Embryonic stem cell–derived RPE (hES-RPE) cells combined with retinal progenitor cells (RPCs) represent a promising therapy for geographic atrophy in age-related macular degeneration. In this study, polarized hES-RPE cells secreted high levels of the growth factor PEDF that promote the survival of RPCs.
Abstract
Purpose.
Human embryonic stem cell–derived RPE (hES-RPE) transplantation is a promising therapy for atrophic age-related macular degeneration (AMD); however, future therapeutic approaches may consider co-transplantation of hES-RPE with retinal progenitor cells (RPCs) as a replacement source for lost photoreceptors. The purpose of this study was to determine the effect of polarization of hES-RPE monolayers on their ability to promote survival of RPCs.
Methods.
The hES-3 cell line was used for derivation of RPE. Polarization of hES-RPE was achieved by prolonged growth on permeable inserts. RPCs were isolated from 16- to 18-week-gestation human fetal eyes. ELISA was performed to measure pigment epithelium–derived factor (PEDF) levels from conditioned media.
Results.
Pigmented RPE-like cells appeared as early as 4 weeks in culture and were subcultured at 8 weeks. Differentiated hES-RPE had a normal chromosomal karyotype. Phenotypically polarized hES-RPE cells showed expression of RPE-specific genes. Polarized hES-RPE showed prominent expression of PEDF in apical cytoplasm and a marked increase in secretion of PEDF into the medium compared with nonpolarized culture. RPCs grown in the presence of supernatants from polarized hES-RPE showed enhanced survival, which was ablated by the presence of anti-PEDF antibody.
Conclusions.
hES-3 cells can be differentiated into functionally polarized hES-RPE cells that exhibit characteristics similar to those of native RPE. On polarization, hES-RPE cells secrete high levels of PEDF that can support RPC survival. These experiments suggest that polarization of hES-RPE would be an important feature for promotion of RPC survival in future cell therapy for atrophic AMD.
Age-related macular degeneration (AMD) is the leading cause of blindness among the elderly and is characterized by progressive degeneration and loss of RPE and photoreceptor cells in the macular region leading to loss of central, high-acuity vision.1,2 As the population continues to age, the number of people with advanced AMD is expected to exceed 2.9 million in the United States by 2020.3 While advances have recently been made in the treatment of the neovascularization found in some late AMD patients, there is no effective treatment for the much more common dry form of AMD.4
Retinal pigment epithelial (RPE) cells in human eyes form a quiescent, polarized epithelial monolayer located between neural retina and the vascular choroid and serve to support and maintain the photoreceptor cells and other outer retinal cells via multiple mechanisms including nutritional support, phagocytosis of the shed outer segments of the photoreceptor cells, participation in the retinoid cycle, and maintenance of the outer blood–retinal barrier. Because of its important function of supporting photoreceptors, the dysfunction and loss of RPE leads to photoreceptor degeneration or apoptosis. Substantial evidence supports the notion that dysfunction and death of RPE cells play a critical role in the pathogenesis of AMD.1,5,6
The emerging strategy of cell replacement therapy provides a new approach to the treatment of AMD. Various types of dissociated RPE cells, such as cultured human RPE cell lines, immortalized adult RPE cell lines, human fetal RPE cells, and RPE cells derived from human embryonic stem (hES) cells and induced pluripotent stem (iPS) cells, have been transplanted into the subretinal space of animal models with retinal degeneration caused by dysfunction of RPE.7–17 Many of these studies demonstrate protection of photoreceptors and even improvement in visual function after transplantation; however, the RPE cells are generally implanted early in the course of disease when most photoreceptors are still intact.15–17
In late AMD, there is loss of both RPE cells and photoreceptors. Several groups have shown that cells derived from postmitotic photoreceptor precursors or retinal progenitor cells (RPCs), or photoreceptor-like cells derived from hES or iPS cells, can migrate and integrate into the retina where they express photoreceptor markers.18–21 Studies showing successful subretinal transplantation of human retina+RPE suggest the possibility that more complex strategies that include both RPE and neurosensory retina may be feasible.22–24 Indeed, cell replacement therapies that include stem cell–derived RPE and photoreceptors have been suggested as a way to rebuild the outer retina.25 In support of this suggestion, three-dimensional constructs of RPE and early retinal progenitor cells have been derived from hES cells.26 However, much needs to be learned about the ability of transplanted RPE to promote survival of existing photoreceptors and survival, differentiation, and integration of transplanted RPCs or photoreceptors.
Pigment epithelium-derived factor (PEDF) was first identified in the conditioned media (CM) of human fetal and adult RPE cell cultures and belongs to the serpin superfamily.27,28 This secreted protein exhibits a broad spectrum of bioactive properties, and at least three activities are known to be critical for the health of normal retinas. First, its neuroprotective activity protects photoreceptor and other retinal neural cells from the damage of cytotoxic injury.29–32 Second, its antiangiogenic effect prevents pathologic neovascularization through inhibiting endothelial cell migration and promoting endothelial apoptosis.33–35 Third, its antisenescent function inhibits the premature senescence of both RPE cells and their neighboring retinal cells.36,37 However, the function that PEDF may play in RPE replacement therapy has not been explored.
Because of their unique properties of cell origin and in vitro proliferation and differentiation, mature RPE cells derived from hES cells (hES-RPE) represent a potentially unlimited resource for cell replacement therapy for AMD.15,16,38 In this study, we developed a two-stage hES-RPE induction procedure and successfully induced the differentiation of hES cells into a large number of functionally polarized hES-RPE cells that are suitable for subretinal transplantation in animal models. Although PEDF has been identified in hES-derived RPE,38 we found that it was secreted in low levels from nonpolarized cultures. When functionally polarized, hES-RPE secreted much larger amounts of PEDF through its apical domain, and this secreted PEDF prominently promoted the survival of human RPCs. These data support the contention that highly polarized hES-RPE would have an advantage over nonpolarized cultures for transplantation, both in providing a neuroprotective environment for existing photoreceptors and in supporting the future co-transplantation of RPE with RPC or photoreceptors for treatment of advanced AMD.
Materials and Methods
Differentiation of Human ES into RPE Cells
The utilization of hES cells was approved by the USC Stem Cell Research Oversight Committee (SCRO). hES-3 or -4 cells grown on mouse feeder cells were mechanically sliced into small pieces of cell aggregates containing 500 to 1000 cells with a 25-gauge ocular microsurgical knife. The ES cell aggregates were cultured in suspension for 3 days to form embryoid bodies in retinal neuronal differentiation medium (DMEM/F12 1:1 with 10% knockout serum replacement [Invitrogen, Carlsbad, CA], 2 mM glutamine (Omega Scientific;), 1× N2 supplement and 1× B27 (Invitrogen); and 1 ng/mL Noggin, 1 ng/mL DKK-1, and 5 ng/mL IGF [all from R&D Systems, Minneapolis, MN]). The embryoid bodies were transferred to gelatin- or growth factors-reduced synthetic matrix (Geltrex; Invitrogen)-coated cell culture dishes and further cultured from 8 to 12 weeks in RPE differentiation medium (DMEM with 10% knockout serum replacement [Invitrogen]). The unwanted nonpigmented cells and embryoid body residues were mechanically dissected with a 25-gauge ocular knife and removed from the culture dishes by a Pasteur pipette. The remaining putative RPE cells were allowed to continuously grow for another 48 hours on the same culture dishes, and then trypsinized and subcultured on gelatin- or fibronectin (Fisher Scientific, Waltham, MA)-coated cell culture plates depending on the total cell yields. The RPE-like cells grow into confluence in about 1 week in hES-RPE medium (DMEM with 10% knockout serum replacement [Invitrogen], 5% FBS [Omega Scientific, Tarzana CA], 10 ng/mL FGF2 [R&D Systems]), and produce melanin pigment and form a hexagonal shape in about 2 to 3 weeks of culture. After RPE-like cells became confluent, FGF was removed from the hES-RPE growth medium. For passage of hES-RPE, the cells were trypsinized for 10 minutes, enzyme activity was neutralized with fresh DMEM containing 10% FBS, and the cells were pelleted and resuspended in fresh RPE growth medium and grown to confluence.
The RPE cells were randomly selected for chromosome karyotype. Chromosomes from hES-RPE cells P2 to P3 were harvested and analyzed by using the GTW (G bands by trypsin and Wright's stain) banding method.39
Production of Polarized hES-RPE Cells
The culture and differentiation of hES-RPE cells to form a polarized RPE monolayer was similar to that used for fetal RPE culture, as described previously.40 Briefly, hES-RPE cells (2 × 105 for each well) at passage 3 were seeded on fibronectin-coated polyester membranes (Transwell System; Corning Costar, Corning, NY) and cultured in RPE growth medium (α-MEM basal medium containing 1% N1 supplement [Invitrogen]; 1× glutamine-penicillin-streptomycin, 1× nonessential amino acids, 2 mM taurine, 55 nM hydrocortisone, and 20 pM triiodo-thyronine [all from Sigma-Aldrich, St. Louis, MO]) supplemented with 1% serum (Omega Scientific) for at least 4 weeks. The polarization of the RPE was determined by the formation of functional tight junctions among the cells, as measured by the transepithelial resistance (TER) with a calomel electrode (EVOM epithelial tissue voltohmmeter; World Precision Instruments, Sarasota, FL) and observed by the immunostaining of representative cells with tight-junction–specific antibodies, such as ZO-1 and occludin.40 We have shown that for fetal human RPE, a total TER of 300 Ω · cm2 was associated with morphologic and functional polarization of the monolayer, including apical localization of microvilli and apical localization of Na-K ATPase.40 Therefore, once the TER reached a level of 300 Ω · cm2, the cell sheets were utilized or removed from the permeable membranes for further study. All experiments were performed on phenotypically similar, separately derived RPE lines from hES-3 cells.
Human Fetal RPE and RPC Isolation and Culture
This study conforms to applicable regulatory guidelines at the University of Southern California and principles of human research subject protection in the Declaration of Helsinki. The Institutional Review Board (IRB) of the University of Southern California approved our use of fetal eyes for the culture of retinal and RPE cells.
Human Fetal RPE Isolation and Culture.
Human fetal eyes (16–18 week gestation) obtained from Advanced Bioscience Resources, Inc. (ABR, Alameda, CA) were dissected to obtain posterior eye cups. After the retina was carefully peeled off, the eye cup was treated with 2% Dispase (Invitrogen-Gibco, Grand Island, NY) in PBS at 37°C for 20 minutes. The RPE cells were then gently scraped from the choroid with a sterile inoculating loop and filtered through a cell strainer to get rid of tissue clumps. The filtered RPE cells were centrifuged to pellet and resuspended with RPE growth medium with 10% FBS (Omega Scientific) and plated on a fibronectin-coated (Fisher Scientific) cell culture flask.40 After the RPE cells became confluent, the medium was changed to RPE growth medium with 1% FBS (Omega Scientific). Polarized fetal RPE were obtained as previously reported and as described for hES-RPE. Continuous culture of fetal RPE for >4 weeks was generally required to reach a TER of greater than 300 Ω · cm2.
Human Fetal RPC Isolation and Culture.
The peeled retina from the same posterior eye cup used for isolation of RPE cells was washed twice in PBS and digested with 0.05% trypsin (Invitrogen-Gibco) in 37°C for 15 minutes. The dispersed RPCs were neutralized with culture medium and pelleted through centrifugation. The cell pellets were resuspended in the RPC medium (DMED/F12 [1:1], with 10% knockout replacement serum (KRS; Invitrogen), 1× N2 supplement (Invitrogen), 1× B-27 (Invitrogen), 20 ng/mL FGF2 (R&D Systems), and 20 ng/mL EGF (R&D Systems), and kept for suspension culture in culture dishes to form neurospheres. The retinal neurospheres in suspension culture were subcultured once a week, and media were changed twice a week.
Treatment of RPCs with Conditioned Media
The retinal neurospheres were evaluated for size under a dissecting microscope. Twenty-five neurospheres of similar size were seeded into each fibronectin-coated well of 24-well culture plates (Fisher Scientific), allowed to grow 24 hours for cell attachment in RPC medium supplemented with FGF2 and EGF (R&D Systems), and then treated with RPC medium only as control; 1:1 ratio of RPC medium mixed with different CM (i.e., 50% 2× RPC medium mixed with 50% medium from polarized hES-RPE culture, polarized fRPE culture, ARPE-19 cell culture, or HEK-293 cell culture); RPC medium plus 50 ng/mL recombinant PEDF (Millipore, Temecula, CA); and the RPC medium plus recombinant PEDF and 20 μg/mL neutralizing monoclonal anti-PEDF antibody (Millipore). RPCs were cultured in CM for 4 weeks and then harvested for cell counting.
For BrdU incorporation or apoptosis assays, RPCs were cultured overnight in CM with or without anti-PEDF (Millipore) antibody, then treated with 10 μM BrdU (Roche, Indianapolis, IN) for 6 hours or with 250 μM hydrogen peroxide (H2O2) overnight and then fixed for further evaluation.
Reverse Transcription-Polymerase Chain Reaction
RT-PCR was used to verify the expression of RPE-specific or RPE-enriched genes. Total RNAs were extracted from confluent hES-RPE (nonpolarized versus polarized), fetal RPE (fRPE; nonpolarized versus polarized), the immortalized human RPE cell line (ARPE19) and undifferentiated hES cells (TRIzol reagent; Invitrogen). The cDNAs were synthesized with first-strand cDNA synthesis kit (Invitrogen). PCR was then performed (PCR Core kit; Qiagen). The sequences of primers for amplifying the four RPE-specific genes are shown in Table 1.
Table 1.
Primer Sequences for Amplification of RPE-Specific Genes
| Gene | Sequences | Length of Amplicon (bp) | |
|---|---|---|---|
| CRALBP | Forward | 5′-GGC AAA GTC AAG AAA TCA CCT T-3′ | 96 |
| Reverse | 5′-AGC CAT TGA TTT GAG TTT CCT C-3′ | ||
| PEDF | Forward | 5′-ACG CTA TGG CTT GGA TTC AG-3′ | 109 |
| Reverse | 5′-GGT CAA ATT CTG GGT CAC TTT C-3′ | ||
| RPE65 | Forward | 5′-GTG ACC GAT TCA AGC CAT CT-3′ | 296 |
| Reverse | 5′-GCA GCA GAG ATC CAC AAT CA-3′ | ||
| Bestrophin VMD2 | Forward | 5′-ATT GGG CCT TGG AAA ACA G-3′ | 324 |
| Reverse | 5′-GAC TGG ATC AGT GTC CTG CTG-3′ | ||
| GAPDH | Forward | 5′-CGA CCA CTT TGT CAA GCT CA-3′ | 110 |
| Reverse | 5′-GGT GGT CCA GGG GTC TTA CT-3′ |
Western Blot
Confluent cultures of hES-RPE, fRPE, ARPE-19, and undifferentiated hES cells were lysed in RIPA buffer (Sima; 50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.02% sodium azide, 0.1% SDS, 100 μg/mL phenylmethylsulfonyl fluoride <PMSF>, 1 μg/mL aprotinin, 1% Nonidet P-40, and 0.5% sodium deoxycholate). Protein concentrations were determined by a protein assay kit (Bio-Rad; Hercules, CA) using bovine serum albumin as the standard. Equal amounts of total proteins (20 μg/well) were electrophoresed on a 10% polyacrylamide gel, and electrically transferred to a PVDF membrane (Millipore Corp., Billerica, MA). The protein-bearing PVDF membranes were immunoblotted with anti-PEDF (1:1000 dilution; Millipore) and anti-RPE65 (1:500 dilution; Novus, Littleton, CO) for overnight at 4°C. An enhanced chemiluminescence (ECL) detection system (Thermo Scientific, Rockford, IL) was used to visualize the protein bands.
ELISA for PEDF
The culture media from confluent hES-RPE, fRPE, ARPE-19, and negative control HEK293 cells were collected. The corresponding cells were lysed in RIPA buffer (Sigma-Aldrich, Inc.), and the protein concentrations of those cell lysates were determined by protein assay (Bio-Rad) for normalization of PEDF measurement in ELISA assays. PEDF secretion levels were measured using human PEDF-ELISA Kit (BioProducts MD LLC, Middletown, MD), according to manufacturer's instructions.
Terminal Deoxynucleotidyl Transferase (TdT) dUTP Nick-End Labeling (TUNEL) and BrdU Incorporation Assay
RPCs cultured on fibronectin-coated culture plates (Fisher Scientific) in various CM, in RPC medium, or in the medium with recombinant PEDF were directly fixed with 2% paraformaldehyde in PBS (pH 7.4) or treated with 10 μM BrdU for 6 hours before fixation. The fixed cells were either incubated with TdT enzyme mixture using a cell viability assay (Cell Death Detection Kit; Roche) or reacted with a BrdU antibody conjugated with peroxidase (BrdU Incorporation Assay Kit; Roche). The numbers of TUNEL or BrdU+ cells were counted under a fluorescence microscope or phase contrast microscope, respectively. The number of positive cells was presented as the percentage of dead or proliferative cells.
Phagocytosis Assay
Preparation of Bovine Rod Outer Segments.
Bovine eyes were obtained from a local abattoir within 4 hours of death.41,42 Retinas were carefully peeled off from RPE layer, suspended in 34% sucrose-10 mM Tris-acetate buffer (pH 7.4, with 65 mM NaCl, and 2 mM MgCl2), and vortexed vigorously. The retina mixture was pelleted by centrifugation at 4000 rpm for 15 minutes (Eppendorf 5810R centrifuge; Fisher Scientific). The supernatant containing crude rod outer segments (ROS) was homogenized, diluted with two volumes of 10 mM Tris-acetate buffer, and centrifuged at 400 rpm with an F28/50 rotor for 5 minutes. The pellet was resuspended in 0.77 M sucrose-Tris-acetate buffer. The crude ROS in sucrose was sheared with a 26-gauge needle, layered on the discontinuously gradient sucrose solutions, 2.5 mL of each at 1.15 (1.14 M), 1.13 (1.00 M), 1.11 (0.84 M), and 1.10 (0.77 M) g/mL buffered with 10 mM Tris-acetate (pH7.4). The gradient sucrose solutions were centrifuged at 300,000 rpm for 30 minutes (SW-40 rotor; Beckman Coulter, Brea, CA). The ROS was carefully collected with a Pasteur pipette, transferred into a centrifuge tube, diluted with 5 mL of HBSS, pelleted by centrifugation at 7700g for 10 minutes at 4°C, resuspended in the buffered salt solution, and pelleted again at 800g for 10 minutes.
Fluorescein Isothiocyanate (FITC) Labeling of ROS.
The ROS pellet was suspended in DMEM and incubated with FITC (Cell Signaling, Danvers, MA) at final concentration of 10 μg/mL for 1 hour at room temperature. The labeled ROS was washed twice with repeated pelleting and suspending in DMEM.
Phagocytosis Assay.
The FITC-labeled ROS was added to the culture medium and incubated with the polarized and nonpolarized hES-RPE cells for 1, 6, and 24 hours. Lysosomes were prelabeled in these cultures (LysoTracker Red; Invitrogen-Molecular Probes, Eugene, OR). The cells were washed three times with PBS, fixed with 2% paraformaldehyde, and observed under the confocal microscope (LSM 510; Carl Zeiss Meditec, Inc., Thornwood, NY).
Immunofluorescence Assays
The polarized and nonpolarized hES-RPE grown on fibronectin-coated membranes (Transwell; Corning Costar) were fixed in freshly prepared 2% paraformaldehyde and blotted with primary antibodies, PEDF (1:200; Millipore Corp.), RPE65 (1:50; Novus Biologicals, Littleton, CO), ZO-1 (1:100; Invitrogen), and occludin (1:100; Invitrogen) overnight at 4°C. After incubation with fluorescein-conjugated second antibodies, the immunofluorescently stained images were examined and digitally recorded with a laser scanning confocal microscope (model 510; Carl Zeiss Meditec, Inc.).
Electron Microscopy
Scanning Electron Microscopy.
The polarized hES-RPE cells were fixed in half-strength Karnovsky's fixative, postfixed with 2% osmium tetroxide, dehydrated with ascending concentrations of ethanol, and infiltrated with hexamethyldisilazane (HMDS). After drying overnight under the hood, the cells were sputter coated for 10 minutes with an intermittent pulse using gold-palladium alloy. The cell surface was visualized in a scanning electron microscope (model 360; Cambridge Instruments, Cambridge, UK).
Transmission Electron Microscopy.
The polarized hES-RPE cells were fixed in half-strength Karnovsky's fixative (Ted Pella, Inc., Redding, CA), postfixed with 2% osmium tetroxide in 0.1 M cacodylate buffer for 1 to 2 hours at 4°C, dehydrated with ascending concentrations of ethanol, and embedded with graduated increased concentrations of Epon (all from Ted Pella) in propylene oxide medium and finally in 100% Epon mixture. The fixed cells were sectioned with a diamond knife on an ultramicrotome (Reichert-Jung [Leica], Bannockburn, IL), The ultrathin sections (6 nm) were double stained with uranyl acetate-lead citrate, and examined under an electron microscope (model EM10; Carl Zeiss Meditec, Inc.).
Results
Derivation of RPE Cells from Human Embryonic Cells
Based on the fact that RPE cells derive from neural ectoderm and share common characteristics with neural retinal cells, we experimented with a novel two-stage induction procedure to produce hES-RPE cells from hES-3 and -4 cell lines in vitro. The hES cell aggregates were first kept in suspension culture in neural differentiation medium supplemented with DKK-1, a Wnt antagonist, noggin, a BMP antagonist, and IGF, to undergo stage 1 induction into neural precursors. The neural precursors were then plated on the gelatin or matrix-gel coated cell culture dishes with RPE differentiation medium for further differentiation into putative RPE-like cells. After these stages of induction and differentiation, the putative pigmented hexagonal RPE cells appeared as early as 4 weeks in our culture conditions and reached a high enough number of cells for subculture at approximately 8 weeks. The hES-RPE cells could be subcultured for five to six passages and still kept their pigmented hexagonal morphology (Fig. 1). In preliminary experiments, similar results were obtained using hES-3 and -4; therefore, complete experimental results are presented in detail for hES-3.
Figure 1.
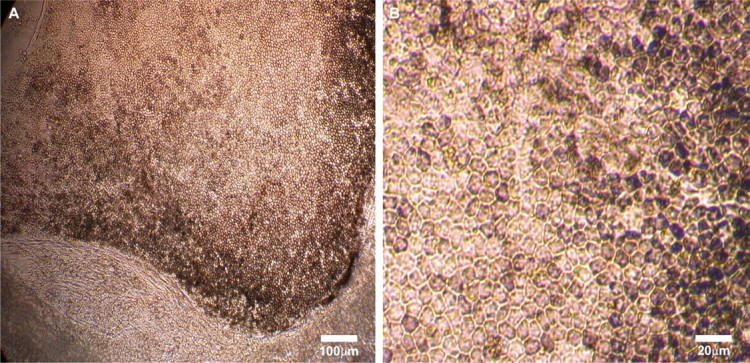
Phase-contrast microscopic images of hES-RPE cells derived from hES-3 stem cell line. After neural precursor induction and RPE spontaneous differentiation, the pigmented hexagonal RPE cells appeared as early as 4 weeks in our culture conditions and reached a high enough cell count for subculture at approximately 8 weeks. The hES-RPE cells could be subcultured for 5 to 6 passages and still retain their pigmented hexagonal morphology. (A) RPE-like cells differentiated from one embryoid body and cultured in the differentiation medium for 8 weeks. (B) Passage 3 hES-RPE cells cultured for 4 weeks.
Confirmation of hES-RPE Cells with RPE-Specific Gene Expression
RPE cells express RPE-specific genes and their protein products, including RPE65 and bestrophin (VMD2), and preferentially express other genes such as cellular retinaldehyde-binding protein (CRALBP) and pigment epithelium–derived factor (PEDF).28,43–46 To determine whether the RPE cells derived from hES cells can express these RPE-specific or related genes, we used RT-PCR, immunofluorescent staining and Western blot analysis to evaluate the expression of these genes in cultured hES-RPE cells. By RT-PCR, Western blot, and immunofluorescent staining, RPE65 was expressed in polarized (differentiated) hES-RPE cultures, but not in nonpolarized (proliferating stage) hES-RPE cultures (Fig. 2). By RT-PCR, VMD2 also showed higher expression in polarized hES-RPE when compared with nonpolarized hES-RPE. Although by RT-PCR, all RPE lines evaluated expressed PEDF and polarized hES-RPE, and polarized fRPE cells expressed much higher levels of PEDF than did the nonpolarized counterpart cells (Fig. 2). By Western blot, prominent reactivity for PEDF was found in polarized hES-RPE and fRPE, but immunoreactive bands were not identified in the nonpolarized hES-RPE or fRPE. Because of the sensitivity differences between RT-PCR and Western blot, the low levels of PEDF expressed in nonpolarized RPE cells were only detected by the more sensitive RT-PCR assay under these conditions. Western blot analysis in nonpolarized hES-RPE and fRPE using increased protein loading and longer exposure times showed low levels of PEDF expression (results not shown).
Figure 2.
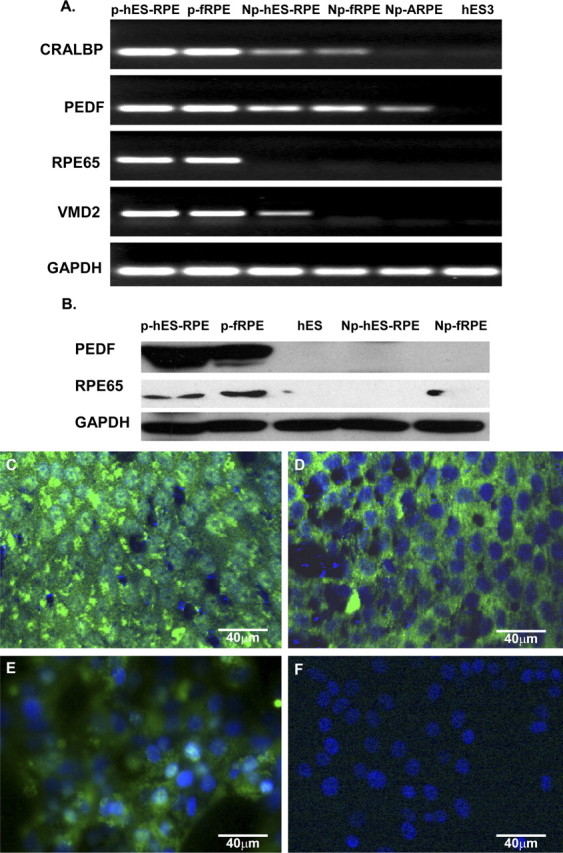
RT-PCR, Western blot analysis, and immunofluorescent staining of RPE-specific genes expressed by hES-RPE derived from hES-3 embryonic stem cell line and control cells. Polarized hES-RPE (p-hES-RPE; passage 3) and polarized fetal human RPE (p-fRPE) were compared with nonpolarized hES-RPE (Np-hES-RPE; passage 3), nonpolarized fetal human RPE (Np-fRPE), nonpolarized ARPE-19 (Np-ARPE), and undifferentiated hES3 cells. The polarized hES-RPE expressed high levels of RPE-specific gene products. (A) RT-PCR for CRALBP, PEDF, RPE65, and VMD2; GAPDH was the loading control. (B) Western blot for PEDF and RPE65 with GAPDH as loading control. (C–F) Immunofluorescent staining. PEDF was highly expressed in polarized hES-RPE cells at passage 3 (C) and less prominently expressed in nonpolarized hES-RPE cells (E). Immunofluorescent staining for RPE65 was found in polarized RPE cells (D), but not in nonpolarized hES-RPE cells (F). Immunoreactivity was detected using secondary antibodies conjugated to FITC (green). Nuclei are counterstained with DAPI (blue).
Differentiation of hES-RPE into a Polarized Monolayer
After confirming that these hES-RPE cells express RPE molecular markers, we proceeded to further induce these cells to form highly polarized RPE cell sheets. The dissociated hES-RPE cells were seeded in the fibronectin-coated inserts and cultured in RPE growth medium with 1% serum for 3 to 5 weeks. The formation of functional tight junctions among the cells was determined by measuring the TER with a calomel electrode. The total tissue resistance started to increase to 300 Ω · cm2 after 2 weeks of culture and could reach more than 500 Ω · cm2 after 5 weeks of culture. These highly polarized hES-RPE cells also exhibited positive immunofluorescent staining for the tight junction specific proteins, such as ZO-1 and occludin (Figs. 3A, 3B), whereas nonpolarized hES-RPE showed only focal staining that was not localized to the cell membrane (Figs. 3C, 3D). Furthermore, the polarized hES-RPE showed characteristic apical microvilli similar to those found on native RPE as shown by scanning electron microscopy (Fig. 3E). Intercellular tight junctions, apical microvilli, and melanin granules were also observed by transmission electron microscopy (TEM) in the polarized hES-RPE (Fig. 3F).
Figure 3.
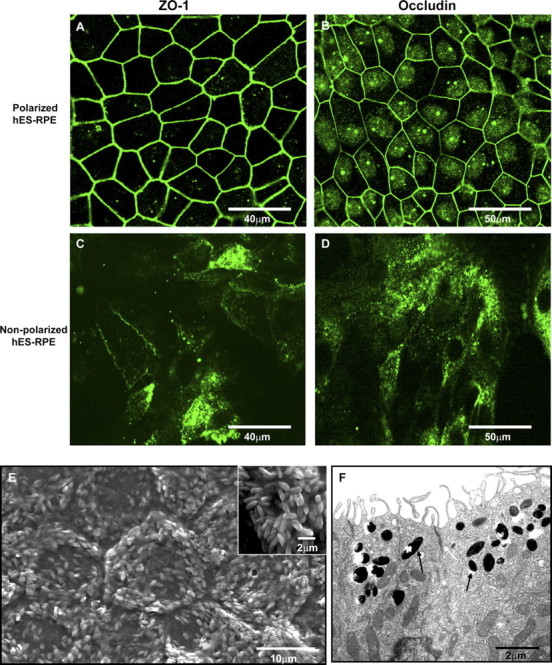
Confocal immunofluorescent, scanning electron microscopic (SEM), and transmission electron microscopic (TEM) images of polarized hES-RPE (passage 3) derived from the hES-3 cell line. The highly polarized hES-RPE cells exhibited positive immunofluorescent staining of the tight junction specific proteins ZO-1 and occludin (A, B). Although focal expression of ZO-1 and occludin was detectable in nonpolarized hES-RPE cells, the proteins did not localize to the cell membrane (C, D). Immunoreactivity was detected using secondary antibodies conjugated to FITC (green). Nuclei are counterstained with DAPI (blue). The polarized sheet of hES-RPE showed apical localization of microvilli on SEM (E; inset: one cell at higher magnification). TEM image of two cells in a polarized hES-RPE sheet shows apical microvilli, melanin granules in apical cytoplasm (black arrows) and apical tight junctions (white arrow) between the two cells (F).
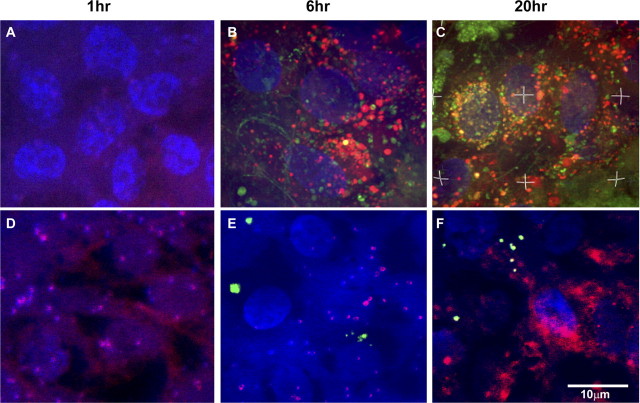
To examine the functional similarity between hES-RPE and the in vivo RPE, we challenged the polarized hES-RPE with FITC-labeled ROS at different time points. The phagocytosis assay revealed that there were no FITC-ROS-containing phagosomes in either polarized or nonpolarized hES-RPE cells after a 1-hour ROS challenge. FITC-positive phagosomes appeared in polarized hES-RPE cells at as early as 6 hours into the ROS treatment, whereas FITC-ROS did not appear in nonpolarized hES-RPE during this 6-hour time period (Fig. 4). After 20 hours of ROS challenge, numerous phagosomes were identified in polarized hES-RPE, and many were fused with lysosomes, whereas few phagosomes were found inside the nonpolarized hES-RPE (Fig. 4). These results suggest that the polarized hES-RPE cells have greater phagocytic function for ROS than do the nonpolarized hES-RPE.
Figure 4.
Confocal images to show the phagocytosis of ROS by polarized hES-RPE (passage 3) derived from hES-3 stem cell line compared to nonpolarized hES-RPE. The polarized and nonpolarized hES-RPE cells were challenged with FITC-labeled ROS (green) at different time points. Lysosomes were labeled with fluorescent dye (red), and nuclei were labeled with DAPI (blue). The phagocytosis assay revealed that there were no FITC-ROS containing phagosomes in either polarized or nonpolarized hES-RPE cells after a 1-hour challenge (A, D). FITC-ROS containing phagosomes appeared in polarized hES-RPE cells as early as 6 hours after ROS challenge (B), whereas they did not appear in nonpolarized hES-RPE at the same time period after ROS challenge (E). After 20 hours of FITC-ROS treatment, the labeled phagosomes reached the highest numbers inside polarized hES-RPE cells and many of them fused with red lysosomes (yellow; C), whereas few FITC-ROS containing phagosomes were found inside nonpolarized hES-RPE cells (F). Images are representative of experiments performed in triplicate.
Since retention of normal karyotype is very important for cell replacement therapy and in vitro culture may result in chromosomal alterations, we karyotyped the chromosomes of passage 2 hES-RPE cells using the GTW banding method. The results showed a normal female karyotype that was consistent during the differentiation and expansion period (data not shown).
Polarized hES-RPE Cells Secreted High Level of PEDF
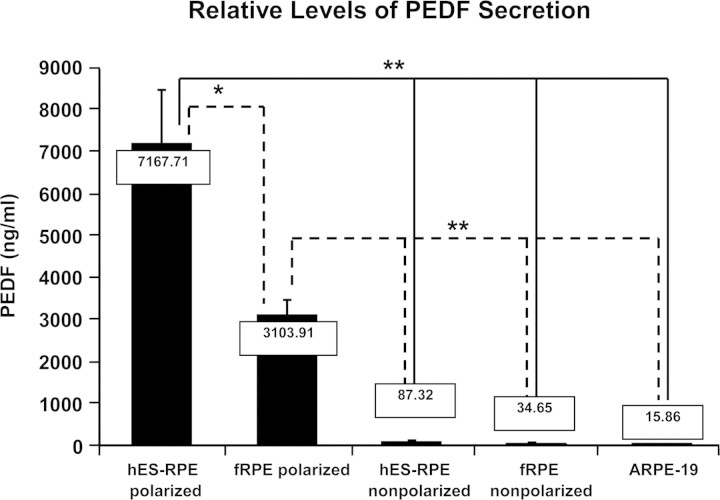
As one of the characteristic secreted RPE-derived growth factors, PEDF has well-established neuronal protective function.27–32 We found that polarized hES-RPE cells expressed much higher levels of PEDF in vitro than nonpolarized hES-RPE cells when evaluated by RT-PCR and Western blot analysis. To further quantify the PEDF secretion among the polarized hES-RPE, polarized fRPE cells and nonpolarized RPE counterparts, we used quantitative ELISA to measure the PEDF level in culture media from different RPE cells normalized to total protein of the cell lysates. The results revealed that both the polarized hES-RPE and polarized fRPE secreted PEDF at the milligrams per milliliter level, whereas nonpolarized hES-RPE, nonpolarized fRPE, and ARPE19 cells secreted PEDF at levels that were approximately 100-fold less (P < 0.001). Direct comparison of polarized hES-RPE versus fetal RPE revealed that polarized hES-RPE secreted twice as much PEDF as polarized fRPE (P < 0.001; Fig. 5). Interestingly, in polarized hES-RPE, most of the PEDF was apically secreted. PEDF immunocytochemistry and confocal microscopy showed that more cytoplasmic PEDF was located in the apical region of polarized hES-RPE RPE cytoplasm (Figs. 6A1–I1), whereas PEDF was reduced in amount and mostly located in the perinuclear region of nonpolarized hES-RPE cells (Figs. 6A2–I2). Furthermore, ELISA assay detected twice as much PEDF protein in the culture medium sampled from the apical side than medium sampled from the basal side and there are no differences between the samples from apical and basal sides of nonpolarized hES-RPE cells (data not shown).
Figure 5.
ELISA analysis of PEDF secretion by hES-RPE (passage 3) derived from hES-3 embryonic stem cell line and control cells. All PEDF values in the supernatants were normalized to total protein levels of the corresponding cellular lysates. Numerical values of PEDF concentration are shown in the box at the top of each bar. The polarized hES-RPE and the polarized fRPE secreted approximately 100-fold greater levels of PEDF than the nonpolarized hES-RPE, fRPE, and ARPE19 cells. ELISA revealed that polarized hES-RPE secreted twice as much PEDF as that of polarized fRPE. Results represent three independent experiments. *P < 0.05; **P < 0.01.
Figure 6.
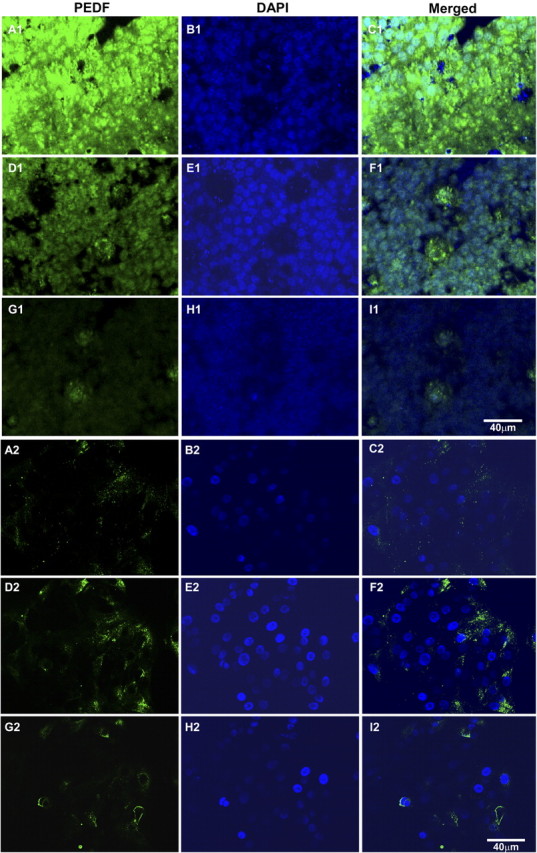
Confocal images of PEDF immunofluorescence in polarized (A1–I1) and nonpolarized (A2–I2) hES-RPE (passage 3) taken from equivalent levels in the apical region (A–C), middle region (D–F), and basal region (G–I). The majority of PEDF is localized to the apical cytoplasm of polarized hES-RPE cells (A1–I1), whereas PEDF was reduced in amount and found more in the central region in the cytoplasm of nonpolarized hES-RPE cells (A2–I2). Immunoreactivity for PEDF was detected using secondary antibodies conjugated to FITC (green). Nuclei are counterstained with DAPI (blue). Merged images show both PEDF immunoreactivity and DAPI nuclear staining. Images are representative of three independent experiments.
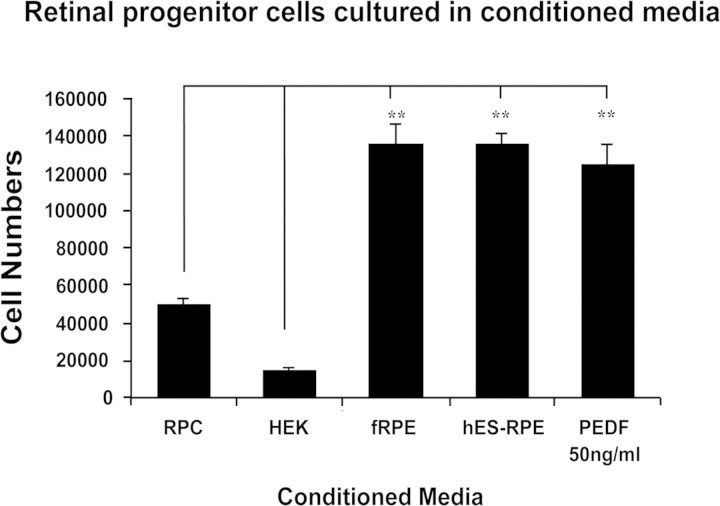
Conditioned Medium from Polarized hES-RPE Culture Promoted RPC Growth
To investigate the effect of PEDF from polarized hES-RPE culture on neural retinal cells, we cultured human RPCs both in the CM from polarized hES-RPE culture and in various control CM. After 4 weeks of culture, the cell-counting results indicated that significantly more RPCs were present in cultures with the polarized hES-RPE CM, polarized fRPE CM, or RPC medium supplemented with 50 ng/mL PEDF, than with control HEK-CM or control RPC medium only (P < 0.01; Fig. 7).
Figure 7.
RPC counting. Human RPCs cultured in the polarized hES-RPE (passage 3) CM, polarized fRPE CM, or RPC medium with 50 ng/mL PEDF supplement had a significantly higher number of cells than those cultured in the control HEK-CM or RPC medium. Results represent three independent experiments; **P < 0.01.
Increased cell number may result from either decreased cell death or increased cell proliferation. We therefore used TUNEL and BrdU incorporation assays to evaluate the rates of RPC death and proliferation after culture with various CM. The result showed that the RPCs cultured in the polarized hES-RPE CM, in the polarized fRPE CM, and in the RPC medium supplemented with 50 ng/mL PEDF had more BrdU-positive cells compared with RPCs cultured in HEK-CM, in RPC medium only (Fig. 8).
Figure 8.
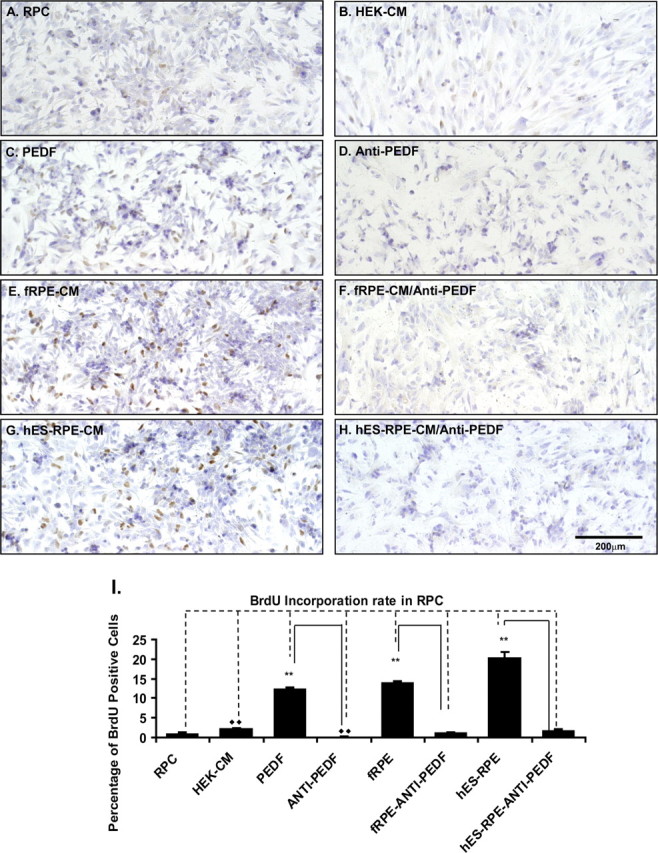
BrdU incorporation analysis of RPC proliferation after culture in various CM. The RPCs cultured in the polarized hES-RPE (passage 3) CM, polarized fRPE CM, or RPC medium with 50 ng/mL PEDF supplement had more proliferating cells (BrdU-positive cells) than those cultured in the regular RPC medium, HEK-CM, polarized fRPE CM, or hES-RPE CM, with anti-PEDF antibody. (A–H) Light microscopy images of BrdU-positive RPCs. (A) RPCs cultured in the regular RPC medium; (B) RPCs in HEK-CM; (C) RPCs in RPC medium with 50 ng/mL PEDF; (D) RPCs in RPC medium with 50 ng/mL PEDF and 20 μg/mL anti-PEDF antibody; (E) RPCs in the polarized fRPE CM; (F) RPCs in the polarized fRPE CM with 20 μg /mL anti-PEDF antibody; (G) RPCs in the polarized hES-RPE CM; (H) RPCs in the polarized hES-RPE CM with 20 μg/mL anti-PEDF antibody. (I) The quantification of BrdU-positive RPCs from three individual experiments. The RPCs cultured in the polarized hES-RPE CM, polarized fRPE CM, or RPC medium with recombinant PEDF supplement had a significantly higher number of BrdU-positive cells than those cultured in the regular RPC medium, HEK-CM, polarized fRPE- CM or hES-RPE CM, and recombinant PEDF with anti-PEDF antibody. **P < 0.01.
There were no differences in cell death of RPC cultured in the different CM, with or without recombinant PEDF (data not shown). However, after challenging with 250 μM H2O2, significantly less cell death was present in RPC cultured in polarized hES-RPE CM, in polarized fRPE CM, and in RPC medium supplemented with 50 ng/mL recombinant PEDF than those cultured in control HEK-CM and RPC medium (Fig. 9).
Figure 9.
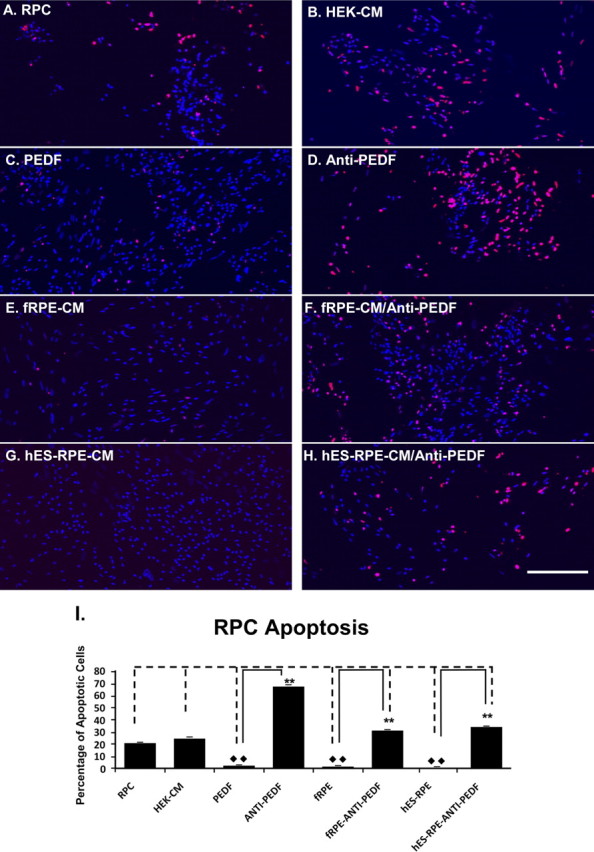
Apoptosis (TUNEL) assay to measure RPC death in various CM after overnight challenge with 250 μM H2O2. The RPCs cultured in the polarized hES-RPE (passage 3) CM, polarized fRPE CM, or RPC medium with 50 ng/mL PEDF supplement showed less cell death than those cultured in the regular RPC medium, HEK-CM, polarized fRPE CM, or hES-RPE CM with anti-PEDF antibody. Images (A–H) Fluorescent microscopic images of TUNEL+ RPCs. (A) RPCs cultured in the regular RPC medium; (B) RPCs in HEK-CM; (C) RPCs in RPC medium with 50 ng/mL PEDF; (D) RPCs in RPC medium with 50 ng/mL PEDF and 20 μg/mL anti-PEDF antibody; (E) RPCs in the polarized fRPE CM; (F) RPCs in the polarized fRPE CM with 20 μg /mL anti-PEDF antibody; (G) RPCs in the polarized hES-RPE CM; (H) RPCs in the polarized hES-RPE CM with 20 μg/mL anti-PEDF antibody. (I) The quantification of apoptotic (TUNEL+) RPCs from three individual experiments. The RPCs cultured in the polarized hES-RPE CM, polarized fRPE CM, or RPC medium with recombinant PEDF supplement had significantly fewer apoptotic cells than those cultured in the regular RPC medium, HEK-CM, and in the polarized fRPE CM, hES-RPE CM, and recombinant PEDF with anti-PEDF antibody; **P < 0.01.
To further confirm the effects of PEDF on the promotion of RPC proliferation and protection, we introduced 20 μg/mL anti-PEDF antibody into CMs to neutralize PEDF. As expected, the BrdU incorporated RPCs were markedly decreased, and apoptotic RPCs after oxidant treatment were significantly increased in hES-RPE CM with anti-PEDF antibody, in fRPE CM with anti-PEDF antibody, and in RPC medium supplemented with 50 ng/mL PEDF, along with anti-PEDF antibody (Fig. 9). These results strongly suggest that PEDF plays a major role in protecting and supporting RPC growth and that PEDF supports RPC growth through increasing RPC proliferation and protecting RPCs from oxidative stress–induced cell death.
Discussion
RPE dysfunction and loss are a pivotal pathologic changes in AMD.4,6,47,48 Because RPE cells in vivo lack the ability to functionally regenerate, cell replacement may be the only efficient way of restoring lost RPE. The RPE derived from ES cells is a potentially good cell resource for cell replacement therapy. In this study, we successfully differentiated hES cells into RPE using a two-step induction procedure. During the first step, we induced differentiation of human embryonic bodies (EBs) into neural precursors with DKK1 and noggin. DKK1, encoded by the DICKKOPF1 gene, is a Wnt antagonist and can promote the neural differentiation of mouse EBs as evidenced by inducing the expression of the neural markers such as nestin, β-III tubulin, and distal-less homeobox gene (DLX2).49–51 Noggin, a BMP signaling antagonist, can induce dorsal development and promote head formation in Xenopus and mammals.52–54 Many studies have shown that noggin alone or DKK1 with lefty (a nodal antagonist) are enough to induce the differentiation of mouse or human EBs into neural precursors.55–57 Others have shown that endogenous level of noggin and DKK1 increase in hES cultures shortly after onset of retinal neural differentiation58 and that treatment of hES cells with noggin, DKK1, and IGF-1 directs cells toward an anterior neural fate.59 Therefore, we decided to first differentiate ES into neural precursors by introducing DKK1 and noggin into our neural differentiation medium, and then induced the formation of pigmented hexagonal RPE-like cells from these neural precursors with RPE differentiation medium.40 We observed that, with this two-step induction procedure, the pigmented hexagonal RPE-like cells appeared as early as 4 weeks in cell culture, whereas with the traditional “spontaneous differentiation method,” the formation of RPE-like cells needed at least 8 weeks of cell culture.38 Although the detailed mechanisms of DKK1 and noggin in the induction of RPE cells from hES cells remain to be elucidated, it is no doubt that DKK1 and noggin can accelerate the formation of RPE cells from ES cells in our two-step induction procedure. Recently, Idelson et al.60 demonstrated that nicotinamide (NIC) promoted the differentiation of hES cells to neural and subsequently to RPE fate. When they cultured hES clusters with a serum-free medium supplemented with NIC, pigmented areas began to appear within the hES cell clusters after 4 weeks. They further revealed that in the presence of NIC, activin A, a member of TGF-β superfamily, significantly augmented pigmented areas within hESC clusters. This demonstration of another signaling pathway that can promote hES differentiation to RPE-like cells with similar differentiation efficiency as the blockage of Wnt and BMP pathways that we showed in this study, suggests that multiple pathways are involved in RPE differentiation in vitro.
RPE cells in vivo form a quiescent, polarized epithelial monolayer with distinct apical and basal characteristics that are essential for their normal function. When cultured on the plastic plates, the RPE cells not only lose their polarization and pigmentation features, but also lose RPE-specific gene expression and RPE functions. We believe that the proper orientation and polarization of the RPE cells, before transplantation, will be one of the important factors for a successful, functional graft. Our group has found that polarized RPE are more resistant to some toxic agents than nonpolarized RPE. For example, the anticancer agent 4-HPR (N-4-hydroxyphenyl retinamide) can induce apoptosis of intralesional RPE cells (activated RPE cells) in experimental choroidal neovascularization (CNV) produced by laser injury of Bruch's membrane and induce the apoptosis of cultured nonpolarized RPE cells in vitro, but does not affect the histology of the normal polarized RPE monolayer in vivo and does not induce the apoptosis of highly polarized RPE cultures.61 Similarly, polarized RPE cultures are much more resistant to the apoptotic effects of C2-ceramide challenge when compared to nonpolarized fRPE cultures.62 These results indicate that the highly polarized in vitro RPE cells are functionally more similar to their normal in vivo counterparts and may be more resistant to certain injuries.
We and other research groups have observed that RPE derived from ES cells have the ability to sequentially expand and differentiate for several cycles.17,60,63,64 At their expanding stage, hES-RPE cells lose their pigment and hexagonal shape. With prolonged culture, hES-RPE cells can differentiate into a polarized monolayer with restored pigmentation and hexagonal shape. However, after a few cycles of alternating expansion and differentiation, some hES-RPE cells will permanently lose their ability to form a polarized cell monolayer. Therefore, it will be problematic to use such dissociated hES-RPE cells for therapeutic transplantation, if they have already lost their ability to differentiate into polarized cells. In our study, we differentiated the hES-RPE into the functionally polarized cell monolayer, which not only expressed RPE-specific marker genes and proteins, formed characteristic RPE-specific morphology with tight junctions and apical microvilli, but also demonstrated an RPE-specific function (i.e., phagocytosis of ROS). This functionally polarized RPE cell monolayer may therefore be more suitable for the purpose of therapeutic cell transplantation for AMD.
Local expression of PEDF is significantly decreased in the eyes of patients with AMD, a finding that is thought to contribute partially to the pathogenesis of the disorder.65,66 On the other hand, with its neuroprotective, antiangiogenic, and antisenescent functions, PEDF is considered to be a promising therapeutic agent for AMD and other retinal degenerations.67,68 We recently reported that polarized fRPE secrete much higher levels of PEDF than nonpolarized RPE from the same donor.69 In the current study, functionally polarized hES-RPE cells secreted even higher levels of PEDF. hES-RPE secreted PEDF at two times the level of polarized fRPE and about 100 times more than that secreted by the nonpolarized hES-RPE. PEDF was localized to the apical region of polarized hES-RPE cells, consistent with the finding that more PEDF protein was secreted apically. This finding indicates that in any cell replacement strategy, using polarized RPE, as opposed to cell suspensions, should improve the growth factor microenvironment of the photoreceptors and that maintaining the correct orientation of the monolayer is essential. When correctly oriented hES-RPE is transplanted into the subretinal space of AMD patients, the abundant PEDF secreted from apical hES-RPE will not only help restore the RPE's own function, but will also exert neuroprotective and antisenescent effects on adjacent photoreceptor and neural retinal cells.
Although PEDF is known to have a broad spectrum of functions including neuroprotection, antiangiogenesis, and antisenescence, its direct and specific effects on photoreceptors and other neural retinal cells remain to be experimentally confirmed in detail. Since it is unlikely that differentiated mature photoreceptors would integrate into host retina, we used human RPCs as the testing surrogate to investigate the influence of PEDF on photoreceptor cells. The results indicated that both the RPC medium supplemented with recombinant PEDF and the hES-RPE CM containing high level of secreted PEDF increased RPC proliferation and supported RPC growth and that adding neutralizing PEDF antibody to the various culture media diminished or completely abolished the enhanced RPC proliferation and growth. Our results were consistent with previous reports that PEDF promoted retinal neurosphere expansion and adult neuronal stem cell renewal in vitro through increasing RPC or NSC proliferation.70–72 Our experiments also showed that the polarized hES-RPE CM, polarized fRPE CM, and the RPC medium containing recombinant PEDF could protect RPCs from oxidative stress–induced cell death. These findings provide new evidence to further justify the use of polarized RPE replacement therapy for AMD.
During the advanced stage of atrophic dry AMD, there is geographic loss of RPE, with secondary loss of photoreceptor cells in the macula. The transplantation of RPE alone may only help to slow the progression of the disease at the edge of the geographic atrophy lesion. More sophisticated replacement procedures such as co-transplantation of RPE with photoreceptor precursors or co-transplantation of RPE with RPCs into the subretinal space may be necessary in this context.25,26 Aramant et al.23,24 has successfully co-transplanted the intact fetal retina with RPE sheets to rescue the vision loss of RCS rats. Their work greatly supports our idea of co-transplantation of polarized RPE monolayer with photoreceptors or RPCs. While cells with photoreceptor phenotype have been differentiated from hES and iPS cells, it is difficult to obtain pure populations of photoreceptor cells in large numbers.73,74 It has been reported that CM from cultured RPE could promote Y79 retinoblastoma cell differentiation to neuronal or photoreceptor-like cells, indicating that secreted factor(s), presumably PEDF, may be important for the differentiation of retinal progenitor cells.75,76 Here, we provide further support for the feasibility of co-transplantation of polarized hES-RPE with RPC. The high level of PEDF secreted by polarized hES-RPE may support the growth and self-renewal of the newly transplanted photoreceptors or RPCs. This notion could be further supported by future studies that directly compare the effect of subretinal injections of nonpolarized versus polarized hES-RPE in animal models.
In summary, we successfully induced the differentiation of hES cells into functionally polarized hES-RPE cells that exhibit characteristics similar to those of native RPE. These hES-RPE cells can be expanded in high yield and purity and are suitable for subretinal transplantation experiments in animal models. In addition, the highly polarized hES-RPE cells secrete a high level of PEDF, which can promote RPC proliferation and survival.
Footnotes
Supported in part by Grants RS1-00222-1 and DR1-01444 from the California Institute for Regenerative Medicine and by the Arnold and Mabel Beckman Foundation.
Disclosure: D. Zhu, None; X. Deng, None; C. Spee, None; S. Sonoda, None; C.-L. Hsieh, None; E. Barron, None; M. Pera, None; D.R. Hinton, P
References
- 1. Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration: emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21 [DOI] [PubMed] [Google Scholar]
- 4. Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293 [DOI] [PubMed] [Google Scholar]
- 5. Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ. Cell loss in the aging retina: relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci. 1989;30:1691–1699 [PubMed] [Google Scholar]
- 6. Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442 [DOI] [PubMed] [Google Scholar]
- 7. Binder S, Krebs I, Hilgers RD, et al. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: a prospective trial. Invest Ophthalmol Vis Sci. 2004;45:4151–4160 [DOI] [PubMed] [Google Scholar]
- 8. Little CW, Castillo B, DiLoreto DA, et al. Transplantation of human fetal retinal pigment epithelium rescues photoreceptor cells from degeneration in the Royal College of Surgeons rat retina. Invest Ophthalmol Vis Sci. 1996;37:204–211 [PubMed] [Google Scholar]
- 9. Lund RD, Adamson P, Sauve Y, et al. Subretinal transplantation of genetically modified human cell lines attenuates loss of visual function in dystrophic rats. Proc Nat Acad Sci USA. 2001;98:9942–9947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lund RD, Kwan AS, Keegan DJ, Sauve Y, Coffey PJ, Lawrence JM. Cell transplantation as a treatment for retinal disease. Prog Retin Eye Res. 2001;20:415–449 [DOI] [PubMed] [Google Scholar]
- 11. Sauve Y, Girman SV, Wang S, Keegan DJ, Lund RD. Preservation of visual responsiveness in the superior colliculus of RCS rats after retinal pigment epithelium cell transplantation. Neuroscience. 2002;114:389–401 [DOI] [PubMed] [Google Scholar]
- 12. Little CW, Cox C, Wyatt J, del Cerro C, del Cerro M. Correlates of photoreceptor rescue by transplantation of human fetal RPE in the RCS rat. Exp Neurol. 1998;149:151–160 [DOI] [PubMed] [Google Scholar]
- 13. Sheedlo HJ, Li L, Turner JE. Photoreceptor cell rescue in the RCS rat by RPE transplantation: a therapeutic approach in a model of inherited retinal dystrophy. Prog Clin Biol Res. 1989;314:645–658 [PubMed] [Google Scholar]
- 14. Sheedlo HJ, Li L, Turner JE. Photoreceptor cell rescue at early and late RPE-cell transplantation periods during retinal disease in RCS dystrophic rats. J Neural Transplant Plasticity. 1991;2:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. da Cruz L, Chen FK, Ahmado A, Greenwood J, Coffey P. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007;26:598–635 [DOI] [PubMed] [Google Scholar]
- 16. Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126–2135 [DOI] [PubMed] [Google Scholar]
- 17. Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. West EL, Pearson RA, Barker SE, et al. Long term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells. 2010;28:1997–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang C, Klassen H, Zhang X, Young M. Laser injury promotes migration and integration of retinal progenitor cells into host retina. Mol Vis. 2010;16:983–990 [PMC free article] [PubMed] [Google Scholar]
- 20. West EL, Oearson RA, MacLaren RE, Snowden JC, Ali RR. Prog Brain Res. 2009;175:3–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol. 2008;146:172–182 [DOI] [PubMed] [Google Scholar]
- 23. Aramant RB, Seiler MJ, Ball SL. Successful cotransplantation of intact sheets of fetal retina with retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1999;40:1557–1564 [PubMed] [Google Scholar]
- 24. Aramant RB, Seiler MJ. Transplanted sheets of human retina and retinal pigment epithelium develop normally in nude rats. Exp Eye Res. 2002;75:115–125 [DOI] [PubMed] [Google Scholar]
- 25. Lee E, Maclaren RE. Sources of RPE for replacement therapy. Br J Ophthalmol. [DOI] [PubMed] [Google Scholar]
- 26. Nistor G, Seiler MJ, Yan F, Ferguson D, Keirstead HS. Three-dimensional early retinal progenitor 3D tissue constructs derived from human embryonic stem cells. J Neurosci Methods. 2010;190:63–70 [DOI] [PubMed] [Google Scholar]
- 27. Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53:411–414 [DOI] [PubMed] [Google Scholar]
- 28. Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Nat Acad Sci USA. 1993;90:1526–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bilak MM, Corse AM, Bilak SR, Lehar M, Tombran-Tink J, Kuncl RW. Pigment epithelium-derived factor (PEDF) protects motor neurons from chronic glutamate-mediated neurodegeneration. J Neuropathol Exp Neurol. 1999;58:719–728 [DOI] [PubMed] [Google Scholar]
- 30. Cao W, Tombran-Tink J, Chen W, Mrazek D, Elias R, McGinnis JF. Pigment epithelium-derived factor protects cultured retinal neurons against hydrogen peroxide-induced cell death. J Neurosci Res. 1999;57:789–800 [PubMed] [Google Scholar]
- 31. Pang IH, Zeng H, Fleenor DL, Clark AF. Pigment epithelium-derived factor protects retinal ganglion cells. BMC Neurosci. 2007;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li H, Tran VV, Hu Y, Mark Saltzman W, Barnstable CJ, Tombran-Tink J. A PEDF N-terminal peptide protects the retina from ischemic injury when delivered in PLGA nanospheres. Exp Eye Res. 2006;83:824–833 [DOI] [PubMed] [Google Scholar]
- 33. Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248 [DOI] [PubMed] [Google Scholar]
- 34. Mori K, Duh E, Gehlbach P, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188:253–263 [DOI] [PubMed] [Google Scholar]
- 35. Simonovic M, Gettins PG, Volz K. Crystal structure of human PEDF, a potent anti-angiogenic and neurite growth-promoting factor. Proc Natl Acad Sci USA. 2001;98:11131–11135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palmieri D, Watson JM, Rinehart CA. Age-related expression of PEDF/EPC-1 in human endometrial stromal fibroblasts: implications for interactive senescence. Exp Cell Res. 1999;247:142–147 [DOI] [PubMed] [Google Scholar]
- 37. Kojima T, Nakahama K, Yamamoto K, Uematsu H, Morita I. Age- and cell cycle-dependent changes in EPC-1/PEDF promoter activity in human diploid fibroblast-like (HDF) cells. Mol Cell Biochem. 2006;293:63–69 [DOI] [PubMed] [Google Scholar]
- 38. Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–245 [DOI] [PubMed] [Google Scholar]
- 39. Erikson J, Finan J, Nowell PC, Croce CM. Translocation of immunoglobulin VH genes in Burkitt lymphoma. Proc Nat Acad Sci USA. 1982;79:5611–5615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protocol. 2009;4:662–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McConnell DG. The isolation of retinal outer segment fragments. J Cell Biol. 1965;27:459–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Papermaster DS, Dreyer WJ. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974;13:2438–2444 [DOI] [PubMed] [Google Scholar]
- 43. Katz ML, Wendt KD, Sanders DN. RPE65 gene mutation prevents development of autofluorescence in retinal pigment epithelial phagosomes. Mech Ageing Dev. 2005;126:513–521 [DOI] [PubMed] [Google Scholar]
- 44. Krill AE, Morse PA, Potts AM, Klien BA. Hereditary vitelliruptive macular degeneration. Am J Ophthalmol. 1966;61:1405–1415 [DOI] [PubMed] [Google Scholar]
- 45. Pang JJ, Chang B, Hawes NL, et al. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA). Mol Vis. 2005;11:152–162 [PubMed] [Google Scholar]
- 46. Bunt-Milam AH, Saari JC. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol. 1983;97:703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schatz H. The senile maculopathies and the retinal pigment epithelium. Int Ophthalmol Clin. 1975;15:169–180 [DOI] [PubMed] [Google Scholar]
- 48. Korte GE, Reppucci V, Henkind P. RPE destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci. 1984;25:1135–1145 [PubMed] [Google Scholar]
- 49. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362 [DOI] [PubMed] [Google Scholar]
- 50. Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–434 [DOI] [PubMed] [Google Scholar]
- 51. Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961 [DOI] [PubMed] [Google Scholar]
- 52. Lamb TM, Knecht AK, Smith WC, et al. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718 [DOI] [PubMed] [Google Scholar]
- 53. Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840 [DOI] [PubMed] [Google Scholar]
- 54. Smith WC, Knecht AK, Wu M, Harland RM. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993;361:547–549 [DOI] [PubMed] [Google Scholar]
- 55. Dottori M, Pera MF. Neural differentiation of human embryonic stem cells. Method Mol Biol. 2008;438:19–30 [DOI] [PubMed] [Google Scholar]
- 56. Zhou JM, Chu JX, Chen XJ. An improved protocol that induces human embryonic stem cells to differentiate into neural cells in vitro. Cell Biol Int. 2008;32:80–85 [DOI] [PubMed] [Google Scholar]
- 57. Ikeda H, Osakada F, Watanabe K, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Nat Acad Sci USA. 2005;102:11331–11336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Nat Acad Sci USA. 2009;106:16698–16703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Nat Acad Sci USA. 2006;103:12769–12774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408 [DOI] [PubMed] [Google Scholar]
- 61. Sreekumar PG, Zhou J, Sohn J, et al. N-(4-hydroxyphenyl) retinamide augments laser-induced choroidal neovascularization in mice. Invest Ophthalmol Vis Sci. 2008;49:1210–1220 [DOI] [PubMed] [Google Scholar]
- 62. Zhu D, Sreekumar PG, Hinton DR, Kannan R. Expression and regulation of enzymes in the ceramide metabolic pathway in human retinal pigment epithelial cells and their relevance to retinal degeneration. Vision Res. 2010;50:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells (Dayton, Ohio). 2009;27:2427–2434 [DOI] [PubMed] [Google Scholar]
- 64. Vugler A, Carr AJ, Lawrence J, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol. 2008;214:347–361 [DOI] [PubMed] [Google Scholar]
- 65. Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004;88:809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bhutto IA, McLeod DS, Hasegawa T, et al. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res. 2006;82:99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miyazaki M, Ikeda Y, Yonemitsu Y, et al. Simian lentiviral vector-mediated retinal gene transfer of pigment epithelium-derived factor protects retinal degeneration and electrical defect in Royal College of Surgeons rats. Gene Ther. 2003;10:1503–1511 [DOI] [PubMed] [Google Scholar]
- 68. Raisler BJ, Berns KI, Grant MB, Beliaev D, Hauswirth WW. Adeno-associated virus type-2 expression of pigmented epithelium-derived factor or Kringles 1-3 of angiostatin reduce retinal neovascularization. Proc Nat Acad Sci USA. 2002;99:8909–8914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sonoda S, Sreekumar PG, Kase S, et al. Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging (Albany, NY). 2009;2:28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. De Marzo A, Aruta C, Marigo V. PEDF promotes retinal neurosphere formation and expansion in vitro. Adv Exp Med Biol. 2010;664:621–630 [DOI] [PubMed] [Google Scholar]
- 71. Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339 [DOI] [PubMed] [Google Scholar]
- 72. Gamm DM, Wright LS, Capowski EE, et al. Regulation of prenatal human retinal neurosphere growth and cell fate potential by retinal pigment epithelium and Mash1. Stem Cells (Dayton, Ohio). 2008;26:3182–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reh TA, Lamba D, Gust J. Directing human embryonic stem cells to a retinal fate. Methods Mol Biol. 2010;636:139–153 [DOI] [PubMed] [Google Scholar]
- 74. Parameswaran S, Balasubramanian S, Babai N, et al. Induced pluripotent stem cells generate both retinal ganglion cells and photoreceptors: therapeutic implications in degenerative changes in glaucoma and age-related macular degeneration. Stem Cells (Dayton, Ohio). 2010;28:695–703 [DOI] [PubMed] [Google Scholar]
- 75. Seigel GM, Tombran-Tink J, Becerra SP, et al. Differentiation of Y79 retinoblastoma cells with pigment epithelial-derived factor and interphotoreceptor matrix wash: effects on tumorigenicity. Growth Factors (Chur, Switzerland). 1994;10:289–297 [DOI] [PubMed] [Google Scholar]
- 76. Tombran-Tink J, Johnson LV. Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest Ophthalmol Vis Sci. 1989;30:1700–1707 [PubMed] [Google Scholar]