Abstract
Studies investigating winter transmission of Eastern equine encephalitis virus (EEEV) were conducted in Hillsborough County, Florida. The virus was detected in Culiseta melanura and Anopheles quadrimaculatus in February 2012 and 2013, respectively. During the winter months, herons were the most important avian hosts for all mosquito species encountered. In collections carried out in the summer of 2011, blood meals taken from herons were still common, but less frequently encountered than in winter, with an increased frequency of mammalian- and reptile-derived meals observed in the summer. Four wading bird species (Black-crowned Night Heron [Nycticorax nycticorax], Yellow-crowned Night Heron [Nyctanassa violacea], Anhinga [Anhinga anhinga], and Great Blue Heron [Ardea herodias]) were most frequently fed upon by Cs. melanura and Culex erraticus, suggesting that these species may participate in maintaining EEEV during the winter in Florida.
Introduction
Eastern equine encephalitis virus (EEEV) is a highly pathogenic arbovirus exhibiting a mortality rate of ∼30–35% in humans and 80–90% in horses.1 Approximately two-thirds of the individuals that recover from the infection suffer from chronic neurological sequelae that can incur lifetime costs upwards of $3 million.2 In the United States, cases occur most frequently in the Atlantic and Gulf Coast states, with ∼6 human cases and 200 horse cases per year.3 Florida has had more human and horse cases than any other state, with an average of one to two human and 70 horse cases per year, respectively.3
EEEV has been shown to infect horses, humans, birds, and other animals.1 The virus is maintained in an enzootic cycle between ornithophilic mosquitoes and avian reservoir hosts.1 Most studies of EEEV have concentrated upon foci in the northern parts of the United States. In the northeastern states, transmission is seasonal, peaking in the late summer months.1 Passerine birds are considered to be the primary reservoir hosts for EEEV in the northeastern United States, with enzootic transmission between avian hosts mediated by Culiseta melanura, particularly in freshwater swamp foci.1,4 Other mosquito species that feed upon mammals and birds, including Aedes vexans, Aedes sollicitans, and Coquillettidia perturbans, have been implicated as bridge vectors, transmitting EEEV from birds to horses and humans.1,5 Shifts in feeding patterns of bridge vectors from feeding on avian to mammalian hosts during the transmission season may help to facilitate transmission to horses and humans.6–8
In the southeastern United States, Culex erraticus is often the most abundant mosquito species in wetlands9–11and are often infected with EEEV.9,10 Laboratory studies also suggest that Cx. erraticus may be a competent vector for EEEV.5 Taken together, these studies suggest that Cx. erraticus may play an important role both as an enzootic and bridge vector in habitats where Cs. melanura is less common.9
Most of the studies examining avian reservoir competence of EEEV have focused on passerine birds.4 Studies have indicated that permanent-resident and summer-resident birds have antibodies to EEEV more often than do transient and winter-resident birds,4,12 and there is some evidence suggesting that migrating birds play a role in transportation of EEEV. Many of these studies have focused on passerine birds; however, recent studies have indicated that wading birds (Ciconiiformes) are preferred hosts of potential bridge vectors of EEEV in the southeast and may play a role in the ecology of EEEV transmission in this region.13,14 In support of this hypothesis, one seroprevalence study conducted in Louisiana found that over 80% of Yellow-crowned Night Herons (Nyctanassa violacea) were seropositive for EEEV,12 a seroprevalence rate that was higher than any of the Passeriformes examined.15 Virus was also isolated from one Yellow-crowned Night Heron nestling, confirming that this species can serve as a host under natural conditions.15 Several other studies have also implicated a wide variety of wading bird species as potential enzootic hosts for EEEV.16,17 In 1962, Herman reported eight species of Ciconiiformes (Great Egret [Ardea alba], Snowy Egret [Egretta thula], Black-crowned Night Heron [Nycticorax nycticorax], Green Heron [Butorides virescens], Little Blue Heron [Egretta caerulea], Tricolored Heron [Egretta tricolor], Yellow-crowned Night Heron and White Ibis [Eudocimus albus]) as either being naturally or experimentally infected with EEEV.17 Experimentally infected wading and water birds have been shown to develop high enough viremias to infect mosquitoes.18,19 In addition, Ciconiiformes, particularly the Night Herons, have been implicated in many other arbovirus transmission cycles including West Nile virus,20 Saint Louis encephalitis virus,16 Venezuelan equine encephalitis virus,21 Western equine encephalitis virus,15 Japanese encephalitis virus,22 and Murray Valley encephalitis virus.23
Florida is unique among states in the United States in that EEEV transmission occurs year-round.24 Furthermore, recent phylogenetic studies have suggested that Florida may serve as a reservoir for EEEV; the virus may periodically be introduced from Florida to the northeastern United States, where it locally amplifies, overwinters, and can remain stable for several years.25–27 If the hypothesis that Florida serves as a reservoir for EEEV for the rest of the country is correct, then transmission during the winter months in Florida (when vector densities are low) may represent a particularly vulnerable point in the viral life cycle. This study was undertaken to investigate the winter ecology of EEEV transmission with respect to relative abundance, virus infection, and host use of potential vector mosquito species, focusing on wading birds as potential amplification and dissemination hosts. In addition, host preference was compared between one peak transmission season (summer) and the following winter season to see how host preference varies between seasons in Florida.
Materials and Methods
Study sites.
Field work was conducted at three county parks in Hillsborough County, Florida. Lettuce Lake Park (28°4′33.875″N, 82°22′35.837″W) is a 240-acre site along the Hillsborough River, with more than half of the park consisting of a hardwood and cypress swamp and the remainder consisting of hardwood hammocks and pine flatwoods. The park is named after a shallow dead-end offshoot of the Hillsborough river, which is dominated by water lettuce (Pistia stratiotes), a floating aquatic plant. Lettuce Lake Park also houses sentinel chickens that are tested by the Florida Department of Health for EEEV and EEEV activity has been detected at this location in the past.3 The second site, John B. Sargeant Park (28°4′57.793″N, 82°17′10.991″W), is a 23-acre park also along the Hillsborough River, dominated by cypress and hardwood swamps. It is located upstream of Lettuce Lake Park and has a similar habitat; however, fewer wading birds appear to frequent this site. The third site, Eureka Springs Park (28°0′22.928″N, 82°20′41.529″W), is a 31-acre park with a floodplain forest of maple, cypress, and tupelo. This site has a slightly different habitat than the others, but can still support Cs. melanura populations.
Mosquito collections.
The mosquito population at each site was sampled weekly during the winter months using three methods; resting shelters, CO2-baited Centers for Disease Control and Prevention (CDC) light traps, and vegetation sweeps. Resting mosquitoes were collected from wire frame shelters that served as artificial resting sites.28 Six resting shelters were sampled at each site, which were spaced ∼50 meters apart along designated trails. Mosquitoes were aspirated during the morning hours (0800–1030 h) from the resting shelters using a modified Dustbuster and a piece of white corrugated plastic cardboard with a 10 cm diameter hole cut in it.29 Mosquitoes resting in vegetation were also sampled during morning hours (0800–1030 h) with vegetation sweeps. Low-growing herbaceous vegetation was swept using a heavy-duty sweep net along two predetermined paths and contents of the net vacuumed. Host-seeking mosquitoes were sampled using two CO2-baited CDC light traps at each site. Traps were set shortly before dusk, and retrieved the following morning, after the resting mosquito collections were completed. Samples were collected once per week at Lettuce Lake Park from January through March 2012 and December 2012 through March 2013. Samples were collected once per week from the other two sites from December 2012 through March 2013. Mosquitoes were also collected from artificial resting shelters at Lettuce Lake Park from June through August 2011 to determine if host feeding patterns differed between summer and winter. Field-collected mosquitoes were returned to the laboratory for identification, pool-screening, and blood meal analysis.
Detection of virus in mosquito pools.
Mosquitoes were sorted by species, collection date, and collection site into pools of 50 individuals or fewer. Females were identified to species using standard keys.30 A copper BB and 1 mL of BFD (biological field diluent; 90% Minimum Essential Medium with Hanks' salts, 10% fetal bovine serum, 200 U/mL penicillin, 200 μg/mL streptomycin, 2.5 μg/mL amphotericin B, and 50 μg/ml kanamycin) were added to each pool and mosquitoes were homogenized using a high-speed mechanical homogenizer (TissueLyser; Qiagen, Valencia, CA). Homogenates were subjected to centrifugation at 10,500 × g for 7 minutes at 4°C. The RNA was prepared from 140 μL of the resulting supernatant using the QIAamp Viral RNA Mini Kit (Qiagen) following the manufacturer's conditions. The Qiacube platform (Qiagen) was used to automate RNA extraction and the isolated RNA (eluted into 60 μL of nuclease free water) was stored at −80°C. A real-time reverse transcriptase-polymerase chain reaction (qRT-PCR) assay was then conducted using the iScript one step RT-PCR kit for probes (Bio-Rad, Hercules, CA) according to the manufacturer's protocol. The primers, probe, and reaction conditions used to detect EEEV RNA were those recommended by Lambert and others,31 with the exception that reactions were performed in a final volume of 25 μL, and used 5 μL of the RNA template. This assay used two primers (5′-ACACCGCACCCTGATTTTACA-3′ and 5′-CTTCCAAGTGACCTGGTCGTC-3′) that produced an amplicon spanning positions 9298–9456 in the EEEV genome sequence (GenBank accession no. X67111).32 Samples producing a signal at a Ct value of 37 or below were considered putatively positive.
The RNA samples found to be putatively positive in the initial assay were subjected to a confirmatory qRT-PCR assay provided by the CDC to state Department of Health Laboratories conducting arboviral surveillance activities.33 The confirmatory assay used two primers (5′-ACCTTGCTGACGACCAGGTC-3′ and 5′-GTTGTTGGTCGCTCAATCCA-3′) that produced an amplicon spanning positions 9428–9497 in the EEEV genome. The 5′ 6-FAM, 3′ BHQ1a-Q probe used in this assay contained the sequence 5′-CTTGGAAGTGATGCAAATCCAACTCGACA-3′, spanning positions 9449–9477 in the genome. Samples giving a Ct value of < 40 were scored as positive in the confirmatory assay. Samples were only considered to be confirmed positive if they were scored as positive in both assays.
Virus isolation was attempted from all confirmed qRT-PCR positive samples by inoculating individual T-25 flasks of confluent Vero cell cultures with 1 mL of filtered mosquito pool supernatant. Flasks were incubated for 2 hours at 37°C, with gentle rocking every 15 minutes. After the incubation, 9 mL of maintenance media (1× Earle's minimal essential medium, 2% fetal bovine serum, 200 U/mL penicillin, 200 μg/mL streptomycin, 2.5 μg/mL amphotericin B) were added to each flask. Cells were then monitored daily for cytopathic effect.
Blood meal analysis.
Individual blood-engorged female mosquitoes were homogenized in 200 μL DNAzol reagent (Molecular Research Center, Inc., Cincinnati, OH) using a disposable plastic pestle. Samples were incubated for 10 minutes at room temperature, subjected to centrifugation at 7000 × g for 10 minutes at room temperature, and the supernatant transferred to a new tube. A total of 80 μL of isopropanol was added to the supernatant. The solution was mixed and incubated for 5 minutes at room temperature. The precipitate was collected by centrifugation at 4,000 × g for 10 minutes. After centrifugation, the pellet was washed twice with 1 mL of 75% ethanol and the DNA dissolved with 50 μL of Tris-EDTA buffer (Tris-EDTA, pH 8.0, Boston Bioproducts, Ashland, MA). Isolated DNA was stored at −80°C.
The identification of the blood meals from the extracted DNA used two PCR-based assays. The initial nested PCR used a set of universal vertebrate primers targeting the vertebrate cytochrome B gene.13 The first PCR reaction used the following primers: 5′-CCCCTCAGAATGATATTTGTCCTCA-3′and 5′-CCATCCAACATCTCAGCATGATGAAA-3′ and followed the reaction conditions previously described.13 This reaction yielded a 383 base pair (bp) product that was used as a template for the nested reaction. The sequence of the forward primer for the nested reaction was 5′-TCWRCHTGATGAAACTTCGG-3′. The reverse primer used was a mixture of four primers with the following sequences: 5′-ACRAARGCRGTTGCTATTAG-3′, 5′-ACRAAGGCAGTKGCTATAAG-3′, 5′-ACGAARGCRGTTGCYATGAG-3′, and 5′-ACGAAGGCMGTKGCTATTAG-3′. The use of four reverse primers in the nested reaction allowed for a greater variety of species to be detected than from one primer set alone and yielded a 296 bp product. Reaction conditions were as previously described.13 A second PCR assay was used to attempt to identify the source of blood meals in samples that yielded a negative result in the nested PCR described previously. This assay, which uses a universal vertebrate primer set targeting 16S rRNA, amplifies blood meals from some mammal and reptile species more efficiently than the cytochrome B gene assay.34 Primers used in the PCR were those of Kitano and co-workers35 and were as follows: 5′-GCCTGTTTACCAAAAACATCAC-3′ and 5′-CTCCATAGGGTCTTCTCGTCTT-3′. Reaction conditions were the same as those described previously and yielded a 244 bp product.34
Amplicons were purified using the QIAquick PCR purification kit (Qiagen) and were then sent to the Eurofins MWG Operon sequencing facility (Huntsville, AL) for analysis. Sequences were entered into the NCBI BLAST database for identification, and only those sequences with a match percentage ≥ 95% were accepted as belonging to the identified blood meal source.
Avian surveys.
Wading birds at each site were counted through visual searches of the study areas from elevated boardwalks maintained by the Parks, Recreation and Conservation Department of Hillsborough County. Observers walked slowly along predetermined paths and recorded individual birds as they were encountered. Each survey lasted roughly 1–1.5 hours. Wading birds were surveyed on each occasion when light traps were set (at dusk) and again the following morning when mosquitoes were collected from the traps. Thus, each park was surveyed for birds roughly eight times per month. This survey method is similar to those used for the Christmas Bird Count, a survey sponsored by the National Audubon Society that is used to monitor winter bird population trends throughout the United States every year, and has been shown to be more effective at identifying wetland species than similar surveys that use point counts (e.g., the North American Breeding Bird Survey).36–38 The abundance of some species of wading and water birds are difficult to estimate; however, an increasing number of visits has been shown to yield significant increases in cumulative species richness in wetland areas.39 In addition, combining morning and evening samplings increases the overall detection probability for these species.40
Data analysis.
To characterize the relative use of wading bird hosts as a blood source, both relative water bird abundances and the proportion of avian blood meals taken by Cx. erraticus were calculated for each water bird species. The maximum count for each bird for each sampling day (dusk or dawn) was used for calculating the water bird relative abundances.41 To account for variations in bird abundances over the season, the average monthly relative abundance was calculated. We calculated 95% confidence intervals (CIs) for the sampling error associated with the estimated proportion of blood meals taken from a given class or species, as described previously.42
Results
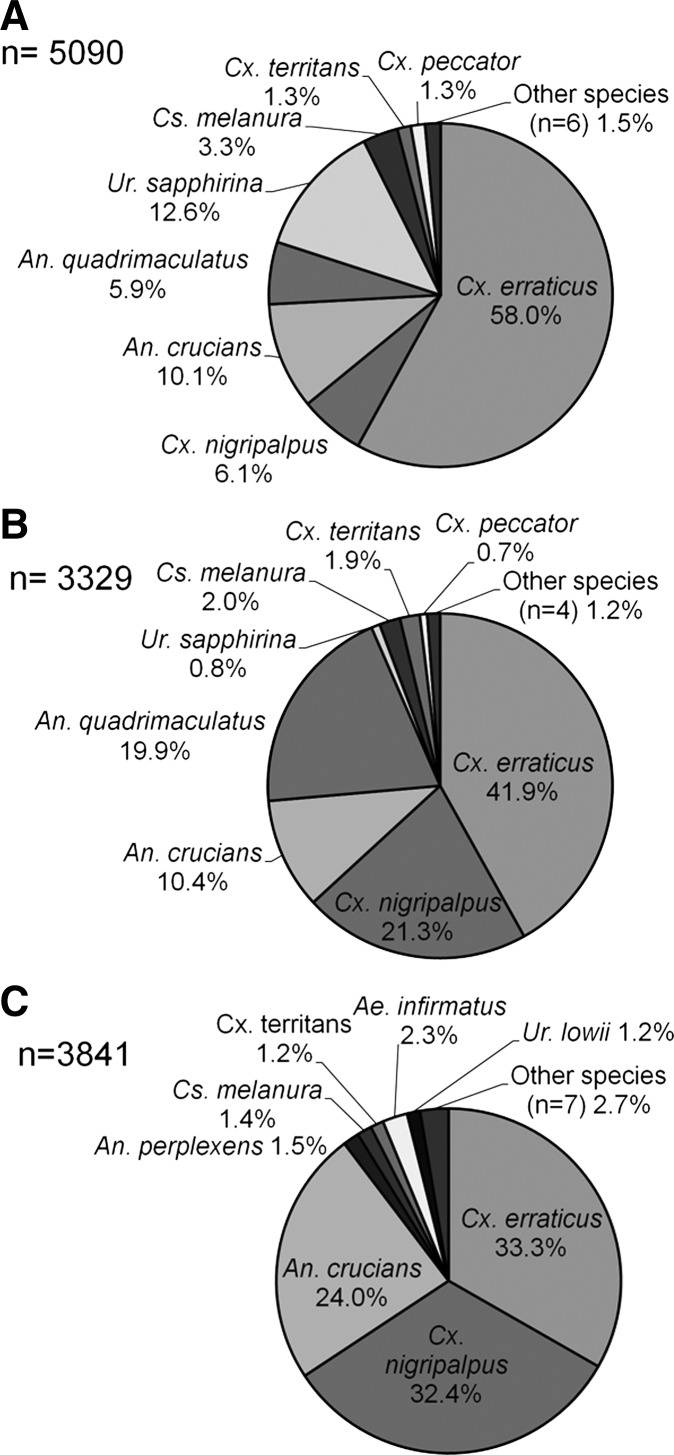
A total of 12,260 mosquitoes representing 16 species were collected from the three sites over 116 trap nights during the winter months. Culex erraticus was the species with the highest abundance at all three sites, representing 58.0% of all mosquitoes collected at Lettuce Lake Park, 41.9% at John B. Sargeant Park, and 33.3% at Eureka Springs Park (Figure 1). Culiseta melanura was present at low numbers at all three study sites, representing < 5% of the overall collections (Figure 1).
Figure 1.
Total relative abundance of mosquitoes (N = 12,260) collected from Lettuce Lake Park (panel A), John B. Sargeant Park (panel B), and Eureka Springs Park (panel C) in Hillsborough County, FL. Mosquitoes were collected during two winter collection periods (2012–2013) for Lettuce Lake Park and one winter period (2013) for both John B. Sargeant Park and Eureka Springs Park.
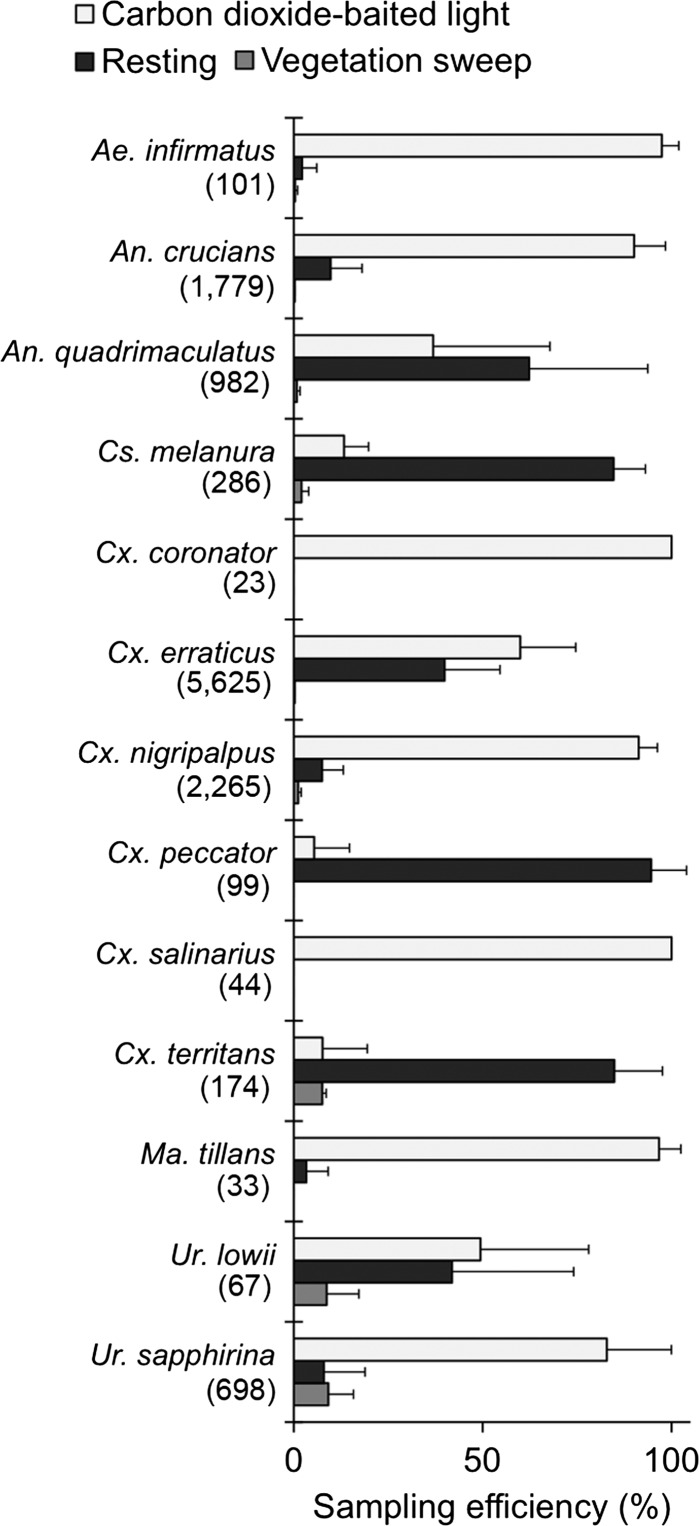
No single collection method was most effective for sampling all mosquito species, although about half (7 of 13) of the species were collected most efficiently from CO2-baited CDC light traps (Figure 2). Four species (Anopheles quadrimaculatus, Culiseta melanura, Culex peccator, and Culex territans) were sampled most efficiently from resting shelters. Vegetation sweeps were the least efficient sampling method (1.57% of total), although females of Uranotaenia sapphirina, Uranotaenia lowii, and Cx. territans were occasionally collected using this method.
Figure 2.
Relative efficiency of sampling methods (CO2-baited CDC light trap, resting site aspiration, and vegetation sweep) for capturing female mosquitoes at three wetland field sites in Hillsborough, County, FL. Data are from weekly sampling during winter months of 2012–2013. Only mosquitoes encountered at more than one site were included. Error bars represent the standard deviation of the mean.
In general, the abundance of host-seeking females was greatest in January in both years (Figure 3), with few mosquitoes collected in weeks leading up to the end of the collection season in March. The one exception to this pattern was a peak of An. crucians that was observed in March 2012 (Figure 3, panel A). Overall, mosquito abundances were higher in the second year of the study (Figure 3).
Figure 3.
Winter abundance of mosquitoes from wetland parks in Hillsborough County, FL; January–March 2012 for Lettuce Lake Park (panel A) and December 2012–March 2013 for all three sites (panel B). Asterisks indicate dates of EEEV-positive mosquito pools.
Quantitative RT-PCR assays to detect EEEV were conducted on all non-engorged mosquitoes collected during both winter seasons. Of 455 total mosquito pools tested for EEEV, two pools were confirmed positive for EEEV; a pool of Cs. melanura collected at Lettuce Lake Park in February 2012 and a pool of An. quadrimaculatus collected at John B. Sargeant Park in February 2013 (Figure 3). The overall seasonal minimum infection rate (MIR) was 22.2/1,000 for Cs. melanura and 1.54/1,000 for An. quadrimaculatus, whereas the February MIRs were 58.82/1,000 for Cs. melanura and 7.19/1,000 for An. quadrimaculatus. The Ct values for the positive pools ranged from 32.7 to 35.2 for the qRT-PCR assays. Attempts to culture virus from both isolates were not successful.
A total of 724 blood-engorged mosquitoes representing eight species were collected during the two winter sampling periods (Table 1). Of these, 701 (96.8%) blood meals were successfully identified to the host species level. The most commonly collected blood-fed species was Cx. erraticus with 500 blood meals identified. Birds were the predominant winter hosts for Cx. erraticus, Cx. nigripalpus, Cx. peccator, and Cs. melanura. Culiseta melanura had the highest proportion of avian blood meals among the eight mosquito species (88.3% of total Cs. melanura blood meals). For An. crucians, Anopheles perplexens and An. quadrimaculatus the majority of blood meals were from mammalian hosts; however, these species also fed occasionally upon avian hosts. Amphibians were the main hosts for Cx. territans. Culex territans and Cx. erraticus were the only mosquito species that fed upon all four host classes (Table 1).
Table 1.
Proportion of blood meals taken from different host classes during the winter months
| Species | ID/tested† | Percentage feeding on* | |||
|---|---|---|---|---|---|
| Avian | Mammalian | Reptile | Amphibian | ||
| Anopheles crucians | 22/22 | 13.6 ± 14.3 | 86.4 ± 14.3 | 0 | 0 |
| Anopheles perplexens | 7/7 | 14.3 | 85.7 | 0 | 0 |
| Anopheles quadrimaculatus | 34/34 | 14.7 ± 11.9 | 85.3 ± 11.9 | 0 | 0 |
| Culex erraticus | 500/518 | 61.0 ± 4.3 | 35.0 ± 4.2 | 3.2 ± 1.5 | 0.8 ± 0.8 |
| Culex nigripalpus | 40/40 | 75.0 ± 13.4 | 20.0 ± 12.4 | 0 | 5.0 ± 6.8 |
| Culex peccator | 7/8 | 71.4 | 28.6 | 0 | 0 |
| Culex territans | 31/35 | 9.7 ± 10.4 | 12.9 ± 11.8 | 6.4 ± 8.6 | 71.0 ± 16.0 |
| Culiseta melanura | 60/60 | 88.3 ± 8.1 | 11.7 ± 8.1 | 0 | 0 |
95% confidence intervals are provided for species where the number of samples identified was greater than or equal to 20.
Only mosquito species for which the number of blood meals identified was > 5 are shown.
A total of nine mammalian species were identified as hosts (Table 2). White-tailed deer (Odocoileus virginianus) was the primary mammalian host representing more than half of the mammalian-derived blood meals for Cx. erraticus (54.9%), An. crucians (63.2%), and An. quadrimaculatus (51.7%). Humans (Homo sapiens) were also important mammalian hosts, making up 29.1% of Cx. erraticus mammal-derived blood meals, and six out of seven (85.7%) of the mammalian-derived blood meals of Cs. melanura.
Table 2.
Blood meals from mammalian hosts during the winter months*
| Host species | Anopheles crucians (N = 19/22) | Anopheles perplexens (N = 6/7) | Anopheles quadrimaculatus (N = 29/34) | Culex erraticus (N = 175/500) | Culex nigripalpus (N = 8/40) | Culex peccator (N = 2/7) | Culex territans (N = 4/31) | Culiseta melanura (N = 7/60) |
|---|---|---|---|---|---|---|---|---|
| Cow (Bos taurus) | 1 | 0 | 5 | 9 | 1 | 0 | 0 | 0 |
| Dog (Canis lupus familiaris) | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Eastern Cottontail Rabbit (Sylvilagus floridanus) | 3 | 3 | 1 | 12 | 2 | 0 | 0 | 0 |
| Human (Homo sapiens) | 2 | 3 | 0 | 51 | 3 | 0 | 3 | 6 |
| Marsh Rabbit (Sylvilagus palustris) | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Raccoon (Procyon lotor) | 0 | 0 | 2 | 3 | 2 | 0 | 0 | 0 |
| Virginia Opossum (Didelphis virginiana) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| White-Tailed Deer (Odocoileus virginianus) | 12 | 0 | 15 | 96 | 0 | 1 | 1 | 1 |
| Wild Boar (Sus scrofa) | 1 | 0 | 5 | 0 | 0 | 1 | 0 | 0 |
The numerator under each mosquito species name indicates the number of blood meals from mammals, whereas the denominator indicates the total number of blood meals identified (from all host classes) for that mosquito species.
A total of seven reptile and amphibian species were used as hosts in this study (Table 3). Culex erraticus, Cx. nigripalpus, and Cx. territans were the only mosquito species to feed upon reptiles and amphibians. Reptilian blood meals were mainly derived from alligators (Alligator mississippiensis) and the green anole (Anolis carolinensis), whereas amphibian hosts included the green tree frog (Hyla cinerea), Southern leopard frog (Lithobates sphenocephalus), Cuban tree frog (Osteopilus septentrionalis), and pine woods tree frog (Hyla femoralis).
Table 3.
Blood meals from reptile and amphibian hosts during the winter months*
| Host species | Culex erraticus (N = 20/500) | Culex nigripalpus (N = 2/40) | Culex territans (N = 24/31) |
|---|---|---|---|
| Alligator (Alligator mississippiensis) | 15 | 0 | 2 |
| American Green Tree Frog (Hyla cinerea) | 1 | 0 | 14 |
| Cuban Tree Frog (Osteopilus septentrionalis) | 0 | 1 | 1 |
| Green Anole (Anolis carolinensis) | 2 | 1 | 5 |
| Pine Woods Tree Frog (Hyla femoralis) | 0 | 0 | 2 |
| Pond Slider Turtle (Trachemys scripta) | 1 | 0 | 0 |
| Southern Leopard Frog (Lithobates sphenocephelus) | 1 | 0 | 0 |
The numerator under each mosquito species name indicates the number of blood meals from reptiles or amphibians, whereas the denominator indicates the total number of blood meals identified (from all host classes) for that mosquito species.
A total of 35 avian species were identified as hosts for the wintertime mosquito community during this study (Table 4). The majority of the avian blood meals came from wading birds, and wading birds made up a significant proportion of all avian-derived blood meals in all species, with the exception of An. perplexens, in which only a single avian-derived blood meal was identified (Table 4). Wading birds were the primary avian hosts for Cx. erraticus, Cs. melanura, and An. quadrimaculatus comprising 82.3%, 39.6%, and 40%, respectively, of the total avian-derived blood meals for these species.
Table 4.
Blood meals from avian hosts during the winter months
| Host species | Anopheles crucians (N = 3/22) | Anopheles perplexens (N = 1/7) | Anopheles quadrimaculatus (N = 5/34) | Culex erraticus (N = 305/500) | Culex nigripalpus (N = 30/40) | Culex peccator (N = 5/7) | Culex territans (N = 3/31) | Culiseta melanura (N = 53/60) |
|---|---|---|---|---|---|---|---|---|
| American Bittern (Botaurus lentiginosus) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Anhinga (Anhinga anhinga) | 0 | 0 | 1 | 41 | 5 | 0 | 0 | 1 |
| Black-crowned Night Heron (Nycticorax nycticorax) | 2 | 0 | 0 | 110 | 1 | 2 | 0 | 12 |
| Eastern Phoebe (Sayornis phoebe) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Great Blue Heron (Ardea herodias) | 1 | 0 | 0 | 33 | 4 | 2 | 0 | 1 |
| Great Egret (Ardea alba) | 0 | 0 | 0 | 23 | 0 | 0 | 0 | 0 |
| Green Heron (Butorides virescens) | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 2 |
| Limpkin (Aramus guarauna) | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 |
| Little Blue Heron (Egretta caerulea) | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 0 |
| Muscovy Duck (Cairina moschata) | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Northern Cardinal (Cardinalis cardinalis) | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 13 |
| Northern Parula (Parula Americana) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Pied-billed Grebe (Podilymbus podiceps) | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Tennessee Warbler (Vermivora peregrina) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 |
| Tufted Titmouse (Baeolophus bicolor) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Turkey Vulture (Cathartes aura) | 0 | 0 | 1 | 1 | 10 | 0 | 0 | 1 |
| White Ibis (Eudocimus albus) | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Wood Stork (Mycteria americana) | 0 | 0 | 1 | 28 | 2 | 1 | 0 | 2 |
| Yellow-crowned Night Heron (Nyctanassa violacea) | 0 | 0 | 1 | 43 | 1 | 0 | 0 | 3 |
| Other Passeriformes species (N = 8)* | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 6 |
| Other avian species (N = 8)† | 0 | 0 | 1 | 2 | 3 | 0 | 0 | 2 |
Only avian species with more than one mosquito blood meal identified are listed. Passeriformes species not included in the table were Carolina Wren (Thryothorus ludovicianus) and Florida Scrub Jay (Aphelocoma coerulescens) for Cx. erraticus; Blue Jay (Cyanocitta cristata), Hermit Thrush (Catharus guttatus), House Wren (Troglodytes aedon), Loggerhead Shrike (Lanius ludovicianus), Pine Warbler (Dendroica pinu), and White breasted Nuthatch (Sitta carolinensis) for Cs. melanura.
Other avian species include Barred Owl (Strix varia) for An. quadrimaculatus; Great Horned Owl (Bubo virginianus), and Wilson's Snipe (Gallinago delicata) for Cx. erraticus; Black Vulture (Coragyps atratus), Mourning Dove (Zenaida macroura), and Osprey (Pandion haliaetus) for Cx. nigripalpus; Chicken (Gallus gallus domesticus), and Wild Turkey (Meleagris gallopavo) for Cs. melanura. The numerator under each mosquito species name indicates the number of blood meals from birds, whereas the denominator indicates the total number of blood meals identified (from all host classes) for that mosquito species.
Culex erraticus fed on 20 different bird species, 12 of which were wading birds. Of the four most commonly fed upon bird species, three were wading birds (Black-crowned Night Heron, Yellow-crowned Night Heron, and Great Blue Heron [Ardea herodias]). Half (50.2%) of all avian-derived blood meals taken by Cx. erraticus came from the two Night Heron species. Culiseta melanura fed on 21 bird species, just over half of which (50.9%) were from passerine birds (Table 4). A total of 39.6% of the avian-derived blood meals taken by Cs. melanura were derived from wading birds, with the Black-crowned Night Heron representing the most commonly fed upon species in this group (Table 4).
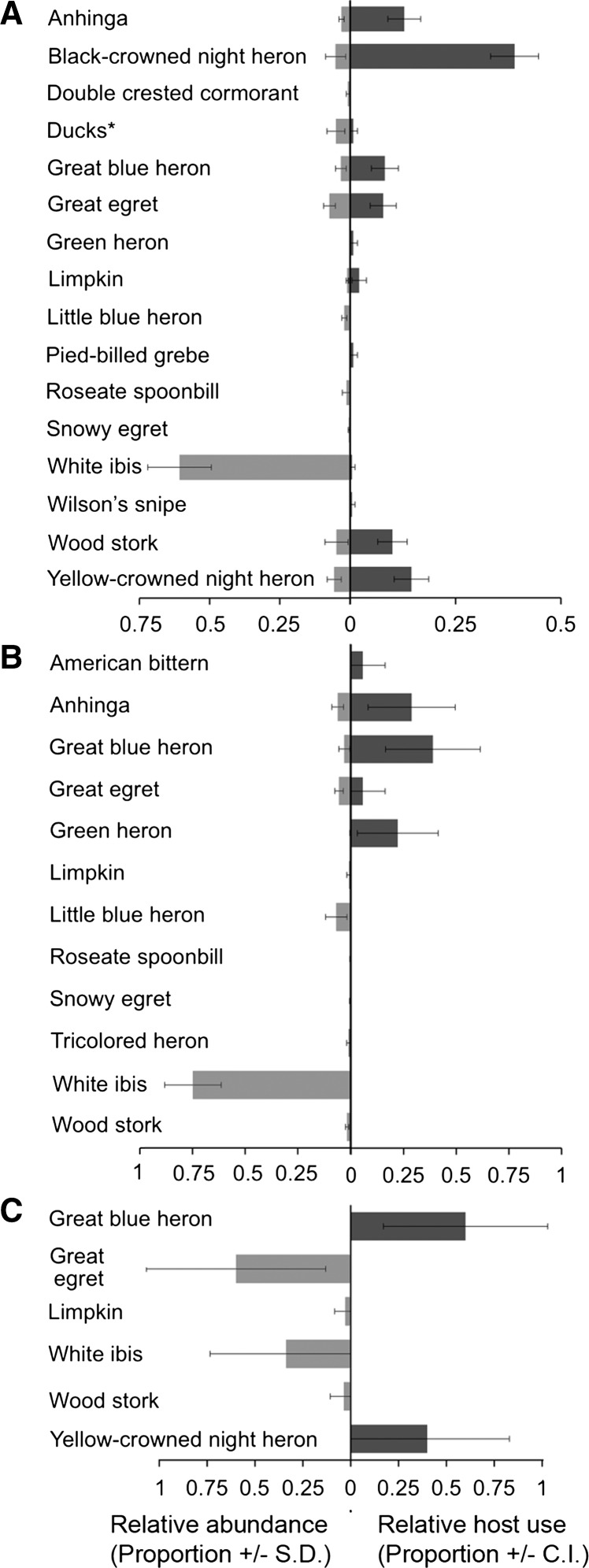
To gain some insight into whether the feeding patterns seen among the wading birds were reflected in their abundance, wading bird counts were conducted at all sites. Wading birds are difficult to detect using standard avian survey techniques,43 and detection probabilities are therefore not available for these species. Thus, it was not possible to calculate forage ratios for these birds. However, it was possible to use the survey data to gain some insights into the feeding behavior of the local mosquito fauna. For example, at Lettuce Lake Park and John B. Sargeant Park, the white ibis was the wading bird species with the highest average monthly relative abundance (0.607 and 0.748, respectively), but it was largely ignored as a host by Cx. erraticus (Figure 4). Similarly, both the Roseate Spoonbill (Platalea ajaja) and the Snowy Egret were present at multiple sites but were not detected in any mosquito blood meals. The four most commonly fed upon wading bird species at Lettuce Lake Park, the Black-crowned Night Heron, Yellow-crowned Night Heron, Anhinga, and Wood Stork (Mycteria americana), were all seen infrequently (Figure 4, panel A). Similarly, at John B. Sargeant Park, the most fed upon species (Great Blue Heron, Anhinga, and Green Heron) were also detected infrequently (Figure 4, panel B). Few avian blood meals were identified from Eureka Springs Park (five total); however, all five Cx. erraticus avian blood meals from this site were from wading birds, even though few wading birds were recorded at this site (Figure 4, panel C).
Figure 4.
Average monthly water bird relative abundance and proportion of Culex erraticus avian blood meals during the winter months from Lettuce Lake Park during two winter collection periods (2012–2013) (panel A), and John B. Sargeant Park (panel B) and Eureka Springs Park (panel C) for one winter collection period (2013). Error bars show the standard deviation for relative bird abundance and confidence intervals for the avian blood meal proportions. * Duck species (Mottled Duck (Anas fulvigula), Muscovy Duck (Cairina moschata), and Whistling Duck (Dendrocygna autumnalis) were combined for Lettuce Lake Park samples.
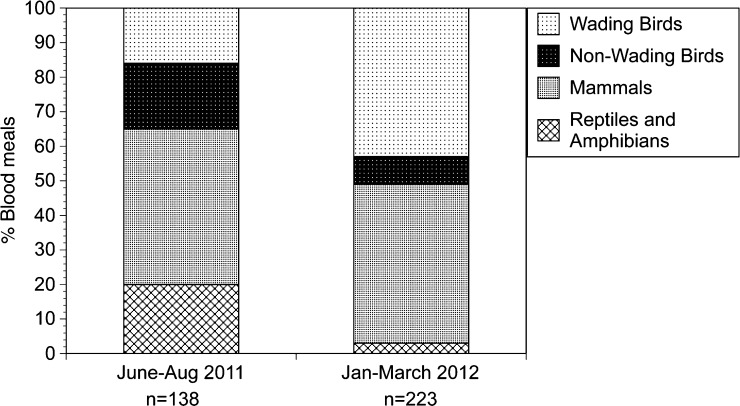
To determine if there were any seasonal shifts in feeding behavior between the winter and summer (peak EEEV transmission) seasons, the data collected from January to March 2012 from Lettuce Lake Park were compared with blood meal data collected at this site during the peak EEEV transmission season of the previous year (June–August 2011). This analysis was restricted to Cx. erraticus, which was the only species for which sufficient data were available to conduct a valid seasonal comparison. Blood fed Cs. melanura were not found in the collections made during the summer of 2011. A total of 145 blood-engorged Cx. erraticus females were collected from Lettuce Lake Park in the summer of 2011 and the blood meal source of 138 (95.2%) of these were identified to the species level. Culex erraticus fed upon seven species of mammals, 13 species of birds, one species of amphibian, and three species of reptiles during the summer months. A total of 35% of Cx. erraticus blood meals came from avian hosts during the summer months, in contrast to the 2012 winter months, when avian hosts made up 51% of the total Cx. erraticus blood meals (Figure 5). Wading birds made up 43% of the meals taken by Cx. erraticus in the winter months and 16% of the meals taken during the summer months (Figure 5). Culex erraticus appeared to feed upon mammals (primarily humans and deer) and on reptiles (primarily alligators) to a greater extent in the summer than winter months (Figure 5).
Figure 5.
Distribution of hosts fed upon by Culex erraticus collected from Lettuce Lake Park, summer 2011 (June–August) compared with samples collected from Lettuce Lake Park, winter 2012 (January–March). Numbers below bars indicate total number of blood meals identified for each host class.
Discussion
Vectors of EEEV were present and often abundant during the winter months and EEEV was detected in mosquitoes twice during the winter months of the study. Both EEEV positive mosquito pools were from collections made during February, when mosquito abundance was relatively low. Both of the positive mosquito species (Cs. melanura and An. quadrimaculatus) have also been shown to be competent vectors of EEEV,5 suggesting that these isolations represented evidence for ongoing winter transmission at the site. Although EEEV was not detected in Cx. erraticus pools during this study, this mosquito is a suspected bridge vector for EEEV, and has been shown to be a competent vector for the virus.5 Isolations of EEEV from Cx. erraticus mosquito pools in Florida have been shown in the past.24,44 Culex erraticus was the most abundant mosquito at these wetland sites during the winter months, a finding that corroborates those of other studies conducted in the southeastern United States.9–11
Avian-derived blood meals also represented a large proportion of blood meals and were the most commonly fed upon host class overall during the winter months. All eight blood-fed mosquito species fed on birds during the winter months, even those species that are known to feed predominantly on mammals (An. crucians and An. quadrimaculatus) or reptiles and amphibians (Cx. territans and Cx. peccator).34 The few avian blood meals for these species were mainly represented by wading or water bird species. Interestingly, An. quadrimaculatus s.l., one of the mosquito species found to be positive for EEEV, fed upon a wide variety of birds, including wading birds (Wood Stork and Yellow-crowned Night Heron).
In this study, a relatively large percentage of the Cs. melanura collected fed on wading birds during the winter months (39.6% of avian-derived blood meals in this species). Other studies conducted in the southeastern United States have shown Cs. melanura feeding on wading birds, but to a smaller extent, representing around 7–15% of blood meals.14 One study that looked at Cs. melanura host preference during the winter months focused their collections in a different part of Florida. They found higher percentages of passerine bird blood meals than we discovered in this study, showing the complex nature of host preference and how much it can vary among sampling locations even within the same state.45 However, that study also concluded that Cs. melanura may feed on Ciconiiformes to a greater extent than would be expected from their relative abundance. Our finding that Cs. melanura took a large fraction of total blood meals (nearly 40% of avian-derived blood meals) from wading birds in the winter is of interest, because this mosquito is considered the primary enzootic vector of EEEV in North America.
Culex erraticus fed upon all four host classes during the winter months; however, the majority fed upon avian hosts. The main avian hosts used by Cx. erraticus in this study were wading and water birds, representing 82.3% and 15.1% of avian-derived blood meals, respectively. Other studies in the southeastern United States conducted during different times of the year have shown a strong preference of Cx. erraticus for wading and water birds.13,14,46 In contrast, passerine birds were fed upon to a much smaller extent by Cx. erraticus (2.0% of avian blood meals). In addition, comparing water bird relative abundance data to relative host use suggests that Cx. erraticus feeds readily on several species of wading and water birds, even when they are present at low abundances. This could perhaps indicate a preference for these species over others, but further research would need to be conducted to confirm this.
Two wading bird species that have been shown to be difficult to survey in the past,43 the Black-crowned and Yellow-crowned Night Herons, were consistently present in our avian surveys, although they were not frequently detected. These species made up a large proportion of the avian-derived blood meals that we found. We believe that it is likely that this finding represents evidence for preferential feeding upon these species and is not a result of the difficulty in detecting these species. Although heterogeneity in the probability of detection can bias the number of birds detected during surveys,47 the length of our counts and our protocol of repeatedly sampling our sites should minimize any bias that might arise because of imperfect detection. Repeatedly sampling an area and combining morning and evening surveys greatly increases the probability of detection of wading birds.39,40 Furthermore, for an individual present within a given survey site to have less than a 95% probability of being detected during at least one of our surveys during a given day, the bird would have to have a probability of detection < 0.77 (1−[1–0.77]2 = 0.95). Given that the only published detection probabilities that we could find for the birds we detected were all > 0.86 for less intensive surveys,48 and that our survey protocols were specifically designed to maximize the probability of detection, we believe that our counts likely represent fairly reliable indices of abundance. If this was indeed the case for the Black-crowned and Yellow-crowned Night Herons, the stalking and nesting behaviors exhibited by these species may influence their ability to serve as potential hosts for EEEV. Night herons, in particular, stand still for long periods of time as they forage for food and have decreased anti-mosquito behavior compared with other species, including other Ciconiiformes.49
Differences in host usage between low (winter) and peak (summer) EEEV transmission seasons were also evident in this study. Although birds were the most commonly fed upon host class for Cx. erraticus during the winter months, mammals were more commonly fed upon during the summer. Almost 46% of Cx. erraticus females fed upon mammalian hosts in the summer months, with humans and deer making up the preferred mammalian hosts in the summer (data not shown). These results indicate a shift in feeding behavior occurring somewhere between the winter and summer months. Such a biphasic pattern of feeding has been seen in other studies of Cx. erraticus50 and other mosquito species,6–8 and has been hypothesized to play an important role in arboviral transmission and amplification. One reason for this shift may be an increased abundance of wading birds at our study sites in the winter months, caused by the in-migration of these species, which augments the year-round resident populations. However, the four most commonly fed upon avian species during the winter months were still among the most often fed upon avian hosts in the summer, although they were targeted to a lesser extent than in the winter. This suggests that these species remain popular hosts year-round, despite the seasonal fluctuations in their numbers.
In summary, the data presented indicate that many mosquito species, notably Cx. erraticus and Cs. melanura, readily feed on wading and water birds during the winter months. Given that previous studies have shown these to be competent enzootic hosts for EEEV, it is possible that these species may play a role in maintaining EEEV transmission during the winter months in Florida, and perhaps in disseminating the virus to the northeastern states during their spring migration. Further research will be needed to investigate the role of wading birds in these processes.
ACKNOWLEDGMENTS
We thank Forest Turbiville of the Hillsborough County Parks, Recreation and Conservation Department for assisting us in obtaining access to the Hillsborough County parks used as field study sites in this project. We also thank Shanna Bolcen, Matthew Anderson, and Jesse Diasparra for assistance with mosquito collection.
Footnotes
Financial support: This project received financial support from the National Institute of Allergy and Infectious Diseases (projects R01AI49724 and R56AI01372 to T.R.U.).
Authors' addresses: Andrea M. Bingham, Hassan K. Hassan, and Thomas R. Unnasch, Global Health Infectious Disease Research Program, Department of Global Health College of Public Health, University of South Florida, Tampa, FL, E-mails: abingha1@health.usf.edu, hhassan@health.usf.edu, and tunnasch@health.usf.edu. Nathan D. Burkett-Cadena, Florida Medical Entomology Laboratory, Entomology and Nematology Department, University of Florida, Vero Beach, FL, E-mail: nburkettcadena@ufl.edu. Christopher J. W. McClure, The Peregrine Fund, Boise, ID, E-mail: cmcclure@peregrinefund.org.
References
- 1.Scott TW, Weaver SC. Eastern equine encephalomyelitis virus: epidemiology and evolution of mosquito transmission. Adv Virus Res. 1989;37:277–328. doi: 10.1016/s0065-3527(08)60838-6. [DOI] [PubMed] [Google Scholar]
- 2.Villari P, Spielman A, Komar N, McDowell M, Timperi RJ. The economic burden imposed by a residual case of eastern encephalitis. Am J Trop Med Hyg. 1995;52:8–13. doi: 10.4269/ajtmh.1995.52.8. [DOI] [PubMed] [Google Scholar]
- 3.US Geological Service ArboNET. 2014. https://wwwn.cdc.gov/arbonet/ Available at. Accessed January 5, 2014.
- 4.Crans WJ, Caccamise DF, McNelly JR. Eastern equine encephalomyelitis virus in relation to the avian community of a coastal cedar swamp. J Med Entomol. 1994;31:711–728. doi: 10.1093/jmedent/31.5.711. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain RW, Sikes RK, Nelson DB, Sudia WD. Studies on the North American arthropod-borne encephalitides. VI. Quantitative determinations of virus-vector relationships. Am J Hyg. 1954;60:278–285. doi: 10.1093/oxfordjournals.aje.a119721. [DOI] [PubMed] [Google Scholar]
- 6.Burkett-Cadena ND, McClure CJ, Ligon RA, Graham SP, Guyer C, Hill GE, Ditchkoff SS, Eubanks MD, Hassan HK, Unnasch TR. Host reproductive phenology drives seasonal patterns of host use in mosquitoes. PLoS ONE. 2011;6:e17681. doi: 10.1371/journal.pone.0017681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:e82. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cupp EW, Klingler K, Hassan HK, Viguers LM, Unnasch TR. Transmission of eastern equine encephalomyelitis virus in central Alabama. Am J Trop Med Hyg. 2003;68:495–500. [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen SB, Lewoczko K, Huddleston DB, Moody E, Mukherjee S, Dunn JR, Jones TF, Wilson R, Moncayo AC. Host feeding patterns of potential vectors of eastern equine encephalitis virus at an epizootic focus in Tennessee. Am J Trop Med Hyg. 2009;81:452–456. [PubMed] [Google Scholar]
- 11.Burkett-Cadena ND, White GS, Eubanks MD, Unnasch TR. Winter biology of wetland mosquitoes at a focus of eastern equine encephalomyelitis virus transmission in Alabama, USA. J Med Entomol. 2011;48:967–973. doi: 10.1603/me10265. [DOI] [PubMed] [Google Scholar]
- 12.Kissling RE, Chamberlain RW, Nelson DB, Stamm DD. Studies on the North American arthropod-borne encephalitides. VIII. Equine encephalitis studies in Louisiana. Am J Hyg. 1955;62:233–254. doi: 10.1093/oxfordjournals.aje.a119776. [DOI] [PubMed] [Google Scholar]
- 13.Hassan HK, Cupp EW, Hill GE, Katholi CR, Klingler K, Unnasch TR. Avian host preference by vectors of eastern equine encephalomyelitis virus. Am J Trop Med Hyg. 2003;69:641–647. [PubMed] [Google Scholar]
- 14.Estep LK, McClure CJ, Burkett-Cadena ND, Hassan HK, Hicks TL, Unnasch TR, Hill GE. A multi-year study of mosquito feeding patterns on avian hosts in a southeastern focus of eastern equine encephalitis virus. Am J Trop Med Hyg. 2011;84:718–726. doi: 10.4269/ajtmh.2011.10-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamm DD. Studies on the ecology of equine encephalomyelitis. Am J Pub Health Nat Health. 1958;48:328–335. doi: 10.2105/ajph.48.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spalding MG, McLean RG, Burgess JH, Kirk LJ. Arboviruses in water birds (Ciconiiformes, Pelecaniformes) from Florida. J Wildl Dis. 1994;30:216–221. doi: 10.7589/0090-3558-30.2.216. [DOI] [PubMed] [Google Scholar]
- 17.Herman CM. The role of birds in the epizootiology of eastern equine encephalitis. Auk. 1962;79:99–103. [Google Scholar]
- 18.Aguirre AA, McLean RG, Cook RS. Experimental inoculation of three arboviruses in black-bellied whistling ducks (Dendrocygna autumnalis) J Wildl Dis. 1992;28:521–525. doi: 10.7589/0090-3558-28.4.521. [DOI] [PubMed] [Google Scholar]
- 19.McLean RG, Crans WJ, Caccamise DF, McNelly J, Kirk LJ, Mitchell CJ, Calisher CH. Experimental infection of wading birds with eastern equine encephalitis virus. J Wildl Dis. 1995;31:502–508. doi: 10.7589/0090-3558-31.4.502. [DOI] [PubMed] [Google Scholar]
- 20.Reisen WK, Wheeler S, Armijos MV, Fang Y, Garcia S, Kelley K, Wright S. Role of communally nesting ardeid birds in the epidemiology of West Nile virus revisited. Vector Borne Zoonotic Dis. 2009;9:275–280. doi: 10.1089/vbz.2008.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powers CD, Dickerman RW. Primary immunoglobulin response of herons to infection with Venezuelan encephalitis virus. Infect Immun. 1975;11:303–308. doi: 10.1128/iai.11.2.303-308.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherer WF, Smith RP. In vitro studies on the sites of Japanese encephalitis virus multiplication in the heron, an important natural host in Japan. Am J Trop Med Hyg. 1960;9:50–55. doi: 10.4269/ajtmh.1960.9.50. [DOI] [PubMed] [Google Scholar]
- 23.Boyle DB, Dickerman RW, Marshall ID. Primary viremia responses of herons to experimental infection with Murray Valley encephalitis, Kunjin and Japanese encephalitis viruses. Aust J Exp Biol Med Sci. 1983;61:655–664. doi: 10.1038/icb.1983.62. [DOI] [PubMed] [Google Scholar]
- 24.Bigler WJ, Lassing EB, Buff EE, Prather EC, Beck EC, Hoff GL. Endemic eastern equine encephalomyelitis in Florida: a twenty-year analysis, 1955–1974. Am J Trop Med Hyg. 1976;25:884–890. doi: 10.4269/ajtmh.1976.25.884. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong PM, Andreadis TG, Anderson JF, Stull JW, Mores CN. Tracking eastern equine encephalitis virus perpetuation in the northeastern United States by phylogenetic analysis. Am J Trop Med Hyg. 2008;79:291–296. [PubMed] [Google Scholar]
- 26.White GS, Pickett BE, Lefkowitz EJ, Johnson AG, Ottendorfer C, Stark LM, Unnasch TR. Phylogenetic analysis of eastern equine encephalitis virus isolates from Florida. Am J Trop Med Hyg. 2011;84:709–717. doi: 10.4269/ajtmh.2011.10-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young DS, Kramer LD, Maffei JG, Dusek RJ, Backenson PB, Mores CN, Bernard KA, Ebel GD. Molecular epidemiology of eastern equine encephalitis virus, New York. Emerg Infect Dis. 2008;14:454–460. doi: 10.3201/eid1403.070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkett-Cadena ND. A wire-frame shelter for collecting resting mosquitoes. J Am Mosq Control Assoc. 2011;27:152–155. doi: 10.2987/10-6076.1. [DOI] [PubMed] [Google Scholar]
- 29.Burkett-Cadena ND, Eubanks MD, Unnasch TR. Preference of female mosquitoes for natural and artificial resting sites. J Am Mosq Control Assoc. 2008;24:228–235. doi: 10.2987/5662.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darsie RF, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Gainesville, FL: University of Florida Press; 2005. p. 383. [Google Scholar]
- 31.Lambert AJ, Martin DA, Lanciotti RS. Detection of North American eastern and western equine encephalitis viruses by nucleic acid amplification assays. J Clin Microbiol. 2003;41:379–385. doi: 10.1128/JCM.41.1.379-385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volchkov VE, Volchkova VA, Netesov SV. Complete nucleotide sequence of the Eastern equine encephalomyelitis virus genome. Mol Gen Mikrobiol Virusol. 1991;5:8–15. [PubMed] [Google Scholar]
- 33.Florida Department of Health . Real-time RT-PCR Arbovirology Protocol–2011. Tallahassee, FL: Florida Department of Health Bureau of Laboratories; 2011. [Google Scholar]
- 34.Burkett-Cadena ND, Graham SP, Hassan HK, Guyer C, Eubanks MD, Katholi CR, Unnasch TR. Blood feeding patterns of potential arbovirus vectors of the genus kCulex targeting ectothermic hosts. Am J Trop Med Hyg. 2008;79:809–815. [PMC free article] [PubMed] [Google Scholar]
- 35.Kitano T, Umetsu K, Tian W, Osawa M. Two universal primer sets for species identification among vertebrates. Int J Legal Med. 2007;121:423–427. doi: 10.1007/s00414-006-0113-y. [DOI] [PubMed] [Google Scholar]
- 36.Butcher GS, Fuller MR, McAllister LS, Geissler P. An evaluation of the Christmas bird count for monitoring population trends of selected species. Wildl Soc Bull. 1990;18:129–134. [Google Scholar]
- 37.Butcher GS, Niven DK, Sauer JR. Using Christmas Bird Count data to assess population dynamics and trends of waterbirds. Am Birds. 2005;59:23–25. [Google Scholar]
- 38.McCrimmon DA, Fryska ST, Ogden JC, Butcher GS. Nonlinear population dynamics of six species of Florida Ciconiiformes assessed by Christmas Bird Counts. Ecol Appl. 1997;7:581–592. [Google Scholar]
- 39.Tozer D, Abraham K, Nol E. Improving the accuracy of counts of wetland breeding birds at the point scale. Wetlands. 2006;26:518–527. [Google Scholar]
- 40.Nadeau CP, Conway CJ, Smith BS, Lewis TE. Maximizing detection probability of wetland-dependent birds during point-count surveys in northwestern Florida. Wilson J Ornithol. 2008;120:513–518. [Google Scholar]
- 41.Colwell MA, Cooper RJ. Estimates of coastal shorebird abundance: the important of multiple counts. J Field Ornithol. 1993;64:293–301. [Google Scholar]
- 42.Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, Farajollahi A, Crans W, Daniels TJ, Falco RC, Benedict M, Anderson M, McMillen L, Unnasch TR. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson DH, Gibbs JP, Herzog M, Lor S, Niemuth ND, Ribic CA, Seamans M, Shaffer TL, Shriver WG, Stehman SV, Thompson WL. A sampling design framework for monitoring secretive marshbirds. Waterbirds. 2009;32:203–215. [Google Scholar]
- 44.Day JF, Stark LM. Eastern equine encephalitis transmission to emus (Dromaius novaehollandiae) in Volusia County, Florida: 1992 through 1994. J Am Mosq Control Assoc. 1996;12:429–436. [PubMed] [Google Scholar]
- 45.Edman JD, Webber LA, Kale HW., 2nd Host-feeding patterns of Florida mosquitoes. II. Culiseta. J Med Entomol. 1972;9:429–434. doi: 10.1093/jmedent/9.5.429. [DOI] [PubMed] [Google Scholar]
- 46.Edman JD. Host-feeding patterns of Florida mosquitoes (Diptera: Culicidae). VI. Culex (Melanoconion) J Med Entomol. 1979;15:521–525. doi: 10.1093/jmedent/15.5-6.521. [DOI] [PubMed] [Google Scholar]
- 47.Nichols J, Thomas L, Conn P. Inferences about landbird abundance from count data: Recent advances and future directions. In: Thomson D, Cooch E, Conroy M, editors. Modeling Demographic Processes in Marked Populations. New York, NY: Springer; 2009. pp. 201–235. [Google Scholar]
- 48.Fletcher RJ, Hutto RL, Haukos DA. Estimating detection probabilities of river birds using double surveys. Auk. 2006;123:695–707. [Google Scholar]
- 49.Webber LA, Edman JD. Anti-mosquito behavior of ciconiiform birds. Anim Behav. 1972;20:228–232. [Google Scholar]
- 50.Oliveira A, Katholi CR, Burkett-Cadena N, Hassan HK, Kristensen S, Unnasch TR. Temporal analysis of feeding patterns of Culex erraticus in central Alabama. Vector Borne Zoonotic Dis. 2011;11:413–421. doi: 10.1089/vbz.2009.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]