Significance
Controlling neuronal activity in live tissue is a long sought-after goal in the neurosciences. Channelrhodopsin-2 (ChR2) is a microbial-type rhodopsin that can be genetically expressed to depolarize neurons with light. Thereby, this “optogenetic tool” delivers cellular specificity and elegant options for studying the neuronal basis of behavior in intact organisms. Unfortunately, low-light transmission through pigmented tissue greatly complicates light delivery to target cells and curtails experiments in freely moving animals. This study introduces a ChR mutant, ChR2-XXL, that gives rise to the largest photocurrents of all ChRs published so far and increases light sensitivity more than 10,000-fold over wild-type ChR2 in Drosophila larvae. As a result, behavioral photostimulation is evoked in freely moving flies using diffuse, ambient light.
Abstract
Channelrhodopsin-2 (ChR2) has provided a breakthrough for the optogenetic control of neuronal activity. In adult Drosophila melanogaster, however, its applications are severely constrained. This limitation in a powerful model system has curtailed unfolding the full potential of ChR2 for behavioral neuroscience. Here, we describe the D156C mutant, termed ChR2-XXL (extra high expression and long open state), which displays increased expression, improved subcellular localization, elevated retinal affinity, an extended open-state lifetime, and photocurrent amplitudes greatly exceeding those of all heretofore published ChR variants. As a result, neuronal activity could be efficiently evoked with ambient light and even without retinal supplementation. We validated the benefits of the variant in intact flies by eliciting simple and complex behaviors. We demonstrate efficient and prolonged photostimulation of monosynaptic transmission at the neuromuscular junction and reliable activation of a gustatory reflex pathway. Innate male courtship was triggered in male and female flies, and olfactory memories were written through light-induced associative training.
Identifying causal relationships between neuronal activity and animal behavior is a fundamental goal of neuroscience. Crucially, this task requires testing whether defined neuronal populations are sufficient for eliciting behavioral modules. The development of light-gated ion channels that can be genetically targeted to specific cells has provided a unique solution to this challenge. In pioneering work, such optogenetic effectors or actuators were originally used as multicomponent approaches (1–3). The introduction of Channelrhodopsin-1 (ChR1) (4) and especially ChR2 as a light-sensitive cation channel (5) dramatically advanced the field by providing an efficient and straightforward single-component strategy for stimulating neuronal activity (6, 7).
Besides cell-specific targeting of appropriate effector elements, precise neuronal control by optogenetics demands efficient light delivery to the neurons of interest. For behavioral studies, photostimulation is ideally accomplished in intact, freely moving organisms and accompanied by functional readouts. The combination of a rich, well-characterized behavioral repertoire and elegant molecular genetics has contributed to Drosophila’s strong impact on behavioral neurogenetics (8, 9). However, low light transmission through the pigmented cuticle presupposes high light intensities for using ChR2 in flies. This obstacle greatly complicates the experimental setup for freely moving animals, and the required light energies can cause heat damage when stimulation is applied over extended time periods. Moreover, limited cellular availability of all-trans-retinal (hereafter retinal for short) demands adding high retinal concentrations as a dietary supplement. If optical access to target cells is not provided by a translucent body wall (e.g., as in nematodes, zebrafish, and Drosophila larvae), an alternative solution is the implantation of an optical fiber directly into the brain. Although this approach has been used successfully in mammals (10), such an invasive procedure is infeasible for the study of intact small organisms.
Due to these restrictions in Drosophila, ChR2 has not reached the popularity attained in other organisms, and instead the field has turned mainly to thermogenetic neuronal stimulation (11–13). As with all techniques, there are also drawbacks to using temperature as a stimulus, such as undesired background activity and a multitude of temperature-sensitive cellular processes and behavioral responses. Photo-liberation of caged ATP, combined with genetic targeting of ATP-gated ion channels, has been introduced as a different optogenetic technique in Drosophila (3, 14). However, its applications are constrained by invasive, time-consuming procedures for injection of caged ATP and a limited experimental time window.
Here, we introduce improved ChR2 variants as an alternative approach to address these shortcomings in Drosophila. Compared with wild-type ChR2 (ChR2-wt), expression of these mutants in target cells led to strongly enhanced photocurrents. We provide the first report, to our knowledge, of ChR2-T159C (15, 16) in flies and describe a ChR2 variant, ChR2-XXL (extra high expression and long open state), that is characterized by an extended open-state lifetime, elevated cellular expression, enhanced axonal localization, and reduced dependence on retinal addition. As a consequence, this mutant does not require dietary retinal supplementation to depolarize cells, evoke synaptic transmission, and activate neuronal networks at very low irradiance. These features enabled behavioral photostimulation in freely moving flies using diffuse low-intensity light.
Results
Biophysical Characterization of ChR2 Variants.
It was recently demonstrated that the efficiency of ChR2 expression depends on retinal availability and the exact amino acid sequence of the apoprotein [i.e., channelopsin-2 (Chop2)] (16). In Xenopus oocytes, Chop2 is degraded more rapidly without its chromophore than in its retinal bound state (ChR2). Moreover, certain point mutations of Chop2 (T159Y/F/W) help to protect the apoprotein from degradation and thereby enable retinal binding several days after Chop2 translation. In fact, when threonine 159 is replaced by aromatic amino acids [tyrosine (Y), phenylalanine (F), or tryptophan (W)], expression levels of Chop2 (“unloaded” protein’) are comparable with ChR2 (“chromophore-loaded” protein). However, only retinal-bound ChR2-T159Y/F/W variants are able to mediate a significant light-gated conductance (16). In contrast, for the related mutant T159C, protein expression levels and photocurrents are greatly enhanced compared with the wt variant both in the presence and in the absence of added retinal (15, 16). This observation has been interpreted as an enhanced binding affinity of Chop2-T159C to the endogenous retinal of oocytes (16).
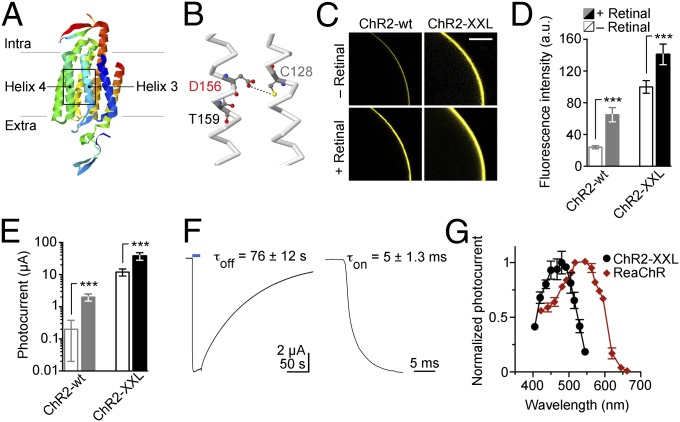
The hypothesized hydrogen bond between residues C128 and D156 of transmembrane helices 3 and 4 is a crucial determinant of the lifetime of ChR2’s conducting state (Fig. 1 A and B) (17, 18). In the course of investigating this structural feature, we discovered that substituting aspartic acid with cysteine at position 156 resulted in exceptionally high protein expression in addition to a long open state. ChR2-D156C, recently studied in terms of primary photoreactions (ps to ns range) (19), was therefore termed ChR2-XXL. Intracellular localization and expression levels were analyzed by expressing yellow fluorescent protein (YFP) fusion constructs of ChR2-wt and ChR2-XXL in Xenopus oocytes. Confocal fluorescence images were recorded, and signal intensity was quantified (Fig. 1 C and D). Subsequently, electrophysiological recordings were used to characterize light-induced photocurrents (Fig. 1E). ChR2-XXL showed very strong expression, as determined by YFP fluorescence (no added retinal, 100 ± 8 a.u. SD, n = 6; plus retinal, 141 ± 13 a.u., n = 6), which indicates increased affinity for retinal (ChR2-wt, no added retinal, 24 ± 2 a.u. SD, n = 6; plus retinal, 65 ± 9 a.u., n = 6). Accordingly, very large photocurrents were recorded without retinal supplementation, and the photocurents were further increased when retinal was added to the medium (Fig. 1 C and E). Adding retinal to oocytes expressing ChR2-XXL resulted in photocurrents of up to −50 µA at −100 mV (Fig. 1E) (no added retinal, 12 ± 3 µA SD, n = 6; plus retinal, 38 ± 10 µA, n = 6), rendering these currents the largest of all ChR variants published so far (ChR2-wt, no added retinal, 0.2 ± 0.2 µA SD, n = 6; plus retinal, 2 ± 0.5 µA, n = 6) (Table S1) (15, 16).
Fig. 1.
Characterization of ChR2-XXL in Xenopus oocytes. (A) Structural illustration of a ChR based on PDB ID code 3UG9 (46). (B) Enlarged boxed region of transmembrane helices 3 and 4 showing the hydrogen bond (dotted line) between residues C128 and D156 of ChR2. (C) Representative confocal images showing retinal-dependent expression of ChR2-wt and ChR2-XXL C-terminally tagged with YFP. (Scale bar: 300 µm.) (D) Comparisons of fluorescence intensities describe enhanced expression of ChR2-XXL both with (filled bars) and without (open bars) retinal supplementation. (E) Consistent with an increased retinal affinity of the mutant, steady-state photocurrent amplitudes of ChR2-XXL were greatly increased irrespective of retinal addition. (F) Example photocurrent and on-kinetics in response to 20-s (Left; 473 nm, 8 mW/mm2, 2 × 1018 photons⋅cm−2⋅s−1) or 5-ns light pulses (Right; 473 nm, pulse energy density 13 mJ/mm2), respectively. Average values for time constants of channel opening (τon), closing (τoff) and data points in figures are given as mean ± SD. Statistical comparisons were performed with the two-tailed Student t test (***P ≤ 0.001). (G) Action spectra of photocurrents mediated by ChR2-XXL and ReaChR.
After excitation, the channel closes with a time constant of about 76 s at 22 °C (Fig. 1F). A deceleration was also observed for the opening of ChR2-XXL with a time constant of ∼5 ms (Fig. 1F), compared with 0.2 ms for ChR2-wt (5). As expected, the slower channel closing is accompanied by a strongly increased light sensitivity (ChR2-XXL, 3.0 ± 0.3 μW/mm2 SD half-saturating light intensity; ChR2-wt, 0.7 ± 0.1 mW/mm2; ChR2-T159C, 1.1 ± 0.2 mW/mm2). For this reason, exposure of oocytes to blue light before measurements had to be absolutely avoided. Importantly, the extraordinarily high photocurrent amplitude mediated by ChR2-XXL clearly distinguishes it from other mutants possessing an extended open state (C128S, C128T, C128A, and D156A) but that do not greatly exceed maximal current amplitudes of ChR2-wt (Fig. S1 and Table S1) (17, 18, 20). Interestingly, ChR2-C128A and D156A also express more efficiently in oocytes than ChR2-wt although strong expression is not a general feature of slow mutants (Fig. S1). It is therefore conceivable that additional factors (such as, e.g., increased single channel conductance and/or open probability) contribute to the large photocurrent amplitude of ChR2-XXL.
Illumination with different wavelengths revealed that the action spectrum of ChR2-XXL was only slightly red-shifted compared with that of ChR2-wt, with a maximum excitation wavelength at ∼480 nm, in good agreement with its previously published absorption spectrum (19). In comparison, the recently published variant red-activatable ChR (ReaChR) (21, 22) displayed a wider action spectrum (Fig. 1G).
Expression and Function at the Drosophila Neuromuscular Junction.
The ability of ChR2 variants to elicit large photocurrents in oocytes correlates with their efficacy of membrane depolarization (5) and neuronal stimulation (6, 7, 15, 23). We therefore turned to Drosophila to test the performance of ChR2-XXL in an intact, complex biological system. To this end, ChR2-wt (consisting of amino acids 1–315 as originally published) (5, 24), ReaChR, and the highly efficient T159C mutant (15, 16), which had previously not been introduced to Drosophila, served as references. ChR2-T159C was chosen for comparison because in oocytes it mediates the largest photocurrents of all heretofore published ChR2 variants, exceeding those of the gain-of-function H134R mutant, the fast E123T/T159C double mutant, and variants possessing an extended open-state lifetime (Table S1) (6, 15, 17, 18, 20). Transgenes were expressed under control of the bipartite GAL4/UAS system for cell-specific targeting (25), and light-triggered outputs of increasingly complex neuronal networks were investigated.
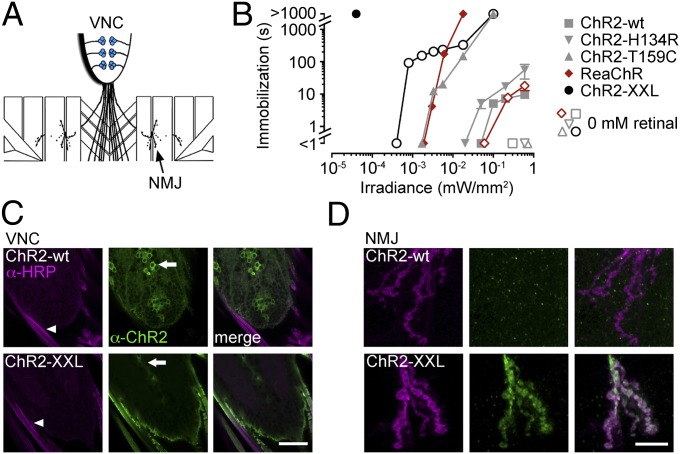
First, we examined photostimulation of monosynaptic transmission at the glutamatergic neuromuscular junction (NMJ) of third instar larvae. ChR2 variants were driven in presynaptic motor neurons (Fig. 2A) (ok6-GAL4 driver) (26), and the postsynaptic muscle response to light-evoked neurotransmitter release was evaluated. Beginning with a simple and optically accessible setting, we assessed the contraction and consequential immobilization of relatively translucent larvae upon exposure to blue light (Fig. 2B). When retinal was not added to the food (“0 mM retinal”), larvae expressing ChR2-wt, ChR2-H134R, or ChR2-T159C displayed no discernible reaction to photostimulation using light intensities up to 0.6 mW/mm2 at 460 nm. Strikingly, ChR2-XXL mediated a pronounced response without retinal supplementation and with nearly three orders of magnitude lower light intensity. When retinal was added (100 µM), light application induced reversible muscle paralysis in all genotypes. Gradual adaptation of motor neurons to small stationary photocurrents enabled larvae to resume crawling despite ongoing photostimulation (27). As a consequence, the duration of immobilization scaled with light intensity during continuous irradiation (Fig. 2B). Remarkably, when larvae expressing ChR2-XXL were fed retinal, light-induced paralysis remained uninterrupted even at light intensities as low as 0.04 µW/mm2. Using red light (623 nm) near its steady-state spectral peak in HEK cells (Table S1) (22), ReaChR required about 500-fold higher irradiance. ChR2-T159C needed about 1,000 times more light and, even with >10,000 times higher light intensity, such prolonged immobilization could not be achieved with ChR2-wt or H134R variants (Fig. 2B).
Fig. 2.
Photostimulation at the larval Drosophila NMJ. (A) Scheme indicating ChR2 expression (blue) in motor neurons leaving the ventral nerve chord (VNC) to innervate muscles at the NMJ. (B) Larvae expressing ChR2 variants (filled symbols, 100 µM retinal; open symbols, no retinal addition) in motor neurons were immobilized during continuous irradiation. The duration of immobilization scaled with light intensity (blue light stimulation, measured at 460 nm; 623 nm for ReaChR). Data are presented as mean ± SEM (no error bars for <1 s and >1,000 s). (C and D) Antibody staining against ChR2 (green) and HRP (horseradish peroxidase, magenta), a marker of neuronal membranes. (C) In the VNC, ChR2-wt was confined to motor neuron cell bodies (arrow) and absent from the efferent nerves (arrowhead), where ChR2-XXL localized strongly. (D) Whereas ChR2-XXL was present at the NMJ, ChR2-wt was not detected in the periphery. (Scale bars: C, 30 µm; D, 10 µm.)
Based on the results in Xenopus oocytes, we investigated whether increased transgene expression in larval motor neurons contributed to the high efficiency of ChR2-XXL in vivo. As previously reported, ChR2-wt localization was restricted to motor neuron cell bodies under these conditions (28). In contrast, ChR2-XXL displayed strong expression in the efferent nerves leaving the ventral nerve chord (VNC) (Fig. 2C). Correspondingly, we detected no signal for ChR2-wt in the axonal endings at the NMJ whereas axonal targeting of ChR2-XXL led to enhanced localization at the NMJ (Fig. 2D). Compatible with its enhanced expression in oocytes (16), ChR2-T159C could also be detected at the NMJ (Fig. S2).
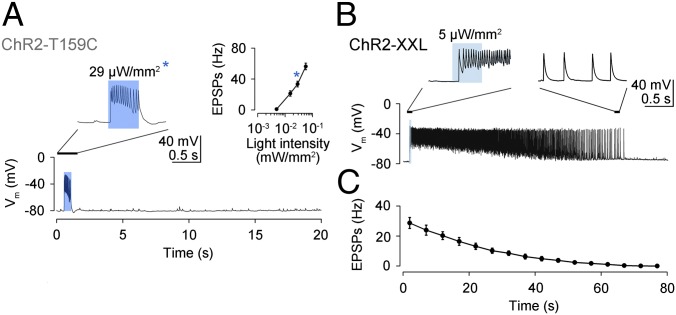
To obtain more quantitative information on how photocurrents shaped neurotransmission at the NMJ, electrophysiological recordings were made from postsynaptic muscles during light-triggered transmitter release from presynaptic motor neurons. ChR2-T159C gave rise to excitatory postsynaptic potentials (EPSPs), which were locked to the light-stimulus (Fig. 3A). The frequency of EPSPs scaled with light intensity, and, notably, 100-fold lower intensity produced a stronger response than ChR2-wt (28). ChR2-T159C was therefore more effective in larvae than the previously introduced H134R variant (27). ChR2-XXL required even lower irradiance to trigger a train of EPSPs, which gradually decayed after the light pulse (Fig. 3 B and C). This phenomenon likely reflects the extended open-state lifetime of ChR2-XXL, and, correspondingly, the rundown of EPSP frequency roughly matched the time constant of current decay in oocytes (Fig. 1F).
Fig. 3.
Electrophysiological characterization of light-evoked neuromuscular transmission. (A) ChR2-T159C elicited EPSPs that terminated with the end of the light pulse. The frequency of EPSPs depended on light intensity (Inset, asterisk indicates 29 µW/mm2). (B) With ChR2-XXL, a brief, low-intensity light pulse triggered EPSPs, which persisted after the end of the stimulus and (C) decayed exponentially (mean ± SEM). Both genotypes were fed retinal.
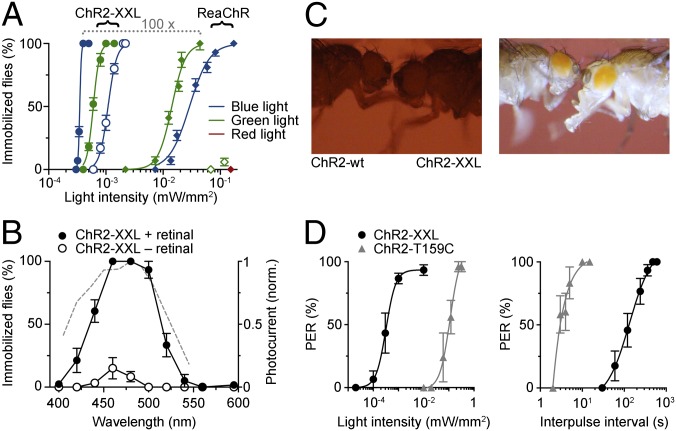
To measure the efficiency of photostimulation in adult Drosophila, ChRs were expressed in motor neurons, and light-induced paralysis was analyzed. As expected, ChR2-XXL immobilized flies very effectively using low-intensity green (520 nm) and blue light (0.35 µW/mm2 half-maximal effective light intensity at 480 nm) (Fig. 4A). For comparison, bright sunlight provides about 500 µW/mm2 of visible light. ReaChR required about two orders of magnitude higher light intensity to fully immobilize flies with green light (535 nm) (Fig. 4A). Blue light (475 nm) was roughly half as effective, and red light (626 nm) produced only a subtle response at 0.33 mW/mm2 (6% of flies immobilized). Wild-type and T159C variants elicited no discernible effect even with very high irradiance (exceeding 3 mW/mm2) (Movies S1–S3). Whereas ChR2-XXL also mediated efficient photostimulation without retinal supplementation, the utility of ReaChR depended on exogenous retinal addition (Fig. 4A).
Fig. 4.
Photostimulation of motor control in adult Drosophila. (A) Light-induced immobilization of adult flies expressing ChR2-XXL (circles) or ReaChR (diamonds) in motor neurons. (B) Spectral dependence (0.28–0.4 µW/mm2) of immobilization by ChR2-XXL. Dashed line, action spectrum of ChR2-XXL in oocytes (Fig. 1G) with axis on the right. (C) Snapshots showing light-induced PER with ChR2-XXL and no reaction with ChR2-wt (2 µW/mm2 at 460 nm). (D) Dependence of PER on light intensity (at 460 nm; 1-s light pulse; gray, ChR2-T159C plus retinal; black, ChR2-XXL plus retinal) and stimulus frequency (1-s light pulse; ChR2-T159C, 0.32 mW/mm2; ChR2-XXL, 8.58 µW/mm2). Data are presented as mean ± SEM.
We used this simple experimental setting to measure the spectral sensitivity of light-gated neuronal control via ChR2-XXL in vivo. In intact flies, the action spectrum of ChR2-XXL closely matched its activity in isolated cells (Figs. 1F and 4B).
Photostimulation of the Proboscis Extension Reflex.
The anatomical location, geometry, and electrical properties of target cells will influence the efficiency of ChR2-mediated depolarization. Further neuron types were therefore studied to characterize ChR2-XXL function in the intact animal. Having examined photostimulation of monosynaptic neurotransmission, we next turned to a polysynaptic reflex pathway. Rather than expressing ChR2 variants directly in motor neurons, transgenes were now driven in gustatory sensory cells in the labellum and distal leg segments. To this end, transcriptional control of a sugar-sensitive taste receptor directed ChR2 expression (Gr5a-GAL4) (29). This approach enabled us to study the behavioral response to optogenetic induction of sugar sensation: the proboscis extension reflex (PER).
Previous work has demonstrated that the PER can be elicited with ChR2-wt when high light intensities are used (6.5 mW/mm2 at 480 nm) and flies are starved before the experiment (30). Both ChR2-T159C and ChR2-XXL triggered a PER in satiated flies using considerably lower light intensities (Fig. 4C and Movies S4 and S5) (no PER via ChR2-wt with 9 mW/mm2 at 460 nm, n = 10 flies). Considering that starvation is required to bring about a responsive state for sugar stimulation of the PER (31), these results emphasize the power of photostimulation. Predictably, ChR2-XXL required less light whereas repetitive stimulation of the PER could be performed at higher frequency with ChR2-T159C (Fig. 4E). The latter is presumably a consequence of the longer open-state lifetime of ChR2-XXL (Figs. 1F and 3B).
Eliciting Male Courtship.
Next, we looked at the utility of using ChR2-XXL to activate neuronal circuits underlying complex behaviors. We chose male courtship as a “hardwired” innate behavior, which is genetically accessible and very robust (32). Male-specific splicing of the fruitless (fru) gene is necessary and sufficient for male courtship. In both sexes, fru is expressed in a network of ∼2,000 cells comprising sensory, central, and motor neurons (33). We used a GAL4 insertion into the fru locus (fru-GAL4) (34) to drive ChR2-XXL in this sex-specific neuronal network.
Activation of ChR2-XXL in fru-expressing neurons triggered distinct behavioral modules of male courtship normally controlled by sensory input, internal states, and experience (Fig. 5). Similar to previous work using the temperature-sensitive cation channel dTRPA1 (Drosophila Transient Receptor Potential A1) (35), courtship behaviors could be elicited in fully intact flies with no need for decapitation (36). Strikingly, however, the courtship ritual induced by ChR2-XXL appeared to unfold in reverse (Fig. 5B and Movie S6).
Fig. 5.
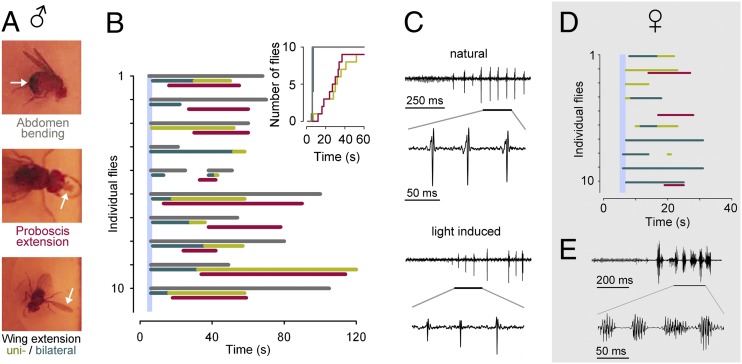
Light-triggered male courtship behavior. (A) Examples of male courtship modules. (B) Ethogram of courtship behaviors (gray, abdomen bending; dark green, bilateral wing extension; light green, unilateral wing extension; red, proboscis extension) evoked by ChR2-XXL in 10 individual male flies (plus retinal) following an ∼2-s light pulse (vertical blue line; 30 µW/mm2 at 460 nm). (Inset) Data as a cumulative plot of courtship behaviors. (C) Example of photostimulated courtship song produced by unilateral wing vibration of a male fly (Lower). The pulse components are similar to the natural courtship song (produced by a male of the same genotype courting a wild-type female in red light (Upper). (D) Ethogram of courtship behaviors evoked by ChR2-XXL in 10 individual female flies (plus retinal) following an ∼2-s light pulse (vertical blue line; 30 µW/mm2 at 460 nm). (E) Example of photostimulated courtship song. The pulse components lack male-specific precision.
Under natural courting conditions, the male will exhibit a characteristic action pattern of consecutive behaviors. This series includes tapping the female’s abdomen, then encircling or following her while extending and vibrating one wing to produce a distinctive “love song,” followed by proboscis extension, licking, abdomen bending, and attempted copulation (32).
When a brief light pulse (2s, 30 µW/mm2 at 460 nm) was used to stimulate fru neurons via ChR2-XXL, male flies responded with abdominal bending, which persisted well after the end of the light pulse. Proboscis extension and unilateral wing extension followed with a delay, and frequently all three behaviors overlapped (Fig. 5B). Moreover, unilateral wing vibration was capable of producing the characteristic pulsatile features of the male courtship song (Fig. 5C) (32, 35, 36). In females, photoactivation of a dormant motor program elicited certain aspects of male courtship although abdomen bending was not observed and song production lacked male-specific precision (Fig. 5 D and E) (36).
With ChR2-XXL, bilateral wing extension always preceded unilateral wing extension. Bilateral wing use is likely suppressed during normal courtship although it can be evoked through strong artificial stimulation of the fru network (35, 36). When dTRPA1 is expressed in fru neurons, a gradual increase in temperature evokes the normal temporal sequence of courtship behaviors and bilateral follows unilateral wing extension (35).
Taken together, these observations indicate that the biophysical properties of ChR2-XXL (Figs. 1F and 3B) partially reversed the normal sequence of courtship modules by triggering rapid onset and gradually decreasing activation of the fru network following a brief light pulse. Conversely, this result suggests that progression from one courtship behavior to the next under physiological conditions is driven by increasing activation of the fru network, possibly through a gradual buildup of sensory inputs. This interpretation is consistent with the notion that progressively higher activation thresholds separate consecutive courtship modules (35).
Triggering Associative Olfactory Memory.
Having tested hardwired, innate, and relatively stereotypic behavior, our final set of experiments focused on experience-dependent, plastic behavior: i.e., learning. Adult and larval fruit flies can be trained to associate an odor stimulus with either a reward or a punishment (37). In Drosophila larvae, ChR2-wt has been used to activate aminergic neurons simultaneously with odorant stimulation. When specific groups of dopaminergic neurons, determined by the TH-Gal4 strain (38), are activated, flies acquire an aversive memory for that odor (24). This finding has been confirmed in adult flies using a thermogenetic TRP channel (12) and UV-dependent liberation of caged ATP in combination with the expression of an ATP-dependent ion channel (14). Optogenetic memory induction using ChR2-wt (or variants thereof) has, however, not been reported for adult fruit flies, most likely due to insufficient photostimulation.
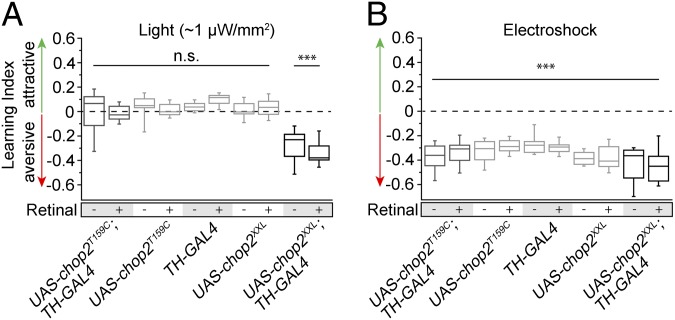
We therefore expressed ChR variants in a large proportion of dopaminergic neurons using TH-Gal4 (38). In the most commonly used aversive olfactory conditioning procedure, an odor is presented simultaneously with electric shocks; a second odor is presented without electric shock punishment. The animals learn to avoid the odor associated with punishment in a subsequent choice situation (39). We modified this learning paradigm and substituted the electric shock punishment with blue-light illumination of ∼1 µW/mm2. Those flies that expressed ChR2-XXL in TH-Gal4–positive dopaminergic neurons acquired a clear aversive short-term memory for the odor paired with the optogenetic activation of dopaminergic neurons whereas the genetic control strains behaved indifferently toward the two odors (Fig. 6A). The artificial induction of an aversive associated memory was independent of dietary retinal supplementation (Fig. 6A), in accordance with the results in motor neurons (Figs. 2B and 4A). All fly strains were able to learn to associate the respective odors with electric shock punishments (Fig. 6B). ChR2-T159C, however, failed to drive the respective dopaminergic neurons such that any light-induced change in behavior could be observed, even when retinal was supplemented (Fig. 6A). Because ChR2-T159C requires significantly higher light intensities to exert an effect in vivo (Fig. 2B), we temporally paired the presentation of an odor with stronger light stimulation (∼0.3 mW/mm2). When combined with retinal supplementation, this protocol resulted in a very slight learning effect (Fig. S3). In conclusion, ChR2-XXL efficiently activated modulatory neurons that mediate the punishment information during associative learning. Moreover, the optogenetically written memory was just as strong as the memory induced using electric shocks as unconditioned stimuli.
Fig. 6.
Light-controlled induction of associative olfactory learning. (A) In adult Drosophila, activation of dopaminergic neurons with light induced an odor-associated aversive memory via ChR2-XXL (UAS-chop2XXL; TH-GAL4), without a requirement for retinal supplementation. Neither control strains (UAS-chop2T159C, UAS-chop2XXL, TH-GAL4) nor flies expressing ChR2-T159C in dopaminergic neurons (UAS-chop2T159C; TH-GAL4) showed an aversive memory after pairing odor and light stimulation. (B) All genotypes associated odors with electric shock punishment, irrespective of retinal addition. n = 8 per experimental group. Learning indices were tested for significant negative differences from 0 using one-tailed Student t test with Bonferroni correction for multiple tests (***P ≤ 0.001).
Discussion
Ultimately, the field of neuroscience strives to provide a scientific description of how the nervous system generates behavior. Building on major advances in recent decades (9), the ability to control neuronal activity in intact, behaving animals promises to deliver significant progress toward this goal. At present, the optogenetic effector ChR2 is arguably the most auspicious choice for this task. However, the difficulty of activating neurons via ChR2-wt in freely moving, opaque animals has hampered its utility for behavioral neuroscience.
Whereas ChR2-wt can be used in translucent larvae fed high concentrations of retinal (24, 27, 28), it functions inefficiently in central brain neurons of intact adult flies (however, for examples of sensory neurons, see refs. 30 and 40). As a consequence, ChR2 has found only limited application in Drosophila, and the community has become somewhat disconnected from technological progress of optogenetic effectors. This development is particularly regretful considering the appeal of Drosophila for behavioral studies and the scientific potential of a synergy with optogenetics.
To date, several ChR variants have been introduced to Drosophila. ChR2-H134R (6) mediates enhanced photocurrents in larvae (27) but has failed to deliver major improvements in adult flies. The long open state of ChR2-C128S (17, 18) has been exploited to investigate the fly visual system using restrained animals and requiring comparable irradiance as ChR2-wt (41). A combination of H134R and ChR2-C128T has been used for laser activation of sensory neurons in restrained flies (42). Very recently, it has been demonstrated that several red-shifted ChRs, ReaChR (which has a broad action spectrum extending well into blue) (Fig. 1G) and Chrimson, deliver important improvements over ChR2-wt for stimulating peripheral and central neurons of adult flies, possibly aided by the improved cuticle penetration of long-wavelength light (21, 22, 43). However, both variants require considerably higher light intensities than ChR2-XXL (Figs. 2B and 4A and Table S1). We observed no major improvement of using long-wavelength light to activate ReaChR. Instead, ReaChR was more responsive to green and blue light stimulation than to red in our in vivo experiments, which is in line with its action spectrum in oocytes and supports previous findings in flies and cultured neurons (21, 43). In summary, ChR2-XXL is most appropriate when maximum light sensitivity is required: e.g., for optically poorly accessible cells, functional genetic screens, especially with weak enhancer lines (44), and during long-term photostimulation experiments where heat damage must be prevented.
Current alternatives to ChR2 have notable drawbacks. UV-dependent liberation of caged ATP is part of a multicomponent strategy, which enables artificial activation of neurons in adult Drosophila. However, this technique cannot be efficiently used for large numbers of animals (3, 14). Thermogenetic effectors, such as dTRPA1 (11, 12) or the mammalian cold-sensitive TRPM8 channel (13), have been very successfully used in Drosophila. Although of undisputed utility, these tools come at the cost of undesired off-target effects, are unsuited for studying temperature-dependent processes (such as, e.g., temperature-preference behavior) (45), and are of limited use in warm-blooded animals. Moreover, acute temperature shifts can induce troublesome movement artifacts during live imaging experiments. Therefore, optogenetic approaches are of obvious value, especially when the effectors can be engaged complementarily: e.g., to stimulate separate neuronal populations using spectrally separated ChRs or using temperature and light with little danger of cross-talk.
In the present study, we introduce a ChR2 variant with beneficial functional properties for low light applications. (i) ChR2-XXL possesses an extended open-state lifetime. This feature contributes to higher light sensitivity than the strongly expressed, but fast, ChR2-T159C variant, because slow channel closure will promote the accumulation of channels in the open state and thereby saturate responsiveness at low light intensities (17). (ii) ChR2-XXL exhibits elevated expression in both vertebrate and invertebrate cells. This property likely confers much larger photocurrent amplitudes than other slow mutants (17, 18, 20). The combination of these characteristics delivers a clear improvement for eliciting neuronal activity in intact animals.
Functionality of ChR2-XXL does not require retinal supplementation (Figs. 1E, 2B, 4A, and 6). Besides simplifying the design of experiments, this important feature strongly influences protein expression. When channelopsin-2 (Chop2) is not bound to its cofactor, the protein is more prone to degradation than in its retinal-bound state (ChR2) (16). We hypothesize that Chop2-XXL has a higher affinity for retinal, which leads to increased protein stability in the presence of endogenous and supplemented retinal.
Accordingly, we observed a significant increase in protein levels of membrane-bound ChR2-XXL over ChR2-wt in spherical Xenopus oocytes (Fig. 1 C and D). In highly polarized Drosophila motor neurons, ChR2-XXL displayed enhanced expression in axonal projections under conditions where ChR2-wt localization is restricted to cell bodies (Fig. 2 C and D). When combined with spatially restricted illumination, such broad expression could be exploited to facilitate photoactivation of distinct subcellular compartments, such as individual synapses.
The long open-state lifetime of ChR2-XXL contributes to its exceptionally high light sensitivity, albeit at the cost of temporal precision. This optogenetic effector is therefore most suited for applications where light delivery is the limiting factor, such as in studies of freely moving animals. In cases where higher temporal precision of stimulus termination is required and alternative variants, such as ChR2-T159C or ReaChR, do not provide sufficient light sensitivity, other measures can be taken. Adjusting light power such that the action potential threshold is barely crossed will narrow the time window of neuronal firing well below the photocurrent decay time constant of ChR2-XXL. Similarly, omitting retinal also accelerates recovery following photostimulation (Movie S3). The temporal precision of ChR2-wt, afforded by its fast current decay, is without doubt an attractive biophysical property. However, the popularity of thermogenetic effectors in Drosophila neurobiology illustrates that many in vivo applications do not in fact require highest temporal resolution. Instead, the usefulness of ChRs for in vivo studies has been limited by insufficient light delivery to target cells.
Strong photostimulation with ChR2-XXL might depress synaptic transmission during prolonged and uninterrupted light application by preventing, e.g., repolarization-dependent recovery from Ca2+ channel inactivation. Correspondingly, larvae expressing the XXL variant in motor neurons appeared to gradually relax while remaining immobilized during continuous irradiation (Movie S7). It may therefore be advantageous to use pulsed light during long-term photostimulation (27).
Conveniently, ChR2-XXL is not activated by long-wavelength light, and, therefore, red foil can simply be used when stimulation is not desired (Movies S1 and S3). Combined with its efficient photostimulation capacity, we anticipate that the properties of this powerful optogenetic tool will be of interest to the Drosophila community and other biologists wishing to control behavior in an intact animal.
Materials and Methods
SI Materials and Methods provides details of experimental procedures. Electrophysiological recordings of photocurrents were made in two-electrode voltage-clamp mode from oocytes in Ringer’s solution (pH 7.6) at a holding potential of −100 mV, and current-clamp mode from ventral longitudinal muscle 6 of Drosophila larvae (28). For associative olfactory learning, groups of ∼100 flies (4–7 d old) were trained as described by Tully and Quinn (39) with modifications. To optogenetically substitute the electric shocks with blue light of ∼1 µW/mm2, transparent training tubes were used that were equipped with 12 blue-light diodes evenly inserted into the tube surface.
Supplementary Material
Acknowledgments
We acknowledge the Bloomington Drosophila Stock Center, C. Helfrich-Förster, and B. Dickson for fly stocks. We thank M. Heckmann and T. Riemensperger for scientific discussions and C. Wirth, M. Oppmann, C. Geiger, J. Heiby, and N. Trinks for technical assistance. This work was supported by Deutsche Forschungsgemeinschaft Grants FI 821/3-1 and SFB 889, B04 (to A.F.), SFB 487, A12 and 1047, A03 (to G.N.), LA 2861/1-1 and SFB 1047, A05 (to T.L.), and KI 1460/1-1 and SFB 1047, A05 (to R.J.K.), and by the Louis-Jeantet-Foundation (G.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408269111/-/DCSupplemental.
References
- 1.Zemelman BV, Lee GA, Ng M, Miesenböck G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33(1):15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 2.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7(12):1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima SQ, Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121(1):141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Nagel G, et al. Channelrhodopsin-1: A light-gated proton channel in green algae. Science. 2002;296(5577):2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 5.Nagel G, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA. 2003;100(24):13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagel G, et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15(24):2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 7.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 8.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4(4):266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 9.Weiner J. Time, Love, Memory: A Great Biologist and His Quest for the Origins of Behavior. New York: Vintage; 1999. [Google Scholar]
- 10.Tsai H-C, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454(7201):217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aso Y, et al. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20(16):1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peabody NC, et al. Characterization of the decision network for wing expansion in Drosophila using targeted expression of the TRPM8 channel. J Neurosci. 2009;29(11):3343–3353. doi: 10.1523/JNEUROSCI.4241-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claridge-Chang A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139(2):405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berndt A, et al. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc Natl Acad Sci USA. 2011;108(18):7595–7600. doi: 10.1073/pnas.1017210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ullrich S, Gueta R, Nagel G. Degradation of channelopsin-2 in the absence of retinal and degradation resistance in certain mutants. Biol Chem. 2013;394(2):271–280. doi: 10.1515/hsz-2012-0256. [DOI] [PubMed] [Google Scholar]
- 17.Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci. 2009;12(2):229–234. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- 18.Bamann C, Gueta R, Kleinlogel S, Nagel G, Bamberg E. Structural guidance of the photocycle of channelrhodopsin-2 by an interhelical hydrogen bond. Biochemistry. 2010;49(2):267–278. doi: 10.1021/bi901634p. [DOI] [PubMed] [Google Scholar]
- 19.Scholz F, Bamberg E, Bamann C, Wachtveitl J. Tuning the primary reaction of channelrhodopsin-2 by imidazole, pH, and site-specific mutations. Biophys J. 2012;102(11):2649–2657. doi: 10.1016/j.bpj.2012.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenenberger P, Gerosa D, Oertner TG. Temporal control of immediate early gene induction by light. PLoS ONE. 2009;4(12):e8185. doi: 10.1371/journal.pone.0008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inagaki HK, et al. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat Methods. 2014;11(3):325–332. doi: 10.1038/nmeth.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: A red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16(10):1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinlogel S, et al. Ultra light-sensitive and fast neuronal activation with the Ca²+-permeable channelrhodopsin CatCh. Nat Neurosci. 2011;14(4):513–518. doi: 10.1038/nn.2776. [DOI] [PubMed] [Google Scholar]
- 24.Schroll C, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16(17):1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 26.Sanyal S. Genomic mapping and expression patterns of C380, OK6 and D42 enhancer trap lines in the larval nervous system of Drosophila. Gene Expr Patterns. 2009;9(5):371–380. doi: 10.1016/j.gep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101(6):3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ljaschenko D, Ehmann N, Kittel RJ. Hebbian plasticity guides maturation of glutamate receptor fields in vivo. Cell Reports. 2013;3(5):1407–1413. doi: 10.1016/j.celrep.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Chyb S, Dahanukar A, Wickens A, Carlson JR. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14526–14530. doi: 10.1073/pnas.2135339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nuwal N, et al. Avoidance of heat and attraction to optogenetically induced sugar sensation as operant behavior in adult Drosophila. J Neurogenet. 2012;26(3-4):298–305. doi: 10.3109/01677063.2012.700266. [DOI] [PubMed] [Google Scholar]
- 31.Shiraiwa T, Carlson JR. 2007. Proboscis extension response (PER) assay in Drosophila. J Vis Exp 2007(3):193.
- 32.Hall JC. The mating of a fly. Science. 1994;264(5166):1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 33.Manoli DS, et al. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436(7049):395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 34.Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121(5):795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 35.Pan Y, Robinett CC, Baker BS. Turning males on: Activation of male courtship behavior in Drosophila melanogaster. PLoS ONE. 2011;6(6):e21144. doi: 10.1371/journal.pone.0021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clyne JD, Miesenböck G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133(2):354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 37.Fiala A. Olfaction and olfactory learning in Drosophila: Recent progress. Curr Opin Neurobiol. 2007;17(6):720–726. doi: 10.1016/j.conb.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Friggi-Grelin F, et al. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54(4):618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 39.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1985;157(2):263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 40.Suh GSB, et al. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol. 2007;17(10):905–908. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 41.Haikala V, Joesch M, Borst A, Mauss AS. Optogenetic control of fly optomotor responses. J Neurosci. 2013;33(34):13927–13934. doi: 10.1523/JNEUROSCI.0340-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang L, et al. GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron. 2013;79(5):917–931. doi: 10.1016/j.neuron.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klapoetke NC, et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11(3):338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenett A, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Reports. 2012;2(4):991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomchik SM. Dopaminergic neurons encode a distributed, asymmetric representation of temperature in Drosophila. J Neurosci. 2013;33(5):2166–76a. doi: 10.1523/JNEUROSCI.3933-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato HE, et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature. 2012;482(7385):369–374. doi: 10.1038/nature10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.