Significance
We have observed more than 200 specimens of Anchiornis, the earliest known feathered dinosaur, and nearly 100 specimens of Sapeornis, one of the basalmost birds, and recognize no sternal ossifications. We propose that the sternum may have been completely lost in these two taxa (and Archaeopteryx as well) based on histological analysis and the excellent preservation of soft-tissue structures, thus suggesting the absence of a sternum could represent the plesiomorphic avian condition. Our discovery reveals an unexpected level of complexity and high degree of inherent developmental plasticity in the early evolution of the avian sternum.
Keywords: Maniraptora, Mesozoic, Jehol, flight, histology
Abstract
Anchiornis (Deinonychosauria: Troodontidae), the earliest known feathered dinosaur, and Sapeornis (Aves: Pygostylia), one of the basalmost Cretaceous birds, are both known from hundreds of specimens, although remarkably not one specimen preserves any sternal ossifications. We use histological analysis to confirm the absence of this element in adult specimens. Furthermore, the excellent preservation of soft-tissue structures in some specimens suggests that no chondrified sternum was present. Archaeopteryx, the oldest and most basal known bird, is known from only 10 specimens and the presence of a sternum is controversial; a chondrified sternum is widely considered to have been present. However, data from Anchiornis and Sapeornis suggest that a sternum may also have been completely absent in this important taxon, suggesting that the absence of a sternum could represent the plesiomorphic avian condition. Our discovery reveals an unexpected level of complexity in the early evolution of the avian sternum; the large amount of observable homoplasy is probably a direct result of the high degree of inherent developmental plasticity of the sternum compared with observations in other skeletal elements.
A hypertrophied ossified sternum characterizes all living birds, and enlarged sterna are also present in other flying vertebrates (Ornithodira: Pterosauria, Mammalia: Chiropterygidae) (1). However, the presence of a sternum in Archaeopteryx, long considered the oldest and most primitive bird known, is controversial—it is absent in every known specimen (2, 3). An ossified sternum is also absent in every reported specimen of Sapeornis chaoyangensis (n = 10), the largest known Early Cretaceous bird and one of the most primitive birds with a shortened tail ending in a pygostyle (Aves: Pygostylia: Sapeornithiformes) (4, 5). The sternum in living birds provides a large surface area for the attachment of the two most important flight muscles: the m. pectoralis and m. supracoracoideus (6). Thus, where an ossified sternum is absent in fossil birds, the element is still inferred to be present but cartilaginous (5, 7). The absence of a large ossified sternum and other skeletal differences (e.g., absence of a procoracoid process) suggest that flight capabilities would be severely limited in basal birds (8, 9).
Sternal ossifications are absent in Troodontidae (Maniraptora: Paraves), a clade of dinosaurs considered closely related to birds (10, 11). In most phylogenetic analyses, Troodontidae and Dromaeosauridae form a clade (Deinonychosauria) that is the sister group of Aves (12). However, the fossil record of troodontids remained highly fragmentary until the recent discovery of a wealth of small, feathered taxa from the Late Jurassic and Early Cretaceous of China. Among these fossils was Anchiornis huxleyi, a small, feathered troodontid from the Jurassic that has alternatively been resolved as an archaeopterygid; however, this conclusion removes this clade from Aves (13, 14). Published specimens of Anchiornis also do not preserve sternal elements of any kind (n = 3) (13–15).
The absence of an ossified sternum in Troodontidae, Archaeopteryx, and Sapeornithiformes is counterintuitive to the inferred importance of the sternum as part of the avian flight apparatus and at odds with the phylogenetic distribution of ossified sterna among maniraptoran theropods. All other groups that are or have been considered closely related to birds (Scansoriopterygidae, Dromaeosauridae, and Oviraptorosauria) possess paired, ossified sternal plates that fuse into a singular element (sternum) late in ontogeny in at least some taxa (e.g., dromaeosaurid Microraptor, oviraptorosaur Ingenia) (16–19). Admittedly, the sternum is not one of the best-known skeletal elements in these clades; the presence of sternal plates is affected by ontogeny and even in adults, these thin, plate-like elements are often not preserved (19). This observation has lead to the assumption that the absence of sternal elements in Archaeopteryx, Sapeornis, and troodontids could potentially be due to preservational or ontogenetic bias in the fossil record (5, 7). To test this hypothesis for troodontids, we use Anchiornis, for which a large number of specimens are available at the Shandong Tianyu Museum of Nature (STM). This museum holds the largest collections of any single dinosaurian taxon in the world (more than 200 specimens each of Anchiornis and Microraptor), providing a unique environment in which we can investigate assumptions based on previously limited material. This study represents the largest published dataset of a single extinct dinosaurian taxon to date. Here, we examine a large number of previously unpublished specimens referable to Anchiornis and Sapeornis for sternal elements and discuss the implications of the absence of sternal ossifications at the base of the avian clade.
Methods
Two hundred twenty-six specimens of Anchiornis sp. (SI Appendix, Table S1) were studied at the STM, Shandong Province, China; 96 specimens of Sapeornis chaoyangensis were studied (SI Appendix, Table S2). We selected a comparably sized taxon with an ossified sternum for comparison and to determine the extent of preservational bias; 88 specimens of Jeholornis (Aves: Jeholornithiformes), a long bony-tailed bird considered only more derived than Archaeopteryx within Aves, were studied (SI Appendix, Table S3). Published data were also included for all three taxa. The quality of preservation, femur length (measured by using stainless hardened digital calipers), and presence of sternal elements (sternal plates and ribs) and gastralia (these delicate elements act as a second proxy for quality of preservation) were noted for each specimen (SI Appendix, Tables S1–S3). Specimens near the lower and upper size limits were sampled histologically to confirm the presence of an ontogenetic series (SI Appendix, SI Methods). Clearly, no very young juveniles are present in the sample; no specimen preserves juvenile features such as small size, a large orbit, or incompletely ossified periosteal surface.
Results
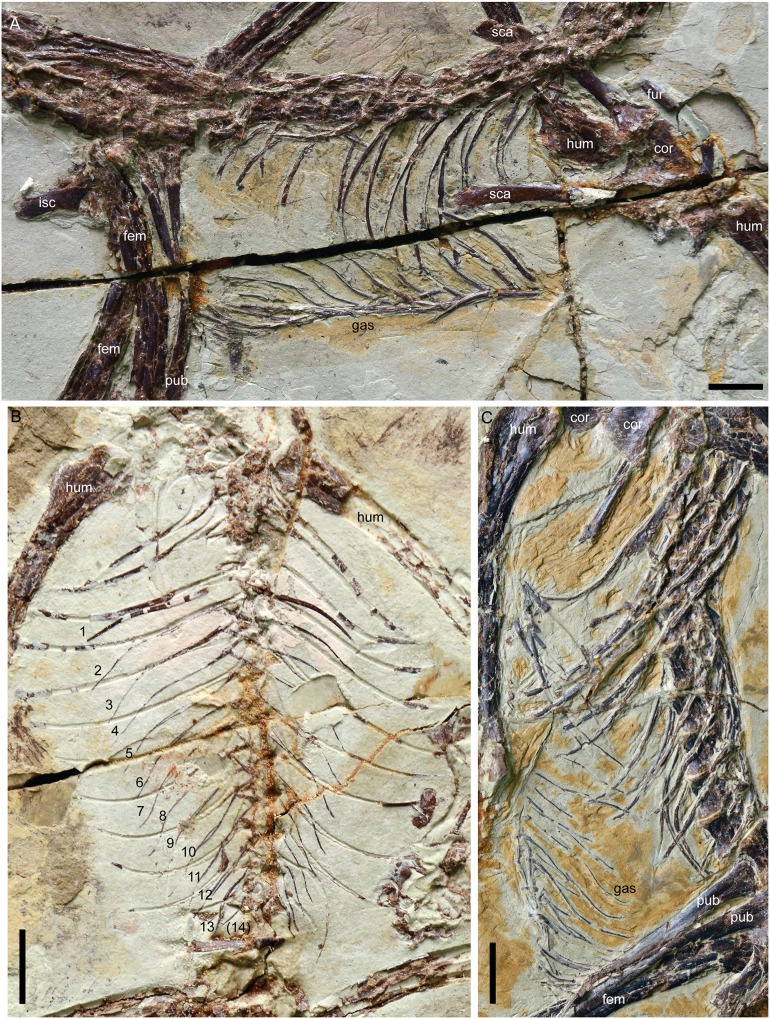
Anchiornis specimens (including previously published material; n = 229) range in femur length (as a proxy for size) from 40 to 93 mm (SI Appendix, Table S1). No specimen preserves sternal ribs or plates (Fig. 1); 78% preserve gastral elements, but in only 10% is the basket even moderately well preserved.
Fig. 1.
Three specimens of Anchiornis sp. preserving the complete or nearly complete gastral basket but no sternal ossifications including sternal ribs: STM0-165 (A); STM0-120 (B); STM0-52 (C). (Scale bars: 1 cm.) cor, coracoid; fem, femur; fur, furcula; gas, gastralia; isc, ischium; pub, pubis; sca, scapula.
Sapeornis (n = 106) specimens range in femoral length from ∼50 to 90 mm (SI Appendix, Table S2). Of the sample, no specimen preserves sternal elements (including sternal ribs), 50% preserve gastralia, but only 13% preserve complete or nearly complete baskets (SI Appendix, Table S2 and Fig. S1).
Jeholornis (n = 95) specimens preserve femora ranging from ∼50 to 85 mm long (SI Appendix, Table S3). Approximately half the specimens (52%) preserve sternal ossifications and slightly less preserve gastralia (44%). The sternum is similar to that of the dromaeosaurid Microraptor gui (SI Appendix, Fig. S2F), except it is proportionately shorter (quadrangular), as in confuciusornithiforms (SI Appendix, Fig. S3C), with small craniolateral processes, and large, simple lateral processes (SI Appendix, Fig. S3A). In some specimens, clefts are located on the rostral and caudal midline, indicating that fusion of the two sternal plates was incomplete (e.g., STM2-16, 2-39). An additional pair of ossifications is preserved, associated with the sternum in multiple specimens (SI Appendix, Fig. S3A) and is inferred to have articulated on the lateral surface, functioning as lateral trabeculae although in no specimen are the “lateral trabeculae” fused to the sternum (20), and their in vivo articulation with the sternal body is unknown (20, 21). Four to five pairs of sternal ribs articulated with the sternum; costal facets appear not to be developed (present in some nonavian theropods, the basal bird Confuciusornis, and ornithuromorphs) (22–24).
Histological samples were taken from both the upper and lower size limit; STM15-32, a larger specimen of Sapeornis, and STM0-8, the largest sampled Anchiornis, both preserve an avascular outer circumferential layer (OCL) marked by multiple lines of arrested growth (LAGs) and a well-developed ICL (SI Appendix, SI Methods and Figs. S4–S9). The first LAG in Sapeornis STM15-32 is a double LAG, separated from the closely packed LAGs in the OCL, indicating that Sapeornis took several years to reach maturity and then continued to grow, albeit slowly (SI Appendix, Fig. S5). No previously sampled specimen of Sapeornis preserves LAGs (25, 26) to our knowledge. STM15-32 preserves a fully fused tibiotarsus and fused proximal carpometacarpus and tarsometatarsus (SI Appendix, Fig. S10), which together with histological data strongly suggests this specimen is a skeletally mature adult (27). Histology confirms that no young juvenile Sapeornis or Anchiornis are present in the sample; even the smallest specimens (Anchiornis STM0-5, Sapeornis STM15-6) preserve an ICL, indicating medullary expansion (bone remodeling) had already occurred at least once (SI Appendix, Figs. S8 and S9). Notably, despite its smaller size relative to STM15-70, the histology of STM15-32 appears to be more mature, with an OCL marked by several LAGs (SI Appendix, Figs. S5B and S7). We suggest that this observation may be indicative of sexual dimorphism in Sapeornis.
Discussion
Based on this study conducted on the largest published sample of specimens referable to a single dinosaurian taxon (Anchiornis, n = 229; Sapeornis, n = 106; Jeholornis, n = 95) (SI Appendix, Tables S1–S3), we consider the absence of sternal elements in Anchiornis and Sapeornis to be a true feature of these taxa and not an artifact of preservation or ontogeny. The known collections of Jehol specimens are heavily biased toward more complete specimens, which should increase the likelihood of sternal elements being preserved in the available material (71% of the Anchiornis specimens sampled are approximately >90% complete, compared with 62% of all Sapeornis specimens and 52% of all Jeholornis specimens; more fragmentary specimens are rarely less than 50% complete). Sternal plates and ribs are absent in all specimens of Anchiornis and Sapeornis regardless of preservational quality. Sternal morphology is affected by ontogeny, but sternal ossifications appear fairly early in basal birds, present in all known enantiornithine hatchlings and subadult jeholornithiform and ornithuromorph specimens (21, 28–30). In the one specimen of subadult confuciusornithiform, although there is no ossified sternum, a chondrified sternum is preserved (31). Sternal ribs ossify even earlier than the sternal plates in paravians, but this feature is also consistently absent in all specimens of Anchiornis and Sapeornis, further indicating a chondrified sternum was most likely not present. Specimens of both Anchiornis and Sapeornis occupy a considerable size range (SI Appendix, Tables S1 and S2); although there is a known bias toward juveniles in the fossil record of larger dinosaurs (32), this observation should not account for their absence in at least Sapeornis because sternal ossifications typically appear fairly early in ontogeny and subadults—not juveniles—appear to dominate the fossil record of Mesozoic birds. Histological analysis confirms our sample includes skeletally mature specimens, indicating that the absence of sternal plates is not an ontogenetic artifact. In light of the large number of specimens used for each taxon in this study, many of which are complete and fully articulated, and some of which boast exceptional preservation including soft-tissue impressions such as feathers and stomach contents, we feel there is sufficient evidence to conclude that a sternum, chondrified or ossified, was truly absent in both Anchiornis and Sapeornis. Ossification is driven by BMP signaling (33); to ossify a cartilaginous element requires only a simple change in transcription factors. The mechanical stress of volant activity would therefore be expected to readily induce the evolution of an ossified sternum in animals with a cartilaginous precursor, especially given that an ossified sternum is present in more basal maniraptorans, indicating the genetic potential for this feature. However, younger Jiufotang Formation sapeornithiforms, separated from their Yixian relatives by a period of ∼8 million years, also do not preserve any evidence for sternal ossifications, further suggesting no cartilaginous element was present at any stage during sapeornithiform evolution. However, given the vagaries of taphonomy and preservation and the delicate nature of the thin sternum even when ossified, we cannot conclude unequivocally that a chondrified sternum was absent in either Anchiornis or Sapeornis.
In contrast, the adult long boney-tailed Jehol bird Jeholornis has fully fused sternal plates (SI Appendix, Fig. S3A). Of the sampled specimens, one-half (52%) preserve a sternum including the subadult holotype of “Jixiangornis” CDPC 02–04-001 (29). Comparison with this comparably sized sympatric taxon supports interpretations that the absence of a sternum in Sapeornis is not preservational or the result of a sampling bias.
Compensatory Morphologies.
It is commonly accepted that Archaeopteryx and Sapeornis were volant (4, 34), thus they may have compensated for the absence of a sternum through other morphologies. In crocodilians, several muscles attach to the gastralia and the cranial pair of gastralia articulates with the sternum (35). This plesiomorphic condition is retained, and the gastralia are observed to articulate with the sternum in some theropods and basal birds (Jeholornis, Confuciusornis, Eopengornis, Parabohaiornis; SI Appendix, Fig. S11 A and B). Gastralia are notably highly modified within Theropoda compared with other groups of amniotes, and proximally fused gastralia have been proposed to function similarly to the sternum in tyrannosaurids (35). Gastralia are absent in living birds, considered functionally redundant in the presence of the large ossified sternum that characterizes Neornithes (35), thus bridging the possibility that the large gastral basket in Sapeornis (5) may have functioned as a compensatory feature in the absence of a sternum and that the extensive gastral basket of basal birds with sterna may also have participated in supporting or reinforcing the sternum and the flight muscles (SI Appendix, Fig. S1). However, the absence of a sternum in Sapeornis and Anchiornis would have left the gastral basket free, suggesting it would have lacked the rigidity to support large flight muscles. If the fused cranial row in tyrannosaurids functioned similar to the sternum of more derived theropods (35), potentially the same may be true regarding Archaeopteryx and Sapeornis. Although the first pair of gastralia in Sapeornis appears to be similarly fused, the gastral basket is otherwise void of modifications to suggest it was the attachment site of robust musculature. We do not fully understand how the gastralia may have supported the musculature necessary for volant activity, but in the absence of a sternum, these muscles clearly would have had to find attachment elsewhere.
The morphology of the coracoid in Sapeornis also differs from that of other basal birds in which this bone definitively articulates with a sternum. Potentially the large coracoid of Sapeornis may also have compensated for the absence of the sternum in some way (7). Compared with other Cretaceous birds, the coracoid is proportionately wide and short (SI Appendix, Fig. S2E). The morphology in Sapeornis, which is similar to that of Anchiornis (SI Appendix, Fig. S2C) and Archaeopteryx, is plesiomorphic to a larger group of theropods, including nonavian paravians with an ossified sternum. Caudipteryx, with its simple oval sternal plates, also has a plesiomorphic proportionately short coracoid (SI Appendix, Fig. S2B). In contrast, the supposedly volant Microraptor has a proportionately more narrow and elongate coracoid relative to other derived maniraptorans, somewhat resembling that of basal birds such as Jeholornis (SI Appendix, Fig. S2 A and D and Table S4). It is interesting to note that in all supposedly volant taxa with sterna (Microraptor, Jeholornis, Confuciusornithiformes, and ornithothoracines), the coracoid is elongated relative to the plesiomorphic condition, suggesting these two features are correlated.
In the plesiomorphic theropod coracoid morphology, present in Archaeopteryx and Sapeornis, the distal margin is convex, especially along the distolateral margin, whereas in Microraptor and more derived avian taxa, the sternal margins are straight for articulation with the sternum (e.g., Jeholornis, Confuciusornis, ornithothoracines). This convexity is particularly pronounced in troodontid taxa without sterna (e.g., Mei long, Anchiornis), which also have proportionately short coracoids (slightly wider than long; SI Appendix, Table S4). Proximally, the sternolateral margin of the coracoid in Sapeornis is expanded along the distal half (SI Appendix, Fig. S2E), which may also have served to provide a larger proximal attachment surface for the pectoral muscles. Unfortunately, this hypothesis is also not supported by any local rugosity, tubercle, or other indicator on the coracoid to suggest that this convexity was, in fact, a site of muscle attachment.
The Absence of Sternum in Archaeopteryx.
The iconic “first bird” Archaeopteryx is known from 10 published skeletal specimens (36). Of these specimens, six are nearly complete and articulated (the Berlin, Eichstätt, London, Munich, Solnhofen, and Thermopolis specimens), four of which also preserve feather impressions. No specimen preserves evidence of sternal plates or ribs, although a complete or nearly complete gastral basket is preserved in several specimens (well preserved in the Berlin, Eichstätt, Munich, Solnhofen, and Thermopolis specimens); preservation of the latter, which is formed of numerous small, delicate bones, suggests that the absence of sternal elements is not taphonomic. What was described as the sternum in the Munich specimen (2) has been reinterpreted as part of the coracoid (3). However, because this element has such a centripetal role in neornithine-powered flight and is present in other nonavian maniraptorans, it was considered that a cartilaginous sternum was present (37). Because of the small sample size (n = 10), the absence of an ossified sternum may potentially be due to preservational or ontogenetic bias. Archaeopteryx is in many ways morphologically similar to the nonavian maniraptorans Anchiornis and Xiaotingia (13). These taxa share more than the absence of a sternum in all known specimens: They have similar skull morphologies with small conical unserrated teeth; a short, quadrangular coracoid; a tetra-radiate ischium; and a gastral basket composed of 12–14 pairs of gastralia. These taxa have even been resolved together in an “archaeopterygid” clade outside of Dromaeosauridae + Troodontidae (10), highlighting the similarity between these taxa. We have provided convincing evidence that the absence of an ossified sternum in Anchiornis is not due to ontogenetic or preservational bias; we further propose that in light of the excellent preservation of soft tissue in numerous specimens, it is safe to conclude a chondrified sternum was also absent. Considering the morphological similarity between Anchiornis and Archaeopteryx and the absence of a sternum in Sapeornis, we argue a chondrified sternum may also have been absent in the earliest bird, Archaeopteryx.
Sternal Morphology and Avian Origins.
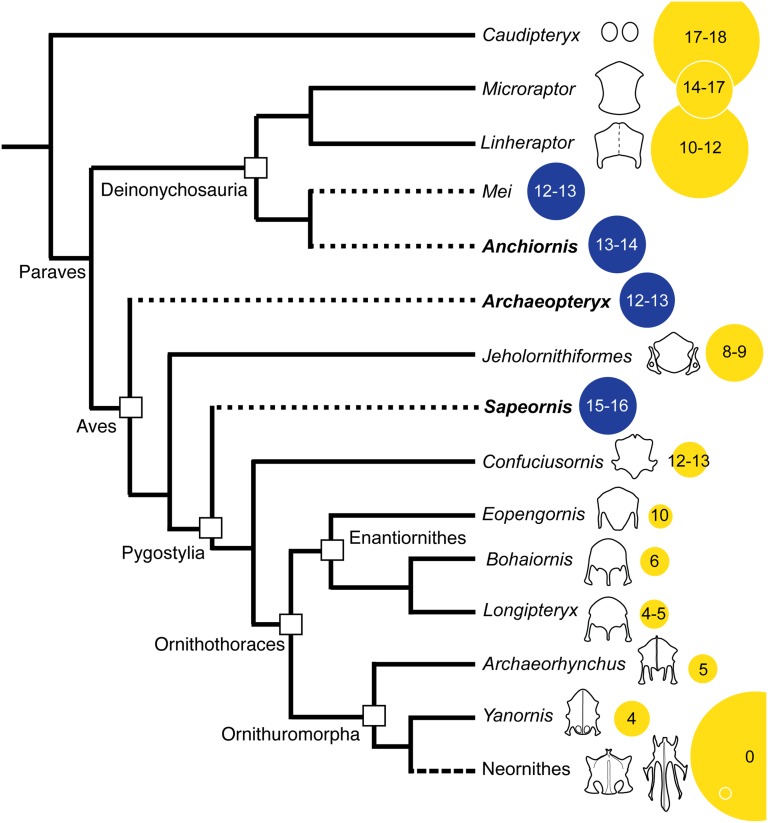
Sternal plates (unfused and cartilaginous) are plesiomorphic for the tetrapod crown group, only known to be lost in turtles and various limbless clades like snakes and caecilians (1). We have provided strong evidence that Anchiornis and Archaeopteryx, taxa inferred to cross the dinosaurian-avian transition (Fig. 2), had no sternum. This observation has important repercussions for theories regarding volant activity in the earliest birds. Given the phylogenetic proximity of Troodontidae to the base of Aves and the absence of a sternum in Archaeopteryx, it is most parsimonious to interpret that this feature was the plesiomorphic avian condition. Its absence in the basal pygostylian Sapeornis may further suggest the avian lineage leading to Ornithothoraces also did not have a sternum; this observation suggests the sterna in Jeholornis and Confuciusornis could have evolved independently and may not be strictly homologous. Alternatively, current popular phylogenetic hypotheses regarding basal bird relationships are incorrect (7); some analyses have resolved Sapeornis as basal to Jeholornis (38, 39), which would suggest a single origin event for the sternum within Aves (but multiple origins for the pygostyle). Placing Archaeopteryx in Troodontidae would also require less “steps” with regards to sternal evolution. However, among derived maniraptorans, the sternum is highly homoplastic, as evidenced by the repeated parallel evolution of features such as a keel and lateral trabeculae; this observation highlights the apparent plasticity of this compound synstotic element (21), exemplified by the novel ossification pattern evolved by some enantiornithines (21).
Fig. 2.
A simplified cladogram of derived maniraptoran theropods showing sternal morphology. The size of the circle reflects body size; blue indicates the absence of sternal ossifications, also indicated by short dashed branches (yellow, present). The number inside the circle indicates the number of gastralia (long dash indicates branch where gastralia are absent). The smaller circle inside the larger circle for Neornithes is meant to indicate the extreme size range occupied by fossil and extant members of this clade.
The absence of a sternum in Archaeopteryx and Sapeornis goes beyond its importance to the flight apparatus: This element is present in all tetrapods with forelimbs (with the unique exception of turtles in which the ribs are enlarged). Intuitively, we must infer a cartilaginous sternum was present not only because of its phylogenetic distribution but also because we can infer no reasonable benefit to volant activity gained through the loss of this feature, which provides the major point of attachment for the primary flight muscles (6). Most researchers tend to agree that Archaeopteryx had extremely limited flight capabilities. Although Sapeornis possesses features that are considerably more advanced such as its small pygostyle, long rectrices arranged into a fan, elongate forelimbs, and carpometacarpus with reduced manual digits (5, 26), derived features are notably absent from the shoulder girdle. This observation may suggest that Sapeornis retained a primitive form of flight and was not capable of a powerful up stroke, as inferred for Archaeopteryx (8).
Conclusions
Using the largest published collection of any dinosaurian taxon to date, we confirm the absence of sternal ossifications in Sapeornis and Anchiornis. Furthermore, despite the bias toward complete or exceptionally preserved material, there is no evidence of a chondrified sternum, widely assumed to be present in the absence of an ossified element in taxa such as Archaeopteryx. Based on this comparison, we suggest that Archaeopteryx, the oldest and most basal bird, also had no sternum, cartilaginous or otherwise. This observation suggests that this feature was the plesiomorphic condition in Aves, which, in turn, suggests that basal bird sterna may not be truly homologous.
Supplementary Material
Acknowledgments
We thank S. Zhang (Institute of Vertebrate Paleontology and Paleoanthropology, IVPP) for assistance with the histology and A-J. Shi (IVPP) for producing the illustrations. This research was supported by National Basic Research Program of China 973 Program Grant 2012CB821906; National Natural Science Foundation of China Grants 41172020, 41372014, and 41172016; and the Chinese Academy of Sciences.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1411070111/-/DCSupplemental.
References
- 1.Parker WK. A Monograph on the Structure and Development of the Shoulder-girdle and Sternum in the Vertebrata. London: The Ray Society; 1867. pp 238. [Google Scholar]
- 2.Wellnhofer P. Das siebte exemplar von Archaeopteryx aus den Solnhofer Schichten [The seventh specimen of Archaeopteryx from the Solnhofen Limestone] Archaeopteryx. 1993;11:1–47. German. [Google Scholar]
- 3.Wellnhofer P, Tischlinger H. Das “Brustbein” von Archaeopteryx bavarica Wellnhofer 1993 - eine Revision [The “sternum” of Archaeopteryx bavarica Wellnhofer 1993: A revision] Archaeopteryx. 2004;22:3–15. German. [Google Scholar]
- 4.Zhou Z, Zhang F. Largest bird from the Early Cretaceous and its implications for the earliest avian ecological diversification. Naturwissenschaften. 2002;89(1):34–38. doi: 10.1007/s00114-001-0276-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z, Zhang F. Anatomy of the primitive bird Sapeornis chaoyangensis from the Early Cretaceous of Liaoning, China. Can J Earth Sci. 2003;40(5):731–747. [Google Scholar]
- 6.Gill FB. Ornithology. 3rd Ed. New York: Freeman; 2007. pp 758. [Google Scholar]
- 7.O'Connor JK, Chiappe LM, Bell A. 2011. Pre-modern birds: Avian divergences in the Mesozoic. Living Dinosaurs: The Evolutionary History of Birds, eds Dyke GD, Kaiser G (Wiley, Hoboken, NJ, pp 39–114.
- 8.Jenkins FA., Jr The evolution of the avian shoulder joint. Am J Sci. 1993;293A:253–267. [Google Scholar]
- 9.Gatesy SM, Dial KP. From frond to fan: Archaeopteryx and the evolution of short-tailed birds. Evolution. 1996;50(5):2037–2048. doi: 10.1111/j.1558-5646.1996.tb03590.x. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, You H, Du K, Han F. An Archaeopteryx-like theropod from China and the origin of Avialae. Nature. 2011;475(7357):465–470. doi: 10.1038/nature10288. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Norell MA. A new troodontid dinosaur from China with avian-like sleeping posture. Nature. 2004;431(7010):838–841. doi: 10.1038/nature02898. [DOI] [PubMed] [Google Scholar]
- 12.Turner AH, Pol D, Clarke JA, Erickson GM, Norell MA. A basal dromaeosaurid and size evolution preceding avian flight. Science. 2007;317(5843):1378–1381. doi: 10.1126/science.1144066. [DOI] [PubMed] [Google Scholar]
- 13.Hu D, Hou L, Zhang L, Xu X. A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature. 2009;461(7264):640–643. doi: 10.1038/nature08322. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, et al. A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin. Chin Sci Bull. 2009;54(3):430–435. [Google Scholar]
- 15.Longrich NR, Vinther J, Meng Q, Li Q, Russell AP. Primitive wing feather arrangement in Archaeopteryx lithographica and Anchiornis huxleyi. Curr Biol. 2012;22(23):2262–2267. doi: 10.1016/j.cub.2012.09.052. [DOI] [PubMed] [Google Scholar]
- 16.Osmólska H, Currie PJ, Barsbold R. Oviraptorosauria. In: Weishampel DB, Dodson P, Osmólska H, editors. The Dinosauria. 2nd Ed. Berkeley: Univ of California Press; 2004. pp. 165–183. [Google Scholar]
- 17.Zhang F, Zhou Z, Xu X, Wang X, Sullivan C. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature. 2008;455(7216):1105–1108. doi: 10.1038/nature07447. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, et al. Four-winged dinosaurs from China. Nature. 2003;421(6921):335–340. doi: 10.1038/nature01342. [DOI] [PubMed] [Google Scholar]
- 19.Norell MA, Makovicky PJ. Important features of the dromaeosaur skeleton: Information from a new specimen. Am Mus Novit. 1997;3215:1–28. [Google Scholar]
- 20.Zhou Z, Zhang F. Jeholornis compared to Archaeopteryx, with a new understanding of the earliest avian evolution. Naturwissenschaften. 2003;90(5):220–225. doi: 10.1007/s00114-003-0416-5. [DOI] [PubMed] [Google Scholar]
- 21.Zheng X, Wang X, O’Connor J, Zhou Z. Insight into the early evolution of the avian sternum from juvenile enantiornithines. Nat Commun. 2012;3:1116. doi: 10.1038/ncomms2104. [DOI] [PubMed] [Google Scholar]
- 22.Chiappe LM, Ji S, Ji Q, Norell MA. Anatomy and systematics of the Confuciusornithidae (Theropoda: Aves) from the Late Mesozoic of northeastern China. Bull Am Mus Nat Hist. 1999;242:1–89. [Google Scholar]
- 23.You H-L, et al. A second ornithuromorph from the Changma Basin, Gansu Province, northwestern China. Acta Palaeontol Pol. 2010;55(4):617–625. [Google Scholar]
- 24.Godfrey SJ, Currie PJ. A theropod (Dromaeosauridae, Dinosauria) sternal plate from the Dinosaur Park Formation (Campanian, Upper Cretaceous) of Alberta, Canada. In: Currie PJ, Koppelhus EB, Shugar MA, Wright JL, editors. Feathered Dragons: Studies on the Transition from Dinosaurs to Birds. Bloomington, IN: Indiana Univ Press; 2004. pp. 144–149. [Google Scholar]
- 25.Erickson GM, et al. Was dinosaurian physiology inherited by birds? Reconciling slow growth in archaeopteryx. PLoS ONE. 2009;4(10):e7390. doi: 10.1371/journal.pone.0007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao C-H, et al. A subadult specimen of the Early Cretaceous bird Sapeornis chaoyangensis and a taxonomic reassessment of sapeornithids. J Vertebr Paleontol. 2012;32(5):1103–1112. [Google Scholar]
- 27.O'Connor JK, Wang M, Zheng X-T, Wang X-L, Zhou Z-H. The histology of two females Early Cretaceous birds. Vertebr Palasiat. 2014;52(1):112–128. [Google Scholar]
- 28.Chiappe LM, Ji S, Ji Q. Juvenile birds from the Early Cretaceous of China: Implications for enantiornithine ontogeny. Am Mus Novit. 2007;3594:1–46. [Google Scholar]
- 29.Ji Q, et al. A new avialian bird - Jixiangornis orientalis gen. et sp. nov. - from the Lower Cretaceous of western Liaoning, NE China. Nanjing Shi Da Xue Bao. 2002;38(6):723–735. [Google Scholar]
- 30.Zhou S, Zhou Z-H, O'Connor JK. Anatomy of the Early Cretaceous Archaeorhynchus spathula. J Vertebr Paleontol. 2013;33(1):141–152. [Google Scholar]
- 31.Zhang F, Zhou Z, Benton MJ. A primitive confuciusornithid bird from China and its implications for early avian flight. Sci China Ser D Earth Sci. 2008;51(5):625–639. [Google Scholar]
- 32.Erickson GM, Currie PJ, Inouye BD, Winn AA. Tyrannosaur life tables: An example of nonavian dinosaur population biology. Science. 2006;313(5784):213–217. doi: 10.1126/science.1125721. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Zheng Y, Chen D, Chen Y. Enhanced BMP signaling prevents degeneration and leads to endochondral ossification of Meckel’s cartilage in mice. Dev Biol. 2013;381(2):301–311. doi: 10.1016/j.ydbio.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgers P, Chiappe LM. The wing of Archaeopteryx as a primary thrust generator. Nature. 1999;399:60–62. [Google Scholar]
- 35.Claessens LPAM. Dinosaur gastralia; origin, morphology, and function. J Vertebr Paleontol. 2004;24(1):89–106. [Google Scholar]
- 36.Wellnhofer P. Archaeopteryx. Der Urvogel von Solnhofen [Archaeopteryx: The first bird of Solnhofen] München: Friedrich Pfeil; 2008. [Google Scholar]
- 37.Chiappe LM. Glorified Dinosaurs: The Origin and Early Evolution of Birds. Hoboken, NJ: Wiley; 2007. pp 263. [Google Scholar]
- 38.Zhou Z-H, Zhang F-C, Li Z-H. A new basal orithurine (Jianchangornis microdonta gen. et sp. nov.) from the Lower Cretaceous of China. Vertebr Palasiat. 2009;47(4):299–310. [Google Scholar]
- 39.Zhou Z-H, Zhang F-C, Li Z-H. 2010. A new lower cretaceous bird from China and tooth reduction in early avian evolution. Proc R Soc Lond B Biol Sci 277(1679):219-227.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.