Abstract
Background
Psychostimulant medication, most commonly the catecholamine agonist methylphenidate, is the most effective treatment for attention-deficit/hyperactivity disorder (ADHD). However, relatively little is known on the mechanisms of action. Acute effects on brain function can elucidate underlying neurocognitive effects. We tested methylphenidate effects relative to placebo in functional magnetic resonance imaging (fMRI) during three disorder-relevant tasks in medication-naïve ADHD adolescents. In addition, we conducted a systematic review and meta-analysis of the fMRI findings of acute stimulant effects on ADHD brain function.
Methods
The fMRI study compared 20 adolescents with ADHD under either placebo or methylphenidate in a randomized controlled trial while performing stop, working memory, and time discrimination tasks. The meta-analysis was conducted searching PubMed, ScienceDirect, Web of Knowledge, Google Scholar, and Scopus databases. Peak coordinates of clusters of significant effects of stimulant medication relative to placebo or off medication were extracted for each study.
Results
The fMRI analysis showed that methylphenidate significantly enhanced activation in bilateral inferior frontal cortex (IFC)/insula during inhibition and time discrimination but had no effect on working memory networks. The meta-analysis, including 14 fMRI datasets and 212 children with ADHD, showed that stimulants most consistently enhanced right IFC/insula activation, which also remained for a subgroup analysis of methylphenidate effects alone. A more lenient threshold also revealed increased putamen activation.
Conclusions
Psychostimulants most consistently increase right IFC/insula activation, which are key areas of cognitive control and also the most replicated neurocognitive dysfunction in ADHD. These neurocognitive effects may underlie their positive clinical effects.
Key Words: ADHD, fMRI, meta-analysis, methylphenidate, review, stimulants
Attention-deficit/hyperactivity disorder (ADHD) is defined by age-inappropriate inattention, impulsiveness, and hyperactivity (1). Attention-deficit/hyperactivity disorder is associated with inhibition, attention, working memory (WM), and timing deficits 2, 3, 4, 5, underpinned by functional magnetic resonance imaging (fMRI) abnormalities in the underlying inferior frontal cortex (IFC) and dorsolateral prefrontal (DLPFC), striatal and parietal regions, and networks 2, 3, 6, 7, which are also structurally abnormal 8, 9, 10.
Psychostimulants, such as methylphenidate, followed by dexamphetamines, are first-line pharmacologic treatment for ADHD and reduce symptoms in about 70% of patients 11, 12. However, their mechanisms of action are poorly understood. At therapeutic doses, methylphenidate blocks 60% to 70% of striatal dopamine transporters (DAT) (13), which are abnormally low in medication-naïve ADHD patients (14). However, in other regions, such as frontal lobes, methylphenidate blocks 70% to 80% of norepinephrine transporters (15), which reuptake both dopamine and norepinephrine, leading to increased extracellular catecholamine levels (15).
Functional magnetic resonance imaging studies of acute effects of psychostimulants reveal true underlying mechanisms of action without confounds of secondary effects of improved behavior under chronic treatment. Randomized placebo-controlled fMRI studies of acute methylphenidate effects in medication-naïve ADHD boys using whole-brain image analyses found increased activation in predominantly right, but also left, IFC during tasks of sustained attention, inhibition, and time discrimination (TD) 4, 16, 17, 18; in parietal regions during sustained attention, error monitoring, and interference inhibition 16, 17, 18; the cerebellum during attention, TD, and interference inhibition 4, 16, 18; and striatum during reward and response inhibition 16, 18. Studies in chronically medicated ADHD patients found that an acute clinical stimulant dose relative to off medication significantly enhanced bilateral medial frontal activation during an emotional Stroop (19), deactivated cingulate default mode regions during a cognitive Stroop task (20), or had no effect during WM (21). Region of interest (ROI) fMRI studies focusing on frontal and striatal regions found that compared with atomoxetine and placebo, methylphenidate had no effect during WM (22) but significantly enhanced right IFC activation during motor inhibition (23) and during time discrimination together with atomoxetine (24). Functional magnetic resonance imaging studies in chronically medicated ADHD patients using the go/no-go task found that an acute dose of methylphenidate in medication responders compared with off medication enhanced activation in inferior, medial frontal, and anterior cingulate cortex (ACC) and striatum 25, 26.
Given these relatively inconsistent findings, we aimed to provide new analyses and to conduct a systematic review and meta-analysis on the available whole-brain fMRI studies to determine the most prominent and replicable areas modulated by acute psychostimulant treatments. Whole-brain analyses do not restrict the search volume unnecessarily and hence do not bias findings toward a priori hypothesized regions (27). For this purpose, we first re-analyzed with a whole-brain analysis three of our previously published ROI analyses of methylphenidate effects relative to placebo and atomoxetine 22, 23, 24, focusing on the contrast of methylphenidate versus placebo only. Second, we performed a voxel-based meta-analysis of whole-brain analysis fMRI studies on the acute effects of methylphenidate/stimulants relative to placebo in medication-naïve ADHD patients or relative to off-medication status in chronically medicated ADHD patients. Based on biochemical mechanisms of action of stimulants on frontal and striatal regions 15, 28, 29, 30 and findings of enhanced right IFC and basal ganglia activation with acute stimulant medication in ADHD 4, 16, 17, 18, 23, 25, 26, we hypothesized that these two regions would be the most prominent and replicable areas that would be modulated by psychostimulants.
Methods and Materials
Whole-Brain Analysis of fMRI Comparison between Methylphenidate and Placebo during Stop, TD, and WM Tasks
Detailed descriptions of participant selection, tasks, and individual fMRI analyses are previously published 23, 22, 24 and in Supplement 1.
In brief, right-handed boys with a diagnosis of hyperactive-impulsive/inattentive combined ADHD between 10 and 17 years (19 for stop; 20 for TD and WM tasks), IQ >70, and no comorbidity except conduct disorder in two patients were scanned in a double-blind placebo-controlled design (Table 1) 1.5 hours after oral administration of either methylphenidate (Equasym [Shire Pharmaceuticals, Dublin, United Kingdom], .3 mg/kg, range 5–20 mg), placebo (vitamin C, 50 mg), or atomoxetine (Strattera [Lilly Pharmaceuticals, Indianapolis, Indiana], 1 mg/kg, range 16–66 mg) (not analyzed). Patients were medication-free between scans, which were 7 days apart. Functional magnetic resonance imaging tasks included a tracking stop task that measured successful and failed stop versus baseline go trials, a TD task measuring the ability to discriminate two time intervals that differed by several hundreds of milliseconds contrasted with an order judgment task, and an n-back WM task that contrasted the function of recognizing letters shown 3/2/1 letters back with the ability to detect a target letter (“Is it X?”). Twenty-nine (stop) or 20 (WM/TD) age-matched healthy control subjects were scanned once (Table 1).
Table 1.
Demographic Data for Healthy Control Subjects and ADHD Patients
| Task | Stop Task |
Time Discrimination Task |
Working Memory Task |
|||
|---|---|---|---|---|---|---|
| Variables | Control Subjects (29) Mean (SD) | ADHD (19) Mean (SD) | Control Subjects (20) Mean (SD) | ADHD (20) Mean (SD) | Control Subjects (20) Mean (SD) | ADHD (20) Mean (SD) |
| Age (Years, Months) | 13, 9 (2, 6) | 13, 1 (1, 7) | 13, 8 (2, 5) | 13, 0 (1, 7) | 13, 8 (2, 5) | 13, 0 (1, 7) |
| IQ | 110 (12) | 92 (11) | 113 (10) | 91 (11) | 114 (11) | 91 (11) |
| SDQ Total | 4 (4) | 22 (7) | 4 (4) | 22 (7) | 4 (4) | 22 (7) |
| SDQ Hyperactive/Inattentive | 1 (2) | 8 (3) | 2 (2) | 8 (2) | 2 (2) | 8 (2) |
| CPRS-R (DSM-IV) Total t | 44 (5) | 79 (11) | 44 (5) | 78 (11) | 44 (5) | 78 (11) |
| CPRS-R Cognitive/Inattention Problems t | 46 (4) | 69 (9) | 45 (4) | 69 (9) | 45 (4) | 69 (9) |
| CPRS-R Hyperactivity t | 45 (4) | 81 (13) | 47 (4) | 79 (14) | 46 (4) | 79 (14) |
| CPRS-R Global Index: Restless Impulsive t | 46 (5) | 78 (11) | 44 (3) | 76 (12) | 44 (3) | 76 (12) |
| CPRS-R ADHD t | 46 (5) | 76 (8) | 44 (4) | 75 (8) | 44 (4) | 76 (8) |
ADHD, attention-deficit/hyperactivity disorder; CPRS-R, Conners’ Parent Rating Scale Revised; SDQ, Strengths and Difficulties Questionnaire; t, t scores.
Participants were paid £50 for each visit. Written informed consent and assent were obtained and the study was approved by the local ethics committee.
Gradient-echo echo-planar imaging magnetic resonance imaging data were acquired on a GD Sigma 3T Horizon DHx system (General Electric, Milwaukee, Wisconsin) at the Centre for Neuroimaging Sciences, Institute of Psychiatry, Kings’ College London (see Supplement 1 for image acquisition details).
Whole-brain fMRI analyses were conducted using XBAM software (http://www.brainmap.co.uk). Individual and group-level analyses are described in detail elsewhere 3, 22, 24 and in Supplement 1. Briefly, fMRI data were realigned to minimize motion-related artifacts and smoothed using a Gaussian filter (full-width at half maximum 8.82 mm) (31). Time-series analyses of individual subject activation were performed with a wavelet-based re-sampling method (31). We convolved the task epoch of each event of interest for each task (i.e., successful/failed stop–go trials for Stop; 3/2/1-back vs. 0-back for WM; time discrimination versus order judgment for TD), with two Poisson model functions (delays of 4 sec and 8 sec). Individual activation maps were recalculated by testing the goodness-of-fit of this convolution with the blood oxygen level-dependent time series that used the ratio of the sum of squares of deviations from the mean intensity value due to the model (fitted time series) divided by the sum of squares due to the residuals (original time series minus model time series). This statistic, the sum of squares ratio, was used in further analyses (32). Using rigid body and affine transformation, the individual maps were registered into Talairach standard space (33). A group brain activation map was then produced for each medication and each experimental condition.
Then, repeated measures analyses of variance (ANOVAs) were conducted with drug condition as the repeated variable (methylphenidate, placebo) for the following contrasts: successful stop–go trials; failed stop–go trials; TD–order judgment; WM: a 2*3 factorial repeated measures design was used, with drug and WM load (1-back, 2-back; 3-back, all contrasted with 0-back) as within-subject variables.
Combined voxel/cluster tests were applied coupled with permutation testing to allow for type I error control at the cluster level 34, 35. For each analysis, <1 false positive three-dimensional cluster per map was expected at p < .05 at the voxel-level and at cluster levels of p < .007 for the TD and p < .006 for the stop and WM tasks.
To test for potential normalization effects, ANOVAs compared performance and brain activation between ADHD boys under either drug condition with healthy control subjects.
Meta-Analysis of Whole-Brain Imaging Studies of Stimulant Effects on ADHD Brain Function
A comprehensive literature search was conducted of task-related fMRI studies up to June 2013 examining the effect of methylphenidate/other stimulants in ADHD children and adults using PubMed, ScienceDirect, Google Scholar, Web of Knowledge, and Scopus electronic search engines using keywords such as attention-deficit/hyperactivity disorder, ADHD, and hyperkinetic, plus fMRI, neuroimaging, and methylphenidate and stimulant. Citations within papers identified additional studies. Studies were excluded based on: 1) ROI analysis; 2) no report of coordinates; 3) no formal statistical comparison; and 4) less than 10 subjects. Meta-Analysis and Systematic Reviews of Observational Studies guidelines for meta-analysis were followed (36). The new whole-brain analysis of our three previously published fMRI ROI datasets 3, 22, 24, comparing methylphenidate relative to placebo only, was included in the meta-analysis.
For each data set, significant peak coordinates were extracted of activation differences between 1) acute dose of methylphenidate versus placebo in medication-naïve ADHD; and 2) on-medication versus off-medication in chronically medicated ADHD patients.
Regional activation differences during cognitive tasks were analyzed using Effect-Size Signed Differential Mapping (ES-SDM) software (http://www.sdmproject.com), a voxel-based meta-analytic approach used in previous meta-analyses of fMRI studies 6, 37 and described elsewhere 38, 39, 40, 41, 42 and briefly here.
First, ES-SDM uses the significant peak coordinates to recreate maps of the effect size of medication condition differences in blood oxygen level-dependent response in ADHD patients for each study. For peak coordinates, the re-creation is based on converting the t value to Hedges effect size and then applying a normalized Gaussian kernel to the voxels close to the peak. Activations and deactivations are recreated in the same map to correctly analyze those regions with higher between-study heterogeneity. Second, studies are combined with a random effects model as in standard meta-analyses, taking into account sample size and intrastudy and between-study heterogeneity (40). Finally, statistical significance is determined using standard permutation tests. Default ES-SDM thresholds were used (voxel p < .005), peak height z = 1, cluster extent = 10 voxels) (39).
Funnel plots were conducted to detect abnormalities such as studies reporting opposite results or publication bias. A jackknife sensitivity analysis (same analysis was repeated excluding one data at a time) established whether results were replicable.
Some studies included overlapping subjects but conducted different fMRI tasks, with hence only partially repeated measures. Nevertheless, this was taken into account by estimating, for each overlapping sample, an effect-size map of the mean brain response to the different fMRI tasks and included only this map in the meta-analysis. See Supplement 1 for details of the estimation assuming a moderate intertask correlation (.3).
Results
Whole-Brain Analysis of fMRI Comparison between Methylphenidate and Placebo During the Stop, TD, and WM Tasks
Multivariate ANOVAs showed no significant differences between control subjects and patients under each drug condition in the extent of maximum rotation and translation movement parameters in the three-dimensional Euclidean space for any of the tasks. Group activation maps are shown in Figures S1 through S3 in Supplement 1.
Stop
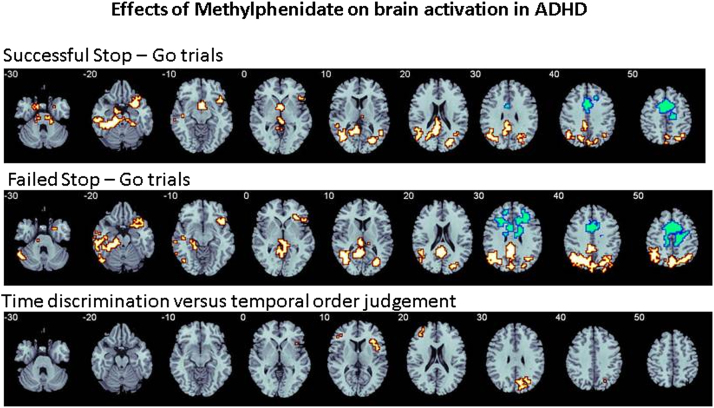
No group differences in performance were observed (Table 2). Analysis of variance showed that for successful stop-go trials, methylphenidate relative to placebo increased activation in right IFC, bordering insula and superior temporal lobe and reaching deep into caudate and rostral anterior cingulate cortex; posterior cingulate cortex (PCC)/precuneus; left medial temporal gyrus; and left midbrain and caudate. For failed stop-go trials, methylphenidate increased activation relative to placebo in a cluster comprising right IFC, insula, and superior temporal lobe; bilateral PCC/precuneus; and a cluster including midbrain, inferior, and medial temporal regions and cerebellum (Table 3; Figure 1). Placebo elicited increased activation relative to methylphenidate in a cluster comprising dorsal anterior cingulate cortex (dACC), precentral gyrus, and supplementary motor area (SMA) for both contrasts (Table 3; Figure 1). This difference, however, was due to this region being activated under placebo for the contrast of failed/successful stop-go but deactivated under methylphenidate, i.e., more activated for go-successful/failed stop trials (Figure S4 in Supplement 1).
Table 2.
Performance Data for Healthy Control Subjects and ADHD Patients
| Task Variable | Control Subjects | ADHD Placebo | ADHD Methylphenidate |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Stop Task SSRT (msec) | 165 (103) | 126 (82) | 93 (110) |
| TD Errors (%) | 22 (22) | 40 (29) | 32 (26) |
| WM Errors (%) | 14 (18) | 17 (16) | 14 (14) |
ADHD, attention-deficit/hyperactivity disorder; SSRT, stop signal reaction time; TD, time discrimination; WM, working memory.
Table 3.
Whole-Brain Image Analysis Showing Differences in Activation between Methylphenidate and Placebo in ADHD Boys
| Task (Contrast) | Brain Regions of Activation | Brodmann Area | Peak Talairach Coordinates (x, y, z) | Voxel Number | Cluster p Value |
|---|---|---|---|---|---|
| Methylphenidate > Placebo | |||||
| STOP (Stop-Go) | R IFC/insula/caudate/ACC/superior temporal | 47/13/38 | 43, 26, −13 | 148 | .001 |
| R PCC/precuneus/thalamus/occipital | 30/31/7/19 | 14, −52, 23 | 761 | .0003 | |
| L Medial temporal gyrus | 39/21 | −39, −55, 23 | 218 | .000006 | |
| L Midbrain/caudate/inferior temporal | −18, 7, −29 | 395 | .0002 | ||
| Placebo > Methylphenidate | |||||
| STOP (Stop-Go) | R ACC/SMA/postcentral | 32/24/6/4 | 7, 11, 43 | 304 | .009 |
| Methylphenidate > Placebo | |||||
| STOP (Failed Stop-Go) | R IFC/superior temporal/insula | 47/38/13 | 43, 25, −13 | 185 | .006 |
| R and L PCC/precuneus/occipital/inferior parietal | 30/31/7/19 | 3, −48, 23 | 1098 | .00004 | |
| L and R Midbrain/medial/inferior temporal/hippocampal gyrus/cerebellum | 21/36 | −25, −22, −26 | 604 | .0009 | |
| Placebo > Methylphenidate | |||||
| STOP (Failed Stop-Go) | R ACC/SMA/postcentral gyrus | 32/24/6/4/5 | 4, 15, 43 | 487 | .00002 |
| Methylphenidate > Placebo | |||||
| TD (Time Discrimination – Order Judgment) | R IFC/insula/putamen | 45/44/13 | 40, 19, 3 | 39 | .007 |
| Placebo > Methylphenidate | |||||
| TD | – | – | – | – | – |
| Methylphenidate > Placebo and Placebo > Methylphenidate | |||||
| WM (1/2/3-back–0-back) | – | – | – | – | – |
ACC, anterior cingulate cortex; ADHD, attention-deficit/hyperactivity disorder; BA, Brodmann area; IFC, inferior prefrontal cortex; L, left; PCC, posterior cingulate cortex; R, right; SMA, supplementary motor area; TD, time discrimination; WM, working memory.
Figure 1.
Increased (orange) and decreased (blue) activation with methylphenidate relative to placebo in adolescents with attention-deficit/hyperactivity disorder (ADHD) during a tracking stop and a time discrimination task.
TD
Methylphenidate relative to placebo significantly decreased time discrimination errors (F1,39 = 4; p < .02) (Table 2). Methylphenidate increased activation relative to placebo in right IFC, insula, and putamen (Table 3; Figure 1). Placebo showed no increased activation relative to methylphenidate. There was a trend-wise significant negative correlation between IFC/insula/putamen activation and TD errors during methylphenidate (r = −.3, p < .11).
WM
No significant medication effects on performance or brain activation were observed.
Normalization Effects
Stop
Control subjects and patients under placebo did not differ in performance. Patients under methylphenidate, however, showed significantly shorter stop-signal reaction time than control subjects (F1,46 = 5.32, p < .026) (Table 2). Compared with control subjects, ADHD boys under placebo had underactivation in bilateral IFC, left middle temporal gyri/inferior temporal gyri reaching into inferior parietal lobe, and right anterior cerebellum/fusiform gyrus. ADHD boys under methylphenidate compared with control subjects showed reduced activation in the same left middle temporal gyri cluster (Figure S5A in Supplement 1) but not in any other previously reduced activation clusters. Patients showed enhanced activation compared with control subjects in left posterior cerebellum/PCC and in right superior temporal gyrus, reaching into posterior insula and putamen.
TD
Healthy control subjects had significantly higher error rates than boys with ADHD when these were under placebo (F1,38 = 5, p < .026) but not when these were under methylphenidate (F1,38 = 2, p < .16) (Table 2). Compared with control subjects, boys with ADHD showed reduced activation under placebo in bilateral IFC, reaching into left insula and putamen; SMA/ACC; and right DLPFC. A small cluster in left medial prefrontal cortex was enhanced in children with ADHD compared with control subjects. Under methylphenidate, compared with control subjects, patients with ADHD only showed reduced activation in the cluster in the ACC, but this was reduced in size and no longer included SMA (Figure S5B in Supplement 1).
WM
No group effects were observed for accuracy. Control subjects compared with patients under placebo showed enhanced activation in bilateral DLPFC (Figure S5C in Supplement 1). Patients had no enhanced activation compared with healthy control subjects. After methylphenidate, patients compared with control subjects showed underactivation in the same left and right DLPFC clusters. However, they showed additional, enhanced activation in a cluster comprising right superior temporal gyrus/premotor cortex, striatum/thalamus, and insula, reaching into the cerebellar vermis, which correlated negatively with the left DLPFC activation cluster (r = −.5, p < .012) and positively with accuracy (r = .37, p < .05).
Meta-Analysis
Included Studies and Characteristics
Fourteen high-quality datasets, all pediatric, were included in the meta-analysis, including 212 children/adolescents with ADHD. Ten datasets compared methylphenidate with placebo on brain function in medication-naïve patients in randomized controlled designs 4, 16, 17, 18, including the new whole-brain re-analyses of four ROI datasets 22, 23, 24 (Table 4) and four datasets comparing single dose effects of methylphenidate/stimulants with off-medication in chronically medicated medication responders 19, 20, 21 (Table 4). Some papers included several tasks or task contrasts and hence more than one independent dataset. All datasets in medicated patients used a washout period of at least 24 hours before fMRI (Table 4).
Table 4.
Whole-Brain Analysis Based fMRI Studies Examining the Acute Effects of Stimulant Medication on Brain Function of ADHD Children and Adolescents
| Study | Task and Contrast | Sample n (% Male Subjects) | Mean Age (Years, Months) (SD) | Comorbidity | Performance Effects | Medication Dose/Time Taken Before ON Scan | Control Condition | On Medication > Placebo/Off Medication | Off Medication/Placebo > On Medication |
|---|---|---|---|---|---|---|---|---|---|
| Placebo-Controlled fMRI Studies in Medication-Naïve ADHD Patients | |||||||||
| Rubia et al. 2009 (16) | CPT: attention contrast (target – non-target) | 13 (100) | 12, 6 (1, 4) | CD = 1 | No | MPH .3 mg/kg/1 hour | Placebo | R IFC/premotor/parietal | – |
| L and R cuneus/precuneus/cingulate/cerebellum | |||||||||
| Rubia et al. 2009 (16) | CPT: reward contrast (reward-non-reward) | 13 (100) | 12, 6 (1, 4) | CD = 1 | No | MPH .3 mg/kg/1 hour | Placebo | R vmPFC/rACC/caudate | R occipital/medial temporal |
| Rubia et al. 2009 (4) | Time discrimination vs. order judgment | 12 (100) | 13 (1) | CD = 1 | No | MPH .3 mg/kg/1 hour | Placebo | L IFC/insula | R superior frontal |
| R dACC | R medial temporal | ||||||||
| R cerebellum | R hippocampus | ||||||||
| R putamen/globus pallidus | |||||||||
| Rubia et al. 2011 (17) | Simon task (incongruent – oddball) | 12 (100) | 13 (1) | CD = 1 | No | MPH .3 mg/kg/1 hour | Placebo | R IFC/premotor/superior temporal/inferior parietal | – |
| L cerebellum/fusiform/middle/inferior temporal | |||||||||
| Rubia et al. 2011 (18) | Stop task: (stop–go) | 12 (100) | 13 (1) | CD = 1 | Trend (MRT go trials, MRT post-error go trials) | MPH .3 mg/kg/1 hour | Placebo | – | – |
| Rubia 2011 (18) | Stop task: failed stop–go | 12 (100) | 13 (1) | CD = 1 | Trend (MRT go trials, MRT post-error go trials) | MPH .3 mg/kg/1 hour | Placebo | L and R IFC insula/putamen/caudate; L DLPFC | – |
| R inferior parietal/precuneus; R occipital cortex | |||||||||
| New analysis of Cubillo et al. 2013 (23) (Table 3)a | Stop task: stop–go | 19 (100) | 13,1 (2, 6) | CD = 2 | No | MPH .3 mg/kg, range 5–20 mg/1.5 hours | Placebo | R IFC, caudate/thalamus | R ACC/SMA/premotor |
| L cerebellum/inferior and medial temporal/inferior superior parietal/precuneus/posterior cingulate | |||||||||
| R precuneus/inferior and superior parietal | |||||||||
| New analysis of Cubillo et al. 2013 (23) (Table 3)a | Stop task: failed stop–go | 19 (100) | 13,1 (2, 6) | CD = 2 | No | MPH .3 mg/kg, range 5–20 mg/1.5 hours | Placebo | R IFC/superior temporal/posterior cingulate/thalamus/L + R inferior/superior parietal/L medial temporal/Cb | R ACC/SMA |
| New analysis of Smith et al. 2013 (24) (Table 3)a | Time discrimination vs. order judgment | 20 (100) | 12, 11 (1, 7) | CD = 2 | Trend (errors) | MPH .3 mg/kg, range 5−20 mg/1.5 hours | Placebo | R IFC/insula | – |
| New analysis of Cubillo et al. 2013 (22) (Table 3)a | WM; Verbal n-back (1/2/3-back versus 0-back) | 20 (100) | 13 (1, 7) | CD = 2 | No | MPH .3 mg/kg, range 5−20 mg/1.5 hours | Placebo | – | – |
| fMRI Studies in Chronically Medicated Medication Responders with ADHD On and Off Their Usual Single Clinical Dose | |||||||||
| Kobel et al. 2009 (21) | WM: 2 & 3-back versus 0-back | 14 (100) | 10.43 (1.34) | ODD/CD = 3, GAD = 2, ODD/CD + GAD = 2 | Yes (3-back) | IR MPH: 2 = 10 mg, 1=15 mg, 6 = 20 mg; ER MPH: 5 = 36–40 mg/? | Off medication/24 hours | – | – |
| Trend (2-back) | |||||||||
| Peterson et al. 2009 (20) | Stroop: incongruent versus congruent | 16 (81) | 14.1 (2.5) | None | No | Effective clinical dose of stimulants/45–60 min | Off medication/72 hours | – | Ventral ACC/PCC |
| Posner 2011 (19) | Stroop: positive valenced incongruent - neutral | 15 (87) | 13.5 (1.2) | ODD/CD = allowed but none reported | No | Effective clinical dose of stimulant/? | Off medication/48 hours | – | – |
| Posner 2011 (19) | Stroop: negative valenced incongruent - neutral | 15 (87) | 13.5 (1.2) | ODD/CD = allowed but none reported | No | Effective clinical dose of stimulant/? | Off medication/48 hours | R and L MFC | – |
ACC, anterior cingulate cortex; ADHD, attention-deficit/hyperactivity disorder; Cb, cerebellum; CD, conduct disorder; CPT, continuous performance task; dACC, dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; ER, extended release; fMRI, functional magnetic resonance imaging; GAD, generalized anxiety disorder; IFC, inferior prefrontal cortex; IR, immediate release; L, left; MFC, medial prefrontal cortex; MPH, methylphenidate; MRT, mean reaction time; ODD, oppositional defiant disorder; PCC, posterior cingulate cortex; R, right; rACC, rostral anterior cingulate cortex; ROI, region of interest; SD, standard deviation; SMA, supplementary motor area; vmPFC, ventromedial prefrontal cortex; WM, working memory.
Data of these studies were newly analyzed using whole-brain image analyses and comparing methylphenidate with placebo only, as opposed to the original publications that used ROI analyses comparing three drugs, i.e., methylphenidate, placebo, and atomoxetine (see Table 3, Methods, and Results sections).
Meta-Analysis Results
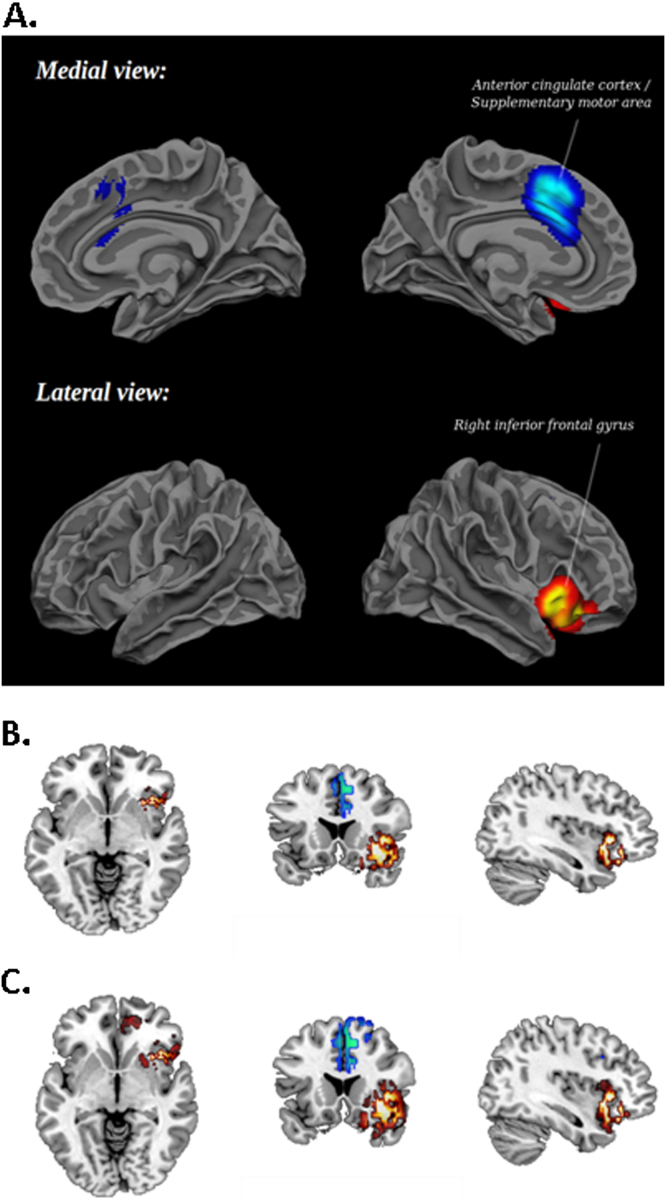
Children/adolescents with ADHD under stimulants relative to when under placebo or off medication showed significantly increased activation in right IFC, insula, and bordering superior temporal lobe (Table 5; Figure 2A,B). To test our hypothesis that stimulants increase basal ganglia activation, which is more difficult to detect in fMRI due to their relatively small size, we explored the data using a more lenient p < .05. As hypothesized, an additional cluster in right putamen was observed (Talairach coordinates: 24, 13, 1; 68 voxels). We also observed a small cluster in rostral anterior cingulate cortex (Brodmann area: 32; Talairach coordinates: 5, 44, 1; 91 voxels) (Figure 2C).
Table 5.
Results of the Meta-Analysis of 14 Whole-Brain fMRI Studies in ADHD: Regional Differences between ADHD Patients on an Acute Dose of Psychostimulants/Methylphenidate Relative to Placebo or Off Their Usual Clinical Dose of Psychostimulants/Methylphenidate
| Contrast | Talairach x, y, z Coordinates | SDM z-Value | p Value | Number of Voxels | Cluster Breakdown (Number Voxels) |
|---|---|---|---|---|---|
| All 14 Datasets | |||||
| Stimulants > Placebo/Off Medication | |||||
| Right inferior frontal gyrus, extending to insula and superior temporal gyrus | 42, 20, −12 | 1.469 | <.000001 | 607 | BA 47 (339); BA 13 (111); BA 38 (68); BA 11 (44); claustrum (19); BA 45 (26) |
| Stimulants < Placebo/Off Medication | |||||
| Right dorsal anterior cingulate/supplementary motor area | 8, 10, 44 | −1.432 | <.000005 | 691 | BA 32 (317); BA 6 (144); BA 8 (103); BA 24 (127) |
| Subgroup Analysis of 10 fMRI Studies that Compared Methylphenidate Effects in Medication-Naïve ADHD Patients | |||||
| MPH > Placebo | |||||
| Right inferior frontal gyrus, extending to insula and superior temporal gyrus | 42, 20, −12 | 1.745 | <.000005 | 632 | BA 47 (356); BA 13 (113); BA 38 (68); BA 11 (48); BA 45 (47) |
| MPH < Placebo | |||||
| Right dorsal anterior cingulate/supplementary motor area | 12, 12, 44 | −1.586 | <.000001 | 728 | BA 32 (293); BA 6 (176); BA 24 (132); BA 8 (127) |
Meta-analysis was conducted at p < .005, z = 1, and a cluster size of >10 voxels.
ADHD, attention-deficit/hyperactivity disorder; BA, Brodmann area; fMRI, functional magnetic resonance imaging; MPH, methylphenidate; SDM, signed differential mapping.
Figure 2.
(A) Meta-analysis results in three-dimension at p < .005 showing brain regions of increased (red/orange) and decreased (blue) activation after a single dose of stimulant medication in children and adolescents with attention-deficit/hyperactivity disorder compared with placebo/off-medication. Relative to placebo, increased activation is shown with acute stimulant medication in right inferior prefrontal cortex extending deep into the insula and bordering superior temporal lobe and decreased activation in anterior cingulate cortex and supplementary motor area. (B) Meta-analysis results in two-dimension at peak Montreal Neurological Institute coordinates: 38, 18, −4 (corresponding to Talairach coordinates: 42, 20, −12) at p < .005, showing right inferior frontal cortex reaching into insula and anterior cingulate cortex/supplementary motor area. (C) Meta-analysis results in two-dimension at peak Montreal Neurological Institute coordinates: 38, 18, −4 (corresponding to Talairach coordinates: 42, 20, −12) at a more lenient p < .05, showing in addition a cluster in right putamen and rostral anterior cingulate.
We tested whether findings remained when we included only the 10 studies in medication-naïve patients that compared specifically methylphenidate versus placebo. The activation cluster in right IFC/insula remained almost identical but became somewhat larger in size and more significant (Table 5).
For both analyses, a cluster in dACC/SMA was significantly decreased in activation in patients under an acute dose of methylphenidate/stimulants compared with placebo/off-stimulant medication (Table 5).
Reliability Analysis
Funnel plots showed that no studies had findings in opposite directions, and there was no detectable publication bias (Egger’s test p > .4 in both cases). A whole-brain jackknife sensitivity analysis showed that the findings on the IFC and ACC activation clusters were moderately replicable and preserved in all but four and three combinations of datasets, respectively (Table S1 in Supplement 1).
Results were identical when sample overlaps were taken into account, though the peak height of right inferior frontal gyrus (IFG) abnormality was only .971 (p < .0001).
Discussion
The whole-brain analysis in 19 to 20 medication-naïve ADHD boys showed that methylphenidate relative to placebo had no significant effect on brain function during WM but increased activation in right IFC/insula during successful and failed response inhibition and TD, which trend-wise correlated with improved TD errors. Methylphenidate also enhanced activation of caudate, parietal, and temporal activation during inhibition. Placebo relative to methylphenidate increased activation in ACC/SMA. The findings replicate and extend previous fMRI findings of methylphenidate enhancing IFG-striatal activation relative to placebo in a different ADHD cohort during the same and similar inhibition and timing tasks 4, 17, 18. Furthermore, the increased right IFC/insula activation with methylphenidate led to significant normalization of its underactivation during stop and TD in ADHD patients when compared with healthy control subjects. The meta-analysis of stimulant effects in 14 fMRI datasets, including the above data, showed that stimulants relative to placebo/off-medication most consistently increase activation in right IFC/insula. The finding remained for the 10 studies that tested specifically for methylphenidate effects relative to placebo in medication-naïve patients. At a more lenient threshold, putamen activation was also increased, suggesting that stimulants most consistently enhance right IFC-insular-striatal activation.
It was not unexpected that methylphenidate/stimulants most consistently increased right IFC/insula activation relative to placebo/off-medication, as this region has been shown to be increased in activation with methylphenidate relative to placebo in several whole-brain and ROI fMRI studies of methylphenidate effects during response inhibition 18, 23, 26, interference inhibition (17), sustained attention (16), and time discrimination 24, 4 (Table 4, Table 6).
Table 6.
ROI-Based Studies Examining the Acute Effects of Stimulant Medication on Brain Function of ADHD Children/Adolescents and Adults
| Study | ROIs | Task (Design; Contrast) | Sample n (% Male Subjects) | Mean Age Years (SD) | Comorbidity | Performance Effects | Medication/Dose | Control Condition/Withdrawal-Time Off Medication | On Medication > Placebo/Off Medication | Placebo/Off Medication > On Medication |
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo-Controlled ROI Studies in Medication-Naïve ADHD Patients | ||||||||||
| Cubillo et al. 2013 (23) | R + L IFC, temporo-parietal, cerebellum | Stop task (ER; successful stop-go) | 19 (100) | 13 (3) | CD = 2 | No | MPH/.3 g/kg | Placebo, Atomoxetine | R IFC | – |
| Smith et al. 2013 (24) | Frontal lobes ACC/SMA, cerebellum striatum | Time discrimination versus order judgment (BD) | 20 (100) | 12 (2) | CD = 2 | Trend (errors) | MPH/.3 mg/kg | Placebo, Atomoxetine | R IFC | – |
| Cubillo et al. 2013 (22) | DLPFC | n-back working memory: (BD; 1/2/3-back–0-back) | 20 (100) | 13 (2) | CD = 2 | No | MPH/.3 mg/kg | Placebo, Atomoxetine | – | – |
| Placebo-Controlled ROI Studies in Chronically Medicated Medication Responders with ADHD | ||||||||||
| Epstein et al. 2007 (26) | Striatum, prefrontal, posterior parietal, cerebellum | go/no-go (ER; no-go – go) | 13 (69) | 17 (1) | MD = 1, Specific phobia = 1, ODD/CD = 2, AUD = 1 | Yes (SD of MRT, d-prime) | MPH/.3 mg/kg | Placebo/washout period of 5 multiplied by the half-life of the medication | ACC, R IFC/OFC, L IFC, L MFC, R + L caudate, R globus pallidus, R inferior parietal, L cerebellum | – |
| Epstein et al. 2007 (26) | Striatum, prefrontal, posterior parietal, cerebellum | Go/no-go (ER; no-go – go) | 15 (33) | 50 (8) | Eating disorder = 1, MD = 7, OCD = 2, PTSD = 1, Social phobia = 1 | Yes (SD of MRT, d-prime) | MPH/.3 mg/kg | Placebo/washout period of 5 multiplied by the half-life of the medication | L caudate, L hippocampus, L cerebellum | L MFC, R inferior parietal |
| Liddle et al. 2011 (74) | DMN (medial frontal, precuneus, PCC, angular gyrus; middle temporal) | Go/no-go (ER; deactivation during go and no-go trials versus rest) | 18 (94) | Not reported (9- to 15-year-old range) | AD = 3, ODD/CD = 13 | Yes (omissions, d-prime) | MPH/.3 g/kg | Placebo/36 hours | – | – |
| On-Off ROI Medication Studies in Chronically Medicated Medication Responders with ADHD | ||||||||||
| Vaidya et al. 1998 (25) | Frontal lobe, ACC, caudate, putamen | Go/no-go (BD; contrasts: no-go – go; stimulus controlled or response controlled) | 10 (100) | 10.5 (1) | NA | Yes (commissions) | MPH/Regular dose (i.e., 7.5–30 mg) | Off medication/36 hours | Stimulus controlled: Caudate and putamen | Stimulus controlled: – |
| Response controlled: – | Response controlled: – | |||||||||
| Posner et al. 2011 (91) | Amygdala | Fearful face processing (BD; fearful - neutral) | 15 (87) | 13.5 (1) | ODD/CD = allowed but none reported | No | Stimulant/effective dose | Off medication/48 hours | – | – |
| Sheridan et al. 2007 (75) | MFC basal ganglia | Working memory delayed match-to-sample (ER; encoding/delay/retrieval - rest) | 5 (0) | 15 (2) | NA | Yes (errors) | Stimulants/effective dose | Off medication/24 hours | Encoding: – | Encoding: MFC, precuneus |
| Retrieval: – | Retrieval: – | |||||||||
| Delay: – | Delay: – | |||||||||
ACC, anterior cingulate cortex; AD, anxiety disorder; ADHD, attention-deficit/hyperactivity disorder; AUD, alcohol use disorder; BD, block design fMRI task design; CD, conduct disorder; DLPFC, dorsolateral prefrontal cortex; DMN, default mode network; ER, event-related fMRI task design; fMRI, functional magnetic resonance imaging; IFC, inferior prefrontal cortex; L, left; MD, mood disorder; MFC, medial prefrontal cortex; MPH, methylphenidate; MRT, mean reaction time; NA, not applicable; OCD, obsessive-compulsive disorder; ODD, oppositional defiant disorder; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; PTSD, posttraumatic stress disorder; R, right; ROI, region of interest; SD, standard deviation; SMA, supplementary motor area; VLPFC, ventrolateral prefrontal cortex.
The area of increased activation, i.e., right IFC, bordering superior temporal lobe and reaching into insula, is a key region of cognitive control, as demonstrated by meta-analyses of fMRI studies of motor, interference inhibition, and switching 43, 44, 45, 46, transcranial magnetic stimulation 47, 48, 49, and lesion studies (50). However, right IFC is also a key region mediating time estimation 51, 52 and part of the ventral attention system mediating selective and sustained attention 53, 54. It has therefore been argued that right IFC/insula have a generic role of updating information (55) and cognitive control (56), including attention allocation to behaviorally relevant salient stimuli 53, 54, 56, 57. The functions mediated by IFC/insula are also most consistently impaired in ADHD, such as cognitive control 58, 59, sustained attention, saliency detection, and timing 4, 5, 58, in line with consistently reduced activation in this region in ADHD during cognitive control 6, 60, attention 16, 61, and timing tasks 24, 6, 62.
This meta-analysis therefore demonstrates that stimulants/methylphenidate most consistently increase activation of a key region of cognitive control/saliency detection that has consistently been found to be underactivated in ADHD patients in the context of disorder-relevant functions of inhibition, attention, and timing 4, 6, 16, 24, 37, 60, 63, 64, 65 and that may be a disorder-specific neurofunctional biomarker of ADHD relative to other childhood disorders 61, 63, 66, 67, 68.
The increase of IFG/insula activation with methylphenidate is likely mediated by both noradrenergic and dopaminergic mechanisms, given that in frontal regions both in humans 15, 69, 70 and in animals 69, 70 methylphenidate increases noradrenaline equally or more than dopamine via reuptake inhibition of noradrenaline transporters, which clear both catecholamines. The explorative analysis at a more lenient threshold revealed an additional increase in right putamen activation, in line with the key mechanism of action of methylphenidate of the blockade of over 50% of striatal DAT, leading to increased striatal dopamine release (13). Caudate and putamen are dysfunctional in ADHD, together with prefrontal regions 2, 3, 7, 37, suggesting that stimulants increase underfunctioning frontostriatal mechanisms in ADHD (71).
Furthermore, acute methylphenidate normalized the underactivation in right IFC that was observed in ADHD patients under placebo compared with healthy control subjects during inhibition and timing processes [see also 23, 24], which has also been observed previously during interference inhibition (17), motor inhibition 18, 23, WM (72), and TD (4) (Table S2 in Supplement 1). Other areas were also normalized, most prominently basal ganglia underfunction during inhibitory control 17, 25, 73 and timing (4) (Table S2 in Supplement 1).
While most fMRI studies found methylphenidate to have a positive effect on brain activation, only some studies observed performance improvement 18, 21, 24, 25, 26, 74, 75, likely reflecting the superior sensitivity of fMRI relative to behavior to detect pharmacologic effects or the relatively small subject numbers of fMRI studies, underpowered for performance effects.
A cluster in ACC/SMA was significantly reduced under stimulants relative to placebo/off-medication. However, a similar cluster in our stop task analysis (Table 3) was partly due to increased deactivation with methylphenidate (i.e., more activation during the go condition) (Figure S4 in Supplement 1), which was also observed in the ROI analysis (23) and other studies 19, 20, 74. This could suggest an impact of methylphenidate on reducing the default mode network or of enhancing activation related to the contrast condition rather than a placebo effect. Alternatively, it is also possible that methylphenidate has differential effects on the saliency network, reducing ACC/SMA and enhancing IFC/insula.
This meta-analysis could only test acute stimulant effects on brain function, given the sparsity of fMRI studies that tested longer term effects. One fMRI study found a trend for ACC reduction to be more pronounced in five nonmedicated versus nine medicated children with ADHD after 1 year methylphenidate treatment, suggesting amelioration but not normalization of activation deficits in ADHD (76). In 10 adults with ADHD, amelioration of dACC underactivation was found, which was associated with treatment response, as well as in right DLPFC and bilateral parietal cortices after 6 weeks OROS methylphenidate (Concerta, Addison, Texas) relative to placebo (77). Meta-regression analyses on fMRI studies found that long-term stimulant medication was linearly associated with more normal basal ganglia function during attention but not inhibition tasks (37).
No prospective studies are published on long-term effects of stimulant treatment on brain structure. Meta-regression analyses show that long-term stimulant medication is associated with more normal basal ganglia structure 8, 78, parallel to the meta-regression findings of an association with more normal striatal function during attention tasks (37). Retrospective structural magnetic resonance imaging (MRI) comparisons found that medicated relative to unmedicated ADHD patients had more normal white matter (79) and cortical thinning development in left IFG, premotor, and parietal regions (80); basal ganglia morphology (81); and sizes of thalamus (82), anterior cingulate (83), cerebellum (84), and corpus callosum (85), suggesting a neuroprotective effect of stimulant medication on ADHD brain development. However, while the experimental within-patient fMRI studies are more likely to reflect causal medication effects, the retrospective structural MRI analyses could be confounded by other discriminating factors than medication status and need corroboration in prospective longitudinal within-patient MRI studies. Our meta-regression analysis of positron emission tomography studies, on the other hand, showed that long-term stimulant medication was associated with abnormally elevated striatal DAT levels (14), which was also found in a prospective 1-year positron emission tomography study 86, 87, suggesting brain adaptation and tolerance to long-term stimulants. Future longitudinal studies using several imaging modalities will need to assess the fundamental question of the impact of stimulant medication on the development of brain structure, function, and biochemistry of ADHD children.
A key relevant clinical question is whether brain structure or function patterns in ADHD patients can predict stimulant response. A recent fMRI study found an association between clinical improvement after 6 to 8 weeks of methylphenidate and brain activation reduction over this time in motor, IFC, and ACC cortices (88). Another study found that methylphenidate response in seven ADHD adolescents was associated with acute stimulant reduction effects in parietal regional homogeneity during rest (89). Future larger powered studies using multivariate pattern recognition analyses are necessary to test whether baseline brain function or structure abnormalities in ADHD can predict stimulant response (90).
A limitation of the re-analyzed fMRI dataset is that clinical ratings from teachers were not obtained. Also, it cannot be ascertained whether stimulant effects on brain activation in ADHD have disorder or diagnostic specificity, as only one fMRI study has tested healthy control subjects under methylphenidate (25) and none in other childhood disorders. The meta-analysis has several limitations inherent to all meta-analyses. Peak-based meta-analyses are based on coordinates from published studies rather than raw statistical brain maps (42). Also, different studies used different statistical thresholds. Third, while voxel-wise meta-analytical methods provide excellent control for false-positive results, false-negative results are more difficult to avoid (42). Last, while most included fMRI studies measured cognitive control functions, there was heterogeneity, with some studies assessing motivational effects within cognitive control tasks and two studies measuring time estimation. Once more studies are available, future meta-analytic studies should subcategorize stimulant effects on homogenous cognitive domains.
In conclusion, our meta-analysis of 14 fMRI datasets in pediatric ADHD shows that the most consistent effect of acute stimulant medication, and of methylphenidate specifically, is the increased activation of right IFC, a key region of cognitive control and the most replicated neurocognitive dysfunction area of ADHD 2, 3, 7, 16, 17, 18, 37, 60. It is likely that increased right IFC activation and its normalization relative to healthy control subjects 4, 16, 17, 18, 23, 24, 72 is underlying the clinical effectiveness of stimulant response on ADHD behaviors.
Acknowledgments
This work was supported by grants by the National Institute of Health Research Biomedical Research Centre for Mental Health at South London and Maudsley National Health Service Foundation Trust and Institute of Psychiatry, Kings College London, and Lilly Pharmaceuticals. Dr. Anna Smith, Dr. Ana Cubillo, and Dr. Andrew Simmons are supported by the National Institute of Health Research Biomedical Research Centre. Ms. Alzamora was supported by an Institute of Psychiatry Ph.D. Excellence award. Lilly Pharmaceuticals had no input into the design, analysis, data interpretation, or write-up.
KR has received speaker’s honoraria from Lilly, Shire, and Novartis. MJB is a consultant for P1Vital, Ltd, Oxford, United Kingdom. The other authors report no biomedical financial interest or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at doi:10.1016/j.biopsych.2013.10.016.
Appendix A. Supplementary Materials
Supplementary Material
References
- 1.American Psychiatric Association . 4th ed. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Rubia K. “Cool” inferior fronto-striatal dysfunction in attention-deficit/hyperactivity disorder (ADHD) versus “hot” ventromedial orbitofronto-limbic dysfunction in conduct disorder: A review. Biol Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Cubillo A., Halari R., Smith A., Giampietro V., Taylor E., Rubia K. Fronto-cortical and fronto-subcortical brain abnormalities in children and adults with ADHD: A review and evidence for fronto-striatal dysfunctions in adults with ADHD followed up from childhood during motivation and attention. Cortex. 2012;48:194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Rubia K., Halari R., Christakou A., Taylor E. Impulsiveness as a timing disturbance: Neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans R Soc Lond B Biol Sci. 2009;364:1919–1931. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noreika V., Falter C., Rubia K. Timing deficits in patients with ADHD. Neuropsychologia. 2012;51:235–266. doi: 10.1016/j.neuropsychologia.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Hart H., Radua J., Mataix D., Rubia K. Meta-analysis of fMRI studies of timing functions in ADHD. Neurosci Biobehav Rev. 2012;36:2248–2256. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., Castellanos F.X. Toward systems neuroscience of ADHD: A meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakao T., Radua C., Rubia K., Mataix-Cols D. Gray matter volume abnormalities in ADHD and the effects of stimulant medication: Voxel-based meta-analysis. Am J Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- 9.Konrad K., Eickhoff S.B. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010;31:904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valera E.M., Faraone S.V., Murray K.E., Seidman L.J. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Wilens T.E. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2008;28:S46–S53. doi: 10.1097/JCP.0b013e318173312f. [DOI] [PubMed] [Google Scholar]
- 12.Hanwella R., Senanayake M., de Silva V. Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: A meta-analysis. BMC Psychiatry. 2011;11:176. doi: 10.1186/1471-244X-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkow N.D., Fowler J.S., Ding Y.S., Wang G.J., Gatley S.J. Positron emission tomography radioligands for dopamine transporters and studies in human and nonhuman primates. Adv Pharmacol. 1998;42:211–214. doi: 10.1016/s1054-3589(08)60730-9. [DOI] [PubMed] [Google Scholar]
- 14.Fusar-Poli P., Rubia K., Rossi G., Sartori G., Ballotin U. Dopamine transporter alterations in ADHD: Pathophysiology or adaptation to psychostimulants? A meta-analysis. Am J Psychiatry. 2012;169:264–272. doi: 10.1176/appi.ajp.2011.11060940. [DOI] [PubMed] [Google Scholar]
- 15.Hannestad J., Gallezot J.D., Planeta-Wilson B., Lin S.F., Williams W.A., van Dyck C.H. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry. 2010;68:854–860. doi: 10.1016/j.biopsych.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubia K., Halari R., Cubillo A., Mohammad M., Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a Rewarded Continuous Performance Task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Rubia K., Halari R., Cubillo A., Smith A., Mohammad M., Brammer M., Taylor E. Methylphenidate normalises fronto-striatal underactivation during interference inhibition in medication-naive boys. Neuropsychopharmacology. 2011;36:1575–1586. doi: 10.1038/npp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubia K., Halari R., Taylor E., Brammer M. Methylphenidate normalises fronto-cingulate underactivation during error processing in children with attention-deficit hyperactivity disorder. Biol Psychiatry. 2011;70:255–262. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posner J., Maia T.V., Fair D., Peterson B.S., Sonuga-Barke E.J., Nagel B.J. The attenuation of dysfunctional emotional processing with stimulant medication: An fMRI study of adolescents with ADHD. Psychiatry Res. 2011;193:151–160. doi: 10.1016/j.pscychresns.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson B.S., Potenza M.N., Wang Z., Zhu H., Martin A., Marsh R. An fMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobel M., Bechtel N., Weber P., Specht K., Klarhofer M., Scheffler K. Effects of methylphenidate on working memory functioning in children with attention deficit/hyperactivity disorder. Eur J Paediatr Neurol. 2009;13:516–523. doi: 10.1016/j.ejpn.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Cubillo A., Smith A., Barrat N., Giampietro V., Brammer M., Simmons A., Rubia K. Drug-specific laterality effects on frontal lobe activation of atomoxetine and methylphenidate in ADHD boys during working memory. Psychol Med. 2013;19:1–14. doi: 10.1017/S0033291713000676. [DOI] [PubMed] [Google Scholar]
- 23.Cubillo A., Smith A.B., Barrett N., Giampietro V., Brammer M.J., Simmons A., Rubia K. Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys [published online ahead of print October 9] Cereb Cortex. 2012 doi: 10.1093/cercor/bhs296. doi:10.1093/cercor/bhs296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith A., Cubillo A., Barrett N., Giampietro V., Simmons A., Brammer M., Rubia K. Neurofunctional effects of methylphenidate and atomoxetine in boys with ADHD during time discrimination. Biol Psychiatry. 2013;74:615–622. doi: 10.1016/j.biopsych.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Vaidya C.J., Austin G., Kirkorian G., Ridlehuber H.W., Desmond J.E., Glover G.H., Gabrieli J.D. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein J.N., Casey B.J., Tonev S.T., Davidson M.C., Reiss A.L., Garrett A. ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. J Child Psychol Psychiatry. 2007;48:899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 27.Friston K.J., Rotshtein P., Geng J.J., Sterzer P., Henson R.N. A critique of functional localisers. Neuroimage. 2006;30:1077–1087. doi: 10.1016/j.neuroimage.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Volkow N., Wang G., Fowler J., Logan J., Angrist B., Hitzemann R. Effects of methylphenidate on regional brain glucose metabolism in humans: Relationship to dopamine D2 receptors. Am J Psychiatry. 1997;154:50–55. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- 29.Volkow N.D., Fowler J.S., Wang G., Ding Y., Gatley S.J. Mechanism of action of methylphenidate: Insights from PET imaging studies. J Atten Disord. 2002;6(suppl 1):S31–S43. doi: 10.1177/070674370200601s05. [DOI] [PubMed] [Google Scholar]
- 30.Volkow N.D., Wang G.J., Fowler J.S., Gatley S.J., Logan J., Ding Y.S. Dopamine transporter occupancy by intravenous methylphenidate: Implications for its reinforcing effects. Abstr Soc Neurosci. 1998;24:778. [Google Scholar]
- 31.Bullmore E., Long C., Suckling J., Fadili J., Calvert G., Zelaya F. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: Resampling methods in time and wavelet domains. Hum Brain Mapp. 2001;12:61–78. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brammer M.J., Bullmore E.T., Simmons A., Williams S.C., Grasby P.M., Howard R.J. Generic brain activation mapping in functional magnetic resonance imaging: A nonparametric approach. Mag Reson Imaging. 1997;15:763–770. doi: 10.1016/s0730-725x(97)00135-5. [DOI] [PubMed] [Google Scholar]
- 33.Talairach J., Tournoux P. Thieme; New York: 1988. Co-planar Stereotaxic Atlas of the Brain. [Google Scholar]
- 34.Bullmore E.T., Brammer M.J., Rabe-Hesketh S., Curtis V.A., Morris R.G., Williams S.C.R. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp. 1999;7:38–48. doi: 10.1002/(SICI)1097-0193(1999)7:1<38::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bullmore E.T., Suckling J., Overmeyer S., Rabe-Hesketh S., Taylor E., Brammer M.J. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- 36.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;293:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 37.Hart H., Radua J., Mataix D., Rubia K. Meta-analysis of fMRI studies of inhibition and attention in ADHD: Exploring task-specific, stimulant medication and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 38.Radua J., Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 39.Radua J., Mataix-Cols D. Heterogeneity of coordinate-based meta-analyses of neuroimaging data: An example from studies in OCD Reply. Br J Psychiatry. 2010;197:77. doi: 10.1192/bjp.197.1.76a. [DOI] [PubMed] [Google Scholar]
- 40.Radua J., Mataix-Cols D., Phillips M.L., El-Hage W., Kronhaus D.M., Cardoner N., Surguladze S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27:605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Radua J., van den Heuvel O.A., Surguladze S., Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 2010;67:701–711. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- 42.Radua J., Via E., Catani M., Mataix-Cols D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 2011;41:1539–1550. doi: 10.1017/S0033291710002187. [DOI] [PubMed] [Google Scholar]
- 43.Nee D.E., Wager T.D., Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Simmonds D.J., Pekar J.J., Mostofsky S.H. Meta-analysis of Go/No-go tasks, demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wager T.D., Jonides J., Reading S. Neuroimaging studies of shifting attention: A meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 46.Buchsbaum B.R., Greer S., Chang W.L., Berman K.F. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chambers C.D., Bellgrove M.A., Gould I.C., English T., Garavan H., McNaught E. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. J Neurophysiol. 2007;98:3638–3647. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- 48.Chambers C.D., Bellgrove M.A., Stokes M.G., Henderson T.R., Garavan H., Robertson I.H. Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- 49.Juan C.-H., Muggleton N.G. Brain stimulation and inhibitory control. Brain Stimul. 2012;5:63–69. doi: 10.1016/j.brs.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Aron A.R., Fletcher P.C., Bullmore E.T., Sahakian B.J., Robbins T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 51.Rubia K., Smith A. The neural correlates of cognitive time management: A review. Acta Neurobiol Exp(Wars) 2004;64:329–340. doi: 10.55782/ane-2004-1517. [DOI] [PubMed] [Google Scholar]
- 52.Wiener M., Turkeltaub P., Coslett H.B. The image of time: A voxel-wise meta-analysis. Neuroimage. 2010;49:1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
- 53.Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shulman G.L., Astafiev S.V., Franke D., Pope D.L.W., Snyder A.Z., McAvoy M.P., Corbetta M. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derrfuss J., Brass M., Neumann J., von Cramon D.Y. Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and Stroop studies. Hum Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willcutt E.G., Doyle A.E., Nigg J.T., Faraone S.V., Pennington B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Lijffijt M., Kenemans J.L., Verbaten M.N., van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: Deficient inhibitory motor control? J Abnorm Psychol. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- 60.Dickstein S.G., Bannon K., Castellanos F.X., Milham M.P. The neural correlates of attention deficit hyperactivity disorder: An ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 61.Rubia K., Halari R., Smith A.B., Mohammad M., Scott S., Brammer M.J. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J Child Psychol Psychiatry. 2009;50:669–678. doi: 10.1111/j.1469-7610.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- 62.Smith A.B., Taylor E., Brammer M., Halari R., Rubia K. Reduced activation in right lateral prefrontal cortex and anterior cingulate gyrus in medication-naive adolescents with attention deficit hyperactivity disorder during time discrimination. J Child Psychol Psychiatry. 2008;49:977–985. doi: 10.1111/j.1469-7610.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 63.Rubia K., Halari R., Smith A.B., Mohammed M., Scott S., Giampietro V. Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. Am J Psychiatry. 2008;165:889–897. doi: 10.1176/appi.ajp.2008.07071084. [DOI] [PubMed] [Google Scholar]
- 64.Rubia K., Overmeyer S., Taylor E., Brammer M., Williams S.C., Simmons A., Bullmore E.T. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: A study with functional MRI. Am J Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 65.Rubia K., Smith A.B., Brammer M.J., Toone B., Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 66.Rubia K., Cubillo A., Smith A.B., Woolley J., Heyman I., Brammer M.J. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum Brain Mapp. 2010;31:287–299. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubia K., Cubillo A., Woolley J., Brammer M.J., Smith A.B. Disorder-specific dysfunctions in patients with attention-deficit/hyperactivity disorder compared to patients with obsessive-compulsive disorder during interference inhibition and attention allocation. Hum Brain Mapp. 2011;32:601–611. doi: 10.1002/hbm.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubia K., Halari R., Cubillo A., Mohammad A., Scott S., Brammer M. Disorder-specific inferior frontal dysfunction in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during cognitive flexibility. Hum Brain Mapp. 2010;31:1823–1833. doi: 10.1002/hbm.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balcioglu A., Ren J.Q., McCarthy D., Spencer T.J., Biederman J., Bhide P.G. Plasma and brain concentrations of oral therapeutic doses of methylphenidate and their impact on brain monoamine content in mice. Neuropharmacology. 2009;57:687–693. doi: 10.1016/j.neuropharm.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berridge C.W., Devilbiss D.M., Andrzejewski M.E., Arnsten A.F., Kelley A.E., Schmeichel B. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 71.Arnsten A., Rubia K. Neurobiological circuits regulating attention, movement and emotion and their disruptions in pediatric neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Prehn-Kristensen A., Krauel K., Hinrichs H., Fischer J., Malecki U., Schuetze H. Methylphenidate does not improve interference control during a working memory task in young patients with attention-deficit hyperactivity disorder. Brain Res. 2011;1388:56–68. doi: 10.1016/j.brainres.2011.02.075. [DOI] [PubMed] [Google Scholar]
- 73.Shafritz K.M., Marchione K.E., Gore J.C., Shaywitz S.E., Shaywitz B.A. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- 74.Liddle E.B., Hollis C., Batty M.J., Groom M.J., Totman J.J., Liotti M. Task-related default mode network modulation and inhibitory control in ADHD: Effects of motivation and methylphenidate. J Child Psychol Psychiatry. 2011;52:761–771. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheridan M.A., Hinshaw S., D’Esposito M. Efficiency of the prefrontal cortex during working memory in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1357–1366. doi: 10.1097/chi.0b013e31812eecf7. [DOI] [PubMed] [Google Scholar]
- 76.Konrad K., Neufang S., Fink G.R., Herpertz-Dahlmann B. Long-term effects of methylphenidate on neural networks associated with executive attention in children with ADHD: Results from a longitudinal functional MRI study. J Am Acad Child Adolesc Psychiatry. 2007;46:1633–1641. doi: 10.1097/chi.0b013e318157cb3b. [DOI] [PubMed] [Google Scholar]
- 77.Bush G., Spencer T.J., Holmes J., Shin L.M., Valera E.M., Seidman L.J. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- 78.Frodl T., Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125:114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- 79.Castellanos F.X., Lee P.P., Sharp W., Jeffries N.O., Greenstein D.K., Clasen L.S. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 80.Shaw P., Sharp W.S., Morrison M., Eckstrand K., Greenstein D.K., Clasen L.S. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sobel L.J., Bansal R., Maia T.V., Sanchez J., Mazzone L., Durkin K. Basal ganglia surface morphology and the effects of stimulant medications in youth with attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167:977–986. doi: 10.1176/appi.ajp.2010.09091259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ivanov I., Bansal R., Hao X.J., Zhu H.T., Kellendonk C., Miller L. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167:397–408. doi: 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pliszka S.R., Lancaster J., Liotti M., Semrud-Clikeman M. Volumetric MRI differences in treatment-naive vs chronically treated children with ADHD. Neurology. 2006;67:1023–1027. doi: 10.1212/01.wnl.0000237385.84037.3c. [DOI] [PubMed] [Google Scholar]
- 84.Bledsoe J., Semrud-Clikeman M., Pliszka S.R. A magnetic resonance imaging study of the cerebellar vermis in chronically treated and treatment-naive children with attention-deficit/hyperactivity disorder combined type. Biol Psychiatry. 2009;65:620–624. doi: 10.1016/j.biopsych.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schnoebelen S., Semrud-Clikeman M., Pliszka S.R. Corpus callosum anatomy in chronically treated and stimulant naive ADHD. J Atten Disord. 2010;14:256–266. doi: 10.1177/1087054709356406. [DOI] [PubMed] [Google Scholar]
- 86.Wang G.J., Volkow N.D., Wigal T., Kollins S., Newcorn J., Telang F. Chronic treatment with methylphenidate increases dopamine transporter density in patients with attention deficit hyperactive disorder. J Nucl Med. 2009;50:1283. [Google Scholar]
- 87.Volkow N.D., Wang G.-J., Tomasi D., Kollins S.H., Wigal T.L., Newcorn J.H. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32:841–849. doi: 10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schulz K.P., Fan J., Bedard A-CV, Clerkin S.M., Ivanov I., Tang C.Y. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69:952–961. doi: 10.1001/archgenpsychiatry.2011.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.An L., Cao X.H., Cao Q., Sun L., Yang L., Zou Q.H. Methylphenidate normalizes resting-state brain dysfunction in boys with attention deficit hyperactivity disorder. Neuropsychopharmacology. 2013;38:1287–1295. doi: 10.1038/npp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Orru G., Pettersson-Yeo W., Marquand A.F., Sartori G., Mechelli A. Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neurosci Biobehav Rev. 2012;36:1140–1152. doi: 10.1016/j.neubiorev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 91.Posner J., Nagel B.J., Maia T.V., Mechling A., Oh M., Wang Z., Peterson B.S. Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:828–837. doi: 10.1016/j.jaac.2011.05.010. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material