Cognitive decline in Alzheimer’s disease (AD) is associated with synaptic loss (1) and network dysfunction (2). The mechanisms involved are not fully understood; however, recent evidence indicate that the neurotoxicity of amyloid-β (Aβ) oligomers is mediated by interactions with synaptic receptors (3). In PNAS, Fu et al. demonstrate that soluble Aβ oligomers trigger synaptic loss and dysfunction via activation of erythropoietin-producing hepatocellular A4 receptor (EphA4) (4). Remarkably, blockade of EphA4 reversed the functional deficits in the hippocampus in animal models of AD. Moreover, based on molecular docking studies, they identify rhynchophylline as a novel EphA4 inhibitor that, following oral administration, rescued the Aβ oligomer-induced deficits in neurotransmission in amyloid precursor protein (APP)/presenilin 1 (PS1) transgenic mice. In agreement with these findings, a recent study by Vargas et al. showed that Eph4 activation via c-Abl mediates the synaptic damage triggered by Aβ oligomers (5). Together, these studies point to EphA4 as a novel mediator of AD-associated synaptic dysfunction and support the notion that Aβ oligomer receptors might be viable therapeutic targets for this disease.
In recent years, several surface proteins were identified as putative Aβ oligomer receptors, including among others prion protein (PrP) (6), metabotropic glutamate receptor 5 (mGluR5) (7, 8), NMDA (9), α7-nicotinic acetylcholine receptor, insulin receptor, NGF receptor, Frizzled, and Wnt-3a (10) (Fig. 1). In the CNS, the Eph family of tyrosine kinase receptors has an important role in axonal guidance, synaptogenesis, and synaptic transmission (11). To date, 10 different subtypes of the EphA and 6 subtypes of EphB receptors have been identified.
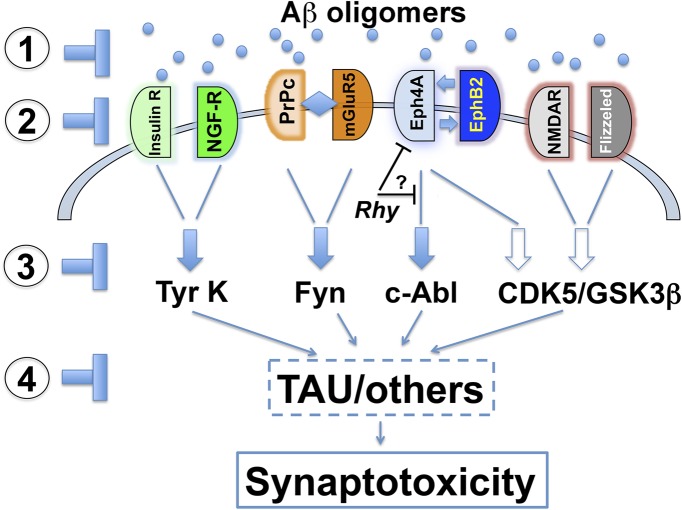
Fig. 1.
Putative Aβ oligomer receptors, signaling pathways, and therapeutic targets. A number of potential cell surface molecules (insulin receptor, NGF receptor, PrPc, mGluR5, EphA4, EphB2, NMDA, and Flizzed) that mediate the synaptotoxic effects of Aβ oligomers have been identified. In general terms, they can be divided into those that signal via tyrosine kinase (tyr k, fyn, and c-Abl) and those that hyperactivate CDK5/glycogen synthase kinase (GSK)3β. Therapeutics for AD might involve (1) directly clearing Aβ oligomers, (2) blocking the surface receptors, (3) interfering with signaling pathways, or (4) decreasing downstream effectors such as Tau. The study by Fu et al. identifies rhynchophylline (Rhy) as an antagonist of EphA4. The mechanisms as to how this compound protects neurons from the toxic effects of Aβ are unclear; however, Rhy might directly block EphA4 or interfere with signaling.
Genome-wide association studies of late-onset AD showed increased susceptibility associated with SNPs at the EphA1 loci (12). Moreover, in AD patients, EphB2 is down-regulated, and rescue of the network dysfunction phenotype in AD-like tg mice was achieved by overexpression of this receptor in the hippocampus (13). In contrast, in AD and APP/Ps1 mice (4) EphA4 is up-regulated, and blocking this receptor genetically or pharmacologically ameliorated the synaptic alterations in AD-like tg mice.
The Eph family has been explored as a therapeutic target for cancer, nerve injury, Parkinson disease (PD), motor neuron disease (MND), arthritis, and reperfusion injury (11). EphA4 inhibitors are currently under consideration for the treatment of spinal cord injury because this promotes axonal outgrowth (14). Interestingly, EphA4, probably through interaction with EphB2 and EphB3, appears to have a role in blocking axonal regeneration after injury, and pharmacological inhibition of these interactions might promote functional recovery (11). For example, in models of spinal cord injury, blocking EphA4–ephrin interactions with antibodies (14) or small peptides (15) resulted in significant functional recovery.
The study by Fu et al. advances the field by identifying a novel EphA4 inhibitor, rhynchophylline, that reverses the synaptic deficits in AD-like models (4). It is of interest to consider that the beneficial effects of rhynchophylline might involve both Aβ-dependent and Aβ-independent mechanisms. Rhynchophylline’s therapeutic mechanisms might be related to its ability to directly block the interaction between Aβ and EphA4, attenuate the activity of glutamate receptors and calcium channels (16), block downstream toxic signaling pathways such as c-Abl, or promote neurite outgrowth by coregulating EphB2 and EphB3 (11) (Fig. 1). The study by Fu et al. also underscores the potential therapeutic usefulness of rhynchophylline and related Eph4A inhibitors in the treatment of other neurological disorders such as PD, motor neuron disease, and nerve injury.
In addition to EphA4, and because of the involvement of other surface molecules as putative Aβ oligomer receptors, recent studies have explored developing inhibitors to PrP (17), mGluR5, NMDA (9), α7-nicotinic acetylcholine receptor, p75, insulin receptor, and others (18) for the treatment of AD. The results of the study by Fu et al. might suggest that blocking just one of the putative receptors might be sufficient, at least in experimental models (4). However, it is likely that several of these receptors cross-talk, interact, and/or have a role at different stages during the progression of AD (Fig. 1). This suggests that a combined therapeutic approach targeting families of these potential oligomer receptors may be needed.
Given that blocking all of the oligomer receptors might be counterproductive, this raises an important, albeit difficult, question to answer: which groups of receptors to target simultaneously and/or in which sequence? One intriguing possibility is to consider compounds with multiple receptor tyrosine kinase inhibitory activity (Fig. 1). Here it is worth noting that several of the mediators of Aβ oligomer synaptotoxicity involve tyrosine kinase signaling; for example, EphA4 engages c-Abl (5), Prp/mGluR5 triggers Fyn (6), and the insulin and NGF receptors are well known to involve tyrosine kinase activation (Fig. 1). The other signaling pathways aberrantly stimulated by Aβ oligomers include cyclin-dependent kinase 5 (CDK5) and GSK3β (19) (Fig. 1). Interestingly, some tyrosine kinase inhibitors were shown to have multiple kinase modulatory activity that includes members of the CDK family (20). Because both tyrosine kinases and CDK5 are deregulated in AD, therapeutic strategies using compounds with dual activity targeting these pathways might be particularly advantageous. The papers by Fu et al. (4) and Vargas et al. (5) identify that one such target, EphA4, which is within the receptor tyrosine kinase family, might have downstream effects on CDK5, and when inhibited, ameliorated the deleterious effects of Aβ in various in vitro and in vivo models of AD (4).
Footnotes
The authors declare no conflict of interest.
See companion article on page 9959 in issue 27 of volume 111.
References
- 1.Terry RD, et al. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 2.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat Neurosci. 2010;13(7):812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2(7):a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu AKY, et al. Blockade of EphA4 signaling ameliorates hippocampal synaptic dysfunctions in mouse models of Alzheimer’s disease. Proc Natl Acad Sci USA. 2014;111(27):9959–9964. doi: 10.1073/pnas.1405803111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vargas LM, et al. EphA4 activation of c-Abl mediates synaptic loss and LTP blockade caused by amyloid-β oligomers. PLoS ONE. 2014;9(3):e92309. doi: 10.1371/journal.pone.0092309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Um JW, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron. 2013;79(5):887–902. doi: 10.1016/j.neuron.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renner M, et al. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66(5):739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Um JW, et al. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat Neurosci. 2012;15(9):1227–1235. doi: 10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talantova M, et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci USA. 2013;110(27):E2518–E2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva-Alvarez C, Arrázola MS, Godoy JA, Ordenes D, Inestrosa NC. Canonical Wnt signaling protects hippocampal neurons from Aβ oligomers: Role of non-canonical Wnt-5a/Ca(2+) in mitochondrial dynamics. Front Cell Neurosci. 2013;7:97. doi: 10.3389/fncel.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov. 2014;13(1):39–62. doi: 10.1038/nrd4175. [DOI] [PubMed] [Google Scholar]
- 12.Naj AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cissé M, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469(7328):47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldshmit Y, et al. EphA4 blockers promote axonal regeneration and functional recovery following spinal cord injury in mice. PLoS ONE. 2011;6(9):e24636. doi: 10.1371/journal.pone.0024636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabes J, Anderson P, Brennan C, Bolsover S. Regeneration-enhancing effects of EphA4 blocking peptide following corticospinal tract injury in adult rat spinal cord. Eur J Neurosci. 2007;26(9):2496–2505. doi: 10.1111/j.1460-9568.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 16.Kang TH, et al. Rhynchophylline and isorhynchophylline inhibit NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 2002;455(1):27–34. doi: 10.1016/s0014-2999(02)02581-5. [DOI] [PubMed] [Google Scholar]
- 17.Laurén J. Cellular prion protein as a therapeutic target in Alzheimer’s disease. J Alzheimers Dis. 2014;38(2):227–244. doi: 10.3233/JAD-130950. [DOI] [PubMed] [Google Scholar]
- 18.Patel AN, Jhamandas JH. Neuronal receptors as targets for the action of amyloid-beta protein (Aβ) in the brain. Expert Rev Mol Med. 2012;14:e2. doi: 10.1017/S1462399411002134. [DOI] [PubMed] [Google Scholar]
- 19.Engmann O, Giese KP. Crosstalk between Cdk5 and GSK3beta: Implications for Alzheimer's Disease. (Translated from eng) Front Mol Neurosci. 2009;2:2. doi: 10.3389/neuro.02.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zapata-Torres G, et al. Effects of natural flavones and flavonols on the kinase activity of Cdk5. J Nat Prod. 2004;67(3):416–420. doi: 10.1021/np034011s. [DOI] [PubMed] [Google Scholar]