Significance
This article defines ecosystem functional properties, which can be derived from long-term observations of gas and energy exchange between ecosystems and the atmosphere, and shows that variations of those cannot be easily explained by classical climatological or biogeographical approaches such as plant functional types. Instead, we argue that plant traits have the potential to explain this variation, and we call for a stronger integration of research communities dedicated to plant traits and to ecosystem–atmosphere exchange.
Keywords: biogeochemistry, plant traits, carbon cycle, eddy covariance, FLUXNET
Abstract
Classical biogeographical observations suggest that ecosystems are strongly shaped by climatic constraints in terms of their structure and function. On the other hand, vegetation function feeds back on the climate system via biosphere–atmosphere exchange of matter and energy. Ecosystem-level observations of this exchange reveal very large functional biogeographical variation of climate-relevant ecosystem functional properties related to carbon and water cycles. This variation is explained insufficiently by climate control and a classical plant functional type classification approach. For example, correlations between seasonal carbon-use efficiency and climate or environmental variables remain below 0.6, leaving almost 70% of variance unexplained. We suggest that a substantial part of this unexplained variation of ecosystem functional properties is related to variations in plant and microbial traits. Therefore, to progress with global functional biogeography, we should seek to understand the link between organismic traits and flux-derived ecosystem properties at ecosystem observation sites and the spatial variation of vegetation traits given geoecological covariates. This understanding can be fostered by synergistic use of both data-driven and theory-driven ecological as well as biophysical approaches.
One of the long-term objectives in global ecology is to understand the multifaceted functions of terrestrial ecosystems in the Earth system. Of particular interest are the salient properties of terrestrial ecosystems as biogeochemical reactors in the Earth system and biogeophysical controls of land–surface atmosphere interactions (1). Limited comprehension and observational uncertainties in the ecosystem functions are hampering the representation of the dynamic interactions of climate and human intervention with the biosphere in current Earth-system models (2, 3). Clearly, the primary tools are models that compute fluxes across a variety of spatial levels (leaves, plants, ecosystems, landscapes, and biomes) and time scales (hours, days, seasons, years, decades, and centuries) (4–6). The implementation of terrestrial ecosystem models requires information on parameters of nonlinear algorithms that produce fluxes of carbon and water at the leaf level and integrate this information up to canopy and landscape scales. Therefore, a fundamental challenge in this context is to identify observations and observational patterns that allow us to parameterize the critical processes.
Today, global ecology is entering the new era of “big data” side-by-side with other areas of science (7). We are better equipped than ever before for exploring observations at a variety of time and space scales that can be integrated into process-based models. Large datasets on both ecosystem and organismic levels are opening new opportunities and allow us to explore challenges that have so far been left untouched. At the ecosystem level, observations of the fluxes of carbon, water, and energy between the biosphere and the atmosphere across a wide range of geoecological conditions (FLUXNET) (8, 9) characterize ecosystem functions, as affected by vegetation, soil, and climate (10–13). By combining these fluxes with remote sensing information, it has become possible to scale-up, i.e., estimate the spatiotemporal variability of biosphere–atmosphere exchange at regional, continental, and global scales (14–17). At the organismic level, information on species occurrence and on plant traits has been assembled in large data bases and analyzed for functional tradeoffs (18–20).
One of today’s scientific challenges is to directly link the observations at organismic and ecosystem levels (21–23) to develop a profound understanding of biotic interactions with environmental constraints: i.e., hydrometeorological and nutritional preconditions. One fundamental question in this context is to what degree the local composition of morphological, anatomical, biochemical, or physiological features measurable at the individual plant (so-called “plant traits”) have an influence on the underlying process chains that may matter for the variation of ecosystem fluxes and properties (24). The future importance of biogeography for an integrated Earth-system science will crucially depend on its capacity to inform which plant traits (and their local composites) matter for the spatiotemporal variations of functions occurring at the ecosystem scale, in addition to, and independently of, climate and environmental factors. A biogeography of ecosystem functional properties has to explore multiple sources of information, from species-distribution maps and satellite remote-sensing data to local trait and flux observations, and foster the incorporation of biotic observations into process-based models (4, 25). An approach of this kind may allow us to scrutinize the emergent behavior of the local plant communities along with their (nonlinear) responses to, and collective feedbacks with, the environment.
In this contribution, we review recent advances of functional biogeography at the ecosystem level as achieved by the global network of biosphere–atmosphere observation and its integration with remote-sensing data and with physiological concepts. Both for assimilatory and dissimilatory functions and properties at ecosystem level, we see large global spatial and temporal variation. Climate or broad conventional vegetation types can explain only a fraction of the observed metabolic variations, indicating that we are confronted with an open scientific puzzle. We propose that trait-based biogeographical approaches will be instrumental for solving this riddle and hypothesize a number of links between vegetation (above- and below-ground) traits, with emphasis on processes that are important for the ecosystems’ carbon balance. However, we have to acknowledge that ecosystem functioning does not simply result from a linear combination of vegetation traits. Rather, we have to consider the nonlinear interactions between organisms and their traits that result in emergent behavior at the ecosystem level and apply appropriate biophysical and ecological, as well as statistical, modeling approaches. Following ref. 26, we structure the text by identifying emergent behavior at the ecosystem level and then consider the underlying processes and traits at the organismic level, followed by a perspective on how the levels can be integrated.
Biogeography and Ecosystem Functional Properties
Biogeography has been defined as the science of documenting and understanding where organisms live, at what abundance, and why (27). This science has classically concentrated on individual species but extends to the study of communities and ecosystems, which emerge from the interaction of the communities with their abiotic environment. The variation of biological structure with space has a long research tradition and is often visually accessible, leading to broad classifications of organisms (such as lifeforms after Raunkiaer) (28) and at ecosystem level (e.g., shrublands, grasslands, forests). Living organisms exchange matter, energy, and information with their environment, which is an expression of their functioning. Ecosystems exchange these quantities with the atmosphere and adjacent ecosystems including rivers, lakes, groundwater, and the subsurface. Consequently, functional biogeography may be defined as the study of the spatial and temporal distribution of the functions of living organisms and of the resulting ecosystems. The emphasis on a functional perspective in biogeography is a comparatively recent development, possibly because “ecological functioning” is harder to observe than structure, in particular at the ecosystem level. However, it is the functioning of ecosystems and organisms therein that influences the environment (e.g., climate) and provides ecosystem services to humankind (23, 29). Therefore, it is of paramount interest to understand how organisms and environmental conditions coshape the variation of ecosystem functions in space and time.
Today considerable progress has been made to establish observing systems and databases to characterize the geographical variation of functioning at both organismic and ecosystem levels, as briefly described in SI Appendix, Fig. S1 and related text. Ecosystem-level quantities are derived from flux and biometric observations, which allow a better characterization and understanding of the ecosystems. Often these quantities are analogous to ecophysiological leaf-level characteristics such as (intrinsic) water-use efficiency, leaf conductance, light-use efficiency, or light-saturated photosynthetic CO2 uptake whereas others relate to physical and ecohydrological characteristics important for land surface–atmosphere interaction (e.g., aerodynamic and surface conductances, albedo, evaporative fraction) (30–32). In addition, and very important for the whole-ecosystem carbon balance, quantities are being explored that entail respiration fluxes and carbon pools (carbon-use efficiency, carbon-turnover times) (33). We define such “ecosystem functional properties” as quantities that characterize ecosystem processes and responses in an integrated and comparable manner. As we will show here, the spatial and temporal variation of ecosystem functional properties is largely unexplained by classical approaches to vegetation (e.g., plant functional types) and remains a major functional biogeographical puzzle to solve, with a trait-based approach being a promising avenue.
Global Functional Biogeographical Knowledge and Questions from Ecosystem-Level Observations
Data-Driven Up-Scaling Approaches.
Site-level measurements of ecosystem–atmosphere exchange, albeit covering footprints between ∼104 to 106 m2, essentially remain point measurements from a global Earth-system perspective. However, combining these flux observations with information from remote-sensing and gridded meteorological drivers via statistical machine-learning approaches (data-driven up-scaling) has allowed us to infer continental-to-global fields of ecosystem functions [gross primary production (GPP) and evapotranspiration (ET)] in recent years (11, 12, 16, 17, 34–36). The seasonal and spatial variation of quantities such as GPP, ET, and sensible heat flux (H) can be estimated with very good performance (r2 > 0.6) as shown in cross-validation exercises (17). Therefore, these fields are used to evaluate whether global carbon-cycle models show the same global behavior as the up-scaled observations (4, 14).
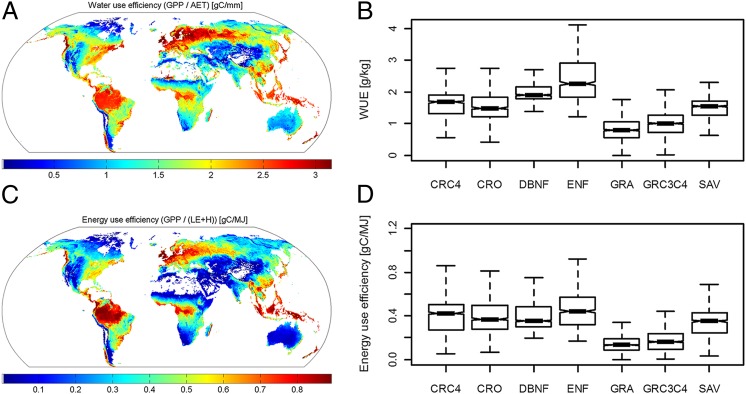
Further research should analyze the functional properties of the ecosystems, which essentially govern the response of ecosystems to changing environmental conditions. Generating such functional properties from globally up-scaled flux fields yields very distinct spatial patterns (Fig. 1) even though these properties are simply derived from annual flux integrals and abstract from temporal variation. For example, the high water-use efficiency (carbon taken up per water transpired) in areas where closed-canopy forests dominate, in the boreal, temperate, and tropical biomes, is evident (Fig. 1A) and corresponds broadly with a high energy-use efficiency (fraction of solar energy converted to chemical energy in photosynthesis). However, within the biomes, the covariation between water and energy-use efficiency is less or even negative (e.g., compare Fig. 1 A and B in the boreal zone). These subtle patterns are propagated to the evaporative fraction (fraction of energy passed to the atmosphere as latent heat) (compare SI Appendix, Fig. S2A). These interrelated ecosystem functional properties can be derived only from the multiple synchronous biogeochemical and biophysical fluxes observed at, and up-scaled from, FLUXNET sites (10, 17) and provide multiple constraints, e.g., to Earth-system models. However, it remains an open question which ecoclimatic and organism-level functional biogeographical patterns underlie the variability of these ecosystem properties. Clearly, there are differences between vegetation types, but the within-vegetation type variability is notable (Fig. 1 B and D and SI Appendix, Fig. S2B). To what extent species identity and plant traits contribute to explain this variability of ecosystem functional properties is an upcoming research topic in functional biogeography research, which will extend results emerging from existing studies on biodiversity–productivity relationships (37) and help their interpretation by integrating more physiological characterizations at the ecosystem level (Fig. 1).
Fig. 1.
Globally distributed ecosystem functional properties derived from integrating FLUXNET, remote sensing, and climate data (A and C) and their within- and between-vegetation type variation for selected vegetation types (B and D). The boxplots show minimum, 25th, 50th, and 75th percentiles, and maximum of the data. The notches approximate the 95% confidence interval for the median [(compare R documentation (87)]. CRO, cropland; CRC4, C4 crops; DBNF, deciduous broad-leaved and needle-leaved forests; ENF, evergreen needle-leaved forest; GRA, C3 grassland; GRC3C4, C3-C4 mixed grassland; SAV, savannah. Computed from Jung et al. (17).
Between-Site and Temporal Patterns of Ecosystem Functional Properties.
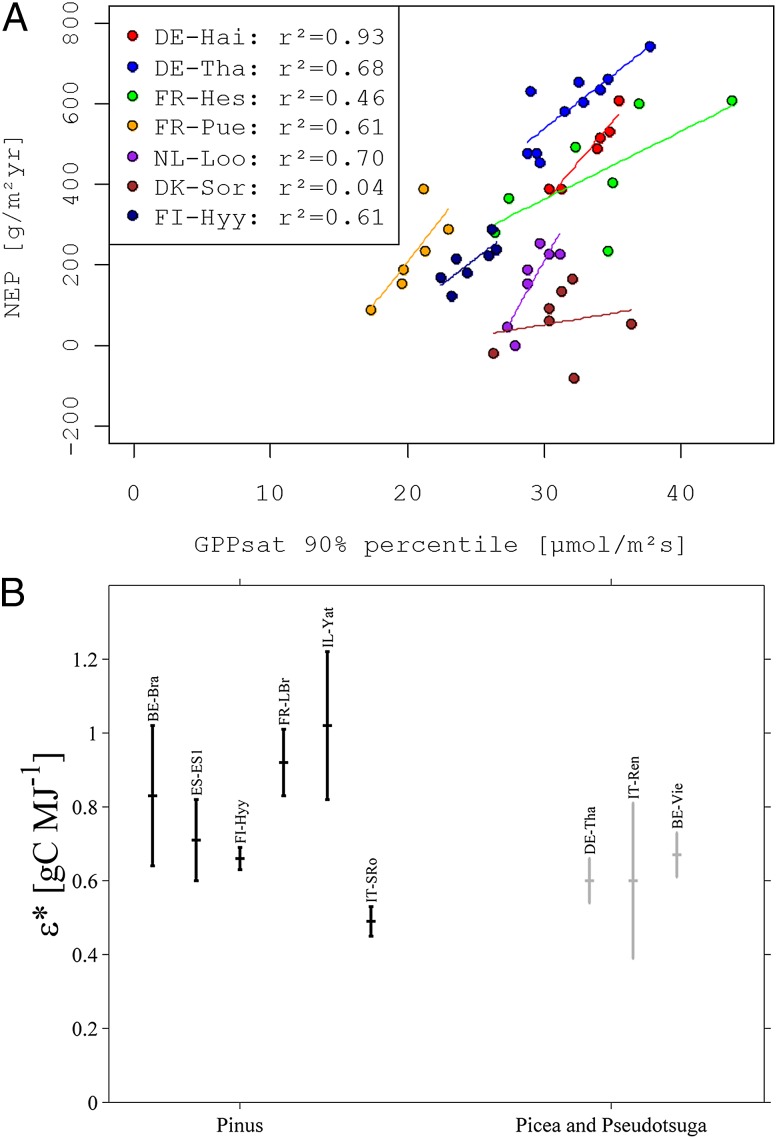
As mentioned in the introduction, the global network of ecosystem observations (FLUXNET) is able to characterize whole-ecosystem behavior or function in terms of their exchange of matter and energy with the atmosphere and its variation in space and time. Whole-ecosystem functional properties can be derived from FLUXNET data by fitting response curves to light, temperature, and vapor-pressure deficit (38–40). Light-saturated gross primary production per ground area (GPPsat), for instance, can be seen as an analog to light-saturated photosynthesis (Amax, also per area) at leaf level (41, 42), which is recorded in trait databases. Ecosystem-level analysis of European FLUXNET data indicates that interannual variation of the net carbon balance (NEP) is significantly related to peak annual GPPsat (Fig. 2A). This ecosystem physiological property correlates more strongly than any climate variable (and even more strongly than peak GPP flux) with annual NEP. This correlation emphasizes how important ecosystem-internal variation of physiology is for the variation of ecosystem functions (here, net carbon fluxes). However, this whole-ecosystem physiological property in turn depends on ecosystem structure and (leaf-level) function because, at the ecosystem level, the light-saturated GPP depends on the fraction of light absorbed (fAPAR) and the efficiency with which the absorbed energy is converted to chemical energy (RUE). This dependency can be easily visualized with the conceptually simple radiation use-efficiency model
| [1] |
where PAR is the photosynthetically active radiation. Also, more complex multilayer soil-vegetation-atmosphere transfer models, where GPP scales with photosynthetic carboxylation capacity (Vcmax) and leaf area index (LAI), show this behavior (43). Nevertheless, neither data-driven models nor process-based models that incorporate only ecosystem structural and climatic effects explain much of the interannual variability of carbon fluxes at ecosystem observation sites (17, 44), suggesting again that interannual variation of ecosystem physiology (for instance, carry-over effects of stress years) governs an important part of the variability (45–47).
Fig. 2.
(A) Covariation of net ecosystem production (NEP) with peak light-saturated GPP (90%ile) at seven European long-term observation sites [(data from Lasslop et al. (40)]. (B) Within- and between-species variation of the Carnegie–Ames–Stanford Approach (CASA) model parameter “maximum light-use efficiency” inferred with a model inversion approach [error bars indicate SEs; data from Carvalhais et al. (48)]. Compare SI Appendix for more details on analysis and sites.
By combining flux measurements with remotely sensed fAPAR and a simple radiation-use efficiency model (as in Eq. 1) with a daily time step, one can infer the ecosystem parameter (RUE), which is independent of the ecosystem structure embedded in the fAPAR observation (48, 49). Such an ecosystem-level parameter links more strongly to ecophysiological parameters in global Earth-system models (e.g., Vcmax and Jmax in the Farquhar model) (50, 51). Classically, the ecophysiological parameters in global vegetation models are assumed constant per each plant functional type (PFT). These functional types are most often defined according to leaf habit (broadleaf, needle leaf), longevity (evergreen, deciduous), and life form (tree, shrub, grass, crop) and relate to vegetation type at the ecosystem level, which are defined based on the dominating PFTs (e.g., evergreen broadleaf forest). Parameterization strategies relying on FLUXNET data offer the chance to analyze the between- and within-vegetation type variability of the essential ecosystem parameters. Fig. 2B shows that there is considerable variation within vegetation types (here, evergreen needle leaf forest). It remains a challenge to explain this between-site variability. The within-vegetation type variability is partly, although not solely, dependent on species and should be able to be traced back to the traits of the dominant individuals of the ecosystem.
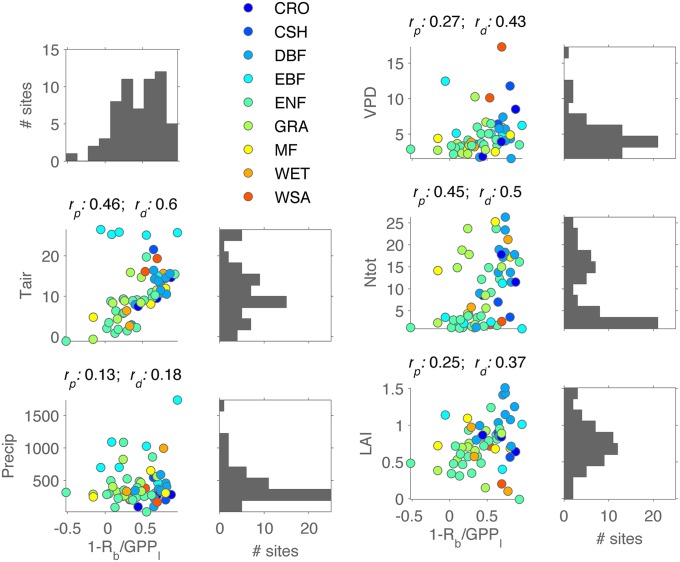
Obviously, the carbon balance of an ecosystem is determined not only by the variation of GPP but with the same importance by processes that determine the mean residence time of the assimilated carbon in the system: for instance, allocation and respiration of carbon, as well as disturbance events, such as fires, that lead to rapid release of carbon (52). Mahecha et al. (33) have found a convergence of the temperature sensitivity of ecosystem respiration. However, they found a considerable variation in the seasonal change of carbon respired versus carbon taken up: i.e., the seasonal carbon-use efficiency. In other words, some ecosystems respire a large proportion of the assimilated carbon within one season whereas others tend to store it. Fig. 3 shows that little of this variability can be explained by climate or conventional site characteristics. The question is whether the seasonal carbon-use efficiency belongs to a syndrome of ecosystem functional properties that might be underlying control by plant, faunal, and microbial traits.
Fig. 3.
Seasonal carbon-use efficiency after Mahecha et al. (see figure 3 and online supporting material in ref. 33) in relation to other ecosystem and climatic characteristics at FLUXNET sites. Shown are histograms of each variable across all sites and their relation with the seasonal carbon-use efficiency. On top of the panels, the (linear) Pearson correlation coefficient and the distance correlation coefficient are shown.
The Potential of Plant Traits for Explaining Ecosystem Functional Properties
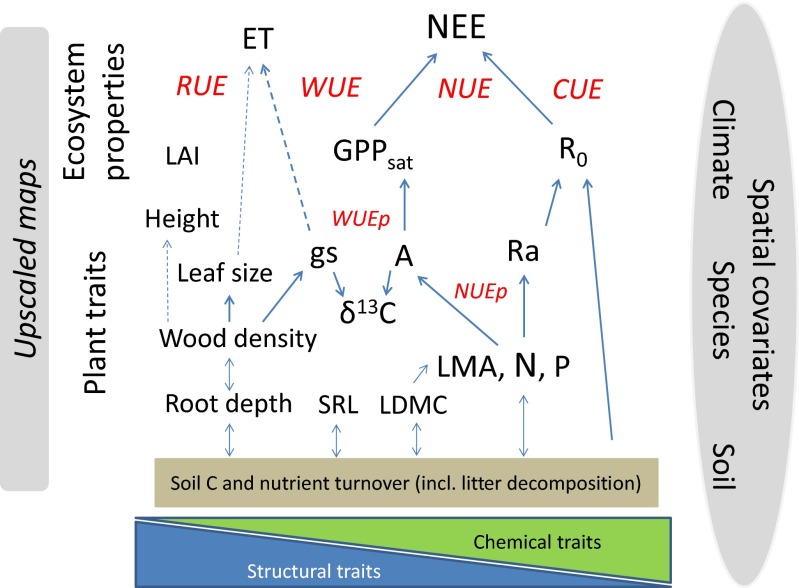
The role of plant traits as important determinants of ecosystem functioning was recognized early on (29). Ultimately, the concept of plant functional types is based on the observation that different trait syndromes result in distinct broad differences in ecosystem functioning. However, the large within-PFT variability of both biogeochemical processes (compare the previous section) and trait values (18) calls for exploring the joint patterns between these two sets of observation. It has been demonstrated that, across different PFTs and biomes, there is a convergence in the relationships of chemical, structural, and physiological leaf traits [the “leaf economics spectrum” (LES)] (20). While the LES includes some variation, partly explained by climate, it is robust in permitting the prediction of leaf physiological parameters based on leaf nitrogen concentrations and leaf mass per area (19, 53). Note, however, that leaf mass per area (LMA) has recently been suggested to reflect primarily its functional relationship with leaf lifespan and biases the correlation of leaf traits, which are functionally often area-proportional (54). Similarly, wood and root N concentrations (and root lifespans) are closely related to wood and root respiration rates (55, 56) although the slopes may differ between functional groups (e.g., grasses versus legumes) (57). Thus, ecosystem properties, such as GPPsat and the autotrophic component of ecosystem respiration, can be in principle derived from plant traits (Fig. 4).
Fig. 4.
Major relationships between plant structural and chemical–physiological traits and ecosystem functional properties related to carbon and water fluxes, embedded in an upscaling framework considering spatial covariates. Tradeoffs related to water-use (WUE), nitrogen-use (NUE), radiation-use (RUE), and carbon-use (CUE) efficiencies at plant (p) and ecosystem scale are printed in italics in red. Note that soil C and nutrient turnover processes are also important ecosystem properties affecting carbon fluxes directly via heterotrophic soil respiration and indirectly via effects of nutrient availability on plant functional traits and ecosystem structure. A, photosynthetic capacity; δ13C, stable carbon isotope ratio; ET, evapotranspiration; GPPsat, gross primary productivity at saturating light; gs, maximum stomatal conductance; LAI, leaf area index; LDMC, leaf dry matter content; LMA, leaf mass per area; N, tissue nitrogen concentration; NEE, net ecosystem exchange of CO2; P, tissue phosphorus concentration; R0, ecosystem respiration at reference temperature; Ra, plant respiration; SRL, specific root length.
The heterotrophic component of ecosystem respiration is more difficult to approximate from plant traits. However, litter decomposition, which contributes substantially to heterotrophic respiration in many ecosystems, can be well-predicted from plant traits such as C, N, dry matter content, and lignin (58–60). Different organs (leaves, stems, roots) exhibit a different, although apparently coordinated, behavior (“plant economics spectrum”) (61). Under similar climates, wood decomposition is also well-correlated with nitrogen and phosphorus concentrations and C/N ratios, with gymnosperms decomposing more slowly than angiosperms (62). Through effects on litter quality, plant traits have also been suggested as important drivers for microbial community composition and shifts between more strongly saprophytic-based to more strongly symbiotic-based C cycling and thus soil organic carbon stabilization (63). Root traits, such as specific length, tissue density, and diameter, are important determinants of soil C turnover (64) and thus may contribute to the variation of carbon-use efficiency (Figs. 3 and 4). Root traits should thus also influence soil N turnover processes although this topic has so far received little attention. By changing tissue N concentrations and allocation patterns, changes in N availability will likely impact plant and ecosystem properties associated with the carbon cycle (compare to this section, above, and Fig. 4). An interesting trait reflecting long-term changes in N availability is the stable N isotope ratio of plant tissues (δ15N) (65) as, e.g., obtained from wood samples or herbarium specimens of leaves (66, 67).
Plant traits related to the water cycle include those affecting plant water uptake (rooting depth, root mass, and specific root length), transport (hydraulic properties), storage, and loss (stomatal and cuticular conductance, leaf size) and thus should strongly link to the spatial variation found in Fig. 1A. At the leaf, plant, and ecosystem scale, water-use efficiency (WUE) can be derived from the carbon-isotope signatures δ13C of plant tissues (e.g., leaves, tree rings), which integrate WUE from daily to decadal timescales (68, 69). For trees, wood density has been found to be an important and well-documented integrative trait related to hydraulic conductivity, leaf traits (including photosynthetic ones), and patterns of water uptake and transpiration (70) and is thus potentially a good proxy for water use and its constraints on the carbon cycle (Fig. 4). Wood density has also been suggested as an indicator of drought resistance as it is positively related to cavitation resistance (71). However, a recent study indicated that, under an extreme drought, species compensated low wood density by drought deciduousness, higher sensitivity of stomata to leaf water potential, and possibly also greater rooting depth (72). Thus, wood density should not be used without considering other traits when predicting resistance and resilience to extreme drought. Similarly, whereas in all biomes trees typically operate at a narrow safety margin for vulnerability to drought (73), species may differ in their strategy of drought avoidance and their capacity of xylem refilling (74). This observation not only reinforces the notion that drought effects on the carbon cycle may be better captured by a combination of plant traits, but also suggests that, in multispecies forests, different species may respond at different thresholds and thus may potentially increase the resistance and resilience of ecosystem processes to extreme events.
Although it has been argued that functional diversity could be the most relevant measure for ecosystem productivity and stability (75), there is still very limited empirical evidence demonstrating the role of functional trait diversity (which could account for both inter- and intraspecific variation) (76, 77). Clearly, targeted studies are needed to explicitly test for such relationships, as well as the effect of species identity or keystone species. Different levels of phylogenetic and spatial integration should be addressed by including specific site-level information and data from trait databases (SI Appendix, Fig. S3).
The Ecosystem Is More than the Sum of Traits: Scale Emergent Properties at the Ecosystem Level
The advantage of investigating data-driven linkages between vegetation traits and ecosystem functional behavior is its directness. However, one should not always expect a simple relation between ecosystem functions and traits at the organismic level (such as the former being a simple linear combination of the latter) because ecosystems are complex dynamic and adaptive systems that may experience scale emergent properties when one transcends scales (78). For example, the reflectivity of a leaf is not the same as the albedo of a canopy (79). Forest canopies are effective at trapping light so they may appear much darker (at 400- to 800-nm wavelengths) than the reflectivity of individual leaves indicates. Nor is the light-use efficiency of a leaf the same as of a canopy (80, 81). The light-response curve of individual plant canopies ranges from being linear for well-watered crops that form a closed canopy and have high nitrogen inputs (82) to being curvilinear with open canopies and those limited in nitrogen resources (80). Similarly to leaves that interact regarding the transfer of radiation through the canopy in a way that needs explicit scaling, other ecosystem processes emerge from interactions between individuals given their specific traits. For example, how strongly physical traits such as leaf toughness will influence the mean-residence time (and thus long-term accumulation) of carbon will depend on the existence of a decomposer community that might be specialized to digest such material. Often in these contexts, biological symbioses are crucially determining the cycling of elements. Examples include termite–microbial symbiosis, without which decomposition of lignin would be much retarded in tropical savannahs, or the influence of ruminant–microbial symbiosis for digesting lichens in high-latitude ecosystems. Similarly, a trait related to fast resprouting may be very important for ecosystem carbon dynamics in a frequently disturbed system, but not necessarily in a more stable environment. Therefore, traits are a necessary but not sufficient condition to understand the ecosystem carbon balance. It is needed to explicitly explore and model the interactions of individuals and to compare the emerging behavior against observed ecosystem functions varying in time and across sites. For an approach applicable at global scale, see ref. 83.
Outlook
We have shown that, based on ecosystem-level observations, we detect large functional biogeographical variation of ecosystem properties, which can only partially by explained by climate and classical vegetation approaches such as plant functional types. Although the gross carbon uptake by vegetation depends on the interactions between leaves generating the microclimate in the canopy, the processing of the photosynthetically fixed carbon depends even more on biological processes that are controlled by traits, including allocation, respiration and decomposition, and stabilization of carbon in the soil. Overall, in the biogeochemical Earth-system context, the challenge is to explore classical biogeographical questions from a functional perspective: The question how to explain spatial patterns of species distribution and diversity has to be rephrased as: What are the determinants and biogeochemical consequences of plant functional attribute and trait variation? How do the inter- and intraspecific variability of plant traits depend on environmental constraints and affect the derived ecosystem functional properties beyond productivity? How are response traits and effect traits coupled with ecosystem functional properties? Can a combined approach of up-scaled traits and down-scaled fluxes help quantifying emergent properties across vegetation types and biogeographic regions? Note that such emergent properties could comprise, e.g., nonadditive effects and/or effects induced by the canopy structure and related microclimatic conditions (76, 84). What is the role of functional diversity for ecosystem functional properties and their resistance and resilience to disturbance and long-term environmental changes?
To our mind, a crucial criterion for future progress in this direction will be whether a stronger integration of largely disjoint activities will be achieved. For example, hypotheses regarding the link between organismic traits and ecosystem flux-derived whole-ecosystem properties can be best tested if the respective observations are made at the same sites. Remote-sensing approaches that have been successful for characterizing ecosystem structural parameters (e.g., leaf area index) and their effect on ecosystem functions need to be extended to detecting plant traits and their diversity. It has recently been demonstrated that hyperspectral remote sensing can be a powerful tool for mapping trait syndromes, which could be used for monitoring functional shifts in ecosystems (85). Finally, trait-based modeling approaches should be linked to the observations via model–data fusion approaches, where the biosphere model parameters (i.e., traits) can be constrained from the ecosystem-level observation via model inversion and compared with observed organismic traits. Once the mechanistic scaling of trait effects on ecosystem functions and response to climate has been successful, such a model would go beyond the projection of species and trait maps under changing conditions and can inform dynamic Earth-system models to calculate effects of changing climate and land use on ecosystem–atmosphere carbon and energy exchange and related feedbacks (86).
Supplementary Material
Acknowledgments
We thank Dario Papale, Martin Jung, and Andrew Richardson for valuable discussions on the topic and Dorothea Frank and Natalia Ungelenk for critical reading and assistance with the literature. We thank Andrew Richardson for his kind, additional language and style check.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216065111/-/DCSupplemental.
References
- 1.Bonan GB. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science. 2008;320(5882):1444–1449. doi: 10.1126/science.1155121. [DOI] [PubMed] [Google Scholar]
- 2.Arneth A, et al. Terrestrial biogeochemical feedbacks in the climate system. Nat Geosci. 2010;3(8):525–532. [Google Scholar]
- 3.Heimann M, Reichstein M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature. 2008;451(7176):289–292. doi: 10.1038/nature06591. [DOI] [PubMed] [Google Scholar]
- 4.Bonan GB, et al. Improving canopy processes in the Community Land Model version 4 (CLM4) using global flux fields empirically inferred from FLUXNET data. J Geophys Res Biogeosci. 2011;116:G02014. [Google Scholar]
- 5.Moorcroft PR. How close are we to a predictive science of the biosphere? Trends Ecol Evol. 2006;21(7):400–407. doi: 10.1016/j.tree.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Prentice IC, Heimann M, Sitch S. The carbon balance of the terrestrial biosphere: Ecosystem models and atmospheric observations. Ecol Appl. 2000;10(6):1553–1573. [Google Scholar]
- 7.Hampton SE, et al. Big data and the future of ecology. Front Ecol Environ. 2013;11(3):156–162. [Google Scholar]
- 8.Baldocchi D, et al. FLUXNET: A new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bull Am Meteorol Soc. 2001;82(11):2415–2434. [Google Scholar]
- 9.Baldocchi D. 'Breathing' of the terrestrial biosphere: Lessons learned from a global network of carbon dioxide flux measurement systems. Aust J Bot. 2008;56(1):1–26. [Google Scholar]
- 10.Law BE, et al. Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric For Meteorol. 2012;113(1-4):97–120. [Google Scholar]
- 11.Reichstein M, et al. Reduction of ecosystem productivity and respiration during the European summer 2003 climate anomaly: A joint flux tower, remote sensing and modelling analysis. Glob Change Biol. 2007a;13(3):634–651. [Google Scholar]
- 12.Reichstein M, et al. Determinants of terrestrial ecosystem carbon balance inferred from European eddy covariance flux sites. Geophys Res Lett. 2007b;34:L01402. [Google Scholar]
- 13.Williams CA, et al. Climate and vegetation controls on the surface water balance: Synthesis of evapotranspiration measured across a global network of flux towers. Water Resour Res. 2012;48:W06523. [Google Scholar]
- 14.Beer C, et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science. 2010;329(5993):834–838. doi: 10.1126/science.1184984. [DOI] [PubMed] [Google Scholar]
- 15.Jung M, Reichstein M, Bondeau A. Towards global empirical upscaling of FLUXNET eddy covariance observations: Validation of a model tree ensemble approach using a biosphere model. Biogeosciences. 2009;6:2001–2013. [Google Scholar]
- 16.Jung M, et al. Recent decline in the global land evapotranspiration trend due to limited moisture supply. Nature. 2010;467(7318):951–954. doi: 10.1038/nature09396. [DOI] [PubMed] [Google Scholar]
- 17.Jung M, et al. Global patterns of land-atmosphere fluxes of carbon dioxide, latent heat, and sensible heat derived from eddy covariance, satellite, and meteorological observations. J Geophys Res Biogeosci. 2011;116:G00J07. [Google Scholar]
- 18.Kattge J, et al. TRY: A global database of plant traits. Glob Change Biol. 2011;17:2905–2935. [Google Scholar]
- 19.Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: Global convergence in plant functioning. Proc Natl Acad Sci USA. 1997;94(25):13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428(6985):821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 21.Violle C, et al. Let the concept of trait be functional! Oikos. 2007;116(5):882–892. [Google Scholar]
- 22.Díaz S, et al. Incorporating plant functional diversity effects in ecosystem service assessments. Proc Natl Acad Sci USA. 2007;104(52):20684–20689. doi: 10.1073/pnas.0704716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Bello F, et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers Conserv. 2010;19(10):2873–2893. [Google Scholar]
- 24.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct Ecol. 2002;16(5):545–556. [Google Scholar]
- 25.Ryu Y, et al. Integration of MODIS land and atmosphere products with a coupled-process model to estimate gross primary productivity and evapotranspiration from 1 km to global scales. Global Biogeochem Cycles. 2011;25:GB4017. [Google Scholar]
- 26.Reichstein M, Beer C. Soil respiration across scales: The importance of a model-data integration framework for data interpretation. J Plant Nutr Soil Sci. 2008;171(3):344–354. [Google Scholar]
- 27.Logue JB. Empirical approaches to metacommunities: A review and comparison with theory. Trends Ecol & Evol. 2010;26(9):482–491. doi: 10.1016/j.tree.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Raunkiaer C. Om biologiske Typer, med Hensyn til Planternes Tilpasninger til at overleve ugunstige Aarstider. Botanisk Tidsskrift. 1904;26:XIV. [Google Scholar]
- 29.Chapin FS, 3rd, et al. Consequences of changing biodiversity. Nature. 2000;405(6783):234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- 30.Kelliher FM, Leuning R, Raupach MR, Schulze E-D. Maximum conductances for evaporation from global vegetation types. Agric For Meteorol. 1994;73:1–16. [Google Scholar]
- 31.Owen KE, et al. Linking flux network measurements to continental scale simulations: Ecosystem CO2 exchange capacity under non-water-stressed conditions. Glob Change Biol. 2007;13:734–760. [Google Scholar]
- 32.Beer C, et al. Temporal and among-site variability of inherent water use efficiency at the ecosystem level. Global Biogeochem Cycles. 2009;23:GB2018. [Google Scholar]
- 33.Mahecha MD, et al. Global convergence in the temperature sensitivity of respiration at ecosystem level. Science. 2010;329(5993):838–840. doi: 10.1126/science.1189587. [DOI] [PubMed] [Google Scholar]
- 34.Papale D, Valentini R. A new assessment of European forests carbon exchanges by eddy fluxes and artificial neural network spatialization. Glob Change Biol. 2003;9:525–535. [Google Scholar]
- 35.Xiao J, et al. Estimation of net ecosystem carbon exchange for the conterminous United States by combining MODIS and AmeriFlux data. Agr Forest Meteorol. 2008;148:1827–1847. [Google Scholar]
- 36.Ichii K, et al. Multi-model analysis of terrestrial carbon cycles in Japan: Limitations and implications of model calibration using eddy flux observations. Biogeosciences. 2010;7(7):2061–2080. [Google Scholar]
- 37.Paquette A, Messier C. The effect of biodiversity on tree productivity: From temperate to boreal forests. Glob Ecol Biogeogr. 2011;20(1):170–180. [Google Scholar]
- 38.Hollinger DY. Optimality and nitrogen allocation in a tree canopy. Tree Physiol. 1996;16(7):627–634. doi: 10.1093/treephys/16.7.627. [DOI] [PubMed] [Google Scholar]
- 39.Gilmanov TG, et al. Partitioning European grassland net ecosystem CO2 exchange into gross primary productivity and ecosystem respiration using light response function analysis. Agric Ecosyst Environ. 2007;121(1-2):93–120. [Google Scholar]
- 40.Lasslop G, et al. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: Critical issues and global evaluation. Glob Change Biol. 2010;16:187–208. [Google Scholar]
- 41.Ollinger SV, et al. Canopy nitrogen, carbon assimilation, and albedo in temperate and boreal forests: Functional relations and potential climate feedbacks. Proc Natl Acad Sci USA. 2008;105(49):19336–19341. doi: 10.1073/pnas.0810021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore DJP, Hu J, Sacks WJ, Schimel DS, Monson RK. Estimating transpiration and the sensitivity of carbon uptake to water availability in a subalpine forest using a simple ecosystem process model informed by measured net CO2 and H2O fluxes. Agric For Meteorol. 2008;148(10):1467–1477. [Google Scholar]
- 43.Baldocchi D, Meyers T. On using eco-physiological, micrometeorological and biogeochemical theory to evaluate carbon dioxide, water vapor and trace gas fluxes over vegetation: a perspective. Agric For Meteorol. 1998;90(1-2):1–25. [Google Scholar]
- 44.Keenan TF, Davidson EA, Moffat AM, Munger W, Richardson AD. Using model-data fusion to interpret past trends, and quantify uncertainties in future projections, of terrestrial ecosystem carbon cycling. Glob Change Biol. 2012;18(8):2555–2569. [Google Scholar]
- 45.Owen KE, et al. Linking flux network measurements to continental scale simulations: ecosystem carbon dioxide exchange capacity under non-water-stressed conditions. Glob Change Biol. 2007;13(4):734–760. [Google Scholar]
- 46.Richardson AD, Hollinger DY, Aber JD, Ollinger SV, Braswell BH. Environmental variation is directly responsible for short- but not long-term variation in forest-atmosphere carbon exchange. Glob Change Biol. 2007;13(4):788–803. [Google Scholar]
- 47.Wu J, et al. Effects of climate variability and functional changes on the interannual variation of the carbon balance in a temperate deciduous forest. Biogeosciences. 2012;9:13–28. [Google Scholar]
- 48.Carvalhais N, et al. Implications of carbon cycle steady state assumptions for biogeochemical modeling performance and inverse parameter retrieval. Global Biogeochem Cycles. 2008;22(2):GB2007. [Google Scholar]
- 49.Garbulsky MF, et al. Patterns and controls of the variability of radiation use efficiency and primary productivity across terrestrial ecosystems. Glob Ecol Biogeogr. 2010;19(2):253–267. [Google Scholar]
- 50.Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol. 1982;33:317–345. [Google Scholar]
- 51.Farquhar GD, von Caemmerer S. Modelling of photosynthetic response to environmental conditions. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, Jr., editors. Physiological Plant Ecology II, Encyclopedia of Plant Physiology: New Series. Vol 12B. Berlin: Springer; 1982. pp. 549–587. [Google Scholar]
- 52.Körner C. Atmospheric science. Slow in, rapid out—carbon flux studies and Kyoto targets. Science. 2003;300(5623):1242–1243. doi: 10.1126/science.1084460. [DOI] [PubMed] [Google Scholar]
- 53.Wright IJ, et al. Modulation of leaf economic traits and trait relationships by climate. Glob Ecol Biogeogr. 2005;14(5):411–421. [Google Scholar]
- 54.Osnas JLD, Lichstein JW, Reich PB, Pacala SW. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science. 2013;340(6133):741–744. doi: 10.1126/science.1231574. [DOI] [PubMed] [Google Scholar]
- 55.Burton AJ, Pregitzer KS, Ruess RW, Hendrik RL, Allen MF. Root respiration in North American forests: Effects of nitrogen concentration and temperature across biomes. Oecologia. 2002;131(4):559–568. doi: 10.1007/s00442-002-0931-7. [DOI] [PubMed] [Google Scholar]
- 56.Reich PB, et al. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol Lett. 2008;11(8):793–801. doi: 10.1111/j.1461-0248.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 57.Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol. 2005;167(2):493–508. doi: 10.1111/j.1469-8137.2005.01428.x. [DOI] [PubMed] [Google Scholar]
- 58.Garnier E, et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85(9):2630–2637. [Google Scholar]
- 59.Cornwell WK, et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett. 2008;11(10):1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- 60.Fortunel C, et al. Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology. 2009;90(3):598–611. doi: 10.1890/08-0418.1. [DOI] [PubMed] [Google Scholar]
- 61.Freschet GT, Aerts R, Cornelissen JHC. A plant economics spectrum of litter decomposability. Funct Ecol. 2012;26(1):56–65. [Google Scholar]
- 62.Weedon JT, et al. Global meta-analysis of wood decomposition rates: A role for trait variation among tree species? Ecol Lett. 2009;12(1):45–56. doi: 10.1111/j.1461-0248.2008.01259.x. [DOI] [PubMed] [Google Scholar]
- 63.De Deyn GB, Cornelissen JHC, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett. 2008;11(5):516–531. doi: 10.1111/j.1461-0248.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 64.Klumpp K, Soussana J-F. Using functional traits to predict grassland ecosystem change: A mathematical test of the response-and-effect trait approach. Glob Change Biol. 2009;15(12):2921–2934. [Google Scholar]
- 65.Hogberg P. Tansley review no. 95: 15N natural abundance in soil–plant systems. New Phytol. 1997;137(2):179–203. doi: 10.1046/j.1469-8137.1997.00808.x. [DOI] [PubMed] [Google Scholar]
- 66.Craine JM, et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009;183(4):980–992. doi: 10.1111/j.1469-8137.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 67.McLauchlan KK, Ferguson CJ, Wilson IE, Ocheltree TW, Craine JM. Thirteen decades of foliar isotopes indicate declining nitrogen availability in central North American grasslands. New Phytol. 2010;187(4):1135–1145. doi: 10.1111/j.1469-8137.2010.03322.x. [DOI] [PubMed] [Google Scholar]
- 68.Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol. 1984;11(6):539–552. [Google Scholar]
- 69.Werner C, et al. Progress and challenges in using stable isotopes to trace plant carbon and water relations across scales. Biogeosci. 2012;9:3083–3111. [Google Scholar]
- 70.Chave J, et al. Towards a worldwide wood economics spectrum. Ecol Lett. 2009;12(4):351–366. doi: 10.1111/j.1461-0248.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- 71.Jacobsen AL, Pratt RB, Davis SD, Ewers FW. Cavitation resistance and seasonal hydraulics differ among three arid Californian plant communities. Plant Cell Environ. 2007;30(12):1599–1609. doi: 10.1111/j.1365-3040.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- 72.Hoffmann WA, Marchin RM, Abit P, Lau OL. Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Glob Change Biol. 2011;17(8):2731–2742. [Google Scholar]
- 73.Choat B, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491(7426):752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- 74.Klein T, Yakir D, Buchmann N, Grünzweig JM. Towards an advanced assessment of the hydrological vulnerability of forests to climate change-induced drought. New Phytol. 2014;201(3):712–716. doi: 10.1111/nph.12548. [DOI] [PubMed] [Google Scholar]
- 75.Hooper D, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75(1):3–35. [Google Scholar]
- 76.Cadotte MW, Cavender-Bares J, Tilman D, Oakley TH. Using phylogenetic, functional and trait diversity to understand patterns of plant community productivity. PLoS ONE. 2009;4(5):e5695. doi: 10.1371/journal.pone.0005695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Messier J, McGill BJ, Lechowicz MJ. How do traits vary across ecological scales? A case for trait-based ecology. Ecol Lett. 2010;13(7):838–848. doi: 10.1111/j.1461-0248.2010.01476.x. [DOI] [PubMed] [Google Scholar]
- 78.Levin SA. In: Highlights of Mathematical Physics. Fokas A, Halliwell J, Kibble T, Zegarlinski B, editors. Providence, RI: American Mathematical Society; 2002. pp. 209–213. [Google Scholar]
- 79.Hollinger DY, et al. Albedo estimates for land surface models and support for a new paradigm based on foliage nitrogen concentration. Glob Change Biol. 2010;16(2):696–710. [Google Scholar]
- 80.Baldocchi DD, Amthor JS. In: Terrestrial Global Productivity. Roy J, Saugier B, Mooney HA, editors. San Diego: Academic; 2001. pp. 245–283. [Google Scholar]
- 81.Knohl A, Baldocchi DD. Effects of diffuse radiation on canopy gas exchange processes in a forest ecosystem. J Geophys Res Biogeosci. 2008;113(G2):G02023. [Google Scholar]
- 82.Monteith JL. Climate and efficiency of crop production in Britain. Philos Trans R Soc Lond B Biol Sci. 1977;281(980):277–294. [Google Scholar]
- 83.Scheiter S, Langan L, Higgins SI. Next-generation dynamic global vegetation models: Learning from community ecology. New Phytol. 2013;198(3):957–969. doi: 10.1111/nph.12210. [DOI] [PubMed] [Google Scholar]
- 84.Gartner TB, Cardon ZG. Decomposition dynamics in mixed-species leaf litter. Oikos. 2004;104(2):230–246. [Google Scholar]
- 85.Schmidtlein S, Feilhauer H, Bruelheide H. Mapping plant strategy types using remote sensing. J Veg Sci. 2012;23(3):395–405. [Google Scholar]
- 86.Van Bodegom PM, et al. Going beyond limitations of plant functional types when predicting global ecosystem-atmosphere fluxes: Exploring the merits of traits-based approaches. Glob Ecol Biogeogr. 2012;21(6):625–636. [Google Scholar]
- 87. R Development Core Team (2010) R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.