Microtubules (MTs) are key components of the cytoskeleton for all eukaryotes and are vital for cellular processes such as mitotic spindle formation, intracellular transport, vesicle formation, cellular signaling, and migration. These functions depend on the dynamic nature of MTs, which arises from the polymerization and depolymerization events of individual heterodimers, made up of α- and β-tubulin subunits (1). Strict control and appropriate regulation of the equilibrium between free tubulin and MTs is therefore critical for cell viability and has provided the basis for the development of several drugs used in treating cancer (2), autoimmune diseases (3), and neurological diseases (4). Understanding how the drugs bind to tubulin has proven invaluable not only in elucidating how they function, but also for designing new lead agents. In PNAS, Prota et al. add significant new insights into this field by defining a site within tubulin that is able to bind clinically relevant anticancer drugs in a manner that has not been previously described (5).
Structurally, MTs are quite complex, consisting of parallel protofilaments formed from α,β-tubulin heterodimers (Fig. 1A). Their organization is highly dynamic, rapidly fluctuating between periods of growth and shortening. Polymerization is linked to GTP hydrolysis at the terminal tubulin β-subunit, and disassociation releases GDP tubulin (6). Because maintaining a balance between the tubulin dimers and the MTs is critical for cell viability, and many small molecules and proteins act through equilibrium (2, 7). Major drugs, consisting of the vinca alkaloids and taxoids, have long been used in chemotherapy, with considerable success in cancer management (8). The field is rapidly evolving, with new antimitotic drugs and antibody drug conjugates comprised of antitubulin agents such as monomethylauristatin E (9) and a maytansinoid known as DM1 (10).
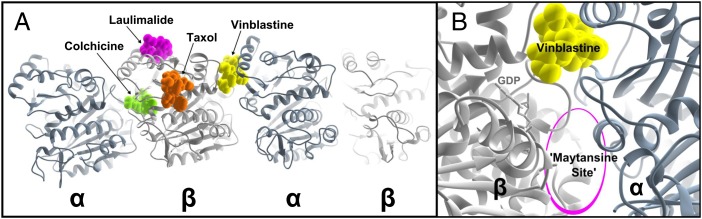
Fig. 1.
Structural overview of MT-binding sites on tubulin heterodimers. (A) Two αβ heterodimers are displayed in the conformation seen in bovine brain tubulin crystal structures (5). The α and β subunits are colored in slate and gray, respectively. Representative MT-binding compounds are depicted as space-filling models, shown in their approximate binding positions. (B) Detail of the vinca site structural features. The bound GDP molecule is shown in stick representation for reference. The approximate location of the maytansine site described by Prota et al. (5) is located below the bound GDP.
The majority of MT-binding drugs are, or have been, derived from natural products, which have evolved to target eukaryotic tubulin with high affinities. Typically, they have been classified as either stabilizers or destabilizers depending on their effect on MT mass (8). Stabilizing agents promote tubulin assembly by increasing lateral protofilament interactions (11–13), whereas destabilizing agents inhibit MT polymerization by promoting a curved conformation or inhibiting a straightened conformation necessary for proper MT formation (14, 15). Each group of compounds is extremely structurally diverse and has historically been defined by their binding site as determined by in vitro competition assays. There are currently four structurally distinct and well-characterized regions on MT where drugs have been known to bind (Fig. 1A): the taxoid site on the luminal face β-subunit (11, 13), the laulimalide/peloruside site on the external face β-subunit (12), the colchicine site at the β-tubulin subunit intradimer interface (14), and the vinca site at α,β-heterodimer interface (15).
The vinca site is a complex drug-binding site located between two heterodimers (Fig. 1B). MT-binding drugs to this site induce heterodimer aggregation, which at high concentrations causes the formation of curved and spiral tubulin assemblies (16). Vinca site binders therefore pose two individual rate constants, binding to the β subunit and induction of heterodimer aggregation (17, 18). A number of different chemical moieties can bind the vinca site, and structural characterization has further subdivided this site into a vinca site and a partially overlapping peptide site that terminates above the bound GDP (19). Although classified as vinca site binders, there has been substantial uncertainty over the actual binding location of the well-known maytansine and rhizoxin antimitotics (20). Both compounds interfere with vinblastine binding, but neither causes the characteristic spiraling or donuts that arise when tubulin is treated with vinca alkaloids. Furthermore, a newer molecule, PM060184, was shown recently to interfere with vinblastine binding, but it was noted that the interaction was not that of a traditional vinca site compound (21).
Using X-ray crystallography to structurally characterize a multicomponent bovine brain tubulin complex, Prota et al. describe a novel tubulin-binding site on the β-subunit capable of binding maytansine, rhizoxin, and PM060104 (5) (Fig. 1B).The binding site of these compounds is in a structurally distinct location to that of the vinca site. Although these compounds are chemically unrelated, their generally shared site of interaction validates this location as a bona fide novel binding site for MT destabilizing compounds. Furthermore, the structures shown in this position shed light on the molecular mechanism by which they destabilize MTs and also how this class of molecule can noncompetitively compete with vinca site compounds to inhibit the spiraling potential. Unlike the vinca site destabilizers, which induce curved aggregation, binding at the maytansine site blocks subunit addition. In this manner, maytansine site binders either sequester soluble tubulin in an unpolymerizable form or poison the ends of growing MTs. When assayed in competition with the vinca compounds, the maytansine site binding compounds partially compete by blocking heterodimer addition and subsequent formation of the complete high-affinity vinca site.
Although MT binders have been hugely successful as anticancer drugs, a number of side effects and resistance mechanisms are associated with their use (2, 8). As a result, new compounds and targeting technologies are needed. The structures presented in this paper illustrate an additional site within the tubulin structure that can be probed for the development of drugs that will complement existing therapeutics and potentially be used in new drug combinations (8). The results illustrate that there is much uncharted territory within tubulin that may be exploitable for novel drug discovery.
Footnotes
The authors declare no conflict of interest.
See companion article on page 13817.
References
- 1.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Dumontet C, Jordan MA. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9(10):790–803. doi: 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Chetrit E, Bergmann S, Sood R. Mechanism of the anti-inflammatory effect of colchicine in rheumatic diseases: A possible new outlook through microarray analysis. Rheumatology. 2006;45:274–282. doi: 10.1093/rheumatology/kei140. [DOI] [PubMed] [Google Scholar]
- 4.Ballatore C, et al. Microtubule stabilizing agents as potential treatment for Alzheimer’s disease and related neurodegenerative tauopathies. J Med Chem. 2012;55(21):8979–8996. doi: 10.1021/jm301079z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prota AE, et al. A new tubulin-binding site and pharmacophore for microtubule-destabilizing anticancer drugs. Proc Natl Acad Sci USA. 2014;111:13817–13821. doi: 10.1073/pnas.1408124111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nogales E. Structural insight into microtubule function. Annu Rev Biophys Biomol Struct. 2001;30:397–420. doi: 10.1146/annurev.biophys.30.1.397. [DOI] [PubMed] [Google Scholar]
- 7.Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol. 2011;8(4):244–250. doi: 10.1038/nrclinonc.2010.228. [DOI] [PubMed] [Google Scholar]
- 8.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 9.Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012;30(7):631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 10.Amiri-Kordestani L, et al. FDA approval: Ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-0012. [DOI] [PubMed] [Google Scholar]
- 11.Prota AE, et al. Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science. 2013;339(6119):587–590. doi: 10.1126/science.1230582. [DOI] [PubMed] [Google Scholar]
- 12.Prota AE, et al. Structural basis of microtubule stabilization by laulimalide and peloruside A. Angew Chem Int Ed Engl. 2014;53(6):1621–1625. doi: 10.1002/anie.201307749. [DOI] [PubMed] [Google Scholar]
- 13.Nogales E, Wolf SG, Downing KH. Structure of the α β tubulin dimer by electron crystallography. Nature. 1998;391(6663):199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 14.Ravelli RB, et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428(6979):198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 15.Gigant B, et al. Structural basis for the regulation of tubulin by vinblastine. Nature. 2005;435(7041):519–522. doi: 10.1038/nature03566. [DOI] [PubMed] [Google Scholar]
- 16.Lobert S, Ingram JW, Correia JJ. The thermodynamics of vinca alkaloid-induced tubulin spirals formation. Biophys Chem. 2007;126(1-3):50–58. doi: 10.1016/j.bpc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Na GC, Timasheff SN. Thermodynamic linkage between tubulin self-association and the binding of vinblastine. Biochemistry. 1980;19(7):1355–1365. doi: 10.1021/bi00548a014. [DOI] [PubMed] [Google Scholar]
- 18.Lobert S, Vulevic B, Correia JJ. Interaction of vinca alkaloids with tubulin: A comparison of vinblastine, vincristine, and vinorelbine. Biochemistry. 1996;35(21):6806–6814. doi: 10.1021/bi953037i. [DOI] [PubMed] [Google Scholar]
- 19.Cormier A, Marchand M, Ravelli RB, Knossow M, Gigant B. Structural insight into the inhibition of tubulin by vinca domain peptide ligands. EMBO Rep. 2008;9(11):1101–1106. doi: 10.1038/embor.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi M, et al. Rhizoxin binding to tubulin at the maytansine-binding site. Biochim Biophys Acta. 1987;926(3):215–223. doi: 10.1016/0304-4165(87)90206-6. [DOI] [PubMed] [Google Scholar]
- 21.Pera B, et al. New interfacial microtubule inhibitors of marine origin, PM050489/PM060184, with potent antitumor activity and a distinct mechanism. ACS Chem Biol. 2013;8(9):2084–2094. doi: 10.1021/cb400461j. [DOI] [PubMed] [Google Scholar]