Significance
We demonstrate that ubiquitin-conjugating enzyme Ubc13, whose expression is elevated in primary and metastatic breast cancer (BCa), promotes metastatic spread of BCa cells by controlling their lung-colonizing ability while having little effect on primary tumor growth. Mechanistically, Ubc13 is required for TGFβ-induced non-SMAD signaling via TAK1 and p38, a pathway that is first activated in the primary tumor. An Ubc13- and p38-dependent metastatic gene signature was identified, explaining how p38 may control metastasis and providing a measure for monitoring the effectiveness of pharmacologic p38 inhibition, which inhibits the growth of established metastatic lesions. We suggest that p38 inhibition should be considered as a potential treatment for metastatic BCa.
Keywords: ubiquitination-mediated signaling, pre-clinical studies
Abstract
Metastatic spread is the leading cause of cancer mortality. Breast cancer (BCa) metastatic recurrence can happen years after removal of the primary tumor. Here we show that Ubc13, an E2 enzyme that catalyzes K63-linked protein polyubiquitination, is largely dispensable for primary mammary tumor growth but is required for metastatic spread and lung colonization by BCa cells. Loss of Ubc13 inhibited BCa growth and survival only at metastatic sites. Ubc13 was dispensable for transforming growth factor β (TGFβ)-induced SMAD activation but was required for activation of non-SMAD signaling via TGFβ-activating kinase 1 (TAK1) and p38, whose activity controls expression of numerous metastasis promoting genes. p38 activation restored metastatic activity to Ubc13-deficient cells, and its pharmacological inhibition attenuated BCa metastasis in mice, suggesting it is a therapeutic option for metastatic BCa.
Breast cancer (BCa) is the leading invasive cancer among women worldwide. BCa-related mortality is usually caused by distant metastases rather than primary tumors (1, 2). The spread of cancer cells from primary tumors to distant organs, termed metastasis, is a multistep process in which cancer cells must (i) invade through the extracellular matrix (ECM), (ii) disseminate into the bloodstream, (iii) survive in the circulation, and (iv) extravasate and successfully colonize distant sites (3). Conventional therapeutic strategies have limited success in preventing and treating metastatic cancer, and BCa metastases can recur many years after removal of the primary tumor. This phenomenon could be due to the complex nature of metastasis itself, and, more realistically, the limitation of current treatments that are effective against primary BCa, i.e., surgical removal and localized radiotherapy, but do little to prevent metastatic recurrence. Even chemotherapy is not very effective against metastatic tumors (4). Unfortunately, the pharmaceutical industry has been reluctant to conduct metastasis prevention trials on patients with early stage cancer using survival and reduction of metastatic load as end points, because such studies are lengthy and require a large number of patients with otherwise relatively good survival prospects (4). Consequently, the development of agents that prevent metastasis from occurring and trigger regression of established metastatic lesions is an urgent unmet need.
It was reported that expression of the ubiquitin conjugating enzyme (E2) Ubc13 is up-regulated in metastatic BCa (5). Ubc13, which heterodimerizes with Uev1a, catalyzes formation of lysine 63 (K63)-linked polyubiquitin chains, which control protein–protein interactions involved in DNA damage repair and protein kinase activation (6, 7). In certain immune cells, Ubc13 is required for IκB kinase (IKK)–nuclear factor κB (NF-κB) activation, but a more ubiquitous role for Ubc13 was observed in the activation of MAPK signaling (8–11). We found that Ubc13 is required for activation of mitogen-activated protein kinase kinase kinase 1 (MEKK1), transforming growth factor β (TGFβ)-activating kinase 1 (TAK1), and downstream MAPK cascades on CD40 engagement in B cells (10). Importantly, MEKK1 and TAK1 are also required for BCa metastasis (12, 13). Of the numerous signaling pathways affecting BCa metastasis, the TGFβ pathway has some of the strongest effects, and it promotes metastasis by inducing migration, intravasation, and epithelial-mesenchymal transition (EMT) of carcinoma cells (14). TGFβ signaling is mediated through SMAD-dependent and -independent (non-SMAD) pathways (15, 16). Non-SMAD TGFβ signaling is positively regulated by multiple molecules including TAK1 (17), tumor necrosis factor receptor-associated factor 6 (TRAF6) (18), and TRAF4 (19). The p38 MAPK also participates in different steps of metastasis, including ECM invasion by primary cancer cells, migration across the surrounding tissue, entry into the circulation, and colonization of distant sites (20). p38 inhibitors are not toxic and were found effective in prevention and attenuation of inflammatory pain in humans (21, 22). Here we show that a Ubc13-controled TAK1–p38 cascade controls BCa metastatic dissemination and that a p38 inhibitor can cause regression of established metastases.
Results
Ubc13 Controls BCa Lung Metastasis.
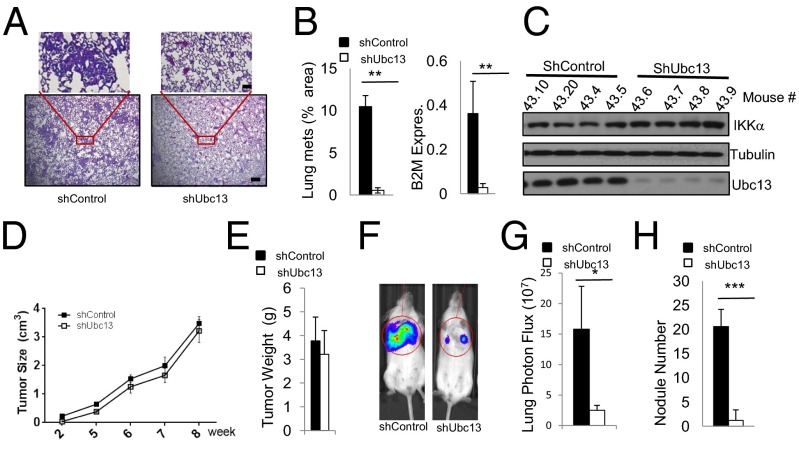
Ubc13 is up-regulated in metastatic BCa (5). Using the Oncomine platform, we confirmed that Ubc13 is up-regulated in various tumor tissues, including breast, pancreas, colon, prostate, and lymphoma (Fig. S1A). A search of The Cancer Genome Atlas (TCGA) datasets showed that Ubc13 (UBE2N) and its targets vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) (see below) are up-regulated in human tumor and metastasis specimens compared with normal tissue (Fig. S1B). Up-regulation was enriched in basal and Her2+ BCa subtypes (Fig. S1B), which are known to be more metastatic. We used a human BCa cell line, LM2 or 4175, which grows in mice and preferentially metastasizes to lung (23), to study the role of Ubc13 in metastatic spread. Ubc13 expression in LM2 cells was silenced by lentiviral shRNA delivery, and resulting shUbc13 and control LM2 cells (shControl) were injected into the #4 mammary fat pads of NOD/SCID recipient mice. After 8 wk, mice were killed, and their lung sections were H&E stained to detect metastases. Lung sections from LM2-shControl cells injected into mice showed large areas containing densely packed cancer cells (Fig. 1A), indicating successful lung colonization. In sharp contrast, LM2-shUbc13 cells formed much smaller areas containing cancer cells (Fig. 1A). LM2-shControl cells gave rise to >10-fold more lung metastases than LM2-shUbc13 cells, based on lung areas occupied by cancer cells or quantitative RT-PCR (qRT-PCR) analysis of human-specific β2-microglobulin (B2M) normalized to mouse β-actin mRNA (Fig. 1B). Immunoblot analysis of cancer cells isolated from xenografts confirmed that Ubc13 expression was stably silenced (Fig. 1C). Importantly, Ubc13 silencing had no detectable effect on primary tumor growth and mass (Fig. 1 D and E). We also inoculated LM2 cells via the tail vein to rule out any effects on the primary tumor and found that Ubc13 silencing dramatically reduced lung metastasis, determined by bioluminescence measurement and surface nodule numbers (Fig. 1 F–H).
Fig. 1.
Ubc13 controls BCa metastatic spread. (A–E) Role of Ubc13 in lung metastasis of orthotopically transplanted BCa cells. (A) H&E staining of lung sections from mice orthotopically transplanted with shControl- and shUbc13-LM2 cells. [Scale bars, 800 μm (top two inlets) and 100 μm (bottom).] (B) Lung metastatic load was determined by calculating the percentage of the lung surface occupied by cancer cells (Left) or by qRT-PCR of human-specific β2-microglobulin (B2M) mRNA normalized to mouse β-actin mRNA (Right). (C) Ubc13 protein expression in cancer cells isolated from primary shControl- and shUbc13-LM2 orthotopic tumors (mouse numbers indicated above the lanes). (D) Tumor growth curve (the whole 8-wk time course) and (E) weights (end point, i.e., 8 wk after injection) of primary tumors formed by shControl- and shUbc13-LM2 cells. (F–H) Requirement of Ubc13 for lung colonization by tail vein injected BCa cells. (F) BLI (bioluminescence) images of NOD/SCID mice injected with luciferase-labeled shControl- and shUbc13-LM2 cells via the tail vein. (G) Quantification of lung photon flux. (H) Quantification of lung surface nodules. Data are averages ± SEM; n = 5 mice. ■, shControl; □, shUbc13. *P < 0.05, **P < 0.01, ***P < 0.001.
To further confirm the requirement of Ubc13 for metastasis, we carried out similar experiments using the mouse metastatic mammary cancer 4T1 cell line. Again, Ubc13 silencing had no effect on primary tumor growth but greatly reduced the ability of 4T1 cells to metastasize to lung in Balb/C mice (Fig. S2A). We also used another metastatic mouse mammary cancer cell line, MT2, derived from MMTV-cNeu/ErbB2–induced tumors in Friend virus B-type (FVB) mice (24). When transplanted into the #2 mammary gland, shControl-MT2 cells formed lung metastases in 60% of transplanted mice, but shUbc13-MT2 cells did not metastasize (Fig. S2B). However, Ubc13 silencing in MT2 cells did partially affect primary tumor growth (Fig. S2B). We also crossed Ubc13F/F mice (8) with MMTV-cNeu mice (25) (both in the FVB background). The resulting MMTV-cNeu;Ubc13F/F mice formed spontaneous mammary tumors starting at 6 mo of age. We isolated cells from these tumors and infected them with Adeno-Cre to delete Ubc13 ex vivo or AdenoGFP as a control (Fig. S3A). The Ubc13-depleted ErbB2 tumor cells failed to form lung metastases after orthotopic transplantation or tail vein injection into FVB mice (Fig. S3A). Last, we reconstituted Ubc13-silenced cells with either WT or a catalytically inactive mutant (C87A) form of Ubc13 (Fig. S3B) and transplanted the resulting cells into mice. Importantly, WT-rescued cells formed lung metastases, whereas cells reconstituted with Ubc13(C87A) did not (Fig. S3B). Thus, Ubc13’s catalytic activity is required for BCa metastatic spread.
Ubc13 Controls BCa Lung Colonization.
To address how Ubc13 controls metastasis, we used an inducible shRNA lentivirus that allows gene silencing on doxycycline (Dox) treatment while labeling transduced cells with red fluorescent protein (RFP) (26). In LM2 cells transduced with this construct, Dox led to stable inhibition of Ubc13 expression within 3 d, and Dox withdrawal restored Ubc13 expression as early as day 3 with full expression on day 5 (Fig. 2A). Flow cytometry and immunofluorescence (IF) microscopy confirmed that both p10-shCtrl– and p10-shUbc13–transduced cells were uniformly RFP positive after Dox addition (Fig. 2B and Fig. S4). To address whether Ubc13 is required for entry of BCa cells into the lung, p10-shControl– and shUbc13-transduced LM2 cells were cultured with Dox for 4 d, and tail vein injected into NOD/SCID mice that were kept on Dox-containing water for 1 wk and switched to regular drinking water for 3 wk. Lung metastasis was monitored weekly by a bioluminescence assay. Curiously, no differences in lung metastasis were observed between the two groups (Fig. 2C), indicating that Ubc13 activity is not required for lung seeding, a process that was probably completed within the first 24 h. We also injected p10-shControl and shUbc13 LM2 cells into NOD/SCID mice that were kept on regular water for 1 wk, allowing the cells to enter the lung and colonize it. The mice were then given Dox-containing water to silence Ubc13 expression. Whereas p10-shControl cells formed detectable lung metastases as early as 2 wk after injection, p10-shUbc13 cells did not form detectable metastases in Dox-treated mice (Fig. 2D). Microscopic analysis under bright field (BF) and red fluorescence (RFP) confirmed that shUbc13-LM2 cells formed much fewer and smaller lung nodules than shControl-LM2 cells (Fig. 2E). To further study how Ubc13 affects metastatic growth, we performed tumorsphere formation assays on control and shUbc13 cells and found that Ubc13 silencing had no effect on these properties (Fig. S5 A and B). These results are consistent with the finding that Ubc13 is generally dispensable for primary tumor growth. Loss of Ubc13 in BCa cells also did not affect their proliferation as evident by carboxyfluorescein succinimidyl ester labeling (Fig. S5C). Importantly, loss of Ubc13 also did not affect LM2 cell intravasation or extravasation quantified by qPCR (Fig. S5D). Through real-time in vivo imaging, we observed no difference in frequencies of circulating tumor cells between shControl and shUbc13 LM2 transplanted mice (Fig. S5 E and F, Table S1, and Movie S1). We therefore reasoned that Ubc13 could specifically control metastatic BCa growth properties. Indeed, shUbc13 BCa cells residing in small lung lesions were less proliferative than shControl cells in lung lesions and were more likely to show caspase 3 activation (Fig. 2F). In keeping with Ubc13 being dispensable for primary tumor growth, we did not observe a difference in proliferation and apoptosis of BCa cells within primary tumors formed by shControl- or shUbc13-LM2 cells (Fig. S6).
Fig. 2.
Ubc13 is required for lung colonization by BCa cells. (A) Dynamic, doxycycline (Dox)-regulated, Ubc13 silencing in p10-shUbc13–infected LM2 cells. (B) Analysis of RFP expression by p10-shCtrl or p10-shUbc13–infected LM2 cells cultured in the absence (−) or presence (+) of Dox for 4 d. (C) BLI measurement of mice injected with Dox-treated p10-Ctrl or p10-shUbc13 LM2 cells, given Dox in water for the first week and switiched to regular water for the following 3 wk. (D) BLI measurement of mice injected with p10-shCtrl or p10-shUbc13 LM2 cells that were not treated with Dox. Mice were given regular water for the first week and switiched to Dox-containing water for the following 3 wk. Data in C and D are averages ± SEM; n = 3 mice. (E) Representative bright field (BF) and RFP images of lungs from mice transplanted with p10-shCtrl (Upper) or p10-shUbc13 (Lower) LM2 cells and treated as in D. (Scale bar, 1 cm.) (F) Ki67 and cleaved caspase 3 staining of lung lesions in mice that were i.v. inoculated with shControl- or shUbc13-LM2 cells (4 wk after injection). Five independent high-power fields (HPFs) were quantitated, and the results are shown on the right as averages ± SEM. (Scale bar, 100 μm.)
Ubc13 Controls BCa Metastasis Through TAK1 and p38 MAPK.
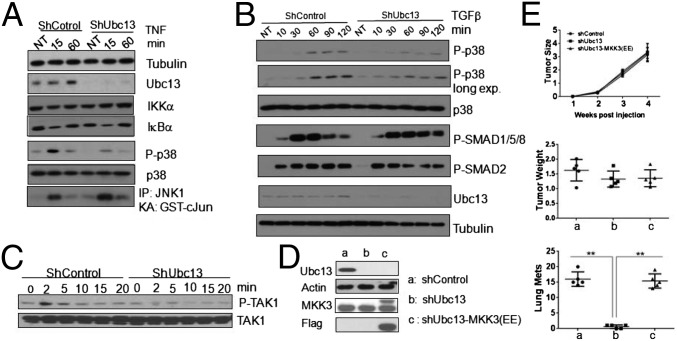
Ubc13 is involved in both NF-κB and MAPK activation, but the dependence of either response on Ubc13 activity is cell type specific (8, 9). To better understand the role of Ubc13 in signaling within BCa cells, we stimulated LM2 cells with TNF. Although Ubc13 silencing had no effect on IκBα degradation and resynthesis, it inhibited p38α phosphorylation (Fig. 3A). However, Ubc13 silencing had no significant effect on JNK activation. Because TGFβ signaling is more relevant to the control of BCa metastasis than TNF (16), we examined the role of Ubc13 in TGFβ-induced SMAD and non-SMAD signaling in LM2 cells. Although Ubc13 silencing had no effect on SMAD phosphorylation, it inhibited TGFβ-induced p38α phosphorylation (Fig. 3B). TNF receptor family members signal to p38 via the MAPK kinase kinases (MAP3K) MEKK1 and TAK1 (10). We found that TGFβ-induced TAK1 phosphorylation was substantially reduced on Ubc13 silencing (Fig. 3C). Silencing of TAK1 or p38α in BCa cells led to dramatically reduced lung metastasis (Fig. S7 A and B). Compared with shControl-LM2 cells, shUbc13-LM2 cells exhibited lower p38 phosphorylation (i.e., activation) in both lung lesions and primary tumors (Fig. S7C). Expression of constitutively active MKK3, which acts between TAK1 and p38, so-called MKK3(EE) (27), in Ubc13-silenced 4T1 cells fully restored their metastatic potential while having no effect on primary tumor growth, which was not influenced by the absence of Ubc13 (Fig. 3 D and E). In conclusion, Ubc13 controls BCa metastasis through TAK1, MKK3 (or MKK6), and p38α.
Fig. 3.
Ubc13 controls BCa metastasis through p38 MAPK. shControl- or shUbc13-LM2 cells were incubated with TNF (20 ng/mL) for the indicated times and assayed for IκBα degradation, p38 phosphorylation, and JNK activation by immunoblotting or in vitro kinase assay at the indicated times (A); or treated with TGFβ1 (10 ng/mL) and analyzed for p38 and SMAD (B) or TAK1 (C) phosphorylation by immunoblotting. (D) Flag-tagged MKK3(EE) was introduced into shUbc13-4T1 cells, and its expression was analyzed by immunoblotting. (E) The indicated derivatives of 4T1 cells were orthotopically (second right mammary gland) transplanted into Balb/C mice. Shown are tumor growth curves (Top), tumor weights (Middle), and lung nodule numbers (Bottom) at 4 wk. Results are averages ± SEM, n = 5 mice.
A Metastatic Gene Signature That Is Controlled by Ubc13 and p38.
To gain an insight to the genes whose expression depends on Ubc13 activity, we performed a gene array analysis on cells isolated from tumors formed by shControl- and shUbc13-LM2 cells. A number of genes, including CNN2 (calponin 2) (28), PLTP (phospholipid transfer protein) (29, 30), and IGFBP3 (insulin-like growth factor binding protein 3) (31), all of which have been previously associated with breast tumorigenesis or metastasis, were found to be down-regulated in shUbc13 cells (Fig. 4A). It is worth noting that Ubc13 also negatively regulates the expression of a number of genes, including FGF13 (fibroblast growth factor 13) and Col3A1 (collagen, type III, α1), whose functions in metastasis remain unexplored. A gene signature that controls BCa metastasis to lung was previously reported (23), and we postulated that loss of Ubc13 can affect this signature. qPCR analysis confirmed the down-regulation of several metastasis-promoting genes, including IL13RA2, CD44, VCAM-1, and ICAM-1 (Fig. 4B). Expression of ICAM-1 protein was also largely dependent on both Ubc13 and p38 (Fig. 4 C and D). As noted above (Fig. S1B), expression of both VCAM-1 and ICAM-1 was up-regulated in human primary tumors and metastasis, including basal and Her2+ BCa, compared with normal tissues. Silencing of ICAM-1 expression in 4T1 cells reduced lung metastasis without affecting primary tumor growth (Fig. 4E). Taken together, these data provide further support to the important metastasis regulating function of Ubc13. A similar change in gene expression pattern was found in p38α-silenced LM2 cells (Fig. S8A). Importantly, expression of MKK3(EE) in Ubc13-silenced cells restored expression of the lung metastasis gene signature (Fig. S8B) in keeping with their regained metastatic potential (Fig. 3E). It is worth noting that mRNA expression of IL-6, a cytokine that plays a key role in BCa metastasis (32), was down-regulated in both shp38α- and shUbc13-LM2 cells, and expression of MKK3(EE) in shUbc13 cells restored its expression (Fig. S8 A and B).
Fig. 4.
Ubc13- and p38-dependent metastasis gene signature. (A) Purified epithelial cells from shControl- and shUbc13-LM2 cells derived xenografts were subjected to transcriptomic analysis. The figures show differentially expressed genes (DEGs) in a heatmap with up-regulated and down-regulated genes in red and green, respectively. Data were z-score normalized by row. (B) Expression of indicated genes was confirmed by qRT-PCR. Results are averages ± SEM, n = 4 each. (C) ICAM-1 protein amounts in primary tumors generated by shControl- and shUbc13-LM2 tumor cells indicated by animal numbers. (D) ICAM-1 protein amounts in p38α-silenced LM2 cells. (E) FACS staining of ICAM-1 in 4T1 cells infected with scrambled or ICAM-1 shRNAs (Left), which were inoculated into the second right mammary fat pad of Balb/C mice. Four weeks later, primary tumors were weighted (Center), and lung metastases were quantified by surface nodule numbers (Right). **P < 0.05 ***P < 0.001. Data are averages ± SEM; n = 5 mice each group.
p38 Inhibition Suppresses Mammary Cancer Metastasis.
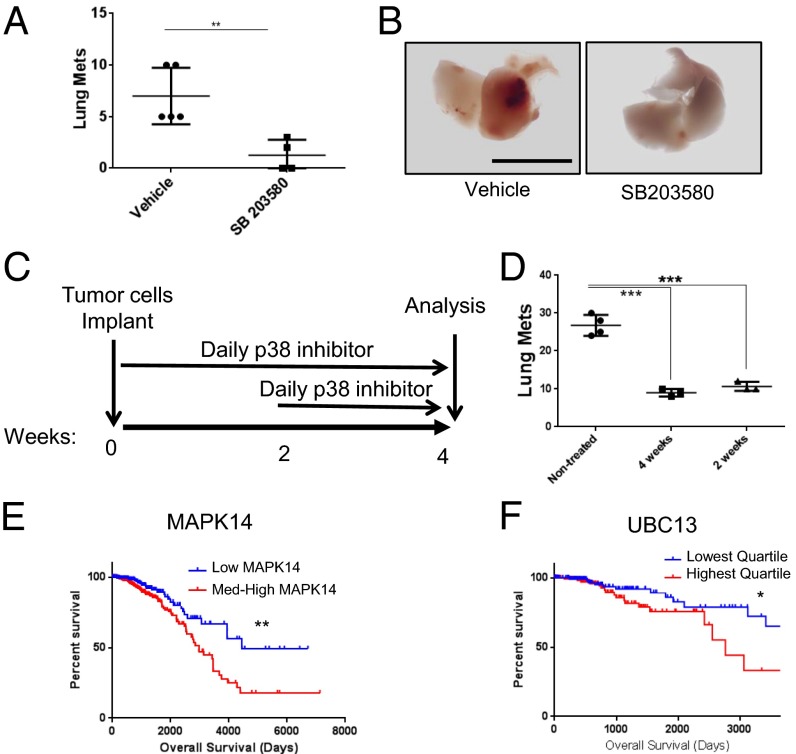
Given the availability of specific and effective p38 kinase inhibitors that are not toxic in humans (21), we examined whether pharmacological inhibition of p38 affects metastatic spread. First, we injected polyomavirus middle T antigen (PyVT) transgene (PyMT) cells (33) into C57BL/6 females via the tail vein, followed by treatment with the p38 inhibitor SB203580 or vehicle via daily oral gavage for 4 wk. Treatment with the p38 inhibitor suppressed formation of lung metastasis (Fig. 5 A and B). To examine whether p38 inhibition leads to regression of established metastases, we started inhibitor treatment 2 wk after cancer cell implantation (Fig. 5C). Importantly, a 2-wk-long treatment with SB203580 was as effective in reducing metastatic load as the 4-wk-long treatment (Fig. 5D). These results suggest that p38 inhibition can be used to treat established BCa metastasis. In support of this hypothesis, we found through a search of the TCGA and BRCA datasets that up-regulation of either p38α (MAPK14) or Ubc13 correlates with worse overall survival in human BCa (Fig. 5 E and F).
Fig. 5.
Inhibition of p38 suppresses BCa metastasis. C57BL/6 females were injected with PyMT (Py8119) cells via the tail vein and treated with the p38 inhibitor SB203580 (15 mg/kg body weight) or vehicle control by daily oral gavage for 4 wk. Lung metastases were quantified by surface nodule numbers (A). Representative lung images are shown in B. (Scale bar, 1 cm.) In another set of experiments, daily p38 inhibitor administration was initiated on either day 0 or 2 wk after BCa cell implantation (C). Lung metastases under both treatment regimens (4 and 2 wk) were quantified and compared by counting surface nodules (D). Results in A and D are averages ± SEM; n = 4–5 mice in each group. (E and F) Correlation between expression of p38 (MAPK14) and Ubc13 and breast cancer patient survival. Kaplan–Meier survival curves for samples classified as indicated. Shown are overall survival percentages for MAPK14 (tertiles) (E) and Ubc13 (quartiles) (F) expression based on clinical information available in the TCGA for the BRCA dataset. **P = 0.0023, *P = 0.0369.
Discussion
The observation that Ubc13 expression is elevated in metastatic BCa raised the suspicion that it may be causally involved in the metastatic process rather than just serve as a marker for BCa with high metastatic potential (5). Our results confirm that in addition to being associated with poor overall survival in human BCa, Ubc13 is directly involved in the metastatic process and is required both for lung colonization and survival and proliferation of established metastatic lesions. Ubc13, however, does not play a significant role in primary tumor development and growth. In addition, Ubc13 is dispensable for intravasation and extravasation of BCa cells, under the experimental conditions we used.
Together with Uev1a, Ubc13 forms an E2 ubiquitin-conjugating enzyme that catalyzes formation of K63-linked polyubiquitin chains in response to activation of diverse cytokine and pattern recognition receptors (7). As such, Ubc13 is involved in activation of a number of signaling pathways where K63-linked polyubiquitin chains mediate protein–protein interactions that control critical protein kinases (7). Among the most thoroughly studied Ubc13-dependent signal transduction pathways are the IKK-NF-κB, JNK, p38 (8, 9), and retinoic acid-inducible protein 1 (RIG-I)–TANK-binding kinase 1 (TBK1) pathways (34), as well as p53 (35), and the mammalian target of rapamycin (mTOR) (36). We previously found that Ubc13 is required for activation of the MAP3Ks TAK1 and MEKK1 downstream to members of the TNF receptor family (10). Ubc13 is also required for TAK1 activation downstream to TGFβ receptors, which, just like TNF receptors, rely on the signaling protein TRAF6 to recruit and direct Ubc13 toward its essential substrates (in this case, the TAB proteins) (17, 18). Although this has not been firmly established in our system, Ubc13 is likely to control BCa metastasis downstream to TGFβ receptors, which are well-established regulators of BCa metastasis (14). TGFβ receptor signaling also leads to activation of TAK1 and p38, both of which act downstream to Ubc13 in the control of BCa metastasis. Importantly, Ubc13 is required for maintaining the expression of numerous BCa metastasis genes, including several genes that are regulated by TGFβ (37–39). Notably, however, Ubc13 is not required for SMAD activation by TGFβ, indicating that if indeed Ubc13 stimulates metastatic growth and survival downstream of TGFβ receptors, this effect is mediated through non-SMAD signaling. Given that TGFβ signaling can either suppress or promote tumor development and metastatic spread depending on context and cell type (40, 41), general inhibition of both SMAD and non-SMAD TGFβ signaling could lead to undesired outcomes. Our results demonstrate that more selective inhibition of non-SMAD signaling through interference with Ubc13 or its downstream effector p38 does lead to effective inhibition of metastatic spread and even compromises the survival of existing metastases. We speculate that Ubc13 exerts its prometastatic activity by controlling expression of cell surface molecules on BCa cells. These molecules play pivotal roles in interacting with the hostile microenvironment, present at metastatic (or premetastatic) sites but are not required for survival and growth at the primary tumor site. Indeed, we found that loss of Ubc13 led to down-regulation of a subset of cell surface molecules, including CD44, ICAM-1, and VCAM-1. VCAM-1 was found to provide a survival advantage to BCa cells by mediating their association with macrophages (42), and soluble ICAM-1 was reported to promote bone metastasis through activation of NF-κB (43).
Although Ubc13-dependent K63-linked polyubiquitination controls the activation of several signaling pathways, as well as DNA repair responses (7, 44), our results indicate that TAK1-dependent p38 activation is the major mediator of the prometastatic effect of Ubc13 in BCa. Importantly, reconstitution of p38 activity by ectopic expression of constitutively active MKK3(EE) in Ubc13-deficient BCa cells restores their metastatic potential. Involvement of p38 MAPK signaling in cancer development, progression, and metastasis has been demonstrated previously (45–48). The p38 MAPK was suggested to overcome ERK signaling to promote survival of dormant cancer cells through activation of the unfolded protein response (49–51), as well as various steps in the process of invasion and metastasis (20). Activation of p38 MAPK was also found to be elevated in BCa cells exposed to chemotherapy (52), and a p38 inhibitor cooperates with cisplatin to induce cancer cell death in PyMT mice (53). In clinical specimens of matched primary and invasive breast carcinomas, p38 phosphorylation was found to correlate with expression of EZH2, a polycomb group protein that functions as an oncogene in BCa and whose overexpression is associated with metastatic disease (54). However, one study has found that the TAK1–p38 pathway inhibits bone metastasis by BCa, acting downstream to hepatocyte growth factor kringle 1 domain and decreasing expression of receptor activator of NF-κB (55). By contrast, we find that either specific ablation of p38 in BCa or mammary cancer cells, as well as systemic inhibition of p38, results in a strong antimetastatic effect. A clinical study had revealed that increased p38 phosphorylation in BCa effusions correlated with shorter overall survival (56). It remains to be examined whether and how Ubc13 and p38 control BCa bone metastasis.
The p38 MAPKs, in particular p38α, exert their protumorigenic activities through transcriptional and posttranscriptional regulation of numerous target genes (48) either through direct phosphorylation or through the p38-dependent kinases: MAPK-activated protein kinase-2 (MAPKAPK2) and MAPKAPK3, p38-related/activated protein kinase (PRAK), mitogen- and stress-activated protein kinase-1( MSK1), and MAP kinase-interacting kinase 1 or 2 (MNK1/2). p38α controls the activation of transcription factors, such as ATF2 and CREB; p38α also controls mRNA turnover and translation through various RNA binding proteins (57, 58). Using gene expression analysis, we identified several target genes, previously shown to affect BCa metastasis, whose expression is p38 dependent, including CNN2, PLTP, IGFBP3, IL-6, IL13RA2, CD44, VCAM-1, and ICAM-1. At least one of these genes, encoding ICAM-1, controls metastatic spread in our system. In addition, p38 and Ubc13 control expression of IL-6 mRNA in BCa cells. Previous studies have shown that autocrine IL-6 signaling controls cancer cell growth, cancer stem cell (CSC) renewal, and metastasis (59–61). IL-6 can stimulate Notch3-dependent up-regulation of Jagged-1 to promote BCa cell growth and maintain an aggressive phenotype (62). It remains to be determined how IL-6 signaling is regulated by the Ubc13 and p38 in the context of BCa.
Importantly, pharmacological inhibition of p38 can block metastatic spread of mammary cancer in mice and can even attenuate the growth or survival of established lung metastases. Given that a number of small molecule p38 inhibitors were found to be effective and safe for the treatment of inflammatory pain in humans (21, 22), these findings suggest that p38 inhibitors should be evaluated as antimetastatic drugs in human BCa. Because bone metastasis is frequently associated with inflammatory and neuropathic pain, such inhibitors can be first evaluated for their ability to alleviate pain in bone metastatic BCa, an application that will facilitate the testing of their antimetastatic potential (63).
Materials and Methods
Female virgin NOD/SCID, Balb/C, FVB, or C57BL/6 mice, 6–7 wk old (from Charles River), were used. For orthotopic inoculation, cells suspended in PBS mixed with Matrigel (100 μL total volume in 1:1 ratio) were injected into the fourth right mammary fat pad of mice unless otherwise indicated. Tumor size was measured using a caliper, and volume was calculated as length × width2 × 0.52. At the end of experiments, mice were killed to harvest tumors and lung tissues for histological and other analyses. For lung colonization assays, mice were i.v. injected with 0.2 × 106 LM2 cells in 100 μL PBS and subjected to BLI imaging by IVIS. For inducible silencing experiments, doxycycline hyclate (Sigma-Aldrich) was added into the drinking water (2 mg/mL). To quantify lung metastasis, visible surface nodules were counted. In some cases, lung tissues were sectioned and stained with H&E. Three sections spaced 100 μm apart were counted for metastases. Mice were maintained under specific pathogen-free conditions, and all experimental protocols were approved by the University of California at San Diego Animal Care Program, following National Institutes of Health Guidelines. Antibody information and shRNA and Q-PCR primer sequences are listed in Tables S2–S4. Please see SI Materials and Methods for more details.
Supplementary Material
Acknowledgments
We thank Santa Cruz Biotechnology, Cell Signaling Technology, and GeneTex for providing antibodies, K. Taniguchi for help with histological analysis, J. Massague (Memorial Sloan Kettering Cancer Center) for sharing MDA-231 LM2 cells, S. Elledge (Harvard Medical School, Brigham and Women’s Hospital) for sharing the inducible lentiviral shRNA vector, P. Sun (The Scripps Research Institute) for pBabeMKK3(EE), F. Zolezzi and J. Lum [Functional Genomics platform, Singapore Immunology Network, Agency for Science, Technology and Research (A*STAR)] for help with the microarray study, Y. Yang and M. Kelliher (University of Massachusetts Medical School) for sharing shUbc13 plasmids, M. Fujimuro (Kyoto Pharmaceutical University) for Ubc13 expression constructs, Jeevisha Bajaj for help with analysis of real time imaging, and J. Xu and E. Theodorakis (University of California, San Diego) for chemical inhibitor synthesis. S.K.B. and M.P. are grateful for funding from the Biomedical Research Council, A*STAR. X.W. was supported by a postdoctoral fellowship from Susan G. Komen for the Cure (KG111506). Funding was provided by National Institutes of Health (NIH) Grants 1R01CA168689 (to J.Y.), 3R01CA168689-02W1 (to T.P.), DP1CA174422 (to T.R.), T32 CA009523-29 (to R.G.F.), T32 GM007752-36 (to N.K.L.). Work was also supported by NIH Grants CA163798 and AI043477 and a San Diego Cancer Center Council Collaborative Translational Grant sponsored by Pedal the Cause San Diego (to M.K.). M.K. is an American Cancer Society Research Professor and holds the Ben and Wanda Hildyard Chair for Mitochondrial and Metabolic Diseases.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE55649).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414358111/-/DCSupplemental.
References
- 1.Desantis C, Ma J, Bryan L, Jemal A. 2014. Breast cancer statistics, 2013. CA Cancer J Clin 64(1):52–62.
- 2.Ferlay J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 4.Wan L, Pantel K, Kang Y. Tumor metastasis: Moving new biological insights into the clinic. Nat Med. 2013;19(11):1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- 5.Pestlin G, et al. 2006. US Patent 20060257950A. [DOI] [PubMed]
- 6.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458(7237):430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 7.Wang G, et al. K63-linked ubiquitination in kinase activation and cancer. Front Oncology. 2012;2:1–13. doi: 10.3389/fonc.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto M, et al. Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat Immunol. 2006;7(9):962–970. doi: 10.1038/ni1367. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, et al. Cutting Edge: Pivotal function of Ubc13 in thymocyte TCR signaling. J Immunol. 2006;177(11):7520–7524. doi: 10.4049/jimmunol.177.11.7520. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzawa A, et al. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science. 2008;321(5889):663–668. doi: 10.1126/science.1157340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng PH, et al. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nat Immunol. 2010;11(1):70–75. doi: 10.1038/ni.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuevas BD, Winter-Vann AM, Johnson NL, Johnson GL. MEKK1 controls matrix degradation and tumor cell dissemination during metastasis of polyoma middle-T driven mammary cancer. Oncogene. 2006;25(36):4998–5010. doi: 10.1038/sj.onc.1209507. [DOI] [PubMed] [Google Scholar]
- 13.Safina A, Ren MQ, Vandette E, Bakin AV. TAK1 is required for TGF-beta 1-mediated regulation of matrix metalloproteinase-9 and metastasis. Oncogene. 2008;27(9):1198–1207. doi: 10.1038/sj.onc.1210768. [DOI] [PubMed] [Google Scholar]
- 14.Padua D, Massagué J. Roles of TGFbeta in metastasis. Cell Res. 2009;19(1):89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- 15.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 16.Massagué J. TGFbeta in Cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorrentino A, et al. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10(10):1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita M, et al. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell. 2008;31(6):918–924. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, et al. TRAF4 promotes TGF-β receptor signaling and drives breast cancer metastasis. Mol Cell. 2013;51(5):559–572. doi: 10.1016/j.molcel.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 20.del Barco Barrantes I, Nebreda AR. Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans. 2012;40(1):79–84. doi: 10.1042/BST20110676. [DOI] [PubMed] [Google Scholar]
- 21.Yong HY, Koh MS, Moon A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin Investig Drugs. 2009;18(12):1893–1905. doi: 10.1517/13543780903321490. [DOI] [PubMed] [Google Scholar]
- 22.Anand P, et al. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur J Pain. 2011;15(10):1040–1048. doi: 10.1016/j.ejpain.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436(7050):518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan W, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470(7335):548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guy CT, et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89(22):10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meerbrey KL, et al. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc Natl Acad Sci USA. 2011;108(9):3665–3670. doi: 10.1073/pnas.1019736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22(10):3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren S, Abuel-Haija M, Khurana JS, Zhang X. D2-40: An additional marker for myoepithelial cells of breast and the precaution in interpreting tumor lymphovascular invasion. Int J Clin Exp Pathol. 2011;4(2):175–182. [PMC free article] [PubMed] [Google Scholar]
- 29.Olayioye MA, et al. The phosphoprotein StarD10 is overexpressed in breast cancer and cooperates with ErbB receptors in cellular transformation. Cancer Res. 2004;64(10):3538–3544. doi: 10.1158/0008-5472.CAN-03-3731. [DOI] [PubMed] [Google Scholar]
- 30.Olayioye MA, et al. StarD10, a START domain protein overexpressed in breast cancer, functions as a phospholipid transfer protein. J Biol Chem. 2005;280(29):27436–27442. doi: 10.1074/jbc.M413330200. [DOI] [PubMed] [Google Scholar]
- 31.Ren Z, et al. IGFBP3 mRNA expression in benign and malignant breast tumors. Breast Cancer Res. 2007;9(1):R2. doi: 10.1186/bcr1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26(1):54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Biswas T, Gu X, Yang J, Ellies LG, Sun LZ. Attenuation of TGF-β signaling supports tumor progression of a mesenchymal-like mammary tumor cell line in a syngeneic murine model. Cancer Lett. 2014;346(1):129–138. doi: 10.1016/j.canlet.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng W, et al. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141(2):315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topisirovic I, et al. Control of p53 multimerization by Ubc13 is JNK-regulated. Proc Natl Acad Sci USA. 2009;106(31):12676–12681. doi: 10.1073/pnas.0900596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linares JF, et al. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol Cell. 2013;51(3):283–296. doi: 10.1016/j.molcel.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schedlich LJ, Yenson VM, Baxter RC. TGF-β-induced expression of IGFBP-3 regulates IGF1R signaling in human osteosarcoma cells. Mol Cell Endocrinol. 2013;377(1-2):56–64. doi: 10.1016/j.mce.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki Y, et al. TGF-beta 1 causes increased endothelial ICAM-1 expression and lung injury. J Appl Physiol (1985) 1994;77(3):1281–1287. doi: 10.1152/jappl.1994.77.3.1281. [DOI] [PubMed] [Google Scholar]
- 39.Park DY, Sol MY, Suh KS, Shin EC, Kim CH. Expressions of transforming growth factor (TGF)-beta1 and TGF-beta type II receptor and their relationship with apoptosis during chemical hepatocarcinogenesis in rats. Hepatol Res. 2003;27(3):205–213. doi: 10.1016/s1386-6346(03)00264-x. [DOI] [PubMed] [Google Scholar]
- 40.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moses H, Barcellos-Hoff MH. TGF-beta biology in mammary development and breast cancer. Cold Spring Harb Perspect Biol. 2011;3(1):a003277. doi: 10.1101/cshperspect.a003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Q, Zhang XH, Massagué J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20(4):538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ell B, et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell. 2013;24(4):542–556. doi: 10.1016/j.ccr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panier S, Durocher D. Regulatory ubiquitylation in response to DNA double-strand breaks. DNA Repair (Amst) 2009;8(4):436–443. doi: 10.1016/j.dnarep.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 46.Nebreda AR, Porras A. p38 MAP kinases: Beyond the stress response. Trends Biochem Sci. 2000;25(6):257–260. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- 47.Tormos AM, Taléns-Visconti R, Nebreda AR, Sastre J. p38 MAPK: A dual role in hepatocyte proliferation through reactive oxygen species. Free Radic Res. 2013;47(11):905–916. doi: 10.3109/10715762.2013.821200. [DOI] [PubMed] [Google Scholar]
- 48.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9(8):537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 49.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ranganathan AC, Adam AP, Zhang L, Aguirre-Ghiso JA. Tumor cell dormancy induced by p38SAPK and ER-stress signaling: An adaptive advantage for metastatic cells? Cancer Biol Ther. 2006;5(7):729–735. doi: 10.4161/cbt.5.7.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sosa MS, Avivar-Valderas A, Bragado P, Wen HC, Aguirre-Ghiso JA. ERK1/2 and p38α/β signaling in tumor cell quiescence: Opportunities to control dormant residual disease. Clin Cancer Res. 2011;17(18):5850–5857. doi: 10.1158/1078-0432.CCR-10-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acharyya S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150(1):165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pereira L, Igea A, Canovas B, Dolado I, Nebreda AR. Inhibition of p38 MAPK sensitizes tumour cells to cisplatin-induced apoptosis mediated by reactive oxygen species and JNK. EMBO Mol Med. 2013;5(11):1759–1774. doi: 10.1002/emmm.201302732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore HM, et al. EZH2 inhibition decreases p38 signaling and suppresses breast cancer motility and metastasis. Breast Cancer Res Treat. 2013;138(3):741–752. doi: 10.1007/s10549-013-2498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao Y, et al. HGFK1 inhibits bone metastasis in breast cancer through the TAK1/p38 MAPK signaling pathway. Cancer Gene Ther. 2012;19(9):601–608. doi: 10.1038/cgt.2012.38. [DOI] [PubMed] [Google Scholar]
- 56.Davidson B, et al. The mitogen-activated protein kinases (MAPK) p38 and JNK are markers of tumor progression in breast carcinoma. Gynecol Oncol. 2006;102(3):453–461. doi: 10.1016/j.ygyno.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 57.Trempolec N, Dave-Coll N, Nebreda AR. SnapShot: p38 MAPK substrates. Cell. 2013;152(4):924–924. doi: 10.1016/j.cell.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 58.Trempolec N, Dave-Coll N, Nebreda AR. SnapShot: p38 MAPK signaling. Cell. 2013;152(3):656–656. doi: 10.1016/j.cell.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 59.Chin AR, Wang SE. Cytokines driving breast cancer stemness. Mol Cell Endocrinol. 2014;382(1):598–602. doi: 10.1016/j.mce.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 60.Dethlefsen C, Højfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat. 2013;138(3):657–664. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- 61.He G, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell. 2013;155(2):384–396. doi: 10.1016/j.cell.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sansone P, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sukhtankar D, et al. Inhibition of p38-MAPK signaling pathway attenuates breast cancer induced bone pain and disease progression in a murine model of cancer-induced bone pain. Mol Pain. 2011;7:81. doi: 10.1186/1744-8069-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.