The development of effective tissue-engineered models of the brain remains an elusive challenge because of its inherent complexity. Such models would be extremely important to understanding brain development, and for exploring therapeutic options for disorders of the CNS, including the treatment of traumatic brain injury (TBI) and related damage to the brain. One million, seven hundred thousand TBIs occur in the United States annually (1). These in vitro models would also be invaluable test beds for drug-discovery investigations and in toxicology evaluations. In PNAS, Tang-Schomer et al. (2) describe a promising model of a cortical tissue mimic and demonstrate its applications to a better understanding of response to TBI.
Several classes of in vitro models of the brain have been described (3), including acute preparations (or explants of CNS tissues), organotypic cultures or thin slices of CNS maintained for greater than 7 d, cerebral organoids (which can be formed from the self-organization of human pluripotent stem cells in 3D cultures) (3), and tissue-engineered constructs (2, 4, 5). Here we focus on the cell-based techniques for organoids and tissue-engineered constructs.
In organoids, the formation of cortex-like structures that are reminiscent of the human developing cerebral cortex have been observed (6). These structures promise to be useful models for brain development and neurodevelopmental disorders. Using human patient-specific induced pluripotent stem cells (iPSC), Lancaster et al. (6) were able to model microcephaly through observations of premature neuronal differentiation. The authors used a spinning bioreactor to grow organoids up to 4 mm in diameter that could be maintained for up to 10 mo. Although this technology is truly impressive, there are key limitations to these models. Currently, adult neuronal behavior is difficult to mimic with iPSC technology and when—and if—this is possible remains an open question. Furthermore, the self-organization in the organoids is incomplete, resulting in a model that lacks the spatial organization observed in natural human tissue. For example, the complex six-layered architecture of the cerebral cortex is not emulated. Although the organoids can grow to a 4-mm diameter, they lack an organized “blood” supply and are starved for nutrients and oxygen in the center of the organoid. In addition, the assembly process for the organoids does not lend itself to ready interrogation with assays or sensors to determine cell health, cellular function, or a higher level of communication in the system. Finally, the interest in combining multiple organs in body-on-a-chip systems would preclude the utilization of organoid models because of the inability to control the structures and growth of the system. Although these organoid models represent exciting progress, the lack of human adult structure and difficulties of integration with sensors or other platforms will limit their use for many disease and injury models.
Tissue-engineered constructs can be formed to address these issues by providing an architecture and environment that emulates key aspects of the organization in the mature brain more completely. Models of the brain using tissue-engineering techniques and relatively mature cells from animals have been constructed and shown to be useful 3D neural cell models (e.g., ref. 4). Typically, such models are based on hydrogels and often use rodent cells (Fig. 1). In early work, Xu et al. used cell printing of hippocampal neurons in hydrogels to form 3D constructs that demonstrated electrical synaptic activity using patch-clamp electrophysiology but lacked a defined architecture (7). Frampton et al. (4) used alginate with rat astroglioma cells, astrocytes, microglia, and neurons, but did not attempt to mimic the layered architecture of the cerebral cortical tissue or demonstrate electrical connections that will be critical to a more complete understanding of brain function. Functional culture has been shown with neuromuscular junction systems for rat (8) and human (9), but without the accompanying 3D architecture.
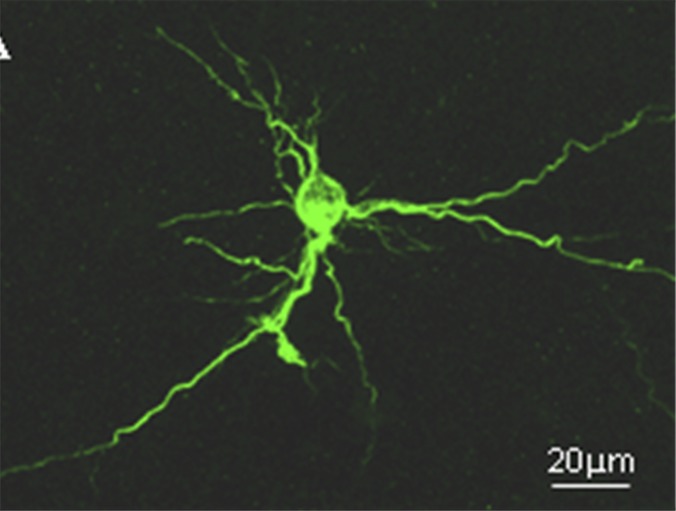
Fig. 1.
An example of the 3D culture of rat hippocampal neurons in a collagen-based hydrogel immunostained with microtubule-associated protein-2. The cells formed signature connections in 3D and had superior survival to 2D-only cultures.
Recently Odawara et al. (5) reported on the construction of a six-layered neuronal system using a reconstructed neuronal tissue mimic based on collagen fibers and polydimethylsiloxane microchambers. These authors used this system to produce alternating layers with somata and neurite outgrowth directed by the orientation of collagen fibrils. Interlayer synchronous firing in the artificial neuronal network was observed using multielectrode arrays from the bottom layer of the structure. Odawara et al. reconstructed these neuronal networks using rat cells and human iPSC-derived neurons. Thus, they were able to mimic some aspects of the 3D layer structure of the cerebral cortex and achieved a cell density approximating the in vivo value, but lacked a true 3D functional characterization of the system.
The work of Tang-Schomer et al. (2) extends beyond these studies to achieve a 3D model of a specific brain structure by mimicking the cerebral cortex with increased authenticity. These constructs seek to provide an effective model of the mechanical, chemical, and electrophysiological environment for the cerebral cortex, along with correlative functional responses to various inputs, such as diffuse axonal injury in brain trauma.
Tang-Schomer et al.’s (2) approach is based on a modular design that emulates the mechanical structure of the brain using silk protein-based scaffolds of high porosity combined with a collagen gel. A unit module consists of neuron-rich regions to emulate “grey matter” and axon-only regions that mimic “white matter”; the composite structure facilitates the formation of 3D axon connections. The neurons were anchored to the silk surface and extended axons into the collagen gel. The silk, coated with polylysine, enabled cortical neuron adhesion, and the stiffness of the material promoted axon outgrowth. This composite, during a 9-wk-long experimental period, promoted in 3D the formation of interconnected mini-networks, increased axon length, and improved viability compared with collagen gel-only controls. Additionally, in vivo-like electrophysiology was monitored in 3D and was sustained better in the composite system than a collagen-only system.
The system was then tested for response (i.e., surrogates for cellular damage, electrophysiological response, and neurochemical changes) to simulated mechanical injury to emulate TBI. In response to simulated injury, there was a rapid increase in both local electrical field potential measurements and glutamate release, which mimic the observed response in animal studies. The composite model demonstrated an increase in neuronal clustering as well as improved cell viability when compared with gels. Tang-Schomer et al. (2) suggest that these composites improve the transport of oxygen and nutrients compared with hydrogels alone, as the hydrogel structure may collapse because of gel degradation. The ability to sustain cellular activity will be important to the study of many disease states and potential response to treatment in future studies.
Tang-Schomer et al. (2) recognize possibilities for further improvement in such models. The current work was mostly focused on reproducing the brain’s in vivo mechanical properties and contains only neurons, whereas the addition of astrocytes, oligodendrocytes, and microglial cells would certainly increase authenticity and allow it to be compared directly to earlier work. As models composed of hydrogels with up to four cell types have been constructed (4), the
Tang-Schomer et al. describe a promising model of a cortical tissue mimic and demonstrate its applications to a better understanding of response to TBI.
addition of other cellular components to this composite model should be straightforward. The authors also suggest the use of human iPSCs, as done in Lancaster et al. (6) and Odawara et al. (5), to transition from a rat to human system. One difficulty with the human iPSC approach is whether a more mature phenotype can be achieved; here the extended period of culture in Tang-Schomer et al. (2) should aid in that goal. The use of human CNS cells would also make it more applicable to creating an in vitro model to verify work being done on the Brain Initiative now being investigated by the National Institutes of Health, the Defense Advanced Research Projects Agency, the National Science Foundation, and the European Union. In addition, the modular approach to construction and development of this system make it an ideal candidate for further integration into body-on-a-chip or organ-on-a-chip systems currently being developed to reduce and eventually eliminate the use of animals in drug discovery and toxicological testing (10).
Overall, during the last few years we have seen a significant improvement in the development of in vitro models of the brain. However, tissue-engineered models of the brain are still in their infancy. A tissue-engineered model demonstrating complex CNS function is a distant goal! What Tang-Schomer et al. (2) have done is to make a superior model emulating response to mechanical insults. This approach is clever, makes effective use of the basic tools of tissue engineering, and has direct applicability to an important human health issue (TBI). However, more complex tissue-engineered models will be required to address the full range of CNS response to exposure to chemicals, pharmaceuticals, and disease. The establishment of an effective tissue-engineered model [such as found in Tang-Schomer et al. (2)] provides a basis upon which more complete models can be constructed, and these models will allow preliminary evaluation of possible therapeutic treatments.
Footnotes
The authors declare no conflict of interest.
See companion article on page 13811.
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006 (National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta)
- 2.Tang-Schomer MD, et al. Bioengineered functional brain-like cortical tissue. Proc Natl Acad Sci USA. 2014;111:13811–13816. doi: 10.1073/pnas.1324214111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison B, III, Elkin BS, Dollé JP, Yarmush ML. In vitro models of traumatic brain injury. Annu Rev Biomed Eng. 2011;13:91–126. doi: 10.1146/annurev-bioeng-071910-124706. [DOI] [PubMed] [Google Scholar]
- 4.Frampton JP, Hynd MR, Shuler ML, Shain W. Fabrication and optimization of alginate hydrogel constructs for use in 3D neural cell culture. Biomed Mater. 2011;6(1):015002. doi: 10.1088/1748-6041/6/1/015002. [DOI] [PubMed] [Google Scholar]
- 5.Odawara A, Gotch M, Suzuki I. A three-dimensional neuronal culture technique that controls the direction of neurite-elongation and the portion of soma to mimic the layered structure of the brain. RSC Adv. 2013;3(45):23620–23630. [Google Scholar]
- 6.Lancaster MA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu T, et al. Electrophysiological characterization of embryonic hippocampal neurons cultured in 3D collagen hydrogel. Biomaterials. 2009;30(26):4377–4383. doi: 10.1016/j.biomaterials.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Das M, et al. Embryonic motor neuron-skeletal muscle co-culture in a defined system. Neuroscience. 2007;146:481–488. doi: 10.1016/j.neuroscience.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 9.Guo X, Gonzalez M, Stancescu M, Vandenburgh H, Hickman JJ. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials. 2011;32(36):9602–9611. doi: 10.1016/j.biomaterials.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esch MB, et al. How multi-organ microdevices can help foster drug development. Adv Drug Deliv Rev. 2014;69-70:158–169. doi: 10.1016/j.addr.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]