Significance
Early detection of cancer positively impacts diagnosis and treatment, ultimately improving patient survival. Using fluorescence imaging offers the promise of safe, noninvasive detection with excellent resolution and guides surgical removal of tumors to improve patient outcomes. However, the success of current optical probes is limited due to high background from tissue autofluorescence, poor penetration depth, and inherently low signal stability. Here, we engineered M13 bacteriophage to stabilize single-walled carbon nanotubes for selective, targeted imaging of ovarian tumors. These nanoprobes fluoresce at longer near-infrared wavelengths than current probes, thereby improving noninvasive detection of small, deep tumors and guidance for surgical removal of submillimeter tumors. This material-based approach may be attractive to guide surgical interventions where deep tissue molecular imaging is informative.
Keywords: cancer imaging, fluorescence-guided surgery, M13 bacteriophage
Abstract
Highly sensitive detection of small, deep tumors for early diagnosis and surgical interventions remains a challenge for conventional imaging modalities. Second-window near-infrared light (NIR2, 950–1,400 nm) is promising for in vivo fluorescence imaging due to deep tissue penetration and low tissue autofluorescence. With their intrinsic fluorescence in the NIR2 regime and lack of photobleaching, single-walled carbon nanotubes (SWNTs) are potentially attractive contrast agents to detect tumors. Here, targeted M13 virus-stabilized SWNTs are used to visualize deep, disseminated tumors in vivo. This targeted nanoprobe, which uses M13 to stably display both tumor-targeting peptides and an SWNT imaging probe, demonstrates excellent tumor-to-background uptake and exhibits higher signal-to-noise performance compared with visible and near-infrared (NIR1) dyes for delineating tumor nodules. Detection and excision of tumors by a gynecological surgeon improved with SWNT image guidance and led to the identification of submillimeter tumors. Collectively, these findings demonstrate the promise of targeted SWNT nanoprobes for noninvasive disease monitoring and guided surgery.
In clinical oncology, in vivo fluorescence imaging has emerged as a valuable tool for improving diagnosis, staging tumors, monitoring response to therapy, and detecting recurrent or residual disease. Compared with existing imaging modalities, fluorescence imaging offers a low-cost, portable, and safe alternative (i.e., nonionizing radiation), with key advantages including real-time imaging, superior resolution, and high specificity for small tumor nodules during diagnostic and intraoperative surgical procedures (1, 2). Although efforts have focused on using visible and short near-infrared (NIR1, 650–900 nm) wavelength fluorescent dyes as contrast agents for delineating tumor margins in both preclinical cancer models (2, 3) and human patients (4), these agents are suboptimal for noninvasive, reflectance-based imaging due to limited penetration depth (3–5 mm) and high tissue autofluorescence. During intraoperative surgery, these dyes may additionally undergo photobleaching, thereby reducing the ability of the surgeon to readily locate and resect tumors. Alternative approaches to specifically permit noninvasive imaging and limited photobleaching would be highly desirable for diagnostic and surgical applications.
Single-walled carbon nanotubes (SWNTs) hold great promise as fluorescence imaging agents due to the large interband difference between their excitation and emission wavelengths, resulting in minimal spectral overlap and tissue autofluorescence. In particular, the low tissue autofluorescence observed with SWNTs greatly enhances target-to-background ratios (TBRs) necessary for improved detection of small tumor nodules in confined anatomic regions. SWNT emission at longer wavelengths in the near-infrared second window (NIR2, 950–1,400 nm) results in less optical scattering and deeper tissue penetration compared with shorter wavelength visible and NIR1 imaging agents. Simulations (5) and experimental results (6) suggest the greatest tissue penetration depth is achieved in the NIR2 regime, which further supports the potential value for use of SWNTs for biological imaging applications. Additionally, unlike visible and near-infrared dyes, well-functionalized SWNTs are less susceptible to photobleaching or quenching effects (7), which make them attractive for continuous, long-term imaging required during many surgical procedures. Previously, we have demonstrated that M13 bacteriophage-stabilized SWNTs can target s.c. prostate tumors in preclinical models for fluorescence imaging in the second optical window (8). SWNTs have also been used for vascular and deep tissue fluorescence imaging (9). Importantly, M13 serves as a scaffold to couple both targeting and imaging moieties while allowing them to retain their functionalities (10); in comparison, more prevalent nanoparticle systems directly conjugated with targeting ligands may require optimization of ligand density for effective targeting (11, 12). Also, the identification of tissue-targeting peptides via phage display has been valuable for enhancing nanoparticle trafficking in vivo (13, 14). However, to date, there has been no report of an affinity-targeted, fluorescence imaging agent capable of noninvasive imaging and guiding diagnosis for surgical resection.
Here we report an M13-stabilized SWNT probe that selectively targets Secreted Protein, Acidic and Rich in Cysteines (SPARC)-expressing tumor nodules in an orthotopic mouse model of human ovarian cancer. Ovarian cancer remains a major health care problem for women. Annually, 225,000 women worldwide are diagnosed with epithelial ovarian cancer (EOC) and ∼140,000 women die as a result (15). Although women with early-stage ovarian cancer [International Federation of Gynecology and Obstetrics (FIGO) stage I/II] can be cured, advanced-stage ovarian cancer (FIGO III/IV) remains considerably more difficult to treat. Unfortunately, 80% of women with EOC have metastatic disease at the time of diagnosis, and many undergo a treatment regimen of surgery and chemotherapy. Our study focused on ovarian cancer because clinical evidence indicates that optimal surgery can significantly prolong the median overall survival of patients as well as reduce disease morbidity (16). Using the NIR2 emission of these fluorescence probes, we determine the detection limit of labeled tumors and their TBRs. We demonstrate that this SWNT-based probe detects tumors that were missed when either visible or NIR1 dyes were used, thereby aiding the discovery of smaller tumors during surgery. Collectively, our results highlight the potential for affinity-targeted NIR2 fluorescence probes to monitor disease processes such as cancer with enhanced sensitivity compared with current state-of-the-art optical probes.
Results
Characterization of the M13-Stabilized SWNT Probe.
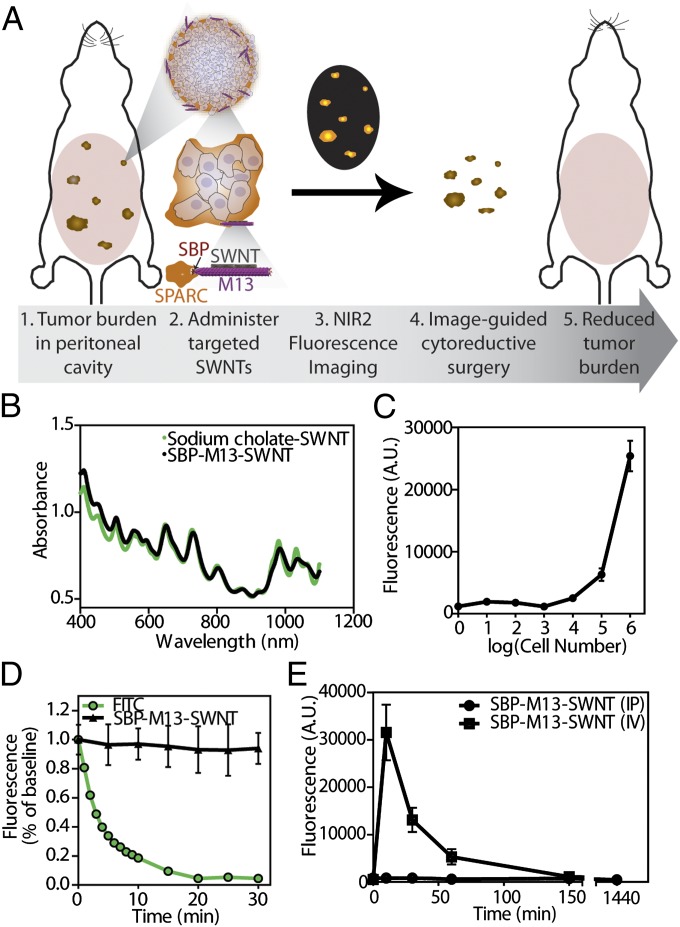
The imaging probe [SPARC binding peptide (SBP)–M13–SWNT] consists of three fundamental components: the SBP, M13 virus, and SWNTs (Fig. 1A). The filamentous M13 virus (6 nm diameter, 880 nm length) is genetically modifiable such that peptides can be incorporated for display on the various coat proteins of the virus. By displaying peptides with varying affinities to materials or biomolecules through an iterative panning and selective enrichment process, M13 is well suited for diverse applications including epitope mapping (17), ligand discovery against cells and tissues (18), and binding and nucleation of materials (19, 20). The modularity of M13 can be further exploited to target various biomarkers in cancers, highlighting its attractiveness as a multifunctional probe. Previously, we used phage display to identify a peptide along the p8 major coat protein of M13 that binds and stabilizes SWNTs (20), while retaining the optical and electronic properties of the nanotubes. Because M13 can be genetically engineered to display 100% of fusion peptides, we further engineered the p3 minor coat protein to display five copies of a targeting peptide that binds SPARC (21). SPARC is a matricellular protein highly expressed in certain subtypes of breast, prostate, and ovarian cancer. SPARC overexpression has been shown to enhance ovarian cancer cell proliferation, invasion, and metastasis. High levels of SPARC expression have been associated with late stages of ovarian carcinoma and correlated with poor clinical prognosis (22), suggesting its relevance as a clinical biomarker. Collectively, these traits have been taken into account in the design of this genetically engineered NIR2 probe in order to enable the localization, detection, and surgical excision of ovarian tumors, as outlined in the schematic presented in Fig. 1A.
Fig. 1.
Characterization of tumor-targeting SBP–M13–SWNT probe. (A) Schematic illustrating association with ovarian tumor nodules for noninvasive detection by NIR2 fluorescence and surgical excision. (B) Absorbance spectra of SWNTs in sodium cholate and as SBP–M13–SWNT probe. (C) In vitro sensitivity of SBP–M13–SWNT fluorescence in ovarian cancer cell culture. n = 3 per group. Error bars represent SE. (D) Photobleaching fluorescence decay of FITC and SBP–M13–SWNTs under continuous excitation. Error bars represent SD. (E) Pharmacokinetic circulation study of SBP–M13–SWNT administered i.v. (IV) and intraperitoneally (IP). n = 3 per timepoint. Errors bars represent SE.
To ensure that our probe retained its functionality following synthesis, we examined the optical properties of SBP–M13–SWNTs. Compared with unmodified SWNTs dispersed in sodium cholate, complexed SBP–M13–SWNTs exhibited similar optical absorbance that was consistent across multiple batches (Fig. 1B and SI Appendix, Fig. S1). Previously, photoluminescence (fluorescence) mapping of the excitation and emission wavelengths of SBP–M13–SWNTs suggests M13-stabilized SWNTs retain their fluorescent properties; nondispersed, aggregating, or bundled SWNTs would quench and not fluoresce and thus not appear in the fluorescence mapping (8).
To establish their use for in vivo applications, we validated the stability of SBP–M13–SWNTs in blood and ascites and at different pH values by measuring the fluorescence over 24 h using a custom-built small-animal NIR2 fluorescence imager (8). SBP–M13–SWNTs retain fluorescence at various dilutions in the blood and ascites fluid from the peritoneal cavity (SI Appendix, Figs. S2 and S3, respectively), and we did not observe quenching of the probe. Previous reports indicate that exposed SWNTs in solution will adsorb serum proteins on their sidewall and subsequently lose fluorescence (8, 23). Here, we observe no loss of fluorescence intensity, indicating the probes are well solubilized by M13 and highly stable for in vivo imaging applications. In addition, the probe is fluorescently stable across a broad pH range, from 4.5 to 8.5 (SI Appendix, Fig. S4), suggesting the probes will be stable in the vascular and lymphatic systems and the peritoneal cavity and for cellular uptake. We also confirmed the targeted probes are not acutely cytotoxic to primary human endothelial cells and ovarian carcinoma cell line 8 (OVCAR8) (SI Appendix, Figs. S5 and S6, respectively), which builds upon previous studies on cell-type dependence of nanomaterial toxicity (24) and underscores their potential for in vivo imaging applications.
We next examined the sensitivity of the probe in terms of its capacity to target OVCAR8 ovarian cancer cells in vitro. Serial 10-fold dilutions of OVCAR8 cells were incubated with SBP–M13–SWNT for 24 h, and cell lysates were collected. Measuring the fluorescence intensity of the SBP–M13–SWNT incubated cells, we observed that as few as ∼10,000 cells incubated with SBP–M13–SWNT exceeded the minimum level of detection (Fig. 1C).
To test SBP–M13–SWNTs for risk of photobleaching, we exposed them to an 808-nm laser for a continuous, 30-min period and measured fluorescence intensity in 5-min intervals up to 30 min postirradiation. No appreciable loss of fluorescence of SBP–M13–SWNTs was observed during this period. However, the intensity of fluorescein isothiocyanate (FITC), a fluorescein derivative that has been used to molecularly image and guide intraoperative resection of ovarian tumors in humans (4), exponentially decreased in response to the same light exposure kinetics (Fig. 1D). The observations that SWNTs do not photobleach and maintain their optical properties illustrate their potential to assist surgeons in visualizing tumors during resection.
Another potential advantage of SWNT-based imaging compared with FITC-based imaging is the potential to detect tumors located at greater depths in the body. To investigate the depth of detection that can be achieved with our probe, we harvested ovarian tumors that had been treated with SBP–M13–SWNTs and imaged the small tumor fragments (∼1-mm diameter) at various depths within a tissue “phantom” construct, which mimics the optical properties of human tissue. Using our NIR2 fluorescence reflectance imaging system (8), we could detect SWNT-containing tumors to depths as great as 9.7–18.2 mm (SI Appendix, Fig. S7). To our knowledge, this is the best reported quantifiable tumor depth using reflectance imaging, relative to previously reported values (3). By permitting deeper imaging, SBP–M13–SWNT therefore offers the potential for noninvasive detection before surgery and improved resection of tumors during surgery.
In Vivo Characterization of the SBP–M13–SWNT Probe.
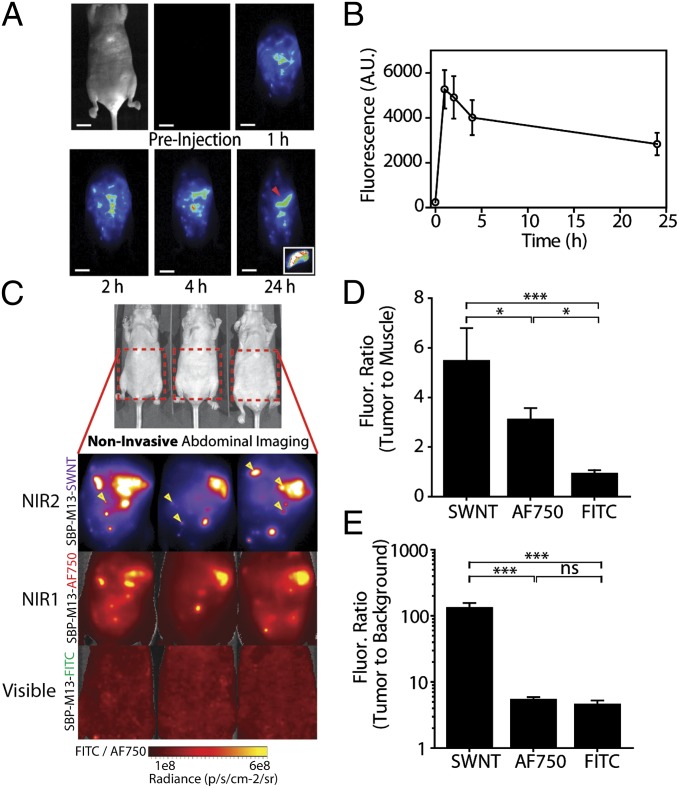
Having demonstrated in vitro stability and fluorescence of SBP–M13–SWNTs, we next characterized their in vivo properties in an orthotopic model of ovarian cancer. The OVCAR8 human cell line was used to create the orthotopic model, as it overexpresses SPARC, as confirmed by ONCOMINE (25) analysis and immunohistochemistry (SI Appendix, Fig. S8). To compare routes of administration, tumor-bearing animals were injected intraperitoneally or i.v., and the circulating probe concentration was monitored via SWNT fluorescence in the blood. The i.v.-administered SBP–M13–SWNTs reached a peak concentration in the circulation ∼10 min after injection, and circulating levels became negligible after 150 min (Fig. 1E). Notably, SBP–M13–SWNTs injected intraperitoneally led to negligible elevations in blood-borne SWNT fluorescence for at least 24 h, suggesting that the majority of SBP–M13–SWNTs remain in the peritoneum postinjection (Fig. 1E). This finding was verified by the observation that, following a transient increase in NIR2 fluorescence in the peritoneum, the overall intensity in this location stabilizes for periods up to 24 h following injection (Fig. 2 A and B). Lastly, we studied the biodistribution of SBP–M13–SWNTs administered into the peritoneal cavity of tumored mice (SI Appendix, Fig. S9). The accumulation of SBP–M13–SWNTs was greatest in tumors at days 1 and 7 postinjection, with additional signal observed in liver, spleen, kidney, and peritoneum in the vicinity of the injection site. At day 7 postinjection, the lowest signals observed were in heart and lung tissue, supporting limited systemic exposure of SBP–M13–SWNT after i.p. administration. Importantly, analysis of serum from tumor-bearing animals following the administration of SBP–M13–SWNTs into the peritoneal cavity revealed no evidence of systemic hepatic, renal, metabolic, or hematologic acute toxicities within the first week following administration (SI Appendix, Fig. S10).
Fig. 2.
Noninvasive tumor imaging with SBP–M13–SWNTs. (A) Representative whole-abdomen NIR2 imaging series following i.p. administration of SBP–M13–SWNTs. (Inset) Surgically excised tumor nodule (denoted by red arrow) observed 24 h postinjection of SBP–M13–SWNTs. (B) NIR2 fluorescence intensity in the abdomen of tumor-bearing animals following IP administration of SBP–M13–SWNTs up to 24 h postinjection (n = 6; error bars represent SE). (C) Noninvasive imaging of ovarian tumors using SBP–M13 conjugated to SWNTs (NIR2), AlexaFluor750 (NIR1), and FITC (Visible) (top to bottom). Arrows in the SWNT panel denote nodules visible only by SWNTs (n = 3 animals). (D and E) Tumor-to-muscle ratio and TBR from noninvasive images obtained with SWNTs, AF750, and FITC (n = 3 per group; ***P < 0.001; *P < 0.05; one-way ANOVA and Tukey posttests). Error bars, s.d. [Scale bar, 1 cm (A).]
Improved Signal-to-Noise Performance in Vivo Using SBP–M13–SWNT in the NIR2 Wavelength Regime.
Signal-to-noise behavior is a critical parameter for sensitive detection of tumors. To directly compare the signal-to-noise ratios between SWNTs and dyes in the NIR1 or visible regimes, SBP–M13 viruses were either complexed to SWNTs or conjugated with AlexaFluor750 dye (SBP–M13–AF750) or FITC (SBP–M13–FITC). Probes were then combined and added in fivefold excess of their minimum detection limits to mice bearing disseminated tumors. Noninvasive images were acquired through the fully intact skin of tumor-bearing animals (Fig. 2C), and fluorescence intensities from tumors in the peritoneal cavity, muscle, and background were determined. The tumor-to-muscle ratio of SBP–M13–SWNTs was 5.5 ± 1.2 (mean ± SD), which was significantly higher than ratios calculated for SBP–M13–AF750 (3.1 ± 0.42) and SBP–M13–FITC (0.96 ± 0.10) (Fig. 2D). The TBR (i.e., intensity of tumor to image background) achieved using SBP–M13–SWNTs was 134.9 ± 21.0, which was a 24- and 28-fold improvement over SBP–M13–AF750 and SBP–M13–FITC, respectively (Fig. 2E). Importantly, as highlighted by the arrows in Fig. 2C, tumor nodules not observed in the visible and NIR1 channels were detectable in the NIR2 regime with SBP–M13–SWNTs, which highlights the sensitivity of the SWNT probe and imaging system.
Selective and Affinity Targeting of SBP–M13–SWNT to Ovarian Tumors in Vivo.
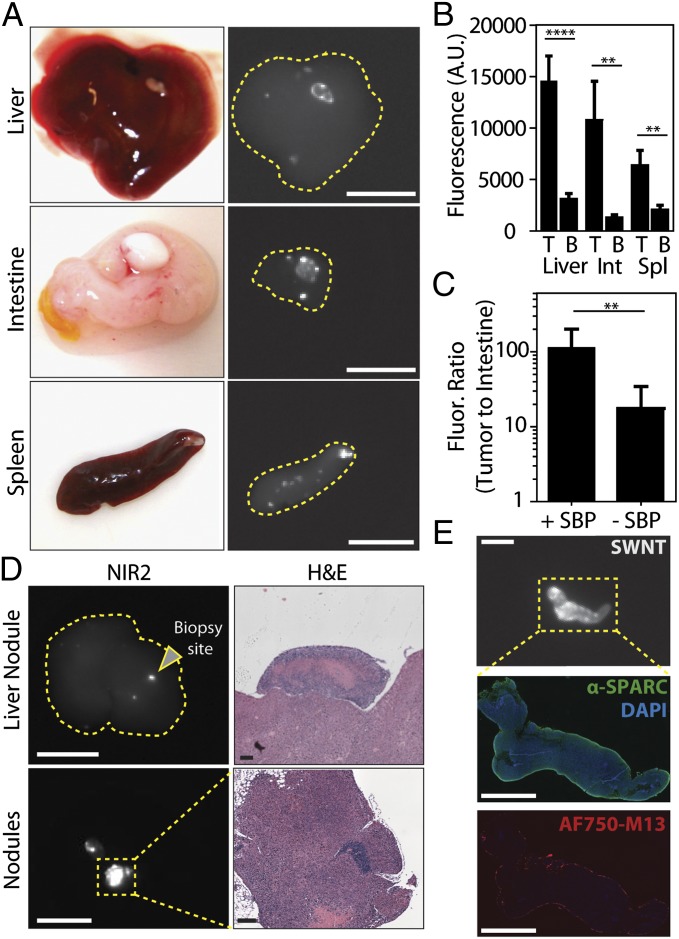
Because many tumor nodules are implanted on the surfaces of peritoneal organs in this model, we also computed organ-specific TBRs for tumor nodules on the liver, intestine, and spleen. Representative photographs of organs containing tumor implants on their surface with their corresponding NIR2 fluorescent images are shown in Fig. 3A. The TBRs (i.e., ratio of surface tumor nodule fluorescence relative to that observed in its underlying organ) calculated for the liver, intestine, and spleen were 4.6, 8.0, and 3.1, respectively (Fig. 3B), highlighting the specificity of the probe toward tumor nodules compared with its underlying organs.
Fig. 3.
Specificity of SBP–M13–SWNTs for OVCAR8 tumor nodules in the abdominal cavity. (A) Photographs and NIR2 fluorescence (10–50-ms exposure) of tumor nodules implanted on several peritoneal organs. (B) Quantification of nodule and organ-specific background for nodules present on the liver, intestine, and spleen (n = 8–11 nodules per organ; **P < 0.01; ****P < 0.0001; two-tailed t tests). (C) Tumor-to-intestine ratio for targeted and untargeted M13–SWNT probes. Intestinal tissue used for background intensity (+SBP, n = 6; –SBP, n = 13; **P < 0.01; two-tailed t test). (D) Representative NIR2 fluorescence and H&E staining of a positive nodule revealing characteristic tumor histology. (E) Immunofluorescence staining reveals colocalization of M13–SBP–SWNTs conjugated to AlexaFluor750 dye with SPARC expression in an NIR2-positive nodule. [Scale bars, 10 mm (A), 10 mm (D, NIR2), 250 μm (D, H&E liver nodule), 125 μm (D, H&E nodules), 5 mm (E, NIR2), and 2.5 mm (E, SPARC, AF750–M13).]
The in vivo sensitivity of targeting conferred by the SBP was assessed by injecting tumor-bearing animals with M13–SWNTs expressing SBPs or untargeted M13–SWNTs. The NIR2 intensities of excised tumor nodules and intestinal tissue of the same animal were used to compute TBRs for the targeted and untargeted probes. SBP–M13–SWNTs showed significant, 10-fold higher TBRs than untargeted M13–SWNTs, likely due to a combination of improved targeting and reduced tissue autofluorescence in the NIR2 window (Fig. 3C).
To verify the specificity of SBP–M13–SWNTs, we assessed the SWNT-positive tumor nodules by immunohistochemistry. Standard hematoxylin and eosin (H&E) staining of SWNT-positive tumor sections revealed histopathological features consistent with ovarian tumor nodules, including a high nuclear-to-cytoplasmic ratio, cellular crowding, a necrotic core, and a distinct architecture from underlying organs (Fig. 3D). Interestingly, the liver exhibits a fluorescent signal where no tumor nodule is visible by the eye, and after biopsy of the indicated area, H&E staining indicated pathology consistent with tumor nodules located on the liver (Fig. 3D, Upper). Additionally, immunohistochemical staining revealed an enrichment of SPARC expression along the periphery of the SWNT-positive tumor nodules (SI Appendix, Fig. S8). Finally, to assess whether our probe specifically colocalizes with SPARC-expressing regions of the tumor nodules, we administered SBP–M13–SWNTs conjugated with AlexaFluor750 to tumor-bearing mice and analyzed the excised tumor nodules by immunofluorescence. In multiple nodules, SPARC was widely expressed, with particularly strong expression at the tumor periphery (Fig. 3E, Middle) in a pattern consistent with our immunohistochemical staining described in SI Appendix, Fig. S8. The AF750-labeled SBP–M13–SWNTs were similarly enriched at the tumor periphery (Fig. 3E, Bottom). These patterns are consistent with an outside–in diffusion model limited both by the hydrodynamic radius and ligand interactions of SBP–M13–SWNTs with the tumor nodule.
Image Guidance Using SBP–M13–SWNT Improves Surgical Resection of Tumors.
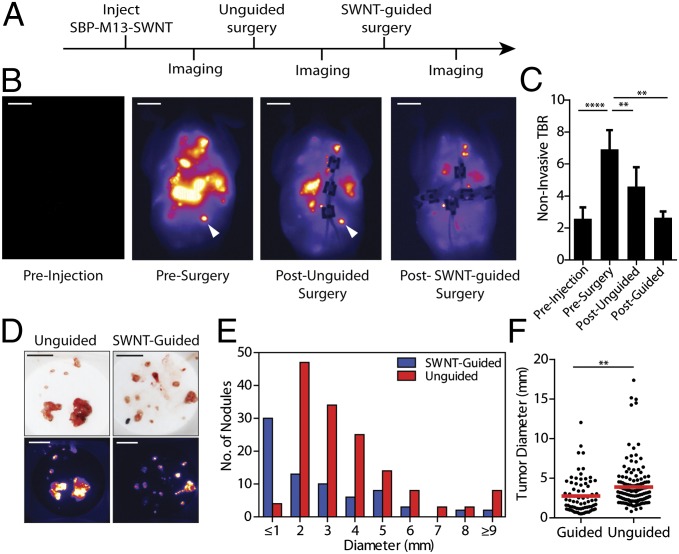
To assess the potential clinical utility of SBP–M13–SWNTs for reduction of tumor burden, a gynecological surgeon performed surgeries on orthotopic models of ovarian cancer that were imaged at various points during the surgical procedures. Approximately 15–25 min were spent on procedures in each experiment, and tumor implants were predominantly distributed in the bowel mesentery, peritoneal wall, subdiaphragmatic surfaces, and surfaces of organs including the liver, spleen, pancreas, and within the pelvic cavity. H&E-stained tissue sections were prepared from all excised nodules and assessed by a pathologist. With the exception of two non–tumor-containing mesenteric lymph nodes, all tissues examined (n = 197) were positive for ovarian tumor tissue, indicating an accuracy of 98.9% of our probe for ovarian tumors. Surgery was first performed with preoperative image guidance to assess whether this addition to the process would be beneficial to the surgical procedure based on the distribution of excised tumor nodule sizes. A comparative analysis of excised tumors revealed that a significantly higher number of submillimeter tumor nodules were discovered in the image-guided cohorts versus the non–image-guided cohorts (12 and 0 nodules, respectively; SI Appendix, Fig. S11). Using image guidance, there were also greater numbers of excised tumors from 1.3 to 3 mm; however, there was no appreciable difference for larger tumors (>3 mm) between image-guided and non–image-guided cohorts. We additionally assessed the impact of performing surgery in a serial manner, with an initial round of non–image-guided surgery, followed by image acquisition and a second round of image-guided surgery (Fig. 4A). We observed reduction of tumor burden from non–image-guided surgery to image-guided surgery (Fig. 4B). We determined the TBR of overall tumor burden to background muscle by region of interest analysis and confirmed reduction of overall tumor burden due to image-guided reduction (Fig. 4C). Using both SWNT imaging (Fig. 4B) and quantification of excised tumor nodule diameters, we observed a greater number of submillimeter excised tumors in the groups assisted by SWNT image guidance (30 versus 4 nodules; Fig. 4 D and E). Overall, significantly more, smaller diameter tumors were excised using SWNT-based image guidance as opposed to unguided surgeries (Fig. 4F).
Fig. 4.
Surgical removal of tumors with SBP–M13–SWNT guidance. (A) Schematic of serial surgical procedure. (B) Representative whole-abdomen NIR2 images before injection of SBP–M13–SWNT, before surgery, after an initial unguided surgery, and after subsequent SBP–M13–SWNT-guided surgery. White arrow indicates an SWNT-positive nodule detected only during image-guided surgery. (C) TBRs during surgery. Muscle from the hind limb was used for background (**P < 0.01; ****P < 0.0001; two-tailed t tests; error bars represent SE). (D) Photographs and NIR2 images of excised tumor nodules following unguided and SWNT-guided surgery. (E) Histogram of tumor diameters removed with and without guidance. (F) Dot plot of individual tumor nodule diameters excised with and without SWNT-guidance (guided, n = 74; unguided, n = 146; **P < 0.01; two-tailed t test). [Scale bars, 1 cm (B), 1 cm (C, photograph), and 1 cm (C, NIR2).]
Discussion
This study describes the development and use of a single fluorescence imaging agent for high-contrast, detection and guidance for surgical removal of disseminated ovarian tumors. NIR2-emitting SWNT probes offer significantly improved signal-to-noise performance compared with visible and near-infrared dyes and detect tumors not visualized using the optical dyes. These targeted, M13-stabilized SWNT probes assist surgical removal of ovarian tumors with excellent sensitivity, as confirmed by subsequent pathological examination. The probe is sensitive for identifying tumor nodules located on several abdominal viscera, the peritoneal wall, and the bowel mesentery. Importantly, compared with fluorescent probes in the visible or NIR1 regimes, the fluorescence of SWNTs is not limited by quenching, allowing for long-term, continuous imaging. With the development of advanced imaging platforms, surgeons may be able to visualize tumors both before and throughout surgical procedures, thereby significantly improving fluorescence-guided tumor resection. This study demonstrates that surgery accompanied by image guidance leads to identification and removal of smaller tumor nodules. Although NIR2 images could not provide 3D localization of the tumor implants, they provided information about the sites of disease burden requiring closer surgical examination. Imaging of regions in which the surgeon was initially reluctant to explore in an effort to minimize morbidity by risking excessive blood loss, but were shown to harbor a positive NIR2 signal, often led to the identification and excision of additional tumor nodules missed on non–image-guided approaches. Although we did not investigate longitudinal survival rates after image-guided surgery due to surgical constraints in our small-animal models, the majority of clinical evidence suggests that optimal surgery, currently defined as the removal of tumors with diameters of 1 cm and larger, is correlated with improved overall survival rates (16). SWNT-based affinity probes may aid in surgical planning and resection to help achieve a reduction in mortality rates in the future.
We achieve detection of submillimeter tumors with excellent TBRs using M13-stabilized SWNTs, in part due to properties of the particles that lead to low tissue scattering and minimal tissue autofluorescence in the NIR2 optical window. In comparing excised tumors with unaffected intestinal tissues as a background measurement, we observed high TBRs of ∼112 using our SPARC-targeted M13–SWNT probes. Following i.p. administration, some uptake is observed using nontargeted SWNT probes, which is most likely due to nonspecific binding interactions or convective flow patterns present within the peritoneal cavity. Whereas many nanoparticles include targeting peptides conjugated directly to the nanoparticle, our nanoprobe takes advantage of the genetically encoded M13 scaffold to spatially uncouple the targeting peptide (SBP) from the imaging probe (SWNT). This separation of targeting and imaging moieties circumvents direct and excessive conjugation, which may abolish the functionality of each component (11, 26); however, how this spatial uncoupling affects both targeting and nanomaterial functionality is an area requiring further study.
Fluorescence imaging in the second optical window offers the promise of imaging at greater penetration depths (>3–5 mm) with reduced optical scattering within the tissue. In comparing the performance of visible, NIR1, and NIR2 probes, the M13–SWNT probe achieved the highest tumor-to-muscle ratios and TBRs, most likely due to less tissue absorbance and autofluorescence in the NIR2 regime. In addition, we were able to see tumors not observed in the visible and NIR channels, highlighting the improved tissue penetration and detection in the NIR2 regime. Using our reflectance imaging system, we can detect 1-mm-diameter tumors up to a maximal depth between 9.7 and 18.2 mm. To our knowledge, this represents the highest reported depth of detection using fluorescence reflectance and is also higher than previous reports that detected mammary tumors labeled with activatable Cy5 probes (3). Future work to enhance the fluorescence of M13–SWNTs using plasmonic nanomaterials (27) and affinity targeting using other ligand–receptor interactions including the folate receptor (4) may offer further improvements on current limits of detection and resolution. These longer wavelength-emitting probes will greatly aid in locating ovarian tumors confined to deep anatomical regions.
SBP–M13–SWNTs injected intraperitoneally colocalized with stromal SPARC expression on the periphery of the ovarian tumor nodules. Tumor nodules labeled with the probes exhibited high signal with low background in the surrounding healthy tissues, including liver, spleen, and intestine. These high organ-specific TBRs contributed to more accurate surgical resection of tumor nodules localized to the organ surfaces. Because our probes can visualize the tumor margins, they have potential to assist the surgeon in delineating tumors from healthy tissue for improved resection of other solid tumors, as also demonstrated by approaches using activatable peptides (3, 28) and dyes (2), fluorescein conjugates (4), and multimodal nanoparticles (29).
The use of nanomaterials as clinical imaging probes is a rapidly evolving area. As new materials emerge from the community, their attractiveness for clinical applications is typically informed first by their in vitro properties, then by their behavior in animal models, and ultimately by their performance in humans. In this context, M13–SWNT probes exhibit many desirable in vitro properties such as solution stability, retention of optical properties under various pH and physiological environments and under constant excitation, and the dual capability of genetically encoding affinity ligands and solubilizing SWNTs. The next step in assessing their attractiveness for clinical translation is to assess their performance in animal models. Thus, this study explores the roles of targeting, acute biocompatibility via i.p. delivery, and optical emission in NIR2 in a preclinical mouse model of ovarian cancer. Our acute toxicity studies are consistent with several other preliminary studies exploring the safety of M13 and SWNTs, in that a panel of serum biomarkers were not elevated (30–33). Collectively, we believe these findings suggest that these materials warrant further consideration for clinical translation, which would include the development of instruments for real-time imaging, outcomes research such as survival studies in preclinical models, and rigorous safety evaluation through the National Characterization Laboratory (National Cancer Institute) and others to assess long-term trafficking and clearance of these materials as well as chronic toxicity studies.
Although in this study we use SWNTs for fluorescence imaging, others have demonstrated their utility as carriers for therapeutic cargoes (reviewed in ref. 34) as well as photothermal ablative therapy (35). We have begun to explore the utility of SBP–M13–SWNTs for in vivo heating of the tumor microenvironment to assess their potential as sensitizing agents to multimodal imaging and therapeutic agents.
To advance our findings closer to clinical translation, new instrumentation will be required to allow for real-time intraoperative guidance and 3D tomography for quantitative analysis and more accurate localization of tumors. Simulations suggest that at near-infrared wavelengths that define the NIR2 window, SWNT-based probes may be detectable at depths up to 10 cm on improved imaging platforms, highlighting the potential utility of these particles in human subjects (36). This new platform would allow for real-time, noninvasive imaging and processing for visualization of tumors during tumor staging, presurgical planning, and procedures. Coupling improved instrumentation with probe development will greatly improve the ability to detect tumors at earlier stages. The instrumentation should also be compatible with other NIR2 window optical probes as they are developed. Early detection of smaller tumors may also provide fundamental insights into tumorigenesis and disease progression, as well as allow clinicians to better monitor therapeutic responses and recurrence of disease.
Materials and Methods
All materials are provided in SI Appendix, SI Materials and Methods. In vitro and in vivo characterization of the SBP–M13–SWNT probe, including optical properties, stability, detection sensitivity, biodistribution, and serum chemistry assays, are described in detail in SI Appendix, SI Materials and Methods. Tumor induction procedure, fluorescence imaging, and analysis of SBP–M13–SWNT targeting in vivo and ex vivo are detailed in SI Appendix, SI Materials and Methods. Comparison of noninvasive imaging between SBP–M13–SWNT with visible and NIR1 imaging probes and image-guided surgeries is described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Dr. Rod Bronson for tumor scoring, Mike Brown and the Koch Institute Tang Histology Facility for assistance with histology, and Heather Fleming for excellent assistance with editing of the manuscript. The authors acknowledge the Koch Institute Swanson Biotechnology Center for DNA sequencing. This work was supported by the National Institutes of Health (NIH) Center for Cancer Nanotechnology Excellence Grants U54-CA119349-04 and U54-CA151884 (to S.N.B. and A.M.B.). This work was supported in part by the Koch Institute Frontier Research Program through the Kathy and Curt Marble Cancer Research Fund. This work was funded in part by Grant P30-ES002109, the Marie D. & Pierre Casimir-Lambert Fund, and a Professor Amar G. Bose Research Grant. A.F.B. is supported by the NIH/Medical Scientist Training Program. S.N.B. is a Howard Hughes Medical Institute Investigator.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.M.A. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400821111/-/DCSupplemental.
References
- 1.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452(7187):580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urano Y, et al. Rapid cancer detection by topically spraying a γ-glutamyltranspeptidase-activated fluorescent probe. Sci Transl Med. 2011;3(110):ra119. doi: 10.1126/scitranslmed.3002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen QT, et al. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci USA. 2010;107(9):4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dam GM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat Med. 2011;17(10):1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 5.Lim YT, et al. Selection of quantum dot wavelengths for biomedical assays and imaging. Mol Imaging. 2003;2(1):50–64. doi: 10.1162/15353500200302163. [DOI] [PubMed] [Google Scholar]
- 6.Troy TL, Thennadil SN. Optical properties of human skin in the near infrared wavelength range of 1000 to 2200 nm. J Biomed Opt. 2001;6(2):167–176. doi: 10.1117/1.1344191. [DOI] [PubMed] [Google Scholar]
- 7.Heller DA, et al. Concomitant length and diameter separation of single-walled carbon nanotubes. J Am Chem Soc. 2004;126(44):14567–14573. doi: 10.1021/ja046450z. [DOI] [PubMed] [Google Scholar]
- 8.Yi H, et al. M13 phage-functionalized single-walled carbon nanotubes as nanoprobes for second near-infrared window fluorescence imaging of targeted tumors. Nano Lett. 2012;12(3):1176–1183. doi: 10.1021/nl2031663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welsher K, Sherlock SP, Dai H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc Natl Acad Sci USA. 2011;108(22):8943–8948. doi: 10.1073/pnas.1014501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh D, et al. M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nat Nanotechnol. 2012;7(10):677–682. doi: 10.1038/nnano.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu F, et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci USA. 2008;105(7):2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poon Z, et al. Ligand-clustered “patchy” nanoparticles for modulated cellular uptake and in vivo tumor targeting. Angew Chem Int Ed Engl. 2010;49(40):7266–7270. doi: 10.1002/anie.201003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akerman ME, Chan WCW, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc Natl Acad Sci USA. 2002;99(20):12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010;188(6):759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 16.Chi DS, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103(2):559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 17.Scott JK, Smith GP. Searching for peptide ligands with an epitope library. Science. 1990;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 18.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380(6572):364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, et al. Fabricating genetically engineered high-power lithium-ion batteries using multiple virus genes. Science. 2009;324(5930):1051–1055. doi: 10.1126/science.1171541. [DOI] [PubMed] [Google Scholar]
- 20.Dang X, et al. Virus-templated self-assembled single-walled carbon nanotubes for highly efficient electron collection in photovoltaic devices. Nat Nanotechnol. 2011;6(6):377–384. doi: 10.1038/nnano.2011.50. [DOI] [PubMed] [Google Scholar]
- 21.Kelly KA, Waterman P, Weissleder R. In vivo imaging of molecularly targeted phage. Neoplasia. 2006;8(12):1011–1018. doi: 10.1593/neo.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, et al. SPARC is a key regulator of proliferation, apoptosis and invasion in human ovarian cancer. PLoS ONE. 2012;7(8):e42413. doi: 10.1371/journal.pone.0042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherukuri P, et al. Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence. Proc Natl Acad Sci USA. 2006;103(50):18882–18886. doi: 10.1073/pnas.0609265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4(1):11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes DR, et al. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hlavacek WS, Posner RG, Perelson AS. Steric effects on multivalent ligand-receptor binding: Exclusion of ligand sites by bound cell surface receptors. Biophys J. 1999;76(6):3031–3043. doi: 10.1016/S0006-3495(99)77456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong G, et al. Metal-enhanced fluorescence of carbon nanotubes. J Am Chem Soc. 2010;132(45):15920–15923. doi: 10.1021/ja1087997. [DOI] [PubMed] [Google Scholar]
- 28.Olson ES, et al. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc Natl Acad Sci USA. 2010;107(9):4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kircher MF, et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med. 2012;18(5):829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krag DN, et al. Selection of tumor-binding ligands in cancer patients with phage display libraries. Cancer Res. 2006;66(15):7724–7733. doi: 10.1158/0008-5472.CAN-05-4441. [DOI] [PubMed] [Google Scholar]
- 31.Kolosnjaj-Tabi J, et al. In vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to Swiss mice. ACS Nano. 2010;4(3):1481–1492. doi: 10.1021/nn901573w. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, et al. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc Natl Acad Sci USA. 2008;105(5):1410–1415. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schipper ML, et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol. 2008;3(4):216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Tabakman S, Welsher K, Dai H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009;2(2):85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kam NW, O’Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA. 2005;102(33):11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22(1):93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.