detailed in SI Text. Given the scale of our study, we could not account for intraspecific variability of traits among life stages.
This set of six functional traits and their respective number of categories yield a theoretical number of 5,670 unique combinations of trait values called functional entities. However, the 6,316 tropical-reef fish species fill only 646 FEs (11.4%).
Fish Functional Space.
Pairwise functional distances between functional entities were computed using the Gower distance, which allows mixing different types of variables while giving them equal weight (37). Then, a Principal Coordinates Analysis (PCoA) was performed using this functional distance matrix (37). Functional entities coordinates on the first four principal axes (PC) of this PCoA were kept to build a multidimensional functional space (22, 38). Euclidean distances between functional entities in this four-dimensional space were strongly correlated with initial Gower distances (Mantel test, r = 0.872, P < 0.001), which illustrates that most of the variability in fish functional strategies is robustly summarized in the synthetic four-dimensional functional space. The fifth PC axis only weakly increased the quality of the functional space (Mantel test, r = 0.905) while making the calculations of functional volumes more time consuming.
Functional Indices.
Functional richness of each fauna was measured as the volume inside the convex hull shaping all of the functional entities present in the region (39).
With S the total number of species in a fauna, FE the total number of functional entities, and ni the number of species in functional entity i, then Functional Redundancy (FR), Functional Vulnerability (FV), and Functional Over-Redundancy (FOR) are expressed as the following ratios:
Sensitivity Analyses.
We first assessed the robustness of our findings to the number of traits and to the potential disproportionate influence of one particular trait by rerunning all analyses using all combinations of five traits out of six. We did not reduce the number of traits lower than five because we might have missed important dimensions of the functional space defining fish niches, thus providing an oversimplistic definition of FEs. Second, we determined whether our results are robust to the extent of categorization of functional traits, a crude categorization potentially inducing high functional redundancy and low functional vulnerability (many species in each FE) whereas a fine categorization would lead to the opposite (few species in each FE). In this analysis, we reduced the number of categories for each trait and reran all analyses with a total of 86 FEs (crude categorization) instead of 646 (fine categorization). More precisely, we used the following crude categories: size classes (0–15 cm, 15.1–50 cm, and >50 cm), schooling behavior [solitary, small groups (2–20 individuals), and gregarious (>20 individuals)], mobility (sedentary vs. mobile), position in the water column (strictly pelagic vs. benthic/bentho-pelagic), period of activity (strictly nocturnal vs. diurnal/diurnal–nocturnal), and main diet [primary consumers (detritivores, herbivores, and omnivores), invertivores (sessile, mobile benthos, and plankton), and piscivores]. See Ecological Relevance of Fish Functional Traits for further details.
Null Models.
Functional vulnerability and functional over-redundancy are influenced by the number of species, the number of FEs and the distribution of species into FEs. We tested whether the observed values of functional vulnerability and FOR were significantly different from the null hypothesis that species are randomly distributed into functional entities. In each of the six regions, we simulated a random assignment of species to FEs while ensuring that each FE had at least one species. We simulated 9,999 random assemblages for each region, and, for each simulation, we computed functional vulnerability and FOR while the number of species and the number of FEs were kept constant. The observed values of functional vulnerability and FOR were compared with the simulated values using a bilateral test (α = 5%). For instance, a functional vulnerability and a FOR significantly higher than expected under the null hypothesis would indicate that the distribution of species within FEs is more densely packed and the distribution of the number of species per FE is more right skewed.
All analyzes were conducted using R software.
Supplementary Material
Acknowledgments
We thank three anonymous reviewers for valuable comments on the manuscript. D.M. has received funding from the Marie Curie International Outgoing Fellowship (FISHECO) with agreement number IOF-GA-2009-236316. We also acknowledge support from the Fondation pour la Recherche sur la Biodiversité project “Center for Synthesis and Analysis of Biodiversity-General Approach to Species-Abundance Relationships” and from the Australian Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.V. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317625111/-/DCSupplemental.
References
- 1.Barnosky AD, et al. Has the Earth’s sixth mass extinction already arrived? Nature. 2011;471(7336):51–57. doi: 10.1038/nature09678. [DOI] [PubMed] [Google Scholar]
- 2.Isbell F, et al. High plant diversity is needed to maintain ecosystem services. Nature. 2011;477(7363):199–202. doi: 10.1038/nature10282. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486(7401):59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 4.Bellwood DR, Hoey AS, Hughes TP. Human activity selectively impacts the ecosystem roles of parrotfishes on coral reefs. Proc Biol Sci. 2012;279(1733):1621–1629. doi: 10.1098/rspb.2011.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naeem S, Duffy JE, Zavaleta E. The functions of biological diversity in an age of extinction. Science. 2012;336(6087):1401–1406. doi: 10.1126/science.1215855. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca CR, Ganade G. Species functional redundancy, random extinctions and the stability of ecosystems. J Ecol. 2001;89(1):118–125. [Google Scholar]
- 7.Yachi S, Loreau M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc Natl Acad Sci USA. 1999;96(4):1463–1468. doi: 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouillot D, et al. Rare species support vulnerable functions in high-diversity ecosystems. PLoS Biol. 2013;11(5):e1001569. doi: 10.1371/journal.pbio.1001569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hidasi-Neto J, Barlow J, Cianciaruso MV. Bird functional diversity and wildfires in the Amazon: The role of forest structure. Anim Conserv. 2012;15(4):407–415. [Google Scholar]
- 10.Gallagher RV, Hughes L, Leishman MR. Species loss and gain in communities under future climate change: Consequences for functional diversity. Ecography. 2013;36(5):531–540. [Google Scholar]
- 11.Wardle DA, Bonner KI, Nicholson KS. Biodiversity and plant litter: Experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos. 1997;79(2):247–258. [Google Scholar]
- 12.Cardinale BJ, et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443(7114):989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- 13.Cardoso P, Pekár S, Jocqué R, Coddington JA. Global patterns of guild composition and functional diversity of spiders. PLoS ONE. 2011;6(6):e21710. doi: 10.1371/journal.pone.0021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martins GM, Arenas F, Neto AI, Jenkins SR. Effects of fishing and regional species pool on the functional diversity of fish communities. PLoS ONE. 2012;7(8):e44297. doi: 10.1371/journal.pone.0044297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lohbeck M, et al. Functional diversity changes during tropical forest succession. Perspect Plant Ecol Evol Syst. 2012;14(2):89–96. [Google Scholar]
- 16.Guillemot N, Kulbicki M, Chabanet P, Vigliola L. Functional redundancy patterns reveal non-random assembly rules in a species-rich marine assemblage. PLoS ONE. 2011;6(10):e26735. doi: 10.1371/journal.pone.0026735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bihn JH, Gebauer G, Brandl R. Loss of functional diversity of ant assemblages in secondary tropical forests. Ecology. 2010;91(3):782–792. doi: 10.1890/08-1276.1. [DOI] [PubMed] [Google Scholar]
- 18.Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429(6994):827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 19.Bellwood DR, Hughes TP, Hoey AS. Sleeping functional group drives coral-reef recovery. Curr Biol. 2006;16(24):2434–2439. doi: 10.1016/j.cub.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Bellwood DR, Hughes TP. Regional-scale assembly rules and biodiversity of coral reefs. Science. 2001;292(5521):1532–1535. doi: 10.1126/science.1058635. [DOI] [PubMed] [Google Scholar]
- 21.Parravicini V, et al. Global patterns and predictors of tropical reef fish species richness. Ecography. 2013;36(12):1254–1262. [Google Scholar]
- 22.Mouillot D, Graham NAJ, Villéger S, Mason NWH, Bellwood DR. A functional approach reveals community responses to disturbances. Trends Ecol Evol. 2013;28(3):167–177. doi: 10.1016/j.tree.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Kulbicki M, et al. Global biogeography of reef fishes: A hierarchical quantitative delineation of regions. PLoS ONE. 2013;8(12):e81847. doi: 10.1371/journal.pone.0081847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson KG, Jackson JBC, Budd AF. Caribbean reef development was independent of coral diversity over 28 million years. Science. 2008;319(5869):1521–1523. doi: 10.1126/science.1152197. [DOI] [PubMed] [Google Scholar]
- 25.Wiens JJ, et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett. 2010;13(10):1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- 26.Comeros-Raynal MT, et al. The likelihood of extinction of iconic and dominant herbivores and detritivores of coral reefs: The parrotfishes and surgeonfishes. PLoS ONE. 2012;7(7):e39825. doi: 10.1371/journal.pone.0039825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham NAJ, et al. Extinction vulnerability of coral reef fishes. Ecol Lett. 2011;14(4):341–348. doi: 10.1111/j.1461-0248.2011.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reecht Y, Rochet MJ, Trenkel VM, Jennings S, Pinnegar JK. Use of morphological characteristics to define functional groups of predatory fishes in the Celtic Sea. J Fish Biol. 2013;83(2):355–377. doi: 10.1111/jfb.12177. [DOI] [PubMed] [Google Scholar]
- 29.Lavergne S, Mouquet N, Thuiller W, Ronce O. Biodiversity and climate change: Integrating evolutionary and ecological responses of species and communities. Annu Rev Ecol Evol Syst. 2010;41:321–350. [Google Scholar]
- 30.Stuart-Smith RD, et al. Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature. 2013;501(7468):539–542. doi: 10.1038/nature12529. [DOI] [PubMed] [Google Scholar]
- 31.Fisher JAD, Frank KT, Leggett WC. Global variation in marine fish body size and its role in biodiversity-ecosystem functioning. Mar Ecol Prog Ser. 2010;405:1–13. [Google Scholar]
- 32.Poff NL, et al. Functional trait niches of North American lotic insects: Traits-based ecological applications in light of phylogenetic relationships. J N Am Benthol Soc. 2006;25(4):730–755. [Google Scholar]
- 33.Cadotte MW, Davies TJ. Rarest of the rare: Advances in combining evolutionary distinctiveness and scarcity to inform conservation at biogeographical scales. Divers Distrib. 2010;16(3):376–385. [Google Scholar]
- 34.Briggs JC, Bowen BW. A realignment of marine biogeographic provinces with particular reference to fish distributions. J Biogeogr. 2012;39(1):12–30. [Google Scholar]
- 35.Allen GR. Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquat Conserv Mar Freshw Ecosyst. 2008;18(5):541–556. [Google Scholar]
- 36.Kulbicki M. Biogeography of reef fishes of the french territories in the south pacific. Cybium. 2007;31(2):275–288. [Google Scholar]
- 37.Legendre P, Legendre L. Numerical Ecology. 2nd Ed. Amsterdam: Elsevier; 1998. [Google Scholar]
- 38.Villéger S, Novack-Gottshall PM, Mouillot D. The multidimensionality of the niche reveals functional diversity changes in benthic marine biotas across geological time. Ecol Lett. 2011;14(6):561–568. doi: 10.1111/j.1461-0248.2011.01618.x. [DOI] [PubMed] [Google Scholar]
- 39.Villéger S, Mason NWH, Mouillot D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology. 2008;89(8):2290–2301. doi: 10.1890/07-1206.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.