Abstract
In the older adult the benefits of vaccination to prevent infectious disease are limited, mainly due to the adaptive immune system’s inability to generate protective immunity. Age-dependent decline in immune competence, often referred to as immunosenescence, results from progressive deterioration of innate and adaptive immune responses and most of the insights into mechanisms of immune aging have derived from studies in murine models. In this Review, we explore how well these models are applicable to understand the aging process throughout the 80–100 years of human life and discuss recent advances in uncovering and characterizing the mechanisms underlying age-associated defective adaptive immunity in humans.
The immune system undergoes profound transformations with age, and response patterns to immune challenges are therefore highly age dependent. Changes that occur after the age of 50 years have received particular attention because of their clinical impact. Such changes have been globally termed immune senescence, which is a categorical label and does not imply a functional and certainly not a mechanistic designation. The most widely appreciated consequence of advanced age is a reduced effectiveness of the immune system1. However, immune senescence is multifaceted and also includes an increased susceptibility for autoimmunity that is conceptually difficult to reconcile with the impaired responsiveness of the adaptive immune system2, 3, and a constitutive low-grade inflammation that may contribute to a plethora of degenerative diseases; including cardiovascular disease, neurodegenerative syndromes and age-specific ailments such as frailty (Table 1)4–6. Mechanistically, immune senescence is only partially explained by organismal and cellular senescence. Equally important are physiologic differentiation programs such as the acquisition of memory-like features by naïve T cells, the cumulative history of antigen exposure as well as the infectious load and the dealings with chronic or latent pathogens and adaptations to environmental stressors of the aging host.
Table 1.
Cardinal features of immunosenescence
| Impaired ability to respond to new antigens |
| Unsustained memory responses |
| Greater propensity for autoimmune responses |
| Lingering, low-grade inflammation |
Prevention of or compensation for age-related immune defects is at the core of healthy aging since the immune system is not only involved in controlling infections and malignancies, but also in tissue homeostasis and repair7–9. Given the accelerating trends in population aging, healthy aging is not only a question of individual well-being but an essential objective to maintain prosperity and political stability of the world community. The elderly population increases in different countries at different speed, but the trend is global and involves economically advanced as well as developing countries. Demographic shifts are currently most pronounced in Eastern Asia and some European countries where the number of elderly individuals has or will soon outnumber the number of children and the number of individuals who are actively working will be shrinking. In the future, 75% of elderly individuals will be living in less-developed countries which lack efficient social networks and support systems. Preventing or ameliorating infectious diseases in the aging population has therefore been identified as a public health priority by the WHO through a joined effort of their aging and life-course and immunization departments10.
Vaccination in the Elderly – a work in progress
Vaccinations against childhood diseases are one of the most successful and cost-effective interventions in medicine and have completely reshaped the landscape of infectious diseases in children and young adults. Since infections are a major cause of morbidity and mortality in the elderly, vaccinations appear to be the optimal tool to promote healthy aging11. Four vaccines are currently recommended for individuals older than 60 years to protect against influenza, pneumococcal, tetanus and pertussis infections and to prevent zoster reactivation. All four of them are against antigens for which immune memory already exists. Three of them induce at least in part recall responses while the current pneumococcal vaccine is a polysaccharide vaccine that mostly induces a T cell-independent B cell response. Of these four vaccines, only the tetanus-reduced diphtheria-acellular pertussis vaccine gives a satisfactory, but reduced protective antibody response compared to young adults12. In contrast, vaccines for influenza or pneumococcal disease fail to induce protective immunity in a large proportion of the elderly population, but at least appear to be able to mitigate disease to some degree13, 14. Similarly, vaccination with the live varicella virus zoster vaccine only partially prevents herpes zoster reactivation or attenuates the severity of postherpetic neuralgia15, 16. Primary immune responses are equally or maybe even more compromised in the elderly than secondary responses, as illustrated by the poor vaccine response to hepatitis B17, the side effects from yellow fever vaccination or the disease severity of West Nile virus infection and severe acute respiratory syndrome (SARS). More than five decades of experience and the recent 2009 H1N1 pandemic have provided an unparalleled understanding of influenza vaccinations18. While most observational studies report a weak increase in hemagglutination antibody inhibitiontiters and mitigation of disease severity, some epidemiological studies have questioned the benefit from the annual influenza vaccination19, 20. Attempts at improving vaccine outcomes have included the use of adjuvant, higher vaccine doses or booster vaccination, but such strategies have been of limited benefit21–23. The live influenza vaccine was not efficacious and is therefore not approved in individuals older than 50 years24. A better understanding of the mechanisms in immune aging is indispensable to optimally target age-related defects and to restore vaccine responses to a level that vaccination is becoming an effective tool to promote healthy aging. While effective vaccine responses depend on the cooperative action of the innate and adaptive immune systems both of which are subject to aging, this review will mainly focus on the adaptive arm. We will first review the impact of age on T and B cell biology in mouse models and then discuss similarities and differences in human aging.
Loss in regenerative capacity in aging mice
Basic principles of immune aging have been widely studied in mouse models given that mice are the preferred system for immunological studies and a vast array of tools are available25. Early studies describe age-acquired defects in antibody responses, in particular germinal center function and somatic hypermutation, providing evidence that the adaptive immune system deteriorates with age26, 27. One obvious culprit is the substantial loss of naïve T cells with age that, together with the recognition of thymic involution, has led to the paradigm that declining regenerative hematopoietic potential is at the core of immune aging28. Indeed, the maintenance of a naïve T cell system in the adult mouse is entirely dependent on the ongoing, but vastly diminished thymic activity29. Loss in regenerative capacity is predicted to account for the loss of naïve T cells and also for a contraction in T cell receptor diversity30. As a result, considerable efforts have focused on understanding the mechanisms of thymic involution and on identifying means of thymic rejuvenation, the subject of several recent excellent reviews31–33. While declining regenerative capacity is most pronounced for the T cell system, other hematopoietic lineages are affected as well. Age is associated with cell-intrinsic changes in the hematopoietic stem cell (HSC) pool, foremost in their differentiation potential. HSCs committed to the myeloid lineage increasingly outnumber lymphoid-prone HSCs with age34, 35. Preferential survival and population expansion of HSCs, DNA damage and defective repair or epigenetic reprogramming as well as extrinsic factors such as the cytokine environment contribute to this increasing imbalance36. The frequency of lymphoid-committed HSCs is rate-limiting for B cell generation since reducing the HSC pool in the young mouse reproduces the B cell subset distribution characteristic of old mice37, 38. In addition, age-associated changes in the microenvironment and the regulation of developmental check points result in quantitative and qualitative changes in B cell generation39. As a consequence, regenerative B cell capacity in old mice is severely reduced to as little as 10% compared to young mice and the naïve B cell compartment is diminished and contracted in diversity. In contrast to T and B cell, the population of plasmacytoid and myeloid dendritic cells (DCs), essential for adaptive immune responses, does not appear to decline, consistent with the finding that the generation of myeloid cells is favored in the aging host. Available studies have the caveat that DC enumeration is tissue dependent, and most studies in the mouse have focused on DC function rather than numbers40.
T and B cell intrinsic defects in murine immune aging
The availability of T cell receptor transgenic mice has made it possible to study age-related defects of antigen-specific responses in naïve and memory T cells. The general consensus is that naïve T cells are increasingly dysfunctional with aging while the function of memory cell populations is preserved25, 41, 42. In vitro studies have mapped defects to T cell synapse formation and early T cell receptor signaling events43. The defects have been attributed to changes in the glycosylation of cell surface molecules or in physicochemical membrane properties, however, the exact mechanisms and in particular the relationship to aging has remained elusive. Diminished interleukin 2 (IL-2) production upon T cell activation was identified as the singular most important consequence, and, indeed, addition of exogenous IL-2 restores many of the age-related defects in T cell activation44. T cell defects could also be improved by addition of proinflammatory cytokines or Toll-like receptor (TLR) ligands, providing support for the current approach in vaccinology to overcome poor vaccine responses by using an optimal adjuvant45.
Several infectious disease models in the mouse have supported the physiological relevance of immune aging to generate insufficient primary T cell responses. Old mice succumb more readily to West Nile virus and Listeria monocytogenes infections than young mice46. Factors that likely contribute to the increased mortality are reduced numbers and T cell receptor diversity of CD8+ T cells, a reduced ability to clonally expand, and a defect to generate CD8+ effector cells that are polyfunctional and produce high amounts of cytokines on a per cell basis46. In contrast, T cell memory responses, once established in the young mouse, are less affected by age and recall responses are generally intact42.
While the aged host environment cannot be ignored, many of these age-related deficiencies have been shown to be T cell intrinsic47. In adoptive transfer experiments, the response to immunization was studied in young and old CD4KO mice reconstituted with young or old naïve TCR-transgenic CD4+ T cells 48. Immune defects correlated with the age of the cell and not the host and closely resembled the in vitro findings. Similarly, adoptive transfer studies with young and old CD8+ T cells have implicated the age of the CD8+ T cell population rather than the host age47.
Mechanisms underlying the preferential dysfunction of naïve cells are difficult to pinpoint. Although the decreasing regenerative potential affects the naïve more than the memory compartment, declining absolute numbers are obviously not the only reason. Peripheral selection that influences CD8+ T cell homeostatic proliferation and/or survival can skew and possibly contract the TCR repertoire49. The preferential occurrence of cell-intrinsic defects in naïve cells, such as defective signaling and loss of replication and differentiation potential, are difficult to explain since naïve T cells have lower homeostatic turnover rates in vivo than memory cells and are therefore less susceptible to replicative senescence. Observations reporting an increased longevity of naïve CD4+ T cells with age due to a decline in BIM expression have led to the hypothesis that this longer lifespan predisposes the cells to accumulate defects25. Naïve T cells can also acquire a semi-memory phenotype with age, indicating that they have entered differentiation pathways. Expression of molecules usually associated with chronic stimulation and exhaustion (such as PD-1 and LAG-3) have been observed in aged naïve T cells47.
Cell-intrinsic defects have also been described for murine B cells including a reduced ability to undergo class switch recombination and decreased expression of the enzyme Activation-Induced Cytidine Deaminase (AID) and the transcription factor E47 (ref. 39). Some of the T cell defects can be overcome by lineage-specific ablation of p16INK4a, an oncogene that has been implicated in cellular senescence50. In contrast, B cell-specific ablation of p16INK4a was tumorigenic. Overall, the molecular mechanisms of the cell-intrinsic defects remain poorly understood for murine T and B cells; in particular it is unclear how they relate to pathways that have been linked to cellular senescence or aberrant cell differentiation.
Immune Aging Concepts – From Mice to Men
While the mouse model has been invaluable to identify immunological principles and to experimentally dissect mechanisms, the evolutionary distance between mouse and men has introduced difference that not always allows for a simple translation of murine findings to the human51, 52. Differences exist in innate and adaptive immunity, most obvious in the species-specific regulation and expression of a large number of molecules that are involved in immune processes53. Studies of immune senescence add additional levels of complexity (Table 2). It cannot be taken for granted that time can be telescoped and 24 months in a mouse is equivalent to 80 years in a human. This point is of particular relevance as consequences of cumulative replication constitute an important cellular aging mechanism. Not only is the replicative history of mouse and human HSCs and lymphocytes over a life time very different, the two species also markedly differ in the telomeric lengths, with telomeres nearly ten times shorter in human cells. Telomeric erosion and the associated p53-mediated induction of cellular senescence are therefore relevant for human but not for murine lymphocyte aging.
Table 2.
Species-specific Similarities and Differences in Immune Aging Studies
| Mouse | Human | |
|---|---|---|
| Lifespan | 2–3 years | 75 –100 years |
| Setting of aging studies | Mostly pathogen free environment | Normal environment |
| Latent viral infection | Few studies on murine cytomegalovirus. Species comparisons difficult because latent viruses have co-evolved with host. | Some but not all latent infections appear to accelerate immune aging. |
| Telomeres | Very long telomeres and constitutive telomerase. Telomeres do not signal replicative aging. | Telomeres shorter and telomerase suppressed. Telomeric erosion in all hematopoietic lineages, leading to DNA damage responses and replicative aging. |
| Cellular senescence | p16INK4a overexpressed in various lineages | p16INK4a overexpressed in various lineages |
| Hematopoietic stem cells | Decline in lymphoid lineage commitment | Decline in lymphoid lineage commitment |
| B cells | Marked decrease in B cell generation | Decrease in B cell generation, extent unknown |
| Repertoire contraction | Repertoire contraction, monoclonal B cell expansions | |
| Defects in class switch recombination and somatic hypermutation, possibly due to decrease in activation-induced cytidine deaminase. | Findings on B cell-intrinsic defects in class switch recombination and somatic hypermutation are conflicting | |
| T cells | Thymic involution | Thymic involution |
| Loss in naïve T cells | T cell generation from peripheral proliferation maintains diverse naïve CD4 T cell compartment up to the 8th decade of life | |
| Oligoclonal T cell expansions, mainly within CD8 central memory T cells | Expansion of terminally differentiated CD8 effector T cells, in part due to latent viral infection | |
| Impaired calcium influx in naïve CD4 T cells after stimulation, defective IL-2 production. | Impaired T cell receptor sensitivity due to loss of mi-R181a and increased DUSP6 in naïve CD4 T cells | |
| Memory T cell responses largely intact | Defective CD4 and CD8 memory T cell responses, inability to provide help to B cells in part due to increased expression of DUSP4 | |
| No equivalent | Loss of CD27 and CD28, gain in negative regulatory receptors (ILT2, KIRs, KLRG), mostly on CD8 effector T cells |
Another major difference is that immune aging is shaped by infections and comorbid conditions that are not truly reflected in most murine aging studies54. By shaping the immune repertoire, previous infections influence later responses to partially related or completely unrelated antigens55. In fact, most of the influenza-specific B cell responses in the elderly are based on mutational modifications of the existing immune B cell repertoire rather than recruitment of new naïve B cells56, 57. Latent infections significantly impact the immune aging process. It has been estimated that clinically healthy humans are inhabited by about 8 to 12 latent viruses including herpes viruses, polyoma virus, Anelloviruses and adeno-associated viruses58. Having co-involved with the host, many of these viruses are highly species-specific and therefore difficult to model in animal models. While they do not cause overt disease (except in immunocompromised individuals), they shape the immune repertoire and the host environment. Cytomegalovirus (CMV) infection has received particular attention because CMV-specific responses encompass large proportions of the entire repertoire in some individuals and then appear to accelerate immune aging and depress responses to unrelated vaccines59–61. Equally important, many comorbid conditions are in a reciprocal relationship with the immune aging process in that each of them accelerate or modified the other. Telomere shortening, a hallmark of cellular aging, has been observed in several autoimmune diseases62. Accelerated immune aging is possibly best studied in rheumatoid arthritis where increased DNA damage and the initiation of deranged DNA repair responses shape T cell homeostasis and function63. Studies in the future may therefore take the reverse approach; rather than studying natural immune aging in the mouse, define age-related immune aberrations in humans and then develop models to study them in the mouse. As one example, the observations on the impact of cytomegalovirus infections have led to several studies in mice demonstrating that chronic infections established early in life influence all-cause mortality and immune responses in later life64–66. These models may now allow longitudinal studies to dissect the mechanisms how infections accelerate immune aging.
Maintenance of the immune repertoire in humans
At first sight, T and B cell development and the decline in regenerative capacity are strikingly similar in mice and men, therefore implying easy extrapolation. HSCs in humans also show a shift to myeloid lineage commitment at the expense of lymphoid precursors34. Recent data indicate that this shift is accentuated under telomeric stress36. In a setting of telomerase deficiency, the basic leucine zipper transcription factor ATF-like (BATF) limits the self-renewal of lymphoid-biased HSCs. The combination of compulsory lymphoid differentiation and diminished self-renewal of lymphoid precursor cells is postulated to lead to a loss of pluripotency and dominance of myeloid lineage HSCs67. This mechanism should be particularly operational for humans where telomeric erosion is relevant and found in HSCs with aging68. Indeed, accumulation of DNA damage and increased expression of BATF are features of human HSC aging. As a consequence of HSC failure, the frequency of early or committed B lymphoid progenitor cells (defined by the expression of CD45RA, CD38, CD10 and CD19) decreases with age69. Diminution of naïve B cells, immunoglobulin repertoire contraction and the emergence of increased frequencies of autoantibodies appears to be at least in part a consequence of declining regenerative potential and associated leakage in central selection mechanisms38, 70. While this limited capacity is already evident in the healthy people and more so in frail elderly71, the defect is of increasing concern for those adult individuals who undergo antibody-mediated B cell depletion, a treatment modality no longer reserved for hematological malignancies but increasingly used as immunosuppressive treatment in various autoimmune diseases.
Similar to mice, the human thymus involutes with the replacement of thymic epithelial cells by fat72. Bone marrow transplantation studies have suggested that thymic function does not sufficiently recover after the age of 40–50 or 50–60 years dependent on the study and the conditioning regimen to rebuild a naïve T cell repertoire73, 74. Given that thymic involution is a very controlled process and already starts in children at the age of 1 year, some investigators consider it as an evolutionarily selected process of an organ that is needed during organism development but not during adult life and therefore not a typical example of tissue aging75. However, in analogy to mice, thymic involution in humans has also been considered detrimental to immune function and a central driver of T cell aging76. How much thymic T cell generation contributes to T cell homeostasis in the healthy adult has been a matter of debate. Frequencies of T cells expressing T cell receptor excision circles (TREC) are frequently considered as a mean to quantify thymic output77 and, indeed, TRECs in peripheral blood T cells exponentially decline throughout adulthood78. However, the age-related loss in TRECs can be explained as a consequence of T cell loss or dilution rather than falling thymic output79, 80. A recent study has challenged the paradigm that the thymus is critical in the adult and provided evidence that the maintenance of naïve T cells fundamentally differs in mice and humans29. While the naïve T cell pool in mice even with old age is highly dependent on thymic output, T cell generation in humans throughout adult life is mostly derived from peripheral division. Thymic output appears only to be important throughout the growth stages of human life, early life thymectomy induces accelerated immune aging, however only in concert with latent CMV infection81, 82. This interpretation is consistent with several observations. Naïve T cell turnover as concluded from the frequency of Ki67-expressing cells does not change significantly between the age of 20 and 65 years suggesting that thymic output is already low during young adulthood with no need for increases in peripheral proliferation with age to compensate for declining output78. The absolute size of the naïve CD4+ T cell compartment in humans is relatively well maintained into the 7th decade of life83. In contrast, the naïve CD8+ T cell compartment largely shrinks46. This differential attrition is unlikely due to declining input which would affect both lineages equally. CD4+ T cells, while still naïve, lose the expression of CD31 when having proliferated84. Compared to CD31+ cells, such CD31− naïve T cells have a skewed TCR repertoire even in young adults consistent with peripheral selection85. However, the global naïve CD4 TCR repertoire remains highly diverse (Fig. 1). Only in the 8th decade of life does cell turnover increase, possibly due to lymphocyte numbers falling below a critical threshold, and the TCR repertoire abruptly contracts78, 86. Similar results have been found in a non-human primate model87. We have used agent-driven stochastic in silico modeling of the human naïve CD4+ T cell repertoire with age88. In this model, even complete thymic demise at the age of 20 years did not have any influence on repertoire diversity. The model only produced a repertoire contraction when multiple inheritable changes in growth behavior were introduced. Restoration of thymic activity in this case could neither prevent nor restore the repertoire collapse. In summary, for vaccine responses in the healthy elderly, thymic activity after the age of 20 years appears to be irrelevant and defects in T cell homeostasis are due to peripheral selection and growth and survival patterns. Nevertheless, thymic activity during adulthood or thymic rejuvenation continues to be important in patients who have depleted their peripheral repertoire during adult life. Obviously, this includes patients with HIV infection who have started highly active antiretroviral therapy in late or mid-adulthood. An increasing segment of the elderly population has undergone medical intervention such as chemotherapy or bone marrow transplantation for cancer treatment. Several recent studies identified that low TREC concentrations after bone marrow transplantation as predictors for increased complications from infections and poor survival74, 89.
Figure 1. Age and the human CD4 T cell repertoire.
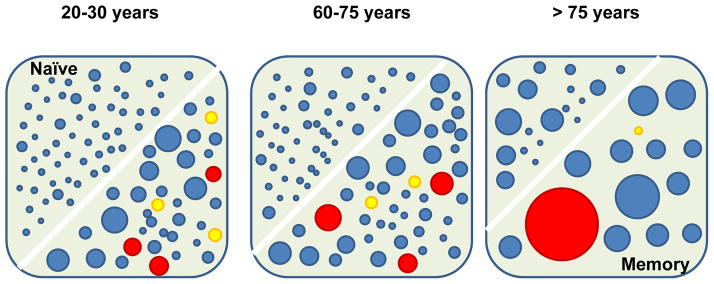
The cartoon illustrates the T cell receptor repertoire within the naïve and memory CD4 compartments with sizes of circles symbolizing different clonal sizes. By the end of the growth period, a diverse naïve repertoire has been established in the young adult. Irrespective of thymic activity, the naïve compartment only moderately decreases in size during the next decades in life while mostly maintaining overall diversity and distribution of clonal sizes. An abrupt contraction is seen in later life. Memory responses to latent infection with cytomegalovirus and herpes zoster virus (VZV) are established in the memory compartment, but behave very differently to aging. While clonal frequencies and sizes of VZV-specific clones decline with age (yellow circles), T cell clones specific for CMV (red circles) dominate the repertoire in the elderly and contribute to the contraction in diversity in the memory compartment.
Aberrant differentiation of naïve CD4+ T cells
Human naïve CD4+ T cells do not develop the severe age-dependent dysfunctionality that is characteristic for murine T cells. Similar amounts of IL-2 were produced when the naïve response of T cells from young and elderly individuals was probed against two new antigens, inactivated rabies virus and recombinant Etr protein of tick-borne encephalitis virus presented by autologous DC90. We have stimulated purified naïve CD4+ T cells with DCs and superantigen and found a high correlation in gene expression between young and elderly adults91. Under suboptimal stimulation conditions, however, reduced responsiveness becomes apparent. Dissection of the TCR signaling cascade revealed that in particular ERK phosphorylation is blunted with age92. The reduced ERK response was due to increased cytoplasmic concentration of the dual-specific phosphatase DUSP6 as individuals progressed in age. DUSP6 is constitutively expressed in T cells and has recently been implicated in attenuating the threshold at which TCR stimulation is translated into a productive signal93. It is one of four phosphatases that are regulated by the microRNA miR-181a, which is highly expressed in double-positive thymocytes. miR-181a concentrations sharply decrease in the transition to single-positive thymocyte and naïve T cells concurrent with decreasing TCR sensitivity to respond to the recognition of autoantigen. miR-181a appears to be similarly important in calibrating TCR responses with advancing age (Fig. 2). miR-181a concentrations in naïve CD4+ T cell decline with age and reconstitution of elderly CD4+ T cells with miR-181a restores responsiveness by repressing DUSP6 translation. The decline in miR-181a may therefore be an example of antagonistic pleiotropy as frequently discussed in the context of aging; beneficial in T cell development in the young to prevent autoimmune disease, but detrimental in the elderly by setting the TCR threshold too high to respond to exogenous antigen. It is currently unclear whether the mechanisms regulating miR-181a expression during thymic development and during peripheral T cell aging are shared. However, the finding that miR-181a expression is lower in memory than naïve CD4+ T cells is consistent with the idea that downregulation is a programmed differentiation process and that aged naïve T cells are semi-differentiated, possibly due to recognition of self-antigen during homeostatic proliferation94. Of note, human CD4+ memory T cells are less dependent on the ERK pathway and phosphorylate ERK less than naïve cells as they channel the signal more through the scaffolding molecule human disks large hDLg to activate the p38 pathway95. But even for memory CD4+ T cells, reconstitution of miR-181a can improve responses in the elderly. DUSP6 is certainly not the only target of miR-181a that is important for T cell biology. In particular, miR-181a has been found to repress negative regulators of the NOTCH pathway and the decline in miR-181a may therefore dampen NOTCH signaling96.
Figure 2. T cell receptor desensitization in the elderly.
One of the important regulators of T cell receptor activation thresholds is the dual-specific phosphatase DUSP6 that controls the initial ERK response and associated positive feedback loops after T cell stimulation. DUSP6 expression is regulated by miR181a. Due to a decline of miR181a, expression of DUSP6 increases with age resulting in the desensitization of the TCR signaling cascade.
Intrinsic T cell defects - a consequence of resource allocation
In contrast to the murine studies cited above, T cell memory established in humans during early age deteriorates during the second half of life. The most obvious example is reactivation of latent VZV infection which manifests as herpes zoster. Also, the reduced vaccine response to influenza is at least in part due to a defective T cell memory response. T cell memory cells to conserved hemagglutinin and neuraminidase epitopes that are not subject to antigenic drift exist in essentially all elderly individuals. Mechanistic defects appear to be highly dependent on the pathogen. For VZV infection, a steady decline of VZV-specific CD4+ T cells over time has been documented, which is only very transiently boosted with zoster vaccination or reactivation16. In contrast, high frequencies of antigen-specific T cells reactive to CMV persist throughout life (Fig. 1). However, contraction in the diversity of CMV-specific TCR repertoire has been associated with diminished viral containment resulting in higher antibody titers and increases antigen-specific clonal expansion97. Polyfunctionality of antigen-specific T cells appears to be one additional factor for effective T cell responses which declines with age with the emergence of more and more monofunctional T cells46.
In addition to quantitative aspects in T cell numbers and repertoire, defects intrinsic to memory T cells develop with age. Interestingly, many of these defects have to do with resource allocation to proper cellular maintenance which can be at odds with the ability to respond to external antigenic stimuli with T cell activation and effector function94. The last decade has seen great progress in our understanding on how metabolic pathways in T cells are connected to function98. In particular, T cell activation and acquisition of effector function is strictly linked to metabolic activity; not only providing sufficient energy in the form of ATP but also precursor metabolites for cellular synthesis. One metabolic master regulator is the AMP-activated protein kinase AMPK which is activated by a low ATP/AMP ratio, increased reactive oxygen species and, in the context of TCR stimulation, by fluxes in cytoplasmic calcium concentrations. We have proposed that functional defects in elderly CD4+ memory T cells are controlled by AMPK (Fig. 3)94. In studying the effect of age on CD4+ T memory cell function, we have identified a negative feedback loop mediated by the dual-specific phosphatase DUSP4 (ref. 99). DUSP4 is a nuclear phosphatase controlling nuclear ERK and JNK activity. Its activity peaks two to four days after T cell activation when it is significantly overexpressed in elderly T cells. DUSP4 curtails the expression of CD40L and several other functional molecules on activated CD4 memory T cells. As a consequence of increased DUSP4 inducibility in elderly T cells, their ability to provide help for T cell-dependent B cell responses is severely impaired, but can be restored by DUSP4 silencing. The upstream mechanism of increased DUSP4 expression is an increased activity of AMPK in aged memory CD4+ T cells that leads to the transcription of EGR1 and eventually DUSP4. This finding raises the interesting possibility that CD4+ T memory T cell function in the elderly can be improved by targeting their metabolic activity.
Figure 3. Defective T-dependent B cell responses in the elderly.
Elderly CD4+ memory T cells respond to T cell stimulation with increased expression of the dual-specific phosphatase DUSP4 because of increased activity of the metabolic master regulator AMPK. DUSP4 is a nuclear phosphatase that curtails sustained pERK and pJNK activity and impairs the ability of the T cell to provide help to B cells. As a consequence of defective CD40-ligand signaling, B cell activation and expression of the transcription factor E47 is impaired. Defective T cell help coincides with an age-associated intrinsic B cell defect to express E47 and as a consequence transcribe AID. As a consequence, B cell clonal expansion, immunoglobulin class switch recombination and hypermutation are impaired.
Even in young cells, a considerable amount of cellular resources are committed to maintaining the integrity of the genome; this is increasingly the case with increasing age. Chronic DNA damage responses are apparent in all hematopoietic lineages in older people including T cells. In part, they are explained by telomeric dysfunction; an increase in frequency of double-strand breaks is also found in peripheral T cells during aging100. Of note, DNA damage in memory T cells far exceeds that found in naïve T cells, at any age. DNA damage responses may in part be responsible for the increased expression for p16INK4a that is found in human T cells as well as other tissues in the elderly101. However, p53-mediated cell cycle arrest, typical for cellular senescence, is not a consistent feature of elderly T cells. Decreased proliferative activity is generally not permanent and can be overcome102. The proliferative ability in terminally differentiated CD8+ T cells can be restored by overexpressing hTERT to increase telomerase activity103. We have found that proliferation of naïve and memory CD4+ T cells from elderly individuals can be significantly improved by providing ionic zinc to upregulate metallothionein expression91.
Chronic DNA damage responses may result from increased genotoxic stress, but may also signal inefficient DNA repair. Such a defect certainly is one of the major driving forces in the accelerated immune aging seen in patients with rheumatoid arthritis. In addition, to expressing reduced telomerase activity similar to healthy immune aging104, expression and function of molecules in the ATM-dependent DNA repair pathway are reduced, leading to increased nontelomeric DNA damage, a failure in p53 activation and the activation of alternative DNA repair pathways100, 105. It will be interesting to examine whether similar mechanisms can also be identified with normal aging.
Expression patterns of T cell regulatory receptors
During aging, the expression patterns of T cell regulatory receptors change markedly (Fig. 4). On balance, expression of various inhibitory receptors is gained, accompanied by a decrease in the expression of costimulatory CD28 family and TNFRSF members106, 107. The changes mostly involve CD8+ memory T cells. Often the majority of CD8+ T cells is affected, while only very small subsets of CD4+ memory T cells show these changes in expression patterns. The l expression of cell surface inhibitory receptors is reminiscent of clonal exhaustion, where chronic high-dose antigen stimulation induces high expression of the inhibitory receptor PD-1, LAG-3 and TIM-3 (refs. 108, 109). In the murine chronic LCMV infection model, in vivo blockade of PD1–PD-L1 signaling rescues interferon-γ production, proliferation and cytotoxic activity of virus-specific CD8+ T cells and reduces viral load. The findings are paralleled in several human chronic viral infections, such as HIV, HBV and HCV. ICompared to exhausted CD8+ T cells, different classes of molecules are expressed with aging which generally do not co-segregate in the same T cell subsets with PD1, LAG-3 and TIM-3. These proteins include members of the killer Ig-like receptors (KIRs), killer cell lectin-like receptors (KLRs), in particular KLRG1 and CD85j of the immunoglobulin-like transcript (ILT) family107. Although T cell exhaustion and age-associated expression of negative regulatory receptors appear to be different programs110, their functional consequences may be similar and blockade of these receptors may be beneficial to improve anti-viral CD8+ T cell responses. Contrary to this model, we have preliminary evidence that negative regulatory receptors expressed during aging selectively inhibit proliferation while keeping effector function intact (own unpublished observation). They therefore may serve a beneficial function to maintain a diverse T cell repertoire by limiting the clonal expansion of selected virus-specific clones.
Figure 4. Age-associated expansion of terminally differentiated CD28-negative T cells.
With increasing age, oligoclonal populations of terminally differentiated effector T cells accumulate that are frequently specific for latent viruses, in particular cytomegalovirus. These T cells differ in phenotype, function and survival from exhausted T cells that develop in response to highly replicating viruses.
B cell responses in the elderly
Similar to T cells, B cell responses in the elderly are determined by the number and diversity of B cells and potential intrinsic defects38, 111. The dependence of many B cell responses on T cell help adds another level of complexity. Regenerative capacity of B cells is compromised due to very similar mechanisms as discussed for the mouse. As a consequence, the number of naïve B cells is reduced. Depending on whether CD19 or CD20 is used to define B cells, total numbers of B cells are slightly or not decreased due to expansion of cells expressing a classical memory or less defined phenotypes69, 70. Repertoire diversity is compressed, in particular in those individuals who are frail and not in good health71. Several lines of evidence exist that limitations in the available B cell repertoire impair the antibody response. Using high throughput parallel sequencing, the lineage structure of prevaccination antibody repertoire in elderly individuals is clearly contracted and the mutation load is higher56. Influenza vaccine responses in the adult highly rely on somatic mutation of the memory repertoire rather than recruitment of new antibody sequences. Even the B cell response against the 2009 pandemic H1N1 influenza virus was dominated by antibodies broadly cross-reactive on hemagglutinin epitopes of multiple influenza strains57, 112. Examination of the circulating antibody-secreting cells after influenza vaccination in ELISPOT assays revealed a nearly tenfold reduction in frequencies while the functionality of plasmablasts were not impaired113. In summary, the data are consistent with the model that the pre-existing repertoire restricts the quantitative response in the elderly. Differences in the quality of the response were less consistent and, if found, may be due to preexisting hypermutations in prevaccination B cells, limiting the ability to optimize the fit of antibodies to newly arising variants. Alternatively, a recent study has correlated a lower induction of AID expression in the elderly with reduced affinity maturation after H1N1 vaccination and production of lower affinity antibodies114. Reduced AID induction may be an intrinsic defect in elderly B cells and caused by a defective expression of the transcription factor E47 and associated with defective immunoglobulin class switch recombination in naïve or IgM memory B cells115. E47 induction is also regulated by CD40L-mediated signals raising the possibility that the reduced expression is caused by a T cell defect. Indeed, we have shown that restoring T cell helper function by silencing DUSP4 in T cells improved E47 induction in B cells99. Obviously, both mechanisms are not mutually exclusive but can be synergistic (Fig. 3).
Vaccination in the Elderly – Where do we go from here?
With changing demographics around the globe, often described as “the grey tsunami”, improving vaccine responses in the elderly is not an option, it is a necessity. Progress over the last decade in optimizing vaccine success has been modest, however, the chances for success are not as dim as sometimes thought. Strategies in creating better vaccines have mostly been modeled on vaccine responses in the young or have relied on studying immune aging in the mouse. We have only just begun to unravel how the immune system ages in humans and to identify molecular pathways that can be targeted to specifically improve the vaccine response in the elderly (Table 3). Responses to vaccines in the older adult will inevitably depend on available resources in terms of T and B cells, yet, repertoire maintenance in the human adult appears to be relatively robust and not heavily reliant on de novo generation of new B and T cells. Restoring B and T precursor cell generation in the bone marrow or rejuvenating thymic function are challenging objectives and certainly not easily applicable, but may not be a primary necessity for healthy immune aging. Exceptions are individuals whose lymphocyte repertoire has been wiped out by medical interventions or by HIV infection. Available data from studying repertoire contraction in CMV infection and anti-H1N1 influenza responses suggest that a diverse memory T and B cell repertoire is as important as maintaining the naïve repertoire. In the very old, severe contraction of the repertoire variably occurs and appears to be a consequence of abnormal peripheral cell growth and survival. Since a multi-hit model best predicts the repertoire collapse in the very old, identifying and preventing single factors may be sufficient to avert substantial loss in diversity. Increased levels of inflammatory cytokines, also termed inflamm-aging, and tissue aging that have been reviewed elsewhere40, 116 certainly contribute to defective vaccine responses. Cell intrinsic changes that impair T and B cell activation and differentiation are rather subtle and may be overcome by temporally limited interventions at the time of vaccination. Negative feedback loops in signaling and cellular metabolism emerge as important pathways to understand and potentially modulate the behavior of aging T cells.
Table 3.
Therapeutic Strategies to Improve Vaccine Responses Prevent repertoire contraction
| Broaden memory B cell repertoire during adult life |
| ? Treat latent viral infections to prevent repertoire skewing |
| Boost miR-181a to increase T cell receptor sensitivity |
| Inhibit DUSP6 to increase T cell receptor sensitivity |
| Diminish expression of DUSP4 to improve T helper function |
| Induce Zn-dependent metallothioneins to improve clonal expansion |
| Optimize DNA and telomeric repair to sustain proliferative capacity |
Acknowledgments
Supported by the US National Institutes of Health (U19-AI057266, U19-AI090019, U01-AI089859 to J.J.G and R01-AR042527, R01-AI44142, R01-EY011916 and P01-HL058000 to C.M.W.).
Footnotes
The authors have no competing interests.
References
- 1.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69:1615–1623. doi: 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther. 2003;5:225–234. doi: 10.1186/ar974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med. 2009;122:605–613. doi: 10.1016/j.amjmed.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavanagh MM, Weyand CM, Goronzy JJ. Chronic inflammation and aging: DNA damage tips the balance. Curr Opin Immunol. 2012;24:488–493. doi: 10.1016/j.coi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boraschi D, et al. Ageing and immunity: addressing immune senescence to ensure healthy ageing. Vaccine. 2010;28:3627–3631. doi: 10.1016/j.vaccine.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Boraschi D, et al. Ageing and Immunity. Sci Transl Med. 2013 In press. [Google Scholar]
- 9.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas-Crusells J, McElhaney JE, Aguado MT. Report of the ad-hoc consultation on aging and immunization for a future WHO research agenda on life-course immunization. Vaccine. 2012;30:6007–6012. doi: 10.1016/j.vaccine.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 11.Chen WH, et al. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30:351–359. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weston WM, Friedland LR, Wu X, Howe B. Vaccination of adults 65 years of age and older with tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Boostrix((R))): results of two randomized trials. Vaccine. 2012;30:1721–1728. doi: 10.1016/j.vaccine.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 13.Jefferson T, et al. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 14.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357:1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 15.Gagliardi AM, Gomes Silva BN, Torloni MR, Soares BG. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst Rev. 2012;10:CD008858. doi: 10.1002/14651858.CD008858.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Levin MJ. Immune senescence and vaccines to prevent herpes zoster in older persons. Curr Opin Immunol. 2012;24:494–500. doi: 10.1016/j.coi.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Denis F, et al. Hepatitis-B vaccination in the elderly. J Infect Dis. 1984;149:1019. doi: 10.1093/infdis/149.6.1019. [DOI] [PubMed] [Google Scholar]
- 18.Dormitzer PR, et al. Influenza vaccine immunology. Immunol Rev. 2011;239:167–177. doi: 10.1111/j.1600-065X.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- 19.Jackson ML, et al. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet. 2008;372:398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- 20.Wong K, Campitelli MA, Stukel TA, Kwong JC. Estimating influenza vaccine effectiveness in community-dwelling elderly patients using the instrumental variable analysis method. Arch Intern Med. 2012;172:484–491. doi: 10.1001/archinternmed.2011.2038. [DOI] [PubMed] [Google Scholar]
- 21.Couch RB, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine. 2007;25:7656–7663. doi: 10.1016/j.vaccine.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khurana S, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3:85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown LE. The role of adjuvants in vaccines for seasonal and pandemic influenza. Vaccine. 2010;28:8043–8045. doi: 10.1016/j.vaccine.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Ambrose CS, Luke C, Coelingh K. Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. Influenza Other Respi Viruses. 2008;2:193–202. doi: 10.1111/j.1750-2659.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes L, Lefebvre JS. Age-related Deficiencies in Antigen-Specific CD4 T cell Responses: Lessons from Mouse Models. Aging Dis. 2011;2:374–381. [PMC free article] [PubMed] [Google Scholar]
- 26.Miller C, Kelsoe G. Ig VH hypermutation is absent in the germinal centers of aged mice. J Immunol. 1995;155:3377–3384. [PubMed] [Google Scholar]
- 27.Yang X, Stedra J, Cerny J. Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice. J Exp Med. 1996;183:959–970. doi: 10.1084/jem.183.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 29.den Braber I, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Blackman MA, Woodland DL. The narrowing of the CD8 T cell repertoire in old age. Curr Opin Immunol. 2011;23:537–542. doi: 10.1016/j.coi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berent-Maoz B, Montecino-Rodriguez E, Dorshkind K. Genetic regulation of thymocyte progenitor aging. Semin Immunol. 2012;24:303–308. doi: 10.1016/j.smim.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixit VD. Impact of immune-metabolic interactions on age-related thymic demise and T cell senescence. Semin Immunol. 2012;24:321–330. doi: 10.1016/j.smim.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Pang WW, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beerman I, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148:1001–1014. doi: 10.1016/j.cell.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 37.Guerrettaz LM, Johnson SA, Cambier JC. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proc Natl Acad Sci U S A. 2008;105:11898–11902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol. 2012;24:342–349. doi: 10.1016/j.smim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Cancro MP, et al. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30:313–318. doi: 10.1016/j.it.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solana R, et al. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24:331–341. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Maue AC, et al. T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 2009;30:301–305. doi: 10.1016/j.it.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang A, Nikolich-Zugich J. Functional CD8 T cell memory responding to persistent latent infection is maintained for life. J Immunol. 2011;187:3759–3768. doi: 10.4049/jimmunol.1100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadighi Akha AA, Miller RA. Signal transduction in the aging immune system. Curr Opin Immunol. 2005;17:486–491. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nikolich-Zugich J, Li G, Uhrlaub JL, Renkema KR, Smithey MJ. Age-related changes in CD8 T cell homeostasis and immunity to infection. Semin Immunol. 2012;24:356–364. doi: 10.1016/j.smim.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Decman V, et al. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J Immunol. 2012;188:1933–1941. doi: 10.4049/jimmunol.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haynes L, Eaton SM. The effect of age on the cognate function of CD4+ T cells. Immunol Rev. 2005;205:220–228. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudd BD, et al. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci U S A. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, et al. Expression of p16(INK4a) prevents cancer and promotes aging in lymphocytes. Blood. 2011;117:3257–3267. doi: 10.1182/blood-2010-09-304402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.High KP, Akbar AN, Nikolich-Zugich J. Translational research in immune senescence: assessing the relevance of current models. Semin Immunol. 2012;24:373–382. doi: 10.1016/j.smim.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis MM. Immunology taught by humans. Sci Transl Med. 2012;4:117fs112. doi: 10.1126/scitranslmed.3003385. 10.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 54.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-Specific CD4(+) Memory-Phenotype T Cells Are Abundant in Unexposed Adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang N, et al. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci Transl Med. 2013;5:171ra119. doi: 10.1126/scitranslmed.3004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li GM, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 59.Pawelec G, McElhaney JE, Aiello AE, Derhovanessian E. The impact of CMV infection on survival in older humans. Curr Opin Immunol. 2012;24:507–511. doi: 10.1016/j.coi.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Goronzy JJ, et al. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saurwein-Teissl M, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 62.Andrews NP, Fujii H, Goronzy JJ, Weyand CM. Telomeres and immunological diseases of aging. Gerontology. 2010;56:390–403. doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goronzy JJ, Shao L, Weyand CM. Immune aging and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36:297–310. doi: 10.1016/j.rdc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smithey MJ, Li G, Venturi V, Davenport MP, Nikolich-Zugich J. Lifelong persistent viral infection alters the naive T cell pool, impairing CD8 T cell immunity in late life. J Immunol. 2012;189:5356–5366. doi: 10.4049/jimmunol.1201867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cicin-Sain L, et al. Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging. PLoS Pathog. 2012;8:e1002849. doi: 10.1371/journal.ppat.1002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mekker A, et al. Immune senescence: relative contributions of age and cytomegalovirus infection. PLoS Pathog. 2012;8:e1002850. doi: 10.1371/journal.ppat.1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandal PK, Rossi DJ. DNA-damage-induced differentiation in hematopoietic stem cells. Cell. 2012;148:847–848. doi: 10.1016/j.cell.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 68.Colmegna I, et al. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 2008;58:990–1000. doi: 10.1002/art.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ademokun A, Wu YC, Dunn-Walters D. The ageing B cell population: composition and function. Biogerontology. 2010;11:125–137. doi: 10.1007/s10522-009-9256-9. [DOI] [PubMed] [Google Scholar]
- 70.Dunn-Walters DK, Ademokun AA. B cell repertoire and ageing. Curr Opin Immunol. 2010;22:514–520. doi: 10.1016/j.coi.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 71.Gibson KL, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 73.Hakim FT, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115:930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castermans E, et al. Thymic recovery after allogeneic hematopoietic cell transplantation with non-myeloablative conditioning is limited to patients younger than 60 years of age. Haematologica. 2011;96:298–306. doi: 10.3324/haematol.2010.029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dowling MR, Hodgkin PD. Why does the thymus involute? A selection-based hypothesis. Trends Immunol. 2009;30:295–300. doi: 10.1016/j.it.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Aspinall R, Pitts D, Lapenna A, Mitchell W. Immunity in the elderly: the role of the thymus. J Comp Pathol. 2010;142 (Suppl 1):S111–115. doi: 10.1016/j.jcpa.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 77.Douek DC, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 78.Naylor K, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 79.Dutilh BE, de Boer RJ. Decline in excision circles requires homeostatic renewal or homeostatic death of naive T cells. J Theor Biol. 2003;224:351–358. doi: 10.1016/s0022-5193(03)00172-3. [DOI] [PubMed] [Google Scholar]
- 80.Hazenberg MD, Borghans JA, de Boer RJ, Miedema F. Thymic output: a bad TREC record. Nat Immunol. 2003;4:97–99. doi: 10.1038/ni0203-97. [DOI] [PubMed] [Google Scholar]
- 81.Sauce D, et al. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119:3070–3078. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sauce D, Appay V. Altered thymic activity in early life: how does it affect the immune system in young adults? Curr Opin Immunol. 2011;23:543–548. doi: 10.1016/j.coi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Czesnikiewicz-Guzik M, et al. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kimmig S, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kohler S, et al. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005;35:1987–1994. doi: 10.1002/eji.200526181. [DOI] [PubMed] [Google Scholar]
- 86.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 87.Cicin-Sain L, et al. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci U S A. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson PL, Yates AJ, Goronzy JJ, Antia R. Peripheral selection rather than thymic involution explains sudden contraction in naive CD4 T-cell diversity with age. Proc Natl Acad Sci U S A. 2012;109:21432–21437. doi: 10.1073/pnas.1209283110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wils EJ, et al. Insufficient recovery of thymopoiesis predicts for opportunistic infections in allogeneic hematopoietic stem cell transplant recipients. Haematologica. 2011;96:1846–1854. doi: 10.3324/haematol.2011.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gomez I, Marx F, Gould EA, Grubeck-Loebenstein B. T cells from elderly persons respond to neoantigenic stimulation with an unimpaired IL-2 production and an enhanced differentiation into effector cells. Exp Gerontol. 2004;39:597–605. doi: 10.1016/j.exger.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 91.Lee WW, et al. Age-dependent signature of metallothionein expression in primary CD4 T cell responses is due to sustained zinc signaling. Rejuvenation Res. 2008;11:1001–1011. doi: 10.1089/rej.2008.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shatynski KE, Chen H, Kwon J, Williams MS. Decreased STAT5 phosphorylation and GATA-3 expression in NOX2-deficient T cells: role in T helper development. Eur J Immunol. 2012;42:3202–3211. doi: 10.1002/eji.201242659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li QJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 94.Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells - a reflection of T cell differentiation, cell senescence and host environment. Semin Immunol. 2012;24:365–372. doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adachi K, Davis MM. T-cell receptor ligation induces distinct signaling pathways in naive vs. antigen-experienced T cells. Proc Natl Acad Sci U S A. 2011;108:1549–1554. doi: 10.1073/pnas.1017340108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fragoso R, et al. Modulating the strength and threshold of NOTCH oncogenic signals by mir-181a-1/b-1. PLoS Genet. 2012;8:e1002855. doi: 10.1371/journal.pgen.1002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang GC, Dash P, McCullers JA, Doherty PC, Thomas PG. T cell receptor alphabeta diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Sci Transl Med. 2012;4:128ra142. doi: 10.1126/scitranslmed.3003647. 10.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maciver NJ, Michalek RD, Rathmell JC. Metabolic Regulation of T Lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu M, et al. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proc Natl Acad Sci U S A. 2012;109:E879–888. doi: 10.1073/pnas.1109797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shao L, et al. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–1449. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, Sharpless NE. Tumor suppressor mechanisms in immune aging. Curr Opin Immunol. 2009;21:431–439. doi: 10.1016/j.coi.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Di Mitri D, et al. Reversible senescence in human CD4+CD45RA+CD27- memory T cells. J Immunol. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- 103.Effros RB. Telomerase induction in T cells: a cure for aging and disease? Exp Gerontol. 2007;42:416–420. doi: 10.1016/j.exger.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shao L, Goronzy JJ, Weyand CM. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med. 2010;2:415–427. doi: 10.1002/emmm.201000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cavanagh MM, Qi Q, Weyand CM, Goronzy JJ. Finding Balance: T cell Regulatory Receptor Expression during Aging. Aging Dis. 2011;2:398–413. [PMC free article] [PubMed] [Google Scholar]
- 108.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 110.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 111.Frasca D, Blomberg BB. Aging impairs murine B cell differentiation and function in primary and secondary lymphoid tissues. Aging Dis. 2011;2:361–373. [PMC free article] [PubMed] [Google Scholar]
- 112.Wrammert J, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sasaki S, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–3119. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khurana S, Frasca D, Blomberg B, Golding H. AID activity in B cells strongly correlates with polyclonal antibody affinity maturation in-vivo following pandemic 2009-H1N1 vaccination in humans. PLoS Pathog. 2012;8:e1002920. doi: 10.1371/journal.ppat.1002920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frasca D, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- 116.Vukmanovic-Stejic M, Rustin MH, Nikolich-Zugich J, Akbar AN. Immune responses in the skin in old age. Curr Opin Immunol. 2011;23:525–531. doi: 10.1016/j.coi.2011.05.008. [DOI] [PubMed] [Google Scholar]





