Abstract
The four serotypes of mosquito-borne dengue virus (DENV-1, -2, -3, and -4) that circulate in humans each emerged from an enzootic, sylvatic cycle in non-human primates. Herein, we present the first study of sylvatic DENV infection dynamics in a primate. Three African green monkeys were inoculated with 105 plaque-forming units (pfu) DENV-2 strain PM33974 from the sylvatic cycle, and one African green monkey was inoculated with 105 pfu DENV-2 strain New Guinea C from the human cycle. All four monkeys seroconverted (more than fourfold rise in 80% plaque reduction neutralization titer [PRNT80]) against the strain of DENV with which they were inoculated; only one (33%) of three monkeys infected with sylvatic DENV showed a neutralizing antibody response against human-endemic DENV. Virus was detected in two of three monkeys inoculated with sylvatic DENV at low titer (≤ 1.3 log10pfu/mL) and brief duration (≤ 2 days). Clinical signs included rash and elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels.
Mosquito-borne dengue virus (DENV; genus Flavivirus) is one of only two arthropod-borne viruses to have established a transmission cycle endemic to humans that is ecologically and evolutionarily distinct from its enzootic ancestors.1 In its human transmission cycle, the virus comprises four antigenically and genetically distinct serotypes (DENV-1, -2, -3, and -4); infection with one serotype conveys lifelong protection against homologous challenge but only transient protection against heterologous infection with another serotype.2,3 Although most infections are subclinical, a fraction results in classical dengue fever (DF), a self-limited febrile illness, and some of these patients progress to severe dengue disease.4 The lack of an animal model that recapitulates human dengue disease has been a major barrier to the development of DENV vaccines and therapeutics. Replication of human-endemic DENV in non-human primates (NHPs)5 is muted in intensity and duration relative to replication in humans,6 and infection with human-endemic DENV produces disease in NHPs only when administered at doses that greatly exceed those delivered by the mosquito.1
Each of four human-endemic DENV serotypes emerged from enzootic ancestors maintained in a sylvatic cycle between NHPs and canopy-dwelling Aedes mosquitoes.1,7 These sylvatic cycles remain active in the forests of southeast Asia and west Africa. Spillover of sylvatic DENV into humans, sometimes causing severe disease, has been repeatedly documented.8–15 Thus, continued circulation of sylvatic DENV may threaten future control of human DENV when a DENV vaccine becomes available.7 Because the replication and immunogenicity of sylvatic DENV in its NHP hosts have not previously been investigated, some mathematical models of sylvatic DENV population dynamics and spillover risk have used infection parameters derived from studies of human-endemic DENV in NHPs.16 However, infection dynamics of arboviruses in reservoir and non-reservoir hosts can differ substantially.17,18 Yellow fever virus (YFV) offers a particularly dramatic example of this difference: YFV infection rarely causes overt illness in African NHPs that serve as reservoir hosts of its ancestral sylvatic cycle, whereas YFV infection of New World monkeys results in high rates of disease and death (reviewed in ref. 1). Mandl and others19 showed that the YFV-17D live-attenuated vaccine strain replicated to lower levels and for shorter duration in YFV reservoir (sooty mangabeys [Cercocebus atys]) than novel (rhesus macaques [Macaca mulatta]) hosts. Moreover, neutralizing antibody responses to YFV-17D were significantly lower in sooty mangabeys than in rhesus macaques 28 days post-infection (pi); neutralizing antibodies waned to undetectable levels by 120 days pi in sooty mangabeys but were maintained at high levels in rhesus macaques. If replication of sylvatic and human-endemic DENV in NHPs differs to a similar degree, models of sylvatic DENV transmission dynamics that rely on infection dynamics of human-endemic DENV in NHPs may be misleading.
In this study, we infected African green monkeys (AGMs; Chlorocebus sabaeus), a known host of sylvatic DENV-2 in West Africa,1 with a West African sylvatic strain of DENV-2. AGMs were provided by Primate Products (Miami, FL) from a colony maintained in St. Kitts. Sixteen adults were first screened by plaque reduction neutralization titer (PRNT) essentially as previously described20,21 to ensure that they had not been exposed to DENV-1, -2, -3, or -4, YFV, or West Nile virus. All 16 NHPs were seronegative (PRNT80 ≤ 20) for all viruses. Four males weighing > 6 kg were then quarantined in the Tulane National Primate Research Center (TNPRC) in Covington, Louisiana. All procedures using these animals were performed by the clinical veterinary staff at the TNPRC under the guidance of veterinarians, and they were approved by the Institutional Animal Care and Use Committee (IACUC) of Tulane University in compliance with the American Association for Laboratory Animal Science (AALAS) “Policy on the Human Care and Use of Laboratory Animals.” Monkeys were anesthetized with ketamine at an intramuscular (i.m.) dose of 10 mg/kg body weight on day 0 of the study; three AGMs were inoculated i.m. with 105 plaque-forming units (pfu) sylvatic DENV-2 strain PM33974, and one AGM was inoculated i.m. with 105 pfu DENV-2 strain New Guinea C (NGC) from the human cycle. The dose was chosen to enable comparisons with previous studies of human DENV22 and other flaviviruses19 in NHPs and DENV vaccine candidates in humans.23 Inocula were administered in a total volume of 1 mL, with 0.5 mL administered to each upper arm.
Sylvatic DENV-2 strain PM33974 was isolated from a pool of Ae. africanus mosquitoes in Guinea in 1981 by inoculation into Toxorhynchites amboinensis mosquitoes and subsequently passaged five times in Ae. albopictus C6/36 cells to create the stock used for infections. This strain has been studied extensively in cell culture and mouse models of human infection.24,25 DENV-2 NGC was derived from the prototype strain (isolated in 1944) without passage in mouse brains.26 DENV-2 NGC was chosen as a control, because in a previous experiment, this strain produced viremia in 100% of rhesus macaques (M. mulatta) injected with 105 pfu.22 The complete genome sequence of the DENV-2 PM33974 lineage used in this study has been determined (Genbank accession no. EF105378.1),24 but only the structural genes (capsid, pre-membrane, and envelope genes; 807 amino acids in total) of the DENV-2 NGC lineage used in this study have been sequenced (AY243468.1).22 The structural genes of the two strains showed 93% amino acid identity. To compare whole genomes, the sequence of a different strain of DENV-2 NGC (AF038403) was aligned to the complete genome of DENV-2 PM33974. The two sequences showed 82% nucleotide identity across the entire genome, 94% amino acid identity in the coding region, and 92% nucleotide identity at both the 5′ and 3′ untranslated regions. To compare the replication of the two viruses in cell culture, plaque size of each virus was measured in C6/36 cells (9 plaques/virus) and AGM kidney Vero cells (30 plaques/virus) as previously described.27 DENV-2 NGC produced substantially smaller plaques than DENV-2 PM33974 in C6/36 cells (mean in millimeters ± 1 SE = 0.29 ± 0.001 versus 0.40 ± 0.001; Student's t test, degree of freedom [df] = 16, P < 0.0001) but slightly larger plaques than DENV-2 PM33974 in Vero cells (0.19 ± 0.002 versus 0.15 ± 0.012; Student's t test, df = 58, P = 0.045).
Serum was collected on days 1–10, 12, 14, 16, and 18 pi to monitor viremia and days −3 and 28 pi to assay neutralizing antibodies by PRNT80 against DENV-2 strains NGC and PM33974. Blood was drawn to conduct a complete blood count and measure components of serum biochemistry (bilirubin, alkaline phosphatase, creatinine, creatinine phosphokinase, glucose, aspartate aminotransferase [AST], alanine aminotransferase [ALT], blood urea nitrogen [BUN], and albumin/globulin ratio) and electrolytes (Na, Cl, and K) on study days −3, 4, 7, and 28 pi. Rectal temperature, behavior, vital signs, weight, and skin condition were monitored on days −3, 0–10, 12, 14, 16, 18, and 28 pi. Viremia was quantified by serial dilution of serum and immunostaining in Vero cells as previously described20,21; virus titers are shown in Table 1. To enhance sensitivity, sera were also passaged one time in 1 well of a 24-well plate of confluent Vero cells, and resulting viral progeny in cell culture supernatants was detected by serial dilution and immunostaining. Detection of virus post-passage is indicated in Table 1 (†).
Table 1.
Viremia and rash in AGMs inoculated with 105 pfu designated DENV-2
| Monkey | DENV-2 transmission cycle | DENV-2 strain | Viremia* (titer [log10pfu/mL]) and detection of rash on a specific day | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 12 | 14 | 16 | 18 | |||
| JT08 | Human | NGC | † | † | 1.0 | 2.0 | ‡ | ‡ | ||||||||
| JT09 | Sylvatic | PM33974 | 1.3 | 1.3 | ‡ | ‡ | ||||||||||
| JT10 | Sylvatic | PM33974 | ‡ | ‡ | ||||||||||||
| JT11 | Sylvatic | PM33974 | † | ‡ | ‡ | ‡ | ||||||||||
Virus was not detected by either method unless noted.
Numbers indicate virus titer from direct assay of serum.
Detection of virus after one passage of serum in Vero cells.
Rash detected.
All four monkeys seroconverted (more than fourfold rise in PRNT80) with high PRNT80 titers against the strain of DENV with which they were inoculated (Table 2), showing that all four had been infected. The monkey infected with DENV-2 NGC showed a substantial neutralizing antibody response to sylvatic DENV-2 PM33974. These data are consistent with our previous finding that sera from 19 vaccinees who had received a live-attenuated DENV-2 vaccine as well as sera from two convalescent DENV-2 patients all effectively neutralized (PRNT80 > 20) four different strains of human-endemic DENV-2 and four strains of sylvatic DENV-2 that originated in both Asia and West Africa.28 However, of the three monkeys infected with sylvatic DENV-2, only one (33%) monkey seroconverted to DENV-2 NGC. Blaney and others29 have also reported variation in neutralization of different strains of DENV within a serotype. Blaney and others29 tested the neutralizing activity of serum from vaccinees who received a live-attenuated tetravalent dengue vaccine against five different DENV strains per serotype, including each of the four strains incorporated into the vaccine. The study found, for each serotype, that (1) the vaccine (infecting) strain was robustly neutralized, (2) at least one strain was more efficiently neutralized than the vaccine strain, and (3) although all strains of DENV-1, -2, and -4 were efficiently neutralized, two of five DENV-3 strains were not.29 The study by Blaney and others29 and other studies30,31 have raised concerns that there could be gaps in vaccine protection caused by strain variation. Similarly, the asymmetry in neutralization of DENV-2 NGC by monkeys infected with DENV-2 PM33971 suggests that NHP populations could be susceptible to some strains of human DENV, even if previously exposed to the homologous serotype of sylvatic DENV. DENV has been shown to spill back from humans into NHPs,32 and such spillback could result in the formation of new sylvatic cycles.1
Table 2.
Neutralizing antibody responses of AGMs 3 days pre-infection and 28 days p.i. with designated strains of DENV-2
| Monkey | Infected with DENV-2 | PRNT80* against designated DENV-2 | |||
|---|---|---|---|---|---|
| Pre-infection | P.i. | ||||
| NGC | PM33974 | NGC | PM33974 | ||
| JT08 | NGC | < 5 | < 5 | 63.2† | 114.4† |
| JT09 | PM33974 | < 5 | < 5 | 116.2† | 301.1† |
| JT10 | PM33974 | < 5 | < 5 | 7.0 | 111.5† |
| JT11 | PM33974 | 15.7 | < 5 | < 5 | 39.2† |
Values < 20 are considered to be seronegative.
Values > 20.
Viremia was detected in two of three monkeys infected with DENV-2 PM33974 after an average of 3.5 days, it persisted for an average of 1.5 days, and it reached a maximum titer of 1.3 log10pfu/mL in the monkey in which viremia could be detected without amplification (Table 1). In contrast, in the monkey infected with human-endemic DENV-2 NGC, viremia was detectable after 4 days, it persisted for 4 days, and it reached a maximum titer of 2.0 log10pfu/mL. Althouse and others5 have recently conducted a meta-analysis of the effects of serotype and host species, among other factors, on time to viremia and duration of viremia during human-endemic DENV replication in NHPs. This analysis included four studies of AGMs. Althouse and others5 detected a significant effect of serotype but not NHP species on these variables; for DENV-2, the median time to viremia was approximately 2.63 days, and the median duration of viremia was 5.13 days. Additionally, Halstead and others33 infected AGMs with 1 × 105 pfu DENV-2 strain 16681 and reported a maximum viremia titer of ≤ 2.0 log10pfu. Thus, the values reported in this study for DENV-2 NGC are consistent with previous studies of human-endemic DENV-2 in AGMs and other NHPs. However, contrary to our initial hypothesis, in AGMs, the magnitude of sylvatic DENV-2 PM33974 viremia was similar to that of human-endemic DENV-2, and the duration of detectable viremia was lower than that of human-endemic DENV-2.
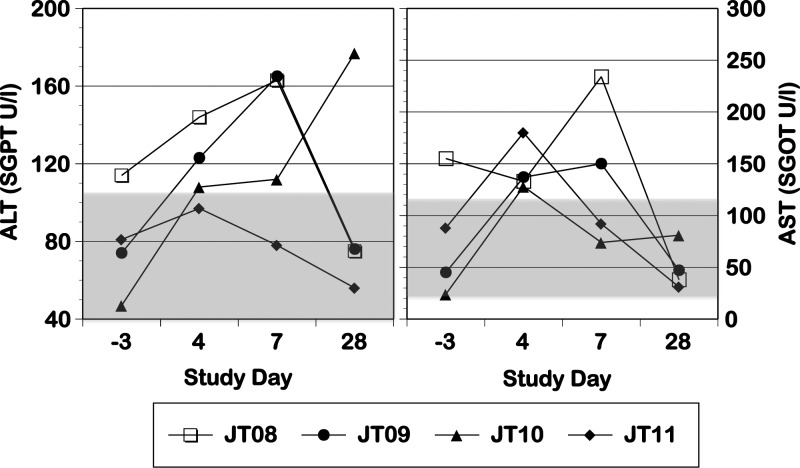
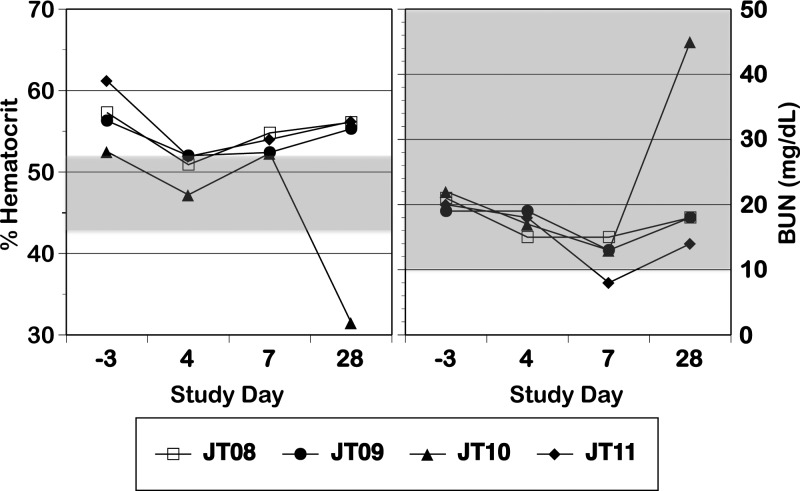
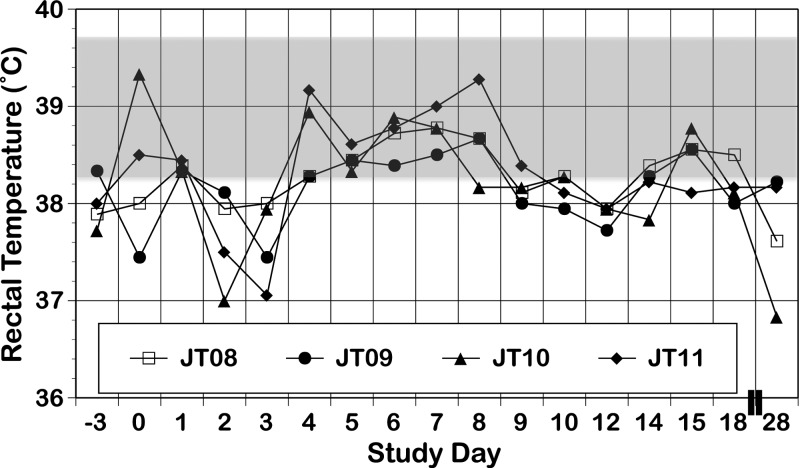
All monkeys exhibited a rash that occurred after viremia had dropped to undetectable levels (Table 1); this rash was characterized as primarily erythema, with some areas of papulas. All four monkeys experienced increases in ALT and AST levels p.i. (Figure 1 ); we did not attempt statistical analysis of this small sample. In one monkey (JT10), hematocrit showed a dramatic decrease on day 28 concurrent with a sharp increase in BUN (Figure 2 ), findings consistent with gastrointestinal bleeding, a sign of dengue disease. Confirmation of such bleeding would require detection of occult blood in stool; unfortunately, stool was not examined in this experiment. There were no other consistent or dramatic changes in any of the other parameters measured between the pre-infection and p.i. periods, and our values were generally in line with previously reported values for free-living St. Kitts AGMs brought into captivity.34 Monkeys exhibited no increase in body temperature (Figure 3) and no change in body condition beyond what was expected for animals being anesthetized daily. There were no dramatic differences between replication of or response to human and sylvatic DENV-2 in any values measured.
Figure 1.
AST and ALT levels in monkeys infected with human DENV-2 NGC (N = 1; open symbol) and sylvatic DENV-2 PM33974 (N = 3; closed symbols) on day −3 pre-infection and days 4, 7, and 28 p.i. The shaded boxes indicate the ranges of values observed for adult male AGMs from St. Kitts within 1 year of captivity (supplemental table 5 in the work by Liddie and others34), including a range of 5–112 units/L for ALT and a range of 22–116 units/L for AST; boxes that touch the top or bottom border represent a range that exceeds the top or bottom value included on the axis. Serum glutamic pyruvic transaminase (SGPT) is synonymous with ALT; serum glutamic oxaloacetic transaminase (SGOT) is synonymous with AST.
Figure 2.
Hematocrit and BUN levels in monkeys infected with human DENV-2 NGC (N = 1; open symbol) and sylvatic DENV-2 PM33974 (N = 3; closed symbols) on day −3 pre-infection and days 4, 7, and 28 p.i. The shaded boxes indicate the ranges of values observed for adult male AGMs from St. Kitts within 1 year of captivity (supplemental tables 5 and 6 in the work by Liddie and others34), including a range of 43.5–51.7% for hematocrit and a range of 10–66 mg/dL for BUN; boxes that touch the top or bottom border represent a range that exceeds the top or bottom value included on the axis.
Figure 3.
Rectal temperature in monkeys infected with human DENV-2 NGC (N = 1; open symbol) and sylvatic DENV-2 PM33974 (N = 3; closed symbols) on designated days between days −3 pre-infection and day 28 p.i. The shaded box indicates the mean ± 1 SD of temperature values (39 ± 0.69) observed for adult male AGMs born and reared in Cuba.36
A National Institute of Allergy and Infectious Diseases (NIAID) workshop convened in 2010 to consider fruitful directions for development of animal models of dengue disease urged researchers to investigate infection dynamics of sylvatic DENV in NHPs.35 Their rationale was that these enzootic viruses may show patterns of replication and pathogenicity in reservoir hosts that more closely reflect those of human-endemic DENV in humans than human-endemic DENVs in NHPs. Contrary to this expectation, our findings, albeit limited by a small sample size, show that levels of sylvatic DENV-2 virus replication in AGMs were substantially lower than those of human-endemic DENV in hospitalized or ambulatory cases of human dengue disease.6 Furthermore, primary infection by sylvatic DENV-2 of AGMs in this study recapitulated infection dynamics and clinical signs, including elevated ALT and rash, observed in humans administered 105 pfu live-attenuated DENV-4 vaccine.23 However, our finding that one monkey exhibited increased BUN coupled with decreased hematocrit is intriguing and warrants additional study. The duration of sylvatic DENV-2 replication in AGMs that we measured was lower than that of human-endemic DENV-2 replication in NHPs.5 Thorough documentation of such differences will be important for refining models of sylvatic DENV population dynamics. Thus, the results of this study provide the impetus to conduct larger studies involving additional sylvatic and human-endemic DENV strains and greater host sample sizes to fully characterize the infection dynamics of sylvatic DENV in reservoir hosts.
ACKNOWLEDGMENTS
The authors thank Paul Burns (New Mexico State University) and Meredith Hunter (Tulane National Primate Research Center) for technical assistance and William Messer (Oregon Health and Science University) for valuable insights into the clinical data.
Footnotes
Financial support: This project was supported by National Center for Research Resources and Office of Research Infrastructure Programs, National Institutes of Health (NIH) Grant P51 RR00164-50, National Center for Research Resources Grant 5P20RR016480-12, and National Institute of General Medical Sciences, NIH Grant 8 P20 GM103451-12. Support was also provided, in part, by the National Institute of Allergy and Infectious Diseases Intramural Research Program, NIH.
Authors' addresses: Kathryn A. Hanley, Department of Biology, New Mexico State University, Las Cruces, NM, E-mail: khanley@nmsu.edu. Mathilde Guerbois, Scott C. Weaver, and Nikos Vasilakis, Center for Tropical Diseases, Department of Pathology and Center for Biodefense and Emerging Infectious Diseases and Institute for Human Infections and Immunity, Center for Tropical Diseases, University of Texas Medical Branch, Galveston, TX, E-mails: mathildeguerbois@yahoo.fr, sweaver@utmb.edu, and nivasila@utmb.edu. Tiffany F. Kautz, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX, E-mail: tfkautz@utmb.edu. Meredith Brown, Banfield Pet Hospital, Las Cruces, NM, E-mail: Meredith.Brown@banfield.net. Stephen S. Whitehead, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, E-mail: swhitehead@niaid.nih.gov. Preston A. Marx, Division of Microbiology, Tulane National Primate Research Center, Covington, LA, E-mail: pmarx@tulane.edu.
References
- 1.Hanley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, Vasilakis N. Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol. 2013;19:292–311. doi: 10.1016/j.meegid.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurane I, Ennis FE. Immunity and immunopathology in dengue virus infections. Semin Immunol. 1992;4:121–127. [PubMed] [Google Scholar]
- 3.Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–528. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 5.Althouse BM, Durbin AP, Hanley KA, Halstead SB, Weaver SC, Cummings DA. Viral kinetics of primary dengue virus infection in non-human primates: a systematic review and individual pooled analysis. Virology. 2014;452-453:237–246. doi: 10.1016/j.virol.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyet MN, Duong TH, Trung VT, Nguyen TH, Tran CN, Long VT, Dui le T, Nguyen HL, Farrar JJ, Holmes EC, Rabaa MA, Bryant JE, Nguyen TT, Nguyen HT, Nguyen LT, Pham MP, Nguyen HT, Luong TT, Wills B, Nguyen CV, Wolbers M, Simmons CP. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 2013;110:9072–9077. doi: 10.1073/pnas.1303395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasilakis N, Cardosa J, Hanley KA, Holmes EC, Weaver SC. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol. 2011;9:532–541. doi: 10.1038/nrmicro2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monlun E, Zeller H, Traore-Lamizana M, Hervy JP, Adam F, Mondo M, Digoutte JP. Caracteres cliniques et epidemiologiques de la dengue 2 au Senegal. Med Mal Infect. 1992;22:718–721. [Google Scholar]
- 9.Robin Y, Cornet M, Heme G, Le Gonidec G. Isolement du virus dela dengue au Senegal. Ann Virol. 1980;131:149–154. [Google Scholar]
- 10.Saluzzo JF, Cornet M, Adam C, Eyraud M, Digoutte JP. Dengue 2 in eastern Senegal: serologic survey in simian and human populations. 1974–85. Bull Soc Pathol Exot. 1986;79:313–322. [PubMed] [Google Scholar]
- 11.Saluzzo JF, Cornet M, Castagnet P, Rey C, Digoutte JP. Isolation of dengue 2 and dengue 4 viruses from patients in Senegal. Trans R Soc Trop Med Hyg. 1986;80:5. doi: 10.1016/0035-9203(86)90182-3. [DOI] [PubMed] [Google Scholar]
- 12.Carey DE. Use of a combined complement-fixing antigen to detect arthropod-borne viral infection. Nature. 1963;200:1024–1025. doi: 10.1038/2001024b0. [DOI] [PubMed] [Google Scholar]
- 13.Vasilakis N, Tesh RB, Weaver SC. Sylvatic dengue virus type 2 activity in humans, Nigeria, 1966. Emerg Infect Dis. 2008;14:502–504. doi: 10.3201/eid1403.070843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardosa J, Ooi MH, Tio PH, Perera D, Holmes EC, Bibi K, Abdul Manap Z. Dengue virus serotype 2 from a sylvatic lineage isolated from a patient with dengue hemorrhagic fever. PLoS Negl Trop Dis. 2009;3:e423. doi: 10.1371/journal.pntd.0000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco C, Hynes NA, Bouri N, Henderson DA. The dengue threat to the United States. Biosecur Bioterror. 2010;8:273–276. doi: 10.1089/bsp.2010.0032. [DOI] [PubMed] [Google Scholar]
- 16.Althouse BM, Lessler J, Sall AA, Diallo M, Hanley KA, Watts DM, Weaver SC, Cummings DA. Synchrony of sylvatic dengue isolations: a multi-host, multi-vector SIR model of dengue virus transmission in Senegal. PLoS Negl Trop Dis. 2012;6:e1928. doi: 10.1371/journal.pntd.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrara AS, Gonzales G, Ferro C, Tamayo M, Aronson J, Paessler S, Anishchenko M, Boshell J, Weaver SC. Venezuelan equine encephalitis virus infection of spiny rats. Emerg Infect Dis. 2005;11:663–669. doi: 10.3201/eid1105.041251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Amo J, Llorente F, Figuerola J, Soriguer RC, Moreno AM, Cordioli P, Weissenbock H, Jimenez-Clavero MA. Experimental infection of house sparrows (Passer domesticus) with West Nile virus isolates of Euro-Mediterranean and North American origins. Vet Res. 2014;45:33. doi: 10.1186/1297-9716-45-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandl JN, Akondy R, Lawson B, Kozyr N, Staprans SI, Ahmed R, Feinberg MB. Distinctive TLR7 signaling, type I IFN production, and attenuated innate and adaptive immune responses to yellow fever virus in a primate reservoir host. J Immunol. 2011;186:6406–6416. doi: 10.4049/jimmunol.1001191. [DOI] [PubMed] [Google Scholar]
- 20.Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, Thumar B, Men R, Lai CJ, Elkins WR, Chanock RM, Murphy BR, Whitehead SS. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am J Trop Med Hyg. 2001;65:405–413. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 21.Hanley KA, Manlucu LR, Manipon GG, Hanson CT, Whitehead SS, Murphy BR, Blaney JE., Jr Introduction of mutations into the non-structural genes or 3′ untranslated region of an attenuated dengue virus type 4 vaccine candidate further decreases replication in rhesus monkeys while retaining protective immunity. Vaccine. 2004;22:3440–3448. doi: 10.1016/j.vaccine.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Whitehead SS, Hanley KA, Blaney JE, Jr, Gilmore LE, Elkins WR, Murphy BR. Substitution of the structural genes of dengue virus type 4 with those of type 2 results in chimeric vaccine candidates which are attenuated for mosquitoes, mice, and rhesus monkeys. Vaccine. 2003;21:4307–4316. doi: 10.1016/s0264-410x(03)00488-2. [DOI] [PubMed] [Google Scholar]
- 23.Durbin AP, Whitehead SS, McArthur J, Perreault JR, Blaney JE, Jr, Thumar B, Murphy BR, Karron RA. rDEN4delta30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J Infect Dis. 2005;191:710–718. doi: 10.1086/427780. [DOI] [PubMed] [Google Scholar]
- 24.Vasilakis N, Fokam EB, Hanson CT, Weinberg E, Sall AA, Whitehead SS, Hanley KA, Weaver SC. Genetic and phenotypic characterization of sylvatic dengue virus type 2 strains. Virology. 2008;377:296–307. doi: 10.1016/j.virol.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasilakis N, Shell EJ, Fokam EB, Mason PW, Hanley KA, Estes DM, Weaver SC. Potential of ancestral sylvatic dengue-2 viruses to re-emerge. Virology. 2007;358:402–412. doi: 10.1016/j.virol.2006.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bray M, Men R, Tokimatsu I, Lai CJ. Genetic determinants responsible for acquisition of dengue type 2 virus mouse neurovirulence. J Virol. 1998;72:1647–1651. doi: 10.1128/jvi.72.2.1647-1651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanley KA, Nelson JT, Schirtzinger EE, Whitehead SS, Hanson CT. Superior infectivity for mosquito vectors contributes to competitive displacement among strains of dengue virus. BMC Ecol. 2008;8:1. doi: 10.1186/1472-6785-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasilakis N, Durbin AP, da Rosa AP, Munoz-Jordan JL, Tesh RB, Weaver SC. Antigenic relationships between sylvatic and endemic dengue viruses. Am J Trop Med Hyg. 2008;79:128–132. [PubMed] [Google Scholar]
- 29.Blaney JE, Jr, Durbin AP, Murphy BR, Whitehead SS. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol. 2006;19:10–32. doi: 10.1089/vim.2006.19.10. [DOI] [PubMed] [Google Scholar]
- 30.Wahala WM, Donaldson EF, de Alwis R, Accavitti-Loper MA, Baric RS, de Silva AM. Natural strain variation and antibody neutralization of dengue serotype 3 viruses. PLoS Pathog. 2010;6:e1000821. doi: 10.1371/journal.ppat.1000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez M, Pavon-Oro A, Rodriguez-Roche R, Bernardo L, Morier L, Sanchez L, Alvarez AM, Guzman MG. Neutralizing antibody response variation against dengue 3 strains. J Med Virol. 2008;80:1783–1789. doi: 10.1002/jmv.21234. [DOI] [PubMed] [Google Scholar]
- 32.Kato F, Ishida Y, Kawagishi T, Kobayashi T, Hishiki T, Miura T, Igarashi T. Natural infection of cynomolgus monkeys with dengue virus occurs in epidemic cycles in the Philippines. J Gen Virol. 2013;94((Pt 10)):2202–2207. doi: 10.1099/vir.0.055343-0. [DOI] [PubMed] [Google Scholar]
- 33.Halstead SB, Shotwell H, Casals J. Studies on the pathogenesis of dengue infection in monkeys. I. Clinical laboratory responses to primary infection. J Infect Dis. 1973;128:7–14. doi: 10.1093/infdis/128.1.7. [DOI] [PubMed] [Google Scholar]
- 34.Liddie S, Goody RJ, Valles R, Lawrence MS. Clinical chemistry and hematology values in a Caribbean population of African green monkeys. J Med Primatol. 2010;39:389–398. doi: 10.1111/j.1600-0684.2010.00422.x. [DOI] [PubMed] [Google Scholar]
- 35.Cassetti MC, Durbin A, Harris E, Rico-Hesse R, Roehrig J, Rothman A, Whitehead S, Natarajan R, Laughlin C. Report of an NIAID workshop on dengue animal models. Vaccine. 2010;28:4229–4234. doi: 10.1016/j.vaccine.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casaco A, Beausoleil I, Gonzalez B, Luaces P, Leon A, Arteaga ME, Prado P, Rodriguez V, Perez A, Guevara G, Bada AM, Ledon N, Fuentes D, Gonzalez C, Hernandez O, Orphee R, Blanco D, Garcia-Osuma M, Ballester-Labrada A. Hematological, biochemical, respiratory, cardiovascular and electroneurophysiological parameters in African green monkeys (Cercopithecus aethiops sabaeus). Its use in non-clinical toxicological studies. J Med Primatol. 2010;39:177–186. doi: 10.1111/j.1600-0684.2010.00410.x. [DOI] [PubMed] [Google Scholar]