Abstract
Our previous study demonstrated that platelet-derived growth factor-BB (PDGF-BB) increased the cell proliferation of primary rat neuronal progenitor cells (NPCs). However, whether PDGF-BB regulates neurogenesis in HIV-associated neurological disorder (HAND) remains largely unknown. In this study we demonstrated that pre-treatment of NPCs with PDGF-BB restored Tat-mediated impairment of cell proliferation via activation of p38 and JNK MAPK pathways. Moreover, treatment with PDGF-BB induced inactivation of glycogen synthase kinase-3β (GSK-3β), evidenced by its phosphorylation at Ser9, this effect was significantly inhibited by the p38 and JNK inhibitors. Level of nuclear β-catenin, the primary substrate of GSK-3β, was also concomitantly increased following PDGF-BB treatment, suggesting that PDGF-BB stimulates NPC proliferation via acting on GSK-3β to promote nuclear accumulation of β-catenin. This was further validated by gain and loss of function studies using cells transfected with either the wild type or mutant GSK-3β constructs. Together these data underpin the role of GSK-3β/β-catenin as a novel target that regulates NPC proliferation mediated by PDGF-BB with implications for therapeutic intervention for reversal of impaired neurogenesis inflicted by Tat.
Keywords: PDGF-BB, HIV Tat, GSK-3beta, Beta-catenin, Neuronal progenitor cell
Introduction
HIV-associated neurological disorders (HAND) comprise a range of disease symptomatology with varying degrees of HIV-related neuropsychiatric impairments. While the advent of anti-retroviral therapy (ART) has decreased the incidence of HAND, its prevalence is actually on a rise (Gonzalez-Scarano and Martin-Garcia 2005). Increasing lines of evidence indicates that brains of patients with HAND exhibit not only neuronal damage/loss, but also exhibit fewer adult neural stem/progenitor cells (NPCs) in the hippocampus. Such a defect could account in part, for the increased prevalence of neurological disorders observed in patients with HAND in the post-ART era.
Neurogenesis includes a process wherein new dentate granule cells that are continuously generated from NPCs are integrated into the existing hippocampal circuitry in the adult mammalian brain (Venkatesan et al. 2007). Both physiological and pathological stimuli are known to regulate adult hippocampal neurogenesis. It is well documented that during HIV infection, early viral protein such as the HIV transactivating protein Tat, is both released by infected cells as well as taken up by neighboring cells. Tat, in turn, has been shown to impair neurogenesis (Mishra et al. 2010). These findings thus raise the concern that cognitive dysfunction in the HIV-infected individuals may, in part, be attributed to impaired hippocampal neurogenesis.
Neurotropic family of growth factors plays key roles in maintaining neuronal homeostasis via regulation of neurogenesis (Almeida et al. 2005; Mohapel et al. 2005). Growth factors belonging to the platelet-derived growth factor (PDGF) family are composed of products of four gene products (A–D) that can dimerize and bind to two receptor tyrosine kinases, PDGF-αR & -βR (Li et al. 2000; Bergsten et al. 2001; Heldin et al. 2002). Our previous study has implicated PDGF-BB as a crucial factor in the regulation of NPC proliferation (Yao et al. 2012b). However, whether PDGF-BB regulates neurogenesis especially in the context of HAND remains largely unknown.
It is well-known that GSK-3β plays a critical role in neuronal apoptosis and neurogenesis (Mao et al. 2009; Qu et al. 2010). Following GSK-3β inactivation, there was accumulation of its substrate-β-catenin in the nucleus. Reciprocally, in the presence of active GSK-3β, there was degradation of β-catenin. Following translocation into the nucleus, β-catenin has the capacity to bind with the transcription factors, T-cell factor/lymphoid enhancer binding factor (TCF/LEF), resulting in the promotion of cell survival and neurogenesis (Gordon and Nusse 2006; Kuwabara et al. 2009). Whether GSK-3β/β-catenin are involved in PDGF-BB-mediated neurogenesis in NPCs remains poorly understood.
In the current study, we demonstrate direct evidence of the involvement of PDGF-BB/PDGF-R axis in regulating NPC proliferation via a previously unidentified role of GSK-3β/β-catenin.
Materials and method
Reagents
Recombinant PDGF-BB was purchased from R&D Systems (Minneapolis, MN, USA) and Tat1–72 was obtained from UK College of Medicine, Lexington, KY. The specific p38 inhibitor SB203580 and JNK inhibitor SP600125 were purchased from Calbiochem (San Diego, CA). Tyrosine kinase inhibitor STI 571 was obtained from Novartis, Basel, Switzerland. The primary antibodies used were: p-p38, p38, p-JNK, JNK, p-GSK-3β (Ser9), GSK-3β, β-Catenin, Lamin B (Cell Signaling, 1:200), and β-actin (Sigma, 1:4000).
Isolation, differentiation & characterization of NPCs
NPCs derived from the hippocampus of embryonic day18 (E18) fetus were cultured in substrate-free tissue culture T75 flasks as reported by Tian et al. and our previous study (Tian et al. 2009; Yao et al. 2012b). Based upon our earlier studies, NPCs were treated with PDGF-BB (100 ng/ml) or HIV-1 Tat (200 ng/ml). Treatment of NPCs with pharmacological inhibitors (STI-571: 1 µM; SB203580: 20 µM; SP600125: 20 µM; GSK-3β VIII: 5 µM) for 1 h followed by exposure with Tat and/or PDGF-BB. Forty eight hours later, cells were examined for cell proliferation.
Cell proliferation
Cell proliferation was measured by CyQUANT cell proliferation assay. NPCs dissociated from neurosphere were seeded in 96-well plates at a density of 104 cells/well for 2 days and were pre-treated with PDGF-BB for 1 h followed by subsequent treatment with Tat for 48 h. Then, 100 µl of Quant® Direct reagent was added into each well and incubated in the CO2 incubator for 15 min. Fluorescence intensity of each well was obtained using a Dynatech MR5000 plate counter at excitation and emission wavelengths of 480 and 520 nm, respectively.
Western blotting (WB)
Treated cells were lysed using the Mammalian Cell Lysis kit (Sigma, St. Louis, MO, USA) and the NE-PER Nuclear and Cytoplasmic Extraction kit (Pierce, Rockford, IL, USA). Equal amounts of the proteins were electrophoresed in a sodium dodecyl sulfate-polyacrylamide gel (12 %) under reducing conditions followed by transfer to PVDF membranes. The blots were blocked with 5 % nonfat dry milk in TBST. Western blots were then probed with antibodies recognizing the p-p38, p38, p-JNK, JNK, p-GSK-3β (Ser9), GSK-3β, β-Catenin, Lamin B (Cell Signaling, 1:200), and β-actin (Sigma, 1:4000). The secondary antibodies were alkaline phosphatase conjugated to goat anti mouse/rabbit IgG (1:5000). Signals were detected by chemiluminescence (SuperSignal West Dura Chemiluminescent Substrate, Thermo Scientific). A single representative immunoblot for all related blots is shown in a given Figure. Immunoblot densitometry was performed using Image J (http://rsb.info.nih.gov/ij/) on blot images.
Immunocytochemistry
For immunocytochemistry, NPCs were plated on cover slips, fixed with 4 % paraformaldehyde and permeabilized with 0.3 % Triton X-100 in PBS. For β-catenin staining, after blocking, cells were then incubated with a blocking buffer followed by incubation with β-catenin antibody (1:200, Cell Signalling) overnight at 4 °C. For BrdU detection, the fixed cells were incubated in 2 N HCl with 0.3 % Triton X-100 followed by neutralization with 0.1 M boric acid (pH 8.0). Cells were then incubated with a blocking buffer followed by incubation with rabbit BrdU (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) antibodies overnight at 4 °C. Secondary AlexaFluor 594 goat anti-rabbit gG and AlexaFluor 488 goat anti-mouse IgG, was added at a 1:500 dilution for 2 h to detect β-catenin and BrdU, followed by mounting of cells with Vectashield onto glass slides (Vector Laboratories, Burlingame, CA). Fluorescent images were acquired at RTon a Zeiss Observer. A Z1 inverted microscope was used; images were processed using AxioVs 40 Version 4.8.0.0 software (Carl Zeiss MicroImaging GmbH). Photographs were acquired using an AxioCam MRm digital camera.
Short interfering (si) RNA transfection
To confirm the roles of GSK-3β and β-Catenin signaling in PDGF-BB-mediated NPC proliferation, NPCs were transfected with either the wild-type or mutant GSK-3β vector (WT or A9) (kind gift from Dr. J. Silvio Gutkind, National Institutes of Health/NIDCR) or siRNA β-catenin (Thermo Scientific Dharmacon) using the rat stem cell Nucleofector Kit (Amaxa, Gaithersburg, MD) according to the manufacturer’s instructions. Briefly, dissociated cells from neurospheres were resuspended in the transfection medium, mixed with A9 construct (0.5 µg/Well for 24-well plate) or siRNAs (100nM), and electroporated following which cells were quickly centrifuged, resuspended and plated. Cells were treated with PDGF-BB for proliferation or WB analyses at 96 h following siRNA delivery.
Statistical analysis
Data were expressed as mean ± SD. Significance of differences between control and samples treated with various drugs was determined by one-way ANOVA followed by post hoc least significant difference (LSD) test. Values of p <0.05 were taken as statistically significant
Results
PDGF-BB reversed impaired NPC proliferation mediated by Tat
Our previous study has reported that there was a concentration-dependent reduction of proliferation of NPCs in presence of Tat, with the maximal effect observed at concentrations of Tat at 200 ng/ml (Yao et al. 2012a). This concentration of Tat was therefore used for all the experiments (Bokhari et al. 2009; Yao et al. 2009) and must be noted is also physiologically relevant since the level of Tat protein in the CSF is about 16 ng/ml (Westendorp et al. 1995), while that in the serum of HIV+ patients is∼40 ng/ml with actual concentration at tissue sites being even higher (Westendorp et al. 1995; Xiao et al. 2000; Toborek et al. 2005; Rumbaugh et al. 2006; Eugenin et al. 2007).
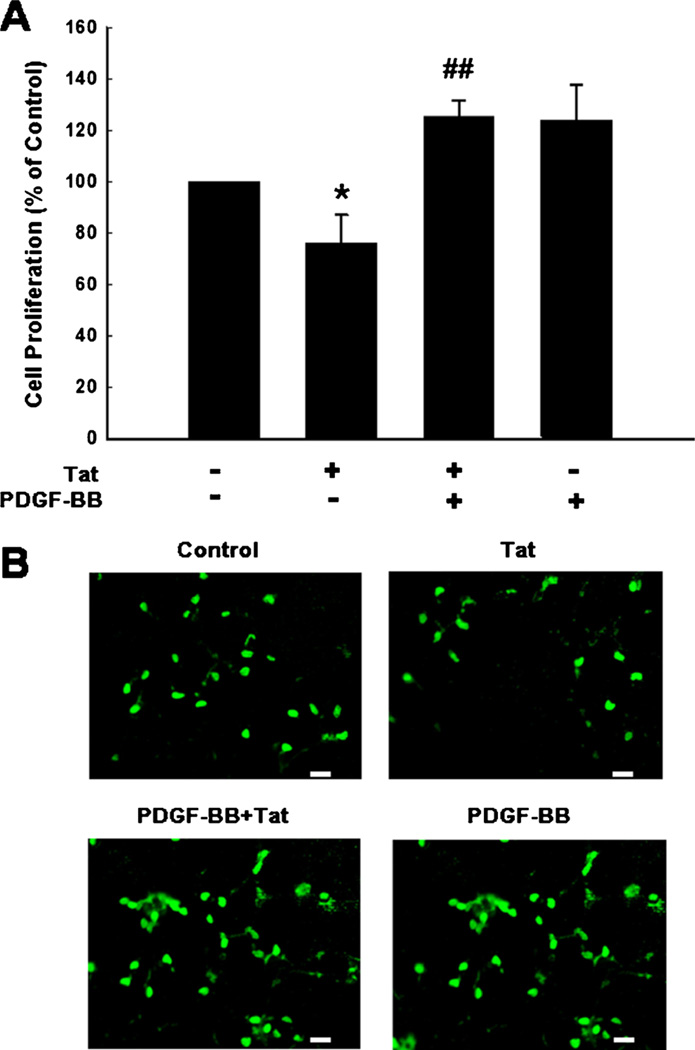
To explore the role of PDGF-BB in restoring cell proliferation, NPCs were pretreated with PDGF-BB followed by exposure of cells to Tat and assessed for proliferation. As shown in Fig. 1a PDGF-BB was able to restore Tat-mediated impairment of NPC proliferation. These results were further validated by immunostaining using the anti-BrdU antibody (Fig. 1b).
Fig. 1.
PDGF-BB reverses HIV-1 Tat-mediated impairment of proliferation of NPCs. a PDGF-BB restored Tat-mediated impairment of NPC proliferation. b Representative microscopic images showing BrdU-labeling in control, Tat, PDGF-BB plus Tat, PDGF-BB alone treated NPCs. BrdU (Green). Scale bar: 20 µm. Data are presented as mean ± SD of four individual experiments. *p <0.05 vs control group; ##p <0.01 vs Tat-treated group
Involvement of p38/JNK MAPKs in PDGF-BB-mediated increase of NPC proliferation
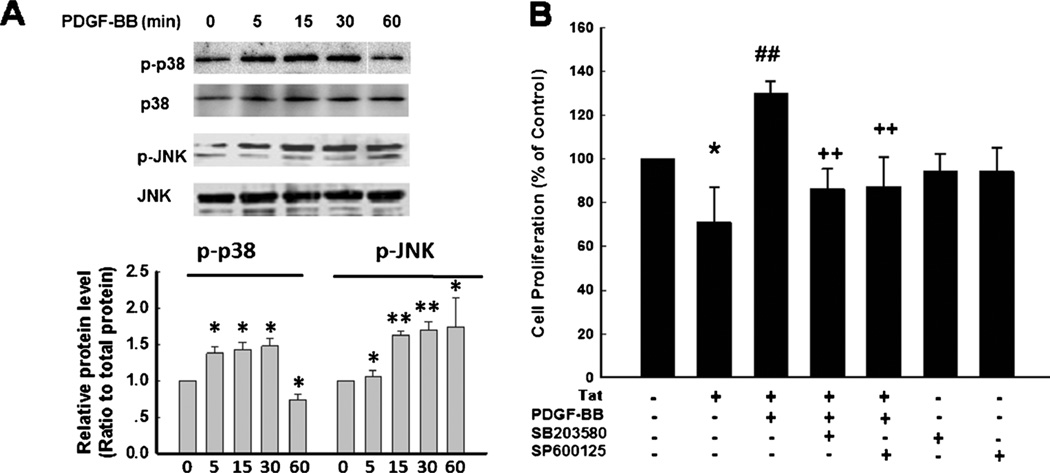
MAPK pathway has been demonstrated to play a crucial role in the proliferation of NPCs (Learish et al. 2000). It was therefore of interest next to examine the effect of PDGF-BB on the activation of p38 and JNK in NPCs. Exposure of NPCs to PDGF-BB resulted in time-dependent activation of p38/ JNK (Fig. 2a). Interestingly, pretreatment of NPCs with either the p38 or JNK inhibitor ameliorated PDGF-BB-mediated increased proliferation of NPCs (Fig. 2b).
Fig. 2.
P38 and JNK MAPKs are involved in PDGF-BB-mediated proliferation of NPCs. a PDGF-BB induced time-dependent phosphorylation of p38 and JNK (Upper panel). Densitometric analyses of p-p38/p38/and p-JNK/JNK from four separate experiments is presented (lower panel). b Pretreatment of NPCs with p38 inhibitor SB203580 (10 µM) or JNK inhibitor SP600125 (10 µM) for 1 h significantly attenuated the effect of PDGF-BB on the restoration of Tat-mediated impairment of proliferation. Data are presented as mean ± SD of four individual experiments. *p <0.05; **p <0.0l vs control group; ##p <0.01 vs Tat-treated group; ++p <0.01 vs both PDGF-BB & Tat treated group
Engagement of PDGF-αR, but not -βR is critical for PDGF-BB-mediated p38 and JNK activation
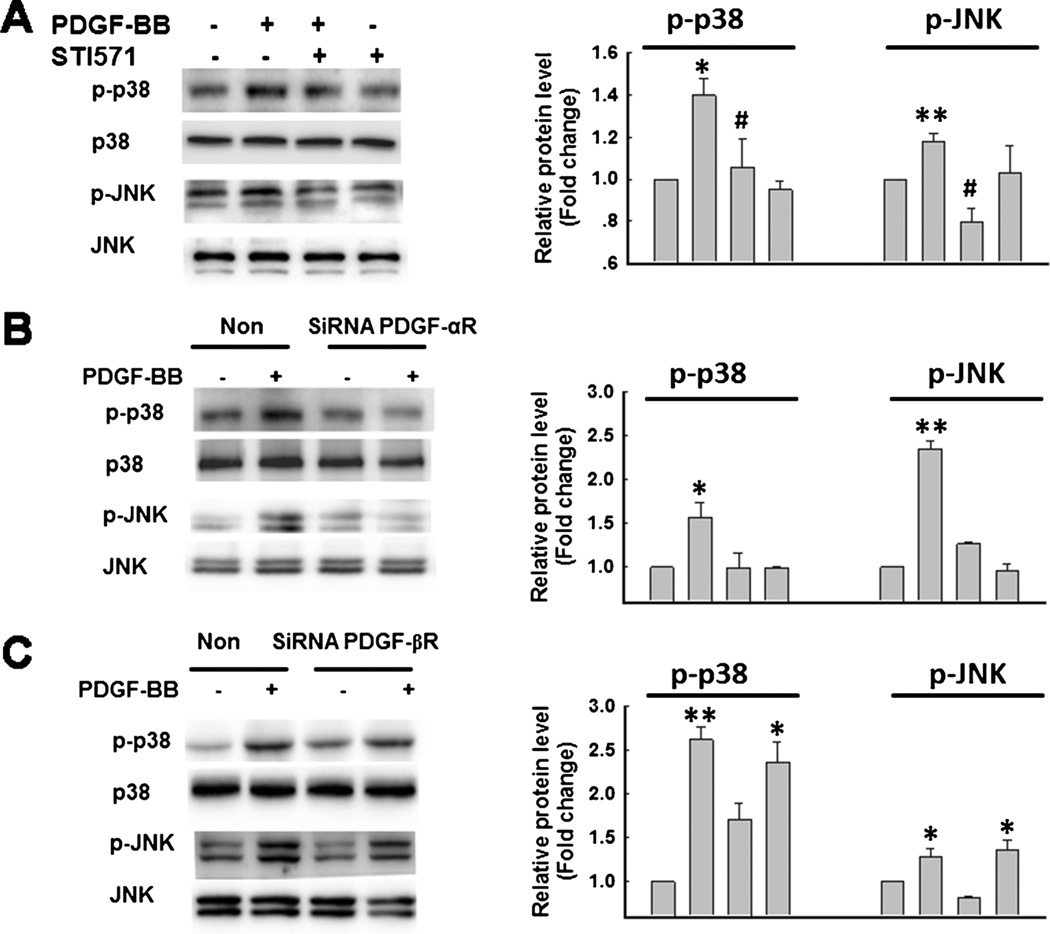
Since PDGF-BB mediates cell signaling through its cognate receptors PDGF-αR and -βR, we sought to examine the involvement of these receptors in PDGF-BB-mediated the increased proliferation of NPCs. Pre-treatment of NPCs with the tyrosine kinase receptor antagonist STI-571 abolished PDGF-BB-mediated increase in p38 and JNK activation, thus confirming the role of PDGF-BB/PDGF-R axis in this process (Fig. 3a).
Fig. 3.
Engagement of PDGF-αR, but not -βR is critical for PDGF-BB-mediated phosphorylation of p38 and JNK MAPKs. a STI-571 abolished PDGF-BB-mediated phosphorylation of p38 and JNK MAPKs (righ panel). Densitometric analyses of p-p38/ p38/and p-JNK/JNK from four separate experiments is presented (left panel). *p <0.05; **p <0.01 vs control group; #p <0.05 vs PDGF-BB-treated group. Transfection of NPCs with siRNAs specific for PDGF-αR (b), but not -βR (c) abolished PDGF-BB-mediated phosphorylation of p38 and JNK MAPKs (right panel). Densitometric analyses of p-p38/ p38/and p-JNK/JNK from four separate experiments is presented (left panel). *p <0.05; **p <0.01 vs Non-siRNA group
It is well recognized that STI-571 is not a specific antagonist for either PDGF-αR/-βR, since it can inhibit other tyrosine kinases as well. Additionally, the antagonist does not allow dissecting the individual contribution of the two receptors. As an alternative approach therefore we sought to knock down the respective receptor expression in NPCs using the siRNA strategy. Intriguingly, transfection of cells with PDGF-αR siRNA but not the -βR siRNA resulted in abrogation of PDGF-BB-mediated p38 and JNK activation (Fig. 3b – c).
GSK-3β plays the critical role in PDGF-BB mediated proliferation of NPCs
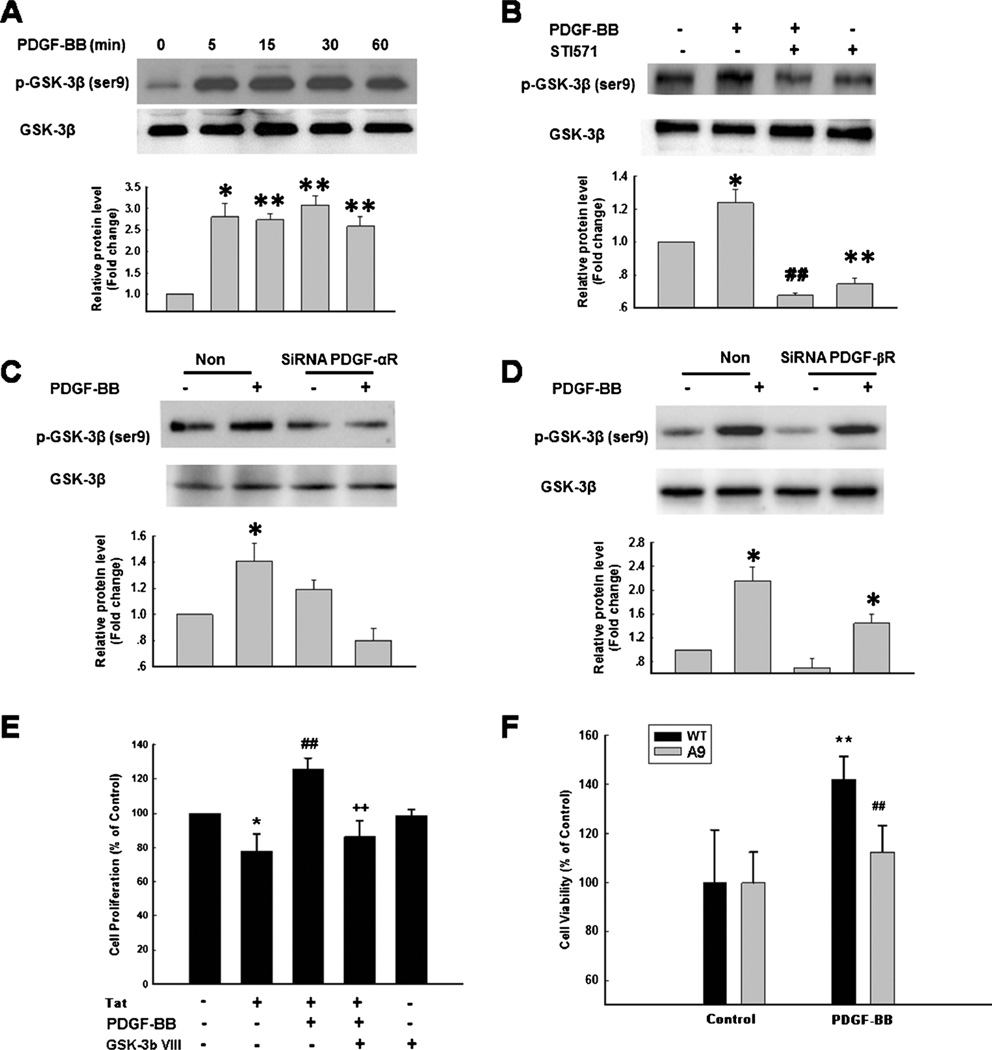
GSK-3β is an important signaling protein implicated in the translocation of several transcription factors that play a key role in the proliferation and differentiation of cells (Brunet et al. 1999). We next sought to explore the role of GSK-3β in PDGF-BB-mediated increased of NPC proliferation. As shown in Fig. 4a, treatment of NPCs with PDGF-BB resulted in inactivation of GSK-3β as evidenced by its phosphorylation at Ser9. This inactivation by PDGF-BB was abolished in cells pretreated with the antagonist for tyrosine kinase receptor STI-571 (Fig. 4b). Furthermore, using another genetic approach, NPCs were transfected with siRNA PDGF-αR or -βR in order to distinguish which type of PDGF receptors was involved in regulation of GSK-3β inactivation. As shown in Fig. 4c–d, transfection of cells with PDGF-αR siRNA but not the -βR siRNA resulted in abrogation of p38 and JNK activation induced by PDGF-BB.
Fig. 4.
Engagement of PDGF-αR, but not -βR is critical for PDGF-BB-mediated phosphorylation of GSK-3β. a PDGF-BB induced time-dependent phosphorylation of GSK-3β (upper panel). Densitometric analyses of p-GSK-3β/GSK-3β from four separate experiments is presented (lower panel). *p <0.05; **p <0.01 vs control group. b STI-571 abolished PDGF-BB-mediated phosphorylation of GSK-3β (upper panel). Densitometric analyses of p-GSK-3β/GSK-3β from four separate experiments is presented (lower panel). *P <0.05; **p <0.01 vs control group; ##p <0.01 vs PDGF-BB-treated group. Transfection of NPCs with siRNAs specific for PDGF-αR (c), but not-βR(d) abolished PDGF-BB-mediated phosphorylation of GSK-3β (upper panel). Densitometric analyses of p-GSK-3β/GSK-3β from four separate experiments is presented (lower panel). *p <0.05 vs Non-siRNA group. e Pretreatment of NPCs with GSK-3β inhibitor GSKΠI (5 µM) for 1 h significantly attenuated the effect of PDGF-BB on the restoration of Tat -mediated impairment of proliferation. Data are presented as mean ± SD of four individual experiments. *p <0.05 vs control group; ##p <0.0l vs Tat-treated group; p <0.0l vs both PDGF-BB & Tat treated group. f Transfection of NPCs with mutant GSK-3β (A9) significantly attenuated the effect of PDGF-BB on the NPC proliferation. Data are presented as mean ± SD of four individual experiments. **p <0.0l vs WT group; ##p <0.01 vs PDGF-BB-treated cells in WT group
The next step was to investigate the functional role of GSK-3β inactivation in PDGF-BB-mediated increase of NPC proliferation using GSK-3β VIII or gain and loss of functions for GSK-3β. Pretreatment of NPCs with GSK-3β VΠI reversed PDGF-BB-mediated increase of NPC proliferation (Fig. 4e). Furthermore, in NPCs transfected with WT GSK-3β (Fig. 4f), as expected, PDGF-BB induced increase of NPC proliferation, however, in cells transfected with the mutant of GSK-3β (A9), PDGF-BB failed to induce inactivation of GSK-3β, thereby resulting in failure of the ability of PDGF-BB to induce increased proliferation of NPCs.
PDGF-mediated activation of GSK-3β involves activation of p38/JNK signal
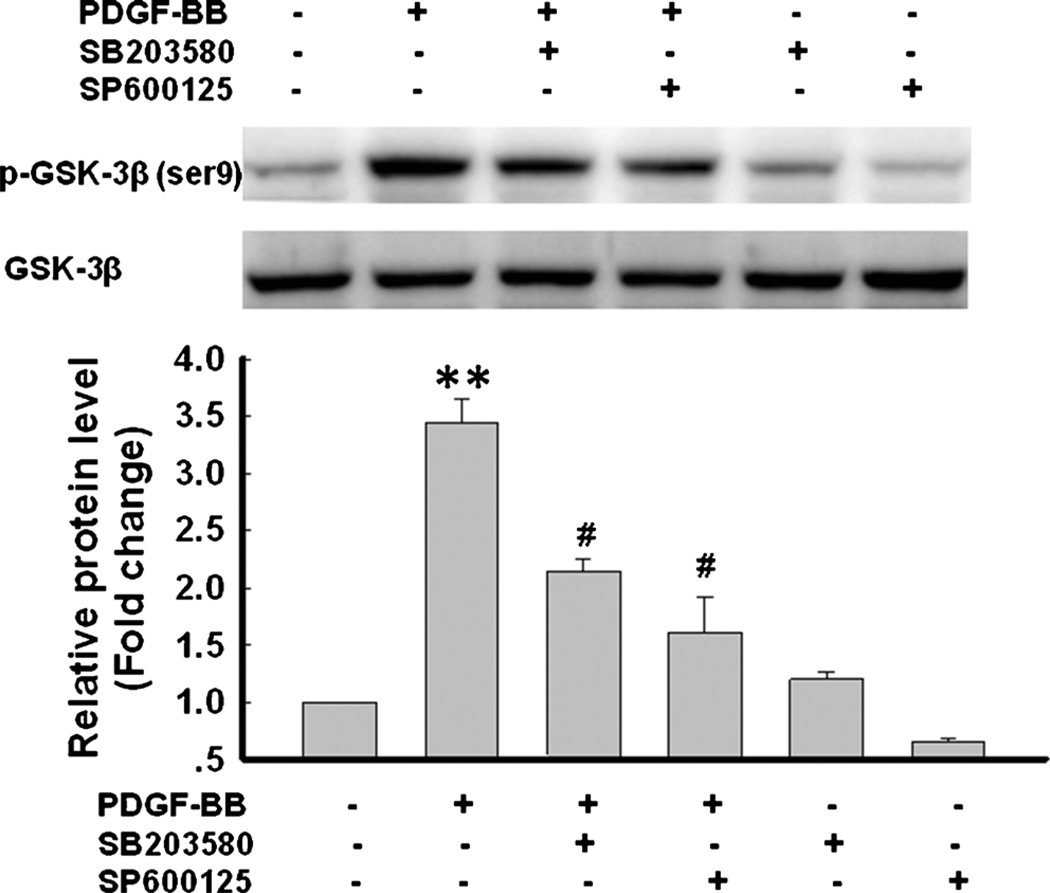
Having determined the role of p38/JNK MAPKs in PDGF-BB-mediated cell proliferation, we next wanted to examine the link of these pathways with PDGF-BB-mediated inactivation of GSK-3β. Pre-treatment of NPCs with either the p38 (SB203580) or JNK inhibitor (SP600125) significantly attenuated PDGF-BB-induced GSK-3β phosphorylation, suggesting thereby that PDGF-BB-mediated inactivation of GSK-3β involved activation of the p38/JNK pathways (Fig. 5).
Fig. 5.
Involvement of p38/JNK MAPKs in PDGF-BB-mediated GSK 3β phosphorylation. Representative immunoblot of NPCs exposed to PDGF-BB in the presence of p38 inhibitor SB203580 (10 µM) or JNK inhibitor SP600125 (10 µM) for 1 h significantly was monitored for GSK-3β phosphorylation. Densitometric analysis of p-GSK-3β/GSK 3β from 4 individual experiments. **p <0.0l vs control group; #p < 0.05 vs PDGF-BB group
Involvement of β-catenin in PDGF-BB-mediated increase of NPC proliferation
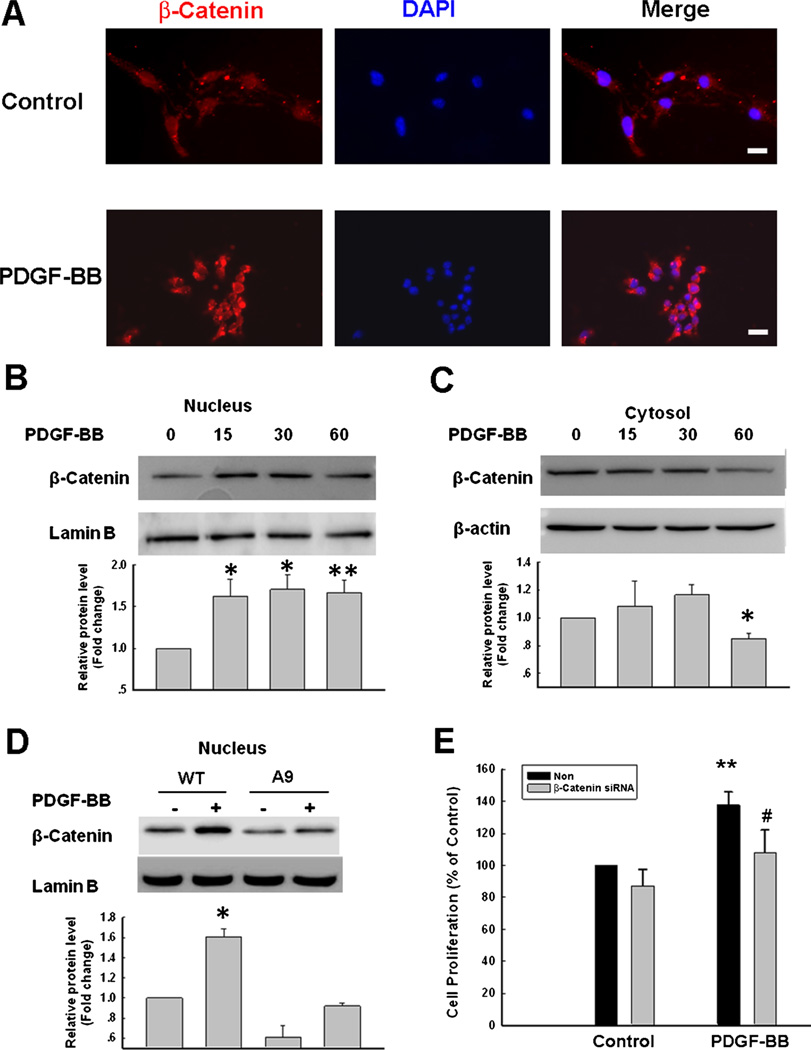
β-catenin is well recognized as an important substrate of GSK-3β signaling with a key role in neuronal survival and proliferation (Li et al. 2007; L’Episcopo et al. 2011). Therefore, the next step was to determine the effect of PDGF-BB on the nuclear translocation of β-catenin. As shown in Fig. 6a, in the untreated cells β-catenin immunoreactivity was primarily localized in the cytosol. Intriguingly, treatment of cells with PDGF-BB resulted in nuclear accumulation of β-catenin (Fig. 6b) with concomitant decrease of β-catenin in the cytosol (Fig. 6c). These findings were also validated by transfection of cells with the mutant GSK-3β construct (A9), which resulted in abrogation of increased accumulation of β-catenin in the nucleus following treatment with PDGF-BB (Fig. 6d). Confirmation of the role of β-catenin in PDGF-BB-mediated NPC proliferation was performed by trasnsfecting the cells with the β-catenin siRNA. In cells transfected with the β-catenin siRNA, PDGF-BB failed to induce NPC proliferation (Fig. 6e).
Fig. 6.
Involvement of β-catenin in PDGF-BB-mediated NPC proliferation. a Representative image demonstrating the immunostaining of β-catenin in the primary cultured NPCs treated with PDGF-BB. β-catenin (Red); DAPI (Blue). Scale bar=20 µm. b PDGF-BB induced time-dependent increase of β-catenin in the nuclear fraction (upper). Densitometric analyses of β-catenin/Lamin B from four separate experiments is presented (lower panel). *p <0.05; **p < 0.01 vs control group. c PDGF-BB decreased β-catenin in the cytosolic fraction (upper). Densitometric analyses of β-catenin/β-actin from four separate experiments is presented (lower panel). *P <0.05 vs control group. d Transfection of NPCs with mutant GSK-3β construct (A9) abolished PDGF-BB-mediated translocation of β-catenin (upper). Densitometric analyses of β-catenin/Lamin B from four separate experiments is presented (lower panel). *p < 0.05 vs WT group. e Transfection of NPCs with siRNA β-catenin significantly attenuated PDGF-BB mediated increase of NPC proliferation. Data are presented as mean ± SD of four individual experiments. **p <0.01 vs Non-siRNA group; #p <0.05 vs PDGF-treated cells transfected with non siRNA group
Discussion
Accumulating evidence has emerged demonstrating the vital role PDGF in the developing and adult brain (Mohapel et al. 2005). Previous studies have demonstrated PDGF-BB mediated neuroprotection against HIV proteins Tat and gp120 as well as its ability to promote NPC proliferation (Peng et al. 2008b; Peng et al. 2008a; Yao et al. 2009; Yao et al. 2012a). Herein we demonstrate that PDGF-BB significantly ameliorated HIV Tat-induced impairment of NPC proliferation via activation of the p38 & JNK MAPK pathways. Moreover, our findings also indicate that treatment of NPCs with PDGF-BB resulted in phosphorylation of GSK-3β on Ser9, a key inhibitory site, leading to the nuclear accumulation of β-catenin, the primary substrate of GSK-3β. The present study has identified a novel molecular target-GSK-3β/β-catenin that underlies the restoration of PDGF-BB-mediated neurogenesis.
Adult neurogenesis is a well orchestrated process involving continuous generation of the dentate granule cells from NPCs and their assimilation into the existing hippocampal circuitry (Venkatesan et al. 2007). Viral proteins such as Tat and gp120 have been shown to negatively affect the self-renewal capacity of the hippocampus by decreasing the proliferative rate of NPCs (Okamoto et al. 2007; Mishra et al. 2010). These findings thus raise the speculation that cognitive impairment due to HIV infection could, in part, be attributed to impaired hippocampal neurogenesis. There is no conclusive evidence of direct neuronal infection by HIV-1, and the decreased cell proliferation of NPSc or neuronal death is considered to be a consequence of the toxic effects of viral and cellular neurotoxins that are released from virus-infected and/or activated cells (Eugenin et al. 2003; Mishra et al. 2010). Among the viral products, HIV Tat has been shown to be neuroexcitatory and neurotoxic, and it continues to be implicated as a causative agent in HAND (Buscemi et al. 2007; Agrawal et al. 2012). Tat can be both secreted from infected cells and can also be taken up by neighboring non-infected cells, including neurons (Liu et al. 2000). It is well-recognized that microglia/macrophages are the most commonly infected cells in the brain and serve as lifelong reservoirs for HIV (Minagar et al. 2002; Gonzalez-Scarano and Martin-Garcia 2005; Kramer-Hammerle et al. 2005). Mounting evidence also suggests the role of astrocytes as cells permissive for virus replication, albeit as low levels (Conant et al. 1994; Brack-Werner 1999; Canki et al. 2001; Li et al. 2011; Atluri et al. 2013).
PDGF is composed of a family of five dimeric ligands that are assembled from four gene products (PDGF A-D) and that dimerize and act via two receptor tyrosine kinases, PDGF-αR & -βR (Zauli et al. 2000; Bergsten et al. 2001; Heldin et al. 2002). PDGF-BB has been implicated as a crucial factor in the developing postnatal rat brains (Smits et al. 1991). Previous reports have indicated that PDGF-BB mediated increased NPC proliferation involved the ERK MAPK pathway (Yao et al. 2012a). Furthermore, PDGF-BB pretreatment was also known to induce striatal neurogenesis in a Parkinsonian rat model of 6-hydroxydopamine lesions (Mohapel et al. 2005). The novel finding of this report is that PDGF-BB via binding to its cognate receptors was able to rescue Tat -mediated impairment of NPC proliferation.
Through the stimulation of PDGF-Rα and PDGF-Rβ, PDGF-BB has been shown to activate various signaling pathways such as ERK, JNK and p38 MAPK in various cell lines and tissues (Seo et al. 2011; Chan et al. 2013). Previous reports have also demonstrated that in NPCs, PDGF-BB ameliorated Tat & cocaine-mediated impairment of NPC proliferation through activation of the ERK MAPK pathway (Yao et al. 2012a). Interestingly, in addition to ERK activation, it was also demonstrated that two other MAPK signals-p38 and JNK, were also activated by PDGF-BB in NPCs. Inhibition of these both pathways at a dose that did not impact basal proliferation of hNPCs, significantly blocked PDGF-BB-induced cell proliferation. These findings thus suggest that activation of p38 & JNK MAPK signaling is essential for mediating the pro-proliferative effects of PDGF-BB in NPCs.
P38, ERK and JNK belong to the MAPK family. It is well-known that ERK cascade can be activated by a variety of growth factors and can transmit signals to promote cell proliferation (Yao et al. 2009; Yao et al. 2012a). The role of p38 and JNK MAPK signaling in NPC regulation however, has been less studied. In this study, we demonstrated that activation of p38MAPK mediated PDGF-BB-induced proliferation of NPCs. Our findings are in agreement with a previous study demonstrating curcumin-mediated stimulation of embryonic neural progenitor cells via the activation of p38 MAPK pathway (Kim et al. 2008). Similar findings on the involvement of p38MAPK activation have also been reported in adiponectin-mediated cell proliferation in the adult rat hippocampal neural stem cells (Zhang et al. 2011). However, there are also contradictory reports indicating that activation of p38MAPK pathway by virus env gp120 leads to suppression of proliferation of adult hNPCs (Okamoto et al. 2007). A possible explanation for the discrepancy in the findings could be attributed to the transient versus sustained activation of p38 MAPK, which, in turn, could be due to distinct ability to recruit downstream signaling mediators.
In addition to PDGF-BB-mediated activation of p38 MAPK, another interesting finding herein was the observation that inhibition of JNK MAPK pathway resulted in reversal of PDGF-BB-mediated NPC proliferation thereby highlighting the potential role of this pathway. Our results are in agreement with previous reports implying the role of JNK activation in FGF2 (Sanalkumar et al. 2010), angionenic factor angioproieth-1 (Rosa et al. 2010), TNF-α (Bernardino et al. 2008) and ampaline CX546(Schitine et al. 2012)-mediated proliferation of NPCs.
Another key finding of this study was the involvement of GSK-3β/β-catenin in PDGF-BB-mediated proliferation, thereby lending credence to previous reports demonstrating GSK-3β as a key regulator of diverse cellular process including neurogenesis (Zhang et al. 2011). Inactivation of GSK-3β resulted in accumulation of its substrate-β-catenin in the nucleus, binding with the TCF/LEF. TCF/LEF is a group of down-stream transcription factors of the Wnt/β-catenin signal pathway that have been implicated in the survival of neuronal and glial cells (Pei et al. 2012; Salins et al. 2007; Korade and Mimics 2011; Henderson et al. 2012), via regulation of various cell survival-related genes, such as neurotension receptor 1 gene (Souaze et al. 2006) and calcium/calmodulin-dependent protein kinase IV (CamK4) (Arrazola et al. 2009). GSK-3β phosphorylation at Ser9 inhibits its apoptotic activity, whereas phosphorylation at Tyr216 promotes its apoptotic activity (Tang et al. 2010). In the present study, we showed that PDGF-BB induced phosphorylation at Ser9 of GSK-3β in NPCs, which was consistent with the previous report that PDGF-CC stimulated GSK-3β phosphorylation at Ser9 (Tang et al. 2010). In our studies we failed to detect phosphorylation of GSK-3β at Tyr216 (data not shown). Furthermore, the effect of GSK-3β phosphorylation induced by PDGF-BB was attenuated by pretreatment of cells with either the p38 or JNK MAPK inhibitors, indicating thereby that PDGF-BB induced phosphorylation of GSK-3β at Ser9 involved activation of the p38 and JNK MAPK pathway. These finding are in agreement with a previous report demonstrating that p38MAPK directly inactivated GSK-3β by phosphorylating Ser389 in the C-terminus of GSK-3β in the brain and thymocytes, resulting in increased accumulation of intracellular β-catenin (Thornton et al. 2008).
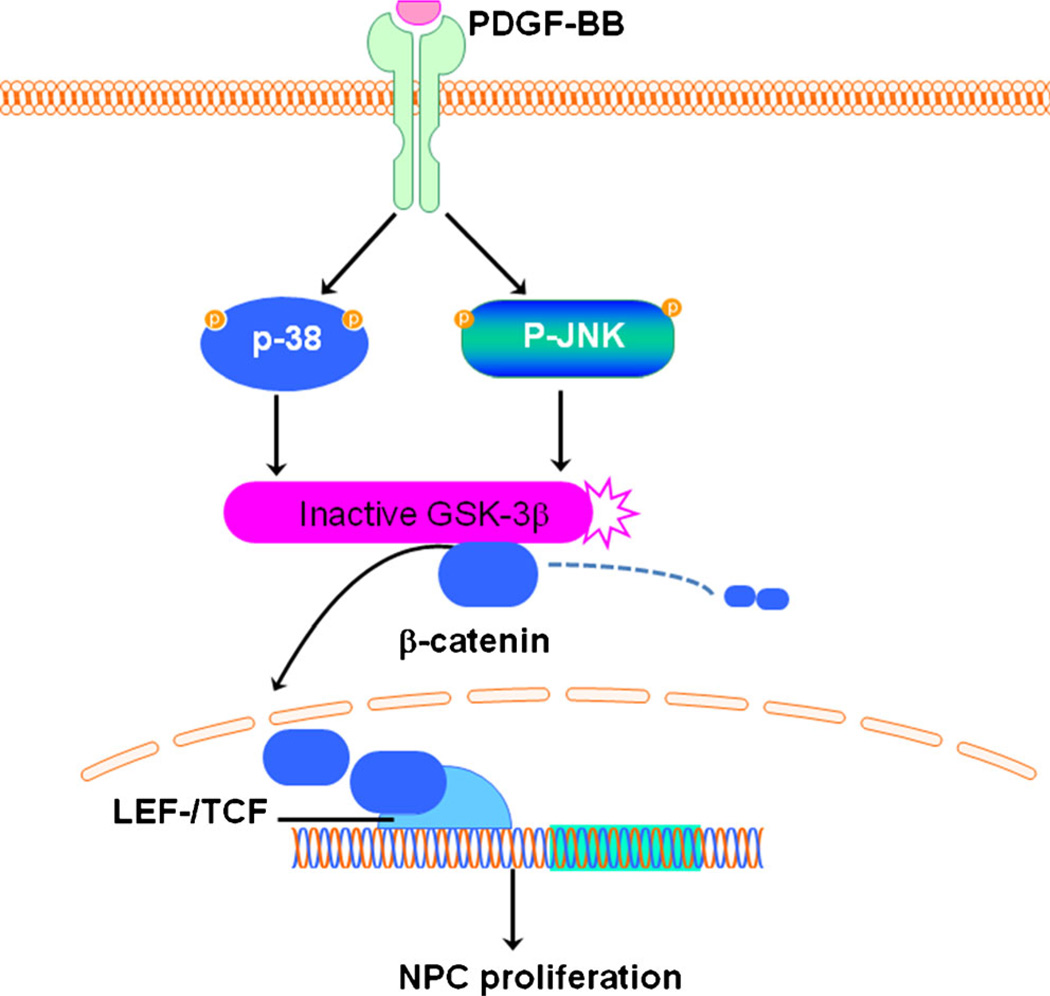
In summary, we provide herein the first evidence that PDGF-BB promoted proliferation of NPCs via activation of the p38 and JNK MAPK/GSK-3β/β-catenin signaling cascade (Fig. 7). Our previous study has indicated that PDGF-BB restored Tat and cocaine-mediated impaired neurogenesis, suggesting thereby that PDGF-BB has the potential to reverse impaired neurogenesis in the context of HIV infection in vivo. It is critical as a next step to investigate the involvement and functionality of PDGF-BB and its signaling pathways in regulating NPC proliferation in vivo. Since impaired hippocampal neurogenesis has been implicated in HAND and other neurological disorders, based on our findings it can be extrapolated that PDGF-BB could serve as a therapeutic target for these disorders.
Fig. 7.
Schematic illustration demonstrating signaling pathways involved in PDGF-BB-mediated increase in proliferation of NPCs. PDGF-BB-mediated engagement of the PDGF-αR stimulated the p38/JNK pathways, which in turn, inactivated GSK-3β resulting in β-catenin translocation into the nucleus, culminating ultimately to increased proliferation of NPCs
Acknowledgments
This work was supported by grants MH-068212, DA020392, DA023397 and DA024442 from the National Institutes of Health.
Footnotes
Conflict of interest disclosure The authors declare no competing financial interests.
Contributor Information
Honghong Yao, Email: hyao@unmc.edu.
Shilpa Buch, Email: sbuch@unmc.edu.
References
- Agrawal L, Louboutin JP, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 Tat neurotoxicity: a model of acute and chronic exposure, and neuroprotection by gene delivery of antioxidant enzymes. Neurobiol Dis. 2012;45:657–670. doi: 10.1016/j.nbd.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- Arrazola MS, Varela-Nallar L, Colombres M, Toledo EM, Cruzat F, Pavez L, Assar R, Aravena A, Gonzalez M, Montecino M, Maass A, Martinez S, Inestrosa NC. Calcium/calmodulin-dependent protein kinase type IV is a target gene of the Wnt/beta-catenin signaling pathway. J Cell Physiol. 2009;221:658–667. doi: 10.1002/jcp.21902. [DOI] [PubMed] [Google Scholar]
- Atluri VS, Kanthikeel SP, Reddy PV, Yndart A, Nair MP. Human synaptic plasticity gene expression profile and dendritic spine density changes in HIV-infected human CNS cells: role in HIV-associated neurocognitive disorders (HAND) PLoS One. 2013;8:e61399. doi: 10.1371/journal.pone.0061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001;3:512–516. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells. 2008;26:2361–2371. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- Bokhari SM, Yao H, Bethel-Brown C, Fuwang P, Williams R, Dhillon NK, Hegde R, Kumar A, Buch SJ. Morphine enhances Tat-induced activation in murine microglia. J Neurovirol. 2009;15:219–228. doi: 10.1080/13550280902913628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Geiger JD. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol Dis. 2007;26:661–670. doi: 10.1016/j.nbd.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canki M, Thai JN, Chao W, Ghorpade A, Potash MJ, Volsky DJ. Highly productive infection with pseudotyped human immunodeficiency virus type 1 (HIV-1) indicates no intracellular restrictions toHIV-1 replication in primary human astrocytes. J Virol. 2001;75:7925–7933. doi: 10.1128/JVI.75.17.7925-7933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CM, Chang HH, Wang VC, Huang CL, Hung CF. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRbeta, PI3K/Akt and MAPK pathways. PLoS One. 2013;8:e56819. doi: 10.1371/journal.pone.0056819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Tornatore C, Atwood W, Meyers K, Traub R, Major EO. In vivo and in vitro infection of the astrocyte by HIV-1. Adv Neuroimmunol. 1994;4:287–289. doi: 10.1016/s0960-5428(06)80269-x. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Eriksson U, Ostman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys. 2002;398:284–290. doi: 10.1006/abbi.2001.2707. [DOI] [PubMed] [Google Scholar]
- Henderson LJ, Sharma A, Monaco MC, Major EO, Al-Harthi L. Human immunodeficiency virus type 1 (HIV-1) transactivator of transcription through its intact core and cysteine-rich domains inhibits Wnt/beta-catenin signaling in astrocytes: relevance to HIV neuropathogenesis. J Neurosci. 2012;32:16306–16313. doi: 10.1523/JNEUROSCI.3145-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, Chung HY, Mattson MP, Lee J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283:14497–14505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Mimics K. Wnt signaling as a potential therapeutic target for frontotemporal dementia. Neuron. 2011;71:955–957. doi: 10.1016/j.neuron.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroDl and retro-elements during adult neurogenesis. NatNeurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learish RD, Bruss MD, Haak-Frendscho M. Inhibition of mitogen-activated protein kinase kinase blocks proliferation of neural progenitor cells. Brain Res Dev Brain Res. 2000;122:97–109. doi: 10.1016/s0165-3806(00)00064-x. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, Serapide MF, Tirolo C, Testa N, Caniglia S, Morale MC, Pluchino S, Marchetti B. AWntl regulated Frizzled-1/beta-Catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: Therapeutical relevance for neuron survival and neuroprotection. Mol Neurodegener. 2011;6:49. doi: 10.1186/1750-1326-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, Wang XC, Chen XQ, Yang Y, Zhang JY, Wang Q, Xu H, Liao FF, Wang JZ. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc Natl Acad Sci U S A. 2007;104:3591–3596. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Henderson LJ, Major EO, Al-Harthi L. IFN-gamma mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the beta-catenin pathway (DKK1) in a STAT 3-dependent manner. J Immunol. 2011;186:6771–6778. doi: 10.4049/jimmunol.1100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KE, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- Mishra M, Taneja M, Malik S, Khalique H, Seth P. Human immunodeficiency virus type 1 Tat modulates proliferation and differentiation of human neural precursor cells: implication in NeuroAIDS. J Neurovirol. 2010;16:355–367. doi: 10.3109/13550284.2010.513028. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132:767–776. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Kang YJ, Brechtel CW, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1:230–236. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Pei Y, Brun SN, Markant SL, Lento W, Gibson P, Taketo MM, Giovannini M, Gilbertson RJ, Wechsler-Reya RJ. WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development. 2012;139:1724–1733. doi: 10.1242/dev.050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Dhillon NK, Yao H, Zhu X, Williams R, Buch S. Mechanisms of platelet-derived growth factor-mediated neuroprotection-implications in HIV dementia. Eur J Neurosci. 2008a;28:1255–1264. doi: 10.1111/j.1460-9568.2008.06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Dhillon N, Callen S, Yao H, Bokhari S, Zhu X, Baydoun HH, Buch S. Platelet-derived growth factor protects neurons against gp120-mediated toxicity. J Neurovirol. 2008b;14:62–72. doi: 10.1080/13550280701809084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q, Sun G, Li W, Yang S, Ye P, Zhao C, Yu RT, Gage FH, Evans RM, Shi Y. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12(sup):31–10. 31–39. doi: 10.1038/ncb2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa AI, Goncalves J, Cortes L, Bernardino L, Malva JO, Agasse F. The angiogenic factor angiopoietin-1 is a proneurogenic peptide on subventricular zone stem/progenitor cells. J Neurosci. 2010;30:4573–1584. doi: 10.1523/JNEUROSCI.5597-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh J, Turchan-Cholewo J, Galey D, St Hillaire C, Anderson C, Conant K, Nath A. Interaction of HIV Tat and matrix metalloproteinase in HIV neuropathogenesis: a new host defense mechanism. FASEB J. 2006;20:1736–1738. doi: 10.1096/fj.05-5619fje. [DOI] [PubMed] [Google Scholar]
- Salins P, Shawesh S, He Y, Dibrov A, Kashour T, Arthur G, Amara F. Lovastatin protects human neurons against Abeta-induced toxicity and causes activation of beta-catenin-TCF/LEF signaling. Neurosci Lett. 2007;412:211–216. doi: 10.1016/j.neulet.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Sanalkumar R, Indulekha CL, Divya TS, Divya MS, Anto RJ, Vinod B, Vidyanand S, Jagatha B, Venugopal S, James J. ATF2 maintains a subset of neural progenitors through CBF1/Notch independent Hes-1 expression and synergistically activates the expression of Hes-1 in Notch-dependent neural progenitors. J Neurochem. 2010;113:807–818. doi: 10.1111/j.1471-4159.2010.06574.x. [DOI] [PubMed] [Google Scholar]
- Schitine C, Xapelli S, Agasse F, Sarda-Arroyo L, Silva AP, De Melo Reis RA, de Mello FG, Malva JO. Ampakine CX546 increases proliferation and neuronal differentiation in subventricular zone stem/progenitor cell cultures. Eur J Neurosci. 2012;35:1672–1683. doi: 10.1111/j.1460-9568.2012.08072.x. [DOI] [PubMed] [Google Scholar]
- Seo J, Lee HS, Ryoo S, Seo JH, Min BS, Lee JH. Tangeretin, a citrus flavonoid, inhibits PGDF-BB-induced proliferation and migration of aortic smooth muscle cells by blocking AKT activation. Eur J Pharmacol. 2011;673:56–64. doi: 10.1016/j.ejphar.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Smits A, Kato M, Westermark B, Nister M, Heldin CH, Funa K. Neurotrophic activity of platelet-derived growth factor (PDGF): Rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci U S A. 1991;88:8159–8163. doi: 10.1073/pnas.88.18.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souaze F, Viardot-Foucault V, Roullet N, Toy-Miou-Leong M, Gompel A, Bruyneel E, Comperat E, Faux MC, Mareel M, Rostene W, Flejou JF, Gespach C, Forgez P. Neurotensin receptor 1 gene activation by the Tcf/beta-catenin pathway is an early event in human colonic adenomas. Carcinogenesis. 2006;27:708–716. doi: 10.1093/carcin/bgi269. [DOI] [PubMed] [Google Scholar]
- Tang Z, Arjunan P, Lee C, Li Y, Kumar A, Hou X, Wang B, Wardega P, Zhang F, Dong L, Zhang Y, Zhang SZ, Ding H, Fariss RN, Becker KG, Lennartsson J, Nagai N, Cao Y, Li X. Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3beta phosphorylation. J Exp Med. 2010;207:867–880. doi: 10.1084/jem.20091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Murrin LC, Zheng JC. Mitochondrial fragmentation is involved in methamphetamine-induced cell death in rat hippocampal neural progenitor cells. PLoS One. 2009;4:e5546. doi: 10.1371/journal.pone.0005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toborek M, Lee YW, Flora G, Pu H, Andras IE, Wylegala E, Hennig B, Nath A. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol. 2005;25:181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A, Nath A, Ming GL, Song H. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci. 2007;64:2120–2132. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Strieker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Peng F, Fan Y, Zhu X, Hu G, Buch SJ. TRPC channel-mediated neuroprotection by PDGF involves Pyk2/ERK/CREB pathway. Cell Death Differ. 2009;16:1681–1693. doi: 10.1038/cdd.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Duan M, Yang L, Buch S. Platelet-derived growth factor-BB restores human immunodeficiency virus Tat-cocaine-mediated impairment of neurogenesis: role of TRPC1 channels. J Neurosci. 2012;32:9835–9847. doi: 10.1523/JNEUROSCI.0638-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli G, Secchiero P, Rodella L, Gibellini D, Mirandola P, Mazzoni M, Milani D, Dowd DR, Capitani S, Vitale M. HIV-1 Tat-mediated inhibition of the tyrosine hydroxylase gene expression in dopaminergic neuronal cells. J Biol Chem. 2000;275:4159–4165. doi: 10.1074/jbc.275.6.4159. [DOI] [PubMed] [Google Scholar]
- Zhang D, Guo M, Zhang W, Lu XY. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3beta (GSK-3beta)/beta-catenin signaling cascade. J Biol Chem. 2011;286:44913–44920. doi: 10.1074/jbc.M111.310052. [DOI] [PMC free article] [PubMed] [Google Scholar]