Abstract
We report outcomes and 12-month survival for the first cohort of patients to undergo multidrug-resistant tuberculosis (MDR-TB) treatment after the earthquake in Haiti. From March 3, 2010 to March 28, 2013, 110 patients initiated treatment of laboratory-confirmed MDR-TB at the Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO) Center in Port-au-Prince, Haiti. Twenty-seven patients (25%) were human immunodeficiency virus (HIV)-positive. As of October 31, 2013, 95 (86%) patients were either cured or alive on treatment, 4 (4%) patients defaulted, and 11 (10%) patients died. Culture conversion occurred by 30 days in 14 (13%) patients, 60 days in 49 (45%) patients, and 90 days in 81 (74%) patients. The probabilities of survival to 12 months were 96% (95% confidence interval [95% CI] = 89–99) and 85% (95% CI = 64–94) for HIV-negative and -positive patients, respectively. Despite adverse conditions, outcomes for patients with MDR-TB are highly encouraging. Major efforts are underway to scale up community directly observed therapy and expand care to other regions of Haiti.
Introduction
Multidrug-resistant tuberculosis (MDR-TB), defined as tuberculosis (TB) resistant to at least isoniazid and rifampin (two of the most effective drugs against TB), represents a major public health problem worldwide. According to the World Health Organization (WHO), an estimated 450,000 people developed MDR-TB and 170,000 people died of MDR-TB in the world in 2013.1 Treatment of MDR-TB is costly, requires 18–24 months before patients can be deemed cured of the disease, results in lower cure rates than the treatment of drug-susceptible TB, and can lead to severe adverse reactions.2 Human immunodeficiency virus (HIV) -positive patients with MDR-TB face higher mortality than HIV-negative patients and tend to experience more adverse events.3,4
At an estimated annual incidence of 213 cases per 100,000 people, Mycobacterium tuberculosis infection in Haiti is among the highest in the world.5 No national survey of MDR-TB prevalence has been conducted yet in Haiti, but a study conducted in 2008 in 1,000 patients in the West Department, where roughly one-half of all TB cases occur, estimated the prevalence of MDR-TB among newly diagnosed cases of TB at 2.9%.6 The WHO estimates national MDR-TB prevalence at 2.2% among new cases or 14% in those receiving category II retreatment regimens.5 HIV-positive patients in Haiti have also been found to have a higher prevalence of MDR-TB.7
In the aftermath of the earthquake of January 12, 2010, most health facilities in Port-au-Prince and its surroundings were damaged or destroyed, and over 1.5 million people settled in makeshift camps, tent cities, and slums.8 Before the earthquake, the only inpatient facility for the treatment of MDR-TB in the West Department was the Ministry of Health–Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO) Hospital in Leogane. The facility was completely destroyed in the earthquake. GHESKIO responded by setting up a field hospital for the management of patients with MDR-TB in Port-au-Prince. Patients were housed separately in isolation tents.
The main objective of this study was to describe treatment outcomes and factors associated with culture conversion and 12-month survival for the first cohort of patients to receive MDR-TB treatment in the aftermath of the earthquake in Port-au-Prince, Haiti. As mycobacterial culture and rapid molecular diagnostics for TB are expanded in Haiti, it is expected that many more MDR-TB patients will be diagnosed and need to be managed. Information about models of care and associated patient outcomes is essential to guide future therapeutic approaches for MDR-TB patients.
Methods
Study setting and patient population.
The study was conducted at GHESKIO, a non-governmental organization based in Port-au-Prince that has provided care to individuals with TB, HIV/acquired immunodeficiency syndrome (AIDS), and sexually transmitted infections for over three decades. GHESKIO is one of two non-governmental organizations in Haiti that provides treatment of patients with MDR-TB; Partners in Health/Zanmi Lasante runs the other program in central Haiti. All patients with microbiologic evidence of resistance to at least isoniazid and rifampin who initiated MDR-TB treatment at GHESKIO from March 3, 2010 to March 28, 2013 were included in this study. Test results included positive sputum smear microscopy and/or Xpert MTB/RIF (Cepheid, Sunnyvale, CA) results showing M. tuberculosis resistant to rifampin or sputum cultures positive for M. tuberculosis and either GenoType MTBDRplus assay (v1.0, Hain Lifescience, Nehren, Baden-W?rttemberg, Germany) or conventional drug susceptibility test (DST). On being informed of the diagnosis of MDR-TB, patients received counseling, expressed verbal understanding of their diagnosis, and signed a consent form to undertake care. Patients were asked to designate a family member or friend to accompany them for support during the course of treatment.
Laboratory procedures.
Mycobacterial cultures were performed at GHESKIO's Rodolphe Mérieux Biosafety Level 3 Laboratory in Port-au-Prince, Haiti. Sputum specimens collected from patients suspected of having TB were stained by the Ziehl–Neelsen method and examined by microscopy for the presence of acid-fast bacilli. The GeneXpert M. tuberculosis and rifampin resistance (Xpert MTB/RIF) detection assay, which became available in May of 2011, was performed concurrently on the sputum specimens. After decontamination with N-acetyl-L-cysteine-sodium hydroxide, specimens were cultured, and first-line DST was performed using the BACTEC MGIT 960 System (Becton-Dickinson, Sparks, MD). The GenoType MTBDRplus assay (v1.0, Hain Life Sciences, Germany) was done for all positive cultures. When first-line DST results confirmed resistance to at least isoniazid and rifampin, second-line DST was performed by the proportion method with 7H10 agar plates. The following drugs were included in the second-line DST at standard concentrations9: ofloxacin, kanamycin, amikacin, capreomycin, ethionamide, cycloserine, and para-aminosalicylic acid (PAS).
Strict internal and external quality assurance standards were established and followed for all laboratory procedures. GHESKIO's Rodolphe Mérieux Laboratory participates in several external quality assurance (EQA) programs. The results in EQA programs for mycobacteriology (College of American Pathologists), Xpert MTB/RIF (two programs: Centers for Disease Control and Prevention [CDC] and Division of Acquired Immunodeficiency Syndrome [DAIDS] Clinical Trials Group), and line probe assays (WHO Stop TB Department) have been 100% successful since 2008.
MDR-TB treatment protocol—drug regimens and follow-up of patients.
Patients with MDR-TB were initiated on an empiric regimen that included at least four drugs to which the M. tuberculosis isolate was likely to be susceptible.2,10 The initial empiric regimen consisted of a later-generation quinolone (levofloxacin or moxifloxacin), a parenteral agent (kanamycin or capreomycin), cycloserine, pyrazinamide, and ethionamide. High-dose isoniazid was added, because 15–20% of patients have isolates resistant to ethionamide.11 Because of funding constraints and the prohibitive cost of PAS, a decision was reached in May of 2011 to limit the use of PAS to HIV-positive patients, patients with advanced pulmonary disease, and patients with intolerance to cycloserine in accordance with Haiti National Tuberculosis Program guidelines. Capreomycin and moxifloxacin were used in HIV-positive patients, patients with diabetes, and patients with advanced pulmonary disease. Kanamycin and capreomycin were dosed one time per day for 6 days per week. Cycloserine and ethionamide were dosed two times per day.
Drug regimens were adjusted on an individual basis on the availability of the second-line DST results. High-dose isoniazid was discontinued if the isolate displayed sensitivity to ethionamide. PAS was used in patients whose isolates were resistant to ethionamide. The parenteral agent was discontinued after six consecutive negative cultures.
Patients were hospitalized in individual tents (Figure 1) during the initial phase of treatment for 3–8 months to ensure optimal treatment response, monitor closely for signs of toxicity, and reinforce adherence. Adverse drug effects of grade 3 or higher were noted. HIV-positive patients who had not yet received antiretroviral therapy (ART) were placed on a regimen consisting of ritonavir-boosted lopinavir and two nucleoside reverse transcriptase inhibitors within 1–2 weeks of starting MDR-TB treatment, regardless of their CD4 T-cell count.12
Figure 1.

The GHESKIO MDR-TB Field Hospital established after the earthquake of January 12, 2010.
On discharge, patients received domiciliary directly-observed therapy (DOT) two times per day from nurse auxiliaires while on parenteral drug therapy and a community health worker after completion of the parenteral drug. Patients had weekly follow-up visits with the medical team for the first 6 months of ambulatory care, then visits every other week for the second 6 months, and visits monthly for the second year of treatment. Sputum specimens were collected for acid-fast smear microscopy and M. tuberculosis culture monthly over the course of treatment. Because audiograms were not routinely available, hearing impairment and vestibular toxicity were assessed clinically. The cause of death was determined after a thorough review of patients' records by the medical team to reach a decision as to the most probable cause of death. Lung resection surgery was not available.
Patients were offered psychosocial support through individual counseling sessions and monthly group support meetings. Family members and friends were strongly encouraged to attend the monthly meetings. Dry food rations were offered to patients in an attempt to obtain a better response to treatment and promote adherence. Incentives, such as mobile phones, phone recharge cards, transportation fees, and an end-of-treatment prize and certificate, were given to patients to reinforce adherence and increase retention.
Outpatient DOT provider's activities were monitored daily by mobile phones equipped with global positioning system (GPS). Nurse auxiliaires and fieldworkers recorded patients' symptoms, vital signs, and weight and took pictures of the patients swallowing the medications. GPS coordinates were recorded automatically. Files were downloaded and examined daily to verify DOT. Patients who missed doses and/or visits were called promptly to be reminded and visited by the nurse. Staff meetings were held with patients who missed doses and/or visits and their designated family member or friend to help identify specific barriers to adherence and offer ways of reinforcing adherence.
Data collection and analysis.
Demographic variables, clinical characteristics, and laboratory and radiographic results were collected through review of medical records and laboratory information and entered into a Microsoft Excel spreadsheet (Microsoft, Redmond, WA). The STATA 12.1 software package (College Station, TX) was used for data analysis.
Patient characteristics and outcomes were compared for HIV-negative and HIV-positive patients. The two primary outcome measures in our study were time to sputum culture conversion and survival. MDR-TB treatment outcomes were based on WHO definitions and included the following categories: cure, completion, default, failure, and death.13 Favorable outcomes included cured, treatment completion, and alive on treatment; adverse outcomes included death, default, and failure.
Categorical variables were summarized as proportions and frequencies, and confidence intervals (CIs) were calculated at the 95% level. Univariate analyses were performed to determine associations between baseline characteristics and outcomes. Kaplan–Meier analysis was used to assess survival probabilities; differences between the two survival curves were determined by the log-rank test. Patients were censored at the time of analysis (October 31, 2013). Cox proportional hazards regression analysis was used to estimate the hazard ratios with 95% CIs. Predictors in the model for death were selected based on prior research experience and factors described in the literature. Odd ratios and P values were two-tailed; P values of 0.05 or lower were considered statistically significant.
This study was approved by the GHESKIO and Weill Cornell Medical College Institutional Review Boards.
Results
Baseline demographic and clinical characteristics of the patient population.
The median time from treatment initiation to data analysis was 22.5 months (interquartile range [IQR] = 11.5–31.0 months). Table 1 summarizes the demographic and clinical characteristics of 110 patients who initiated MDR-TB treatment in the GHESKIO MDR-TB program from March 3, 2010 to March 28, 2013. Sixty (55%) patients were female. The median age was 28 years (IQR = 23–37 years). Eighty-six (79%) patients lived on less than $1 per day; 74 (67%) patients either lived or had access to a residence in the Port-au-Prince metropolitan area.
Table 1.
Baseline characteristics of 110 MDR-TB patients according to HIV status
| Characteristic | Entire population (N = 110) | HIV-negative (N = 83) | HIV-positive (N = 27) | P value |
|---|---|---|---|---|
| Age (years), median (IQR) | 28 (23–37) | 28 (22–36) | 30 (26–39) | 0.268 |
| Female sex, no. (%) | 60 (54.6) | 45 (54.2) | 15 (55.6) | 0.903 |
| Lives on < $1/day, no. (%) | 86 (78.2) | 67 (80.7) | 19 (70.4) | 0.258 |
| Education at primary level or lower, no. (%) | 49 (44.6) | 32 (38.6) | 17 (63.0) | 0.027 |
| Lives in Port-au-Prince area, no. (%) | 74 (67.3) | 55 (66.3) | 19 (70.4) | 0.693 |
| Initial weight (kg), median (IQR) | 48.0 (41.8–53.6) | 48.3 (41.0–54.0) | 46.0 (44.0–50.4) | 0.318 |
| Previous TB treatment, no. (%) | 95 (88.8) | 69 (86.3) | 26 (96.3) | 0.153 |
| Time from diagnosis to start of treatment, median days (IQR) | 46 (20.8–77.3) | 48 (17–82) | 42 (23–66) | 0.182 |
| Radiographic findings (N = 99) | ||||
| Bilateral lung disease | 85 (79.4) | 63 (75.9) | 22 (95.7) | 0.084 |
| Chronic changes (fibrosis, cysts, cavities, or bullae) | 77 (72.0) | 60 (72.3) | 17 (72.8) | 0.889 |
| Hemoglobin, median (IQR) | 11.0 (9.5–12.7) | 11.4 (9.7–12.9) | 9.7 (6.6–11.1) | 0.011 |
| Hemoptysis | 22 (20.0) | 21 (25.3) | 1 (3.7) | 0.015 |
Ninety-eight (89%) patients had received first-line anti-TB drugs before initiating MDR-TB treatment, and no patients had prior exposure to second-line drugs. The median weight was 50.2 kg (IQR = 45.0–55.0 kg) for men and 45.8 kg (IQR = 40.0–52.0 kg) for women. Eighty-five (79%) patients had bilateral lung involvement on baseline chest radiographs. Lung parenchymal tissue damage in the form of cavitary disease, fibrosis, and cysts was seen in 77 (72%) patients. The median hemoglobin level at baseline was 11.0 g/dL (IQR = 9.5–12.7 g/dL). The median delay in initiating treatment was 42.5 days in patients with Xpert MTB/RIF results and 48.5 days in patients without results.
Twenty-seven (25%) patients were HIV-positive, with a median CD4 T-cell count of 329 (IQR = 53–608). There were no significant differences in baseline characteristics between HIV-negative and HIV-positive patients, except for the level of education and median hemoglobin level (Table 1).
Mycobacterial culture and DST results.
Mycobacterial culture and DST results were positive for M. tuberculosis in 107 patients. Two patients had Xpert MTB/RIF results indicating rifampin-resistant M. tuberculosis, but the cultures did not grow. One patient had a mixture of M. tuberculosis and M. avium complex, but the M. tuberculosis isolate showed resistance to isoniazid and rifampin by the GenoType MTBDRplus assay (v.1.0).
DST results for 107 patients are summarized in Table 2. Patients were resistant to a mean of 3.8 (SD = 1.4) drugs; 55 (51%) patients had isolates resistant to streptomycin, 83 (78%) patients had isolates resistant to ethambutol, and 54 (51%) patients had isolates resistant to pyrazinamide. One hundred (93%) patients had isolates with the S315T katG mutation associated with high-level resistance to isoniazid. As for resistance to second-line drugs, 17 (16%) patients had isolates resistant to ethionamide, and 1 (1%) patient had resistance to ofloxacin. No isolates were found to be resistant to kanamycin, capreomycin, cycloserine, or PAS. No cases of extremely drug-resistant TB were found.
Table 2.
Drug resistance profiles of M. tuberculosis isolates cultured from sputum specimens of 107 patients with culture and drug susceptibility results
| Resistance | Entire population (N = 107) | HIV-negative (N = 83) | HIV-positive (N = 24) | P value |
|---|---|---|---|---|
| Number of drugs to which isolate was resistant, mean | 3.84 | 3.84 | 3.81 | 0.925 |
| Pyrazinamide resistance, no. (%) | 54 (50.5) | 43 (51.8) | 11 (45.8) | 0.605 |
| Ethambutol resistance, no. (%) | 83 (77.6) | 61 (73.5) | 22 (91.7) | 0.060 |
| Streptomycin resistance, no. (%) | 55 (51.4) | 39 (47.0) | 16 (66.7) | 0.089 |
| Ofloxacin resistance, no. (%) | 1 (0.9) | 1 (1.2) | 0 (0) | 0.567 |
| Ethionamide resistance, no. (%) | 17 (15.9) | 11 (13.3) | 6 (25.0) | 0.168 |
MDR-TB drug regimens and ART.
The median time from diagnosis to initiation of MDR-TB treatment was 46 days (IQR = 21–77 days) for the entire population, 63 days (IQR = 37–90 days) for HIV-negative patients, and 50 days (IQR = 41–72 days) for HIV-positive patients. Table 3 summarizes the empiric MDR-TB drug regimens initiated for 110 patients in the study according to HIV status. Moxifloxacin, capreomycin, and PAS were more frequently included in the empiric regimens for HIV-positive patients.
Table 3.
Second-line MDR-TB drugs used in the empiric regimens for 110 patients in the study
| Entire population (N = 110) | HIV-negative (N = 83) | HIV-positive (N = 27) | P value | |
|---|---|---|---|---|
| Kanamycin | 96 (87.3) | 78 (94.0) | 18 (66.7) | < 0.0005 |
| Capreomycin | 14 (12.7) | 5 (6.0) | 9 (33.3) | < 0.0005 |
| Levofloxacin | 86 (78.2) | 73 (88.0) | 13 (48.2) | < 0.0005 |
| Moxifloxacin | 14 (12.7) | 10 (12.1) | 14 (51.9) | < 0.0005 |
| Cycloserine | 108 (98.2) | 81 (97.6) | 27 (100.0) | 0.416 |
| PAS* | 64 (58.2) | 39 (47.0) | 25 (92.6) | < 0.0005 |
| Pyrazinamide | 106 (96.4) | 82 (98.8) | 24 (88.9) | 0.017 |
| High-dose isoniazid | 44 (40.0) | 42 (50.6) | 2 (7.4) | < 0.0005 |
| Amoxicillin/clavulanate | 2 (0.2) | 2 (2.4) | 0 (0) | 0.416 |
As of May of 2011, PAS was used only in HIV-positive patients, patients with advanced pulmonary disease, and patients with intolerance to cycloserine per the Haiti National Tuberculosis guidelines.
All 27 HIV-positive patients received ART within 2 weeks of starting MDR-TB treatment. Twelve (44%) patients had received ART before staring treatment of MDR-TB; 15 (56%) patients had a diagnosis of HIV concomitant with the initiation of MDR-TB treatment and had never received ART before.
Adverse events in patients receiving MDR-TB treatment.
Adverse drug-related events were noted in 61 (56%) of 110 patients. Table 4 shows the adverse drug reactions found in patients receiving treatment. Among patients with the most frequently noted reactions, the following reactions were found: peripheral neuropathy (14%), arthralgia (13%), neuropsychiatric disturbance (psychosis, anxiety, and seizure) (16%), gastrointestinal distress (11%), and hearing impairment (11%). Two patients experienced permanent hearing loss and had to be fitted for hearing aids. Discontinuation of cycloserine occurred in six (5%) patients with psychosis, which was the most frequent cause of drug change for adverse drug-related events. There were no cases of drug-induced hepatitis. Overall, the occurrence of any adverse drug event was not significantly higher in HIV-positive patients than HIV-negative patients (12 [44%] versus 49 [59%] patients, P = 0.185). Depression was significantly more common in HIV-positive patients than HIV-negative patients (5 [19%] versus 2 [2%] patients, P = 0.003).
Table 4.
Adverse drug reactions in 110 MDR-TB patients in the study
| Entire population (N = 110) | HIV-negative (N = 83) | HIV-positive (N = 27) | P value | |
|---|---|---|---|---|
| Gastrointestinal distress | 12 (10.9) | 9 (10.8) | 3 (11.1) | 0.969 |
| Neuropsychiatric disturbance | 18 (16.4) | 11 (13.3) | 7 (25.9) | 0.122 |
| Ototoxicity | 12 (10.9) | 11 (13.3) | 1 (3.7) | 0.167 |
| Nephrotoxicity | 3 (2.7) | 1 (1.2) | 2 (7.4) | 0.086 |
| Hypothyroidism | 5 (4.5) | 5 (6.0) | 0 (0) | 0.192 |
| Arthralgia | 14 (12.7) | 12 (14.5) | 2 (7.4) | 0.34 |
| Gynecomastia | 4 (3.6) | 4 (4.8) | 0 (0) | 0.245 |
| Peripheral neuropathy | 15 (13.6) | 13 (15.7) | 2 (7.4) | 0.278 |
| Dermatologic | 5 (4.6) | 5 (6.0) | 0 (0) | 0.192 |
The course of treatment was complicated by a requirement for oxygen supplementation and hemoptysis in 18 (16%) and 22 (20%) patients, respectively.
Sputum culture conversion.
Sputum culture conversion was achieved by 30 days in 14 (13%) patients, 60 days in 49 (45%) patients, and 90 days in 81 (74%) patients; 14 (52%) HIV-positive patients had culture conversion by 60 days compared with 35 (42%) HIV-negative patients (P = 0.379). Factors associated with achieving culture conversion by 60 days were having an isolate resistant to only isoniazid and rifampin (odds ratio [OR] = 3.5, 95% CI = 1.3–10.4, P = 0.011) and having a diagnosis of MDR-TB by the Xpert MTB/RIF assay (OR = 3.3, 95% CI = 1.5–7.2, P = 0.003). Patients with isolates resistant to pyrazinamide were less likely to achieve culture conversion by 60 days (OR = 0.4, 95% CI = 0.2–0.9, P = 0.021).
MDR-TB treatment outcomes.
The median time spent on treatment was 17.8 months for the cohort (IQR = 10.3–24.0 months). Overall, 95 (86%) patients were either cured or alive on treatment over the course of the analysis, with 43 (39%) patients cured and 52 (47%) patients alive on treatment at the time of the analysis (Table 5). Among HIV-negative patients, 74 (89%) patients were either cured or alive on treatment, with 34 (41%) patients cured and 40 (48%) patients alive on treatment, whereas among HIV-positive patients, 21 (78%) patients were cured and alive on treatment, with 9 (33%) patients cured and 12 (44%) patients alive on treatment. Four patients (4%) defaulted on treatment: two patients at 17 months, one patient at 7 months, and one patient at 5 months of treatment.
Table 5.
MDR-TB treatment outcome for 110 patients in the study
| Entire population (N = 110) | HIV-negative (N = 83) | HIV-positive (N = 27) | P value | |
|---|---|---|---|---|
| MDRTB treatment outcome | 0.398 | |||
| Cured | 43 (39.1) | 34 (41.0) | 9 (33.3) | |
| Alive on treatment | 52 (47.3) | 40 (48.2) | 12 (44.4) | |
| Died | 11 (10.0) | 6 (7.2) | 5 (18.5) | |
| Defaulted | 4 (3.6) | 3 (3.6) | 1 (3.7) |
Eleven (10%) patients died. The causes of death were related to MDR-TB and its complications in seven patients (respiratory failure in five patients and pneumothorax in two patients) and unrelated to TB in four patients (one patient died of advanced HIV/AIDS, one patient died of of cholera and HIV/AIDS, one patient died of severe anemia, and one patient committed suicide). Deaths occurred within a median of 3.4 months (IQR = 1.4–16.7 months) after treatment was initiated; 5 (46%) of 11 deaths occurred among HIV-positive patients. No significant associations were found between chest radiograph findings and outcome.
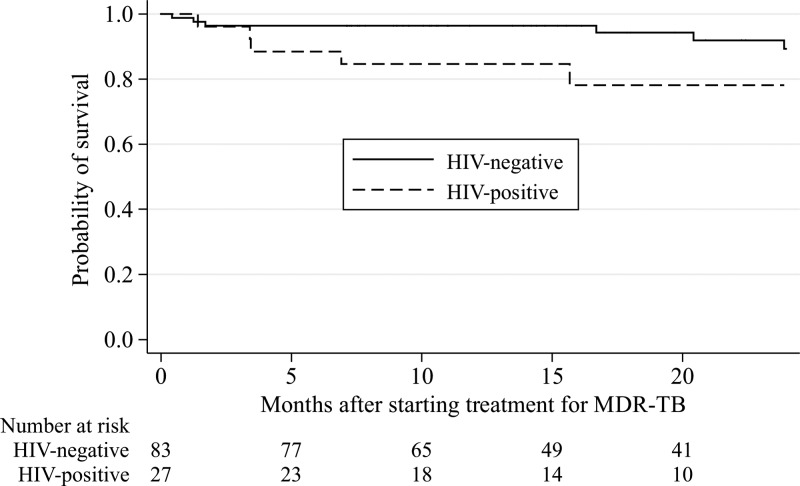
According to Kaplan–Meier survival analysis (Figure 2 ), the probability of survival to 12 months after initiating treatment of MDR-TB was estimated at 96% (95% CI = 89–99) for HIV-negative patients and 85% (95% CI = 64–94) for HIV-positive patients. There was a trend toward greater survival for HIV-negative patients than HIV-positive patients (P = 0.055 by the log-rank test). The unadjusted hazard ratio (HR) for the risk of death for HIV-positive patients compared with that for HIV-negative patients was 3.1 (95% CI = 0.9–10.1, P = 0.068). For HIV-positive patients, a CD4 T-cell count < 100 was a predictor of death (unadjusted HR = 9.8, 95% CI = 2.8–33.9, P < 0.0005). Predictors of mortality (Table 6) were occurrence of adverse neuropsychiatric events in the first 1 month of treatment, hemoglobin level of 9.5 g/dL or lower at baseline, and failure to achieve sputum culture conversion by 60 days. There was no association found between either weight loss within the first 6 months or pyrazinamide resistance and mortality.
Figure 2.
Kaplan–Meier estimates of the probability of survival after starting treatment of MDR-TB in HIV-negative and HIV-positive patients.
Table 6.
Predictors of death in 110 patients receiving MDR-TB treatment
| Predictor | Adjusted HR* (95% CI) | Unadjusted HR (95% CI) | P value |
|---|---|---|---|
| Occurrence of adverse neuropsychiatric event in the first 1 month of treatment | 5.0 (2.3–95.3) | 4.8 (1.3–16.8) P = 0.015 | 0.004 |
| Hemoglobin in lowest quartile (≤ 9.5 g/dL) | 4.8 (1.3–17.9) | 5.5 (1.6–18.7) P = 0.007 | 0.018 |
| Culture conversion after 60 days | 10.1 (1.1–90.0) | 6.9 (0.9–54.3) P = 0.067 | 0.039 |
HRs have been adjusted for age, sex, education level, economic status, baseline weight, and the other variables listed.
Discussion
Outcomes for patients with MDR-TB have been outstanding for the first 110 patients to receive treatment after the devastating earthquake in Port-au-Prince, Haiti; 86% of patients are cured or remain on treatment. Although patients are hospitalized in tents during inpatient therapy, these outcomes rival the most successful treatment cohorts from other more favorable settings worldwide.
Most patients (72%) in our study had advanced parenchymal tissue destruction, involving both lungs in 79% of patients. Some of these patients posed major challenges for medical management in the setting of a field hospital under tents: 18% of patients had hemoptysis, and 20% of patients required supplemental oxygen. Factors that contributed to the success of treatment in our cohort included the low mean resistance to first-line drugs (19% of the patients had isolates resistant to isoniazid and rifampin only), the aggressive empiric drug regimens used,14,15 and the dedication of the staff.
HIV-positive patients tended to have a lower survival than HIV-negative patients, which has been reported elsewhere,16–18 but they were no less likely to be lost to follow-up. Also, they did not have delays in culture conversion compared with HIV-negative patients.
The immediate availability of ART and the relatively preserved immunologic status of the patients also contributed to the relatively high survival of HIV-positive patients over the course of the study.
Our results are highly encouraging given the low treatment success rates that have been reported from other treatment cohorts.19–21 In a recent individual patient data meta-analysis of 9,153 patients, the pooled treatment success was only 54% (95% CI = 53–55%).22 In that study, treatment success was associated with use of later-generation quinolones, ethionamide, or prothionamide, use of four or more likely effective drugs in the initial intensive phase, and use of three or more likely effective drugs in the continuation phase. In addition, the greatest odds of success were seen in patients who had 7.0–8.5 months for the intensive phase and a total length of treatment of 24.6–27.5 months. Because of the low rate of second-line drug resistance and our long duration of injectable therapy, our treatment cohort received these favorable treatment conditions.
Anemia, defined as hemoglobin in the lowest quartile for the entire MDR-TB cohort (≤ 9.5 g/dL), was a predictor for mortality in our study, indicating that undernutrition may be a major factor for death in these poor patients. Indeed, undernutrition is a risk factor for TB and can adversely impact response to TB treatment.23,24 Nearly 80% of patients in our cohort lived on less than $1 a day, and many required food supplements over the course of treatment. According to the International Food Policy Research Institute,25 Haiti's global hunger index of 23.3 is still considered disquietingly high, indicating that “more than 44 percent of Haiti's population is undernourished and more than half of the households live on less than one dollar a day.” Successful scale-up of MDR-TB care in Haiti will require the support of the National Tuberculosis Program and its partners to implement a complete care package, including robust psychosocial support for patients, nutritional support throughout the course of treatment, and development of poverty-reducing programs, such as microcredit, skills workshops, and adult education.
Over one-half of the patients in our cohort reported at least one adverse drug effect. Among the most severe adverse drug-related events were neuropsychiatric disturbance, hearing impairment, and renal toxicity. The occurrence of neuropsychiatric events in our study was higher than has been reported elsewhere.26–28 There was one suicide in our cohort in a 27-year-old male patient who had been receiving treatment for 38 days. This event underscores the need for effective tools to screen for psychiatric illness and early signs of adverse neuropsychiatric complications to avert these preventable deaths. There are few psychiatrists in Haiti and other resource-poor settings, and therefore, it is essential to train medical staff to recognize early warning signs of neuropsychiatric illness to effectively prevent its devastating consequences in this population. Because cycloserine levels are not measured because of financial and logistical challenges, it is possible that high cycloserine levels may pre-dispose patients to psychiatric complications. The use of high-dose isoniazid in the empiric regimen may also contribute to psychiatric side effects.
Most patients (74%) in the cohort reached culture conversion by 90 days, similar to reported percentages for other cohorts.29–31 Interestingly, patients with isolates resistant to pyrazinamide were found to be less likely to achieve culture conversion by 60 days. The introduction of the Xpert MTB/RIF assay allowed for rapid diagnosis of MDR-TB treatment, particularly in patients with no overt signs of failure. However, even when molecular diagnostics allowed for prompt diagnosis, it still took a relatively long time to mobilize resources and convince patients and family members of the necessity of initiating treatment immediately. Patients who were relatively healthy at the time of diagnosis tended to refuse hospitalization on the grounds that they had other responsibilities to attend to and could not afford to be hospitalized. Extending MDR-TB education and management to the community will be important to enroll more patients in care more expeditiously. Despite these impediments, patients who had a diagnosis of MDR-TB by the Xpert MTB/RIF assay took a shorter amount of time to achieve culture conversion.32,33 Indeed, because we implemented a systematic approach of initiating MDR-TB treatment based solely on the Xpert MTB/RIF result, more patients have been initiated on treatment sooner, and the time to culture conversion has been reduced to 45 days. All three major predictors of mortality (adverse neuropsychiatric events in the first 1 month of treatment, low hemoglobin level, and failure to achieve sputum conversion by 60 days) can be known within the first 2 months and can help identify patients at greatest risk of death so that they can receive more attention to try to reverse their projected bad outcomes.
Our study has some limitations. First, we cannot entirely exclude the potential for selection bias, because many patients were referred to our center for evaluation of failure of first-line drugs. It could be that patients who were seriously ill could not be referred for evaluation and treatment. Second, adverse events could have been underestimated, because patients could have underreported their symptoms; also, sporadic lack of funds precluded systematic laboratory investigation of patients' symptoms.
In conclusion, despite these limitations, our study shows that outstanding outcomes can be achieved in MDR-TB treatment, even in the aftermath of a devastating natural catastrophe. As treatment is expanded countrywide, it will be crucial to train health professionals and community health workers and strengthen social support networks for patients to ensure continued success.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Becton-Dickinson for equipment and reagent support, Dr. Barbara Marston for critical review of the manuscript, and the entire team of community health workers at Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO) who work tirelessly under trying conditions to ensure directly observed therapy for the patients. We also thank the patients, family members, and friends for their support.
Footnotes
Financial support: Support for the Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO) Multidrug-Resistant Tuberculosis Program was obtained from the Centers for Disease Control and Prevention, the Global Fund for AIDS, Tuberculosis, and Malaria, the US President's Emergency Fund for AIDS Relief, and the Fogarty International Center. M.C. received support from the Mentored Patient-Oriented Research Career Development Award (K23 AI073190) and from the Robert Wood Johnson's Amos Medical Faculty Development Award (63526). S.P.K. received support from Research Project Grant R01AI104344. O.O. received support from the International Training and Education Center for Health (I-TECH) Grant Number HA000047.
Authors' addresses: Macarthur Charles, Division of Global Health Protection, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: makchuk@gmail.com. Stalz Charles Vilbrun, Lauren M. Hashiguchi, Marie Marcelle Mabou, and Jean W. Pape, Les Centres Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO), Port-au-Prince, Haiti, E-mails: stalzsog@yahoo.com, mmabou@gheskio.org, lhashiguchi@gmail.com, and jwpape@gheskio.org. Serena P. Koenig, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, E-mail: skoenig@partners.org. Oksana Ocheretina, Center for Global Health, Division of Infectious Diseases, Weill Cornell Medical College, New York, NY, E-mail: ocheretina@yahoo.com.
References
- 1.World Health Organization . Global Tuberculosis Report 2013. Geneva: World Health Organization; 2013. [Google Scholar]
- 2.World Health Organization . Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Emergency Update 2008. Geneva: World Health Organization; 2008. [Google Scholar]
- 3.Friedland G. Tuberculosis, drug resistance, and HIV/AIDS: a triple threat. Curr Infect Dis Rep. 2007;9:252–261. doi: 10.1007/s11908-007-0039-7. [DOI] [PubMed] [Google Scholar]
- 4.Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, Castro KG, Weyer K. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196((Suppl)):S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Tuberculosis Country Profiles. 2013. http://www.who.int/tb/country/data/profiles/en/index.html Available at: Accessed January 12, 2014.
- 6.Ocheretina O, Morose W, Gauthier M, Joseph P, D'Meza R, Escuyer VE, Rastogi N, Vernet G, Pape JW, Fitzgerald DW. Multidrug-resistant tuberculosis in Port-au-Prince, Haiti. Rev Panam Salud Publica. 2012;31:221–224. doi: 10.1590/s1020-49892012000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph P, Severe P, Ferdinand S, Goh KS, Sola C, Haas DW, Johnson WD, Rastogi N, Pape JW, Fitzgerald DW. Multidrug-resistant tuberculosis at an HIV testing center in Haiti. AIDS. 2006;20:415–418. doi: 10.1097/01.aids.0000206505.09159.9a. [DOI] [PubMed] [Google Scholar]
- 8.Pape JW, Johnson WD, Fitzgerald DW. The earthquake in Haiti–dispatch from Port-au-Prince. N Engl J Med. 2010;362:575–577. doi: 10.1056/NEJMp1001015. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization World Health Organization Policy Guidance on Drug-Susceptibility Testing (DST) of Second-Line Antituberculosis Drugs. 2008. http://www.who.int/tb/publications/2008/whohtmtb_2008_392/en/ Available at. Accessed January 12, 2014. [PubMed]
- 10.Falzon D, Jaramillo E, Schünemann HJ, Arentz M, Bauer M, Bayona J, Blanc L, Caminero JA, Daley CL, Duncombe C, Fitzpatrick C, Gebhard A, Getahun H, Henkens M, Holtz TH, Keravec J, Keshavjee S, Khan AJ, Kulier R, Leimane V, Lienhardt C, Lu C, Mariandyshev A, Migliori GB, Mirzayev F, Mitnick CD, Nunn P, Nwagboniwe G, Oxlade O, Palmero D, Pavlinac P, Quelapio MI, Raviglione MC, Rich ML, Royce S, Rüsch-Gerdes S, Salakaia A, Sarin R, Sculier D, Varaine F, Vitoria M, Walson JL, Wares F, Weyer K, White RA, Zignol M. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38:516–528. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 11.Katiyar SK, Bihari S, Prakash S, Mamtani M, Kulkarni H. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2008;12:139–145. [PubMed] [Google Scholar]
- 12.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, Khan M, Pienaar J, El-Sadr W, Friedland G, Abdool Karim Q. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laserson KF, Thorpe LE, Leimane V, Weyer K, Mitnick CD, Riekstina V, Zarovska E, Rich ML, Fraser HSF, Alarcón E, Cegielski JP, Grzemska M, Gupta R, Espinal M. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9:640–645. [PubMed] [Google Scholar]
- 14.Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis. 2010;10:621–629. doi: 10.1016/S1473-3099(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 15.Mitnick CD, Franke MF, Rich ML, Alcantara Viru FA, Appleton SC, Atwood SS, Bayona JN, Bonilla CA, Chalco K, Fraser HSF, Furin JJ, Guerra D, Hurtado RM, Joseph K, Llaro K, Mestanza L, Mukherjee JS, Muñoz M, Palacios E, Sanchez E, Seung KJ, Shin SS, Sloutsky A, Tolman AW, Becerra MC. Aggressive regimens for multidrug-resistant tuberculosis decrease all-cause mortality. PLoS ONE. 2013;8:e58664. doi: 10.1371/journal.pone.0058664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farley JE, Ram M, Pan W, Waldman S, Cassell GH, Chaisson RE, Weyer K, Lancaster J, Van der Walt M. Outcomes of multi-drug resistant tuberculosis (MDR-TB) among a cohort of South African patients with high HIV prevalence. PLoS ONE. 2011;6:e20436. doi: 10.1371/journal.pone.0020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaakidis P, Varghese B, Mansoor H, Cox HS, Ladomirska J, Saranchuk P, Da Silva E, Khan S, Paryani R, Udwadia Z, Migliori GB, Sotgiu G, Reid T. Adverse events among HIV/MDR-TB co-infected patients receiving antiretroviral and second line anti-TB treatment in Mumbai, India. PLoS ONE. 2012;7:e40781. doi: 10.1371/journal.pone.0040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palacios E, Franke M, Munoz M, Hurtado R, Dallman R, Chalco K, Guerra D, Mestanza L, Llaro K, Bonilla C, Sebastian J, Bayona J, Lygizos M, Anger H, Shin S. HIV-positive patients treated for multidrug-resistant tuberculosis: clinical outcomes in the HAART era. Int J Tuberc Lung Dis. 2012;16:348–354. doi: 10.5588/ijtld.11.0473. [DOI] [PubMed] [Google Scholar]
- 19.Bassili A, Fitzpatrick C, Qadeer E, Fatima R, Floyd K, Jaramillo E. A systematic review of the effectiveness of hospital- and ambulatory-based management of multidrug-resistant tuberculosis. Am J Trop Med Hyg. 2013;89:271–280. doi: 10.4269/ajtmh.13-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espinal M, Kim S, Suarez P, Kam K, Khomenko A, Migliori G, Baéz J, Kochi A, Dye C, Raviglione M. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 21.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, Becerra MC, Benedetti A, Burgos M, Centis R, Chan ED, Chiang C-Y, Cox H, D'Ambrosio L, DeRiemer K, Dung NH, Enarson D, Falzon D, Flanagan K, Flood J, Garcia-Garcia ML, Gandhi N, Granich RM, Hollm-Delgado MG, Holtz TH, Iseman MD, Jarlsberg LG, Keshavjee S, Kim H-R, Koh W-J, Lancaster J, Lange C, de Lange WCM, Leimane V, Leung CC, Li J, Menzies D, Migliori GB, Mishustin SP, Mitnick CD, Narita M, O'Riordan P, Pai M, Palmero D, S-k Park, Pasvol G, Peña J, Pérez-Guzmán C, Quelapio MID, Ponce-de-Leon A, Riekstina V, Robert J, Royce S, Schaaf HS, Seung KJ, Shah L, Shim TS, Shin SS, Shiraishi Y, Sifuentes-Osornio J, Sotgiu G, Strand MJ, Tabarsi P, Tupasi TE, van Altena R, Van der Walt M, Van der Werf TS, Vargas MH, Viiklepp P, Westenhouse J, Yew WW, Yim J-J. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhargava A, Chatterjee M, Jain Y, Chatterjee B, Kataria A, Bhargava M, Kataria R, D'Souza R, Jain R, Benedetti A, Pai M, Menzies D. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLoS ONE. 2013;8:e77979. doi: 10.1371/journal.pone.0077979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8:286–298. [PubMed] [Google Scholar]
- 25.International Food Policy Research Institute Global Hunger Index—Country Case Study: Haiti. 2013. http://www.ifpri.org/publication/2013-global-hunger-index-country-case-study-haiti Available at. Accessed January 12, 2014.
- 26.Furin JJ, Mitnick CD, Shin SS, Bayona J, Becerra MC, Singler JM, Alcantara F, Castañieda C, Sanchez E, Acha J, Farmer PE, Kim JY. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001;5:648–655. [PubMed] [Google Scholar]
- 27.Shin SS, Pasechnikov AD, Gelmanova IY, Peremitin GG, Strelis AK, Mishustin S, Barnashov A, Karpeichik Y, Andreev YG, Golubchikova VT, Tonkel TP, Yanova GV, Yedilbayev A, Rich ML, Mukherjee JS, Furin JJ, Atwood S, Farmer PE, Keshavjee S. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis. 2007;11:1314–1320. [PubMed] [Google Scholar]
- 28.Vega P, Sweetland A, Acha J, Castillo H, Guerra D, Smith Fawzi M, Shin S. Psychiatric issues in the management of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8:749–759. [PubMed] [Google Scholar]
- 29.Gler MT, Guilatco R, Caoili JC, Ershova J, Cegielski P, Johnson JL. Weight gain and response to treatment for multidrug-resistant tuberculosis. Am J Trop Med Hyg. 2013;89:943–949. doi: 10.4269/ajtmh.13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heller T, Lessells RJ, Wallrauch CG, Bärnighausen T, Cooke GS, Mhlongo L, Master I, Newell ML. Community-based treatment for multidrug-resistant tuberculosis in rural KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2010;14:420–426. [PubMed] [Google Scholar]
- 31.Holtz TH, Sternberg M, Kammerer S, Laserson KF, Riekstina V, Zarovska E, Skripconoka V, Wells CD, Leimane V. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med. 2006;144:650–659. doi: 10.7326/0003-4819-144-9-200605020-00008. [DOI] [PubMed] [Google Scholar]
- 32.Kwak N, Choi SM, Lee J, Park YS, Lee C-H, Lee S-M, Yoo C-G, Kim YW, Han SK, Yim J-J. Diagnostic accuracy and turnaround time of the Xpert MTB/RIF assay in routine clinical practice. PLoS ONE. 2013;8:e77456. doi: 10.1371/journal.pone.0077456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Riordan P, Schwab U, Logan S, Cooke G, Wilkinson RJ, Davidson RN, Bassett P, Wall R, Pasvol G, Flanagan KL. Rapid molecular detection of rifampicin resistance facilitates early diagnosis and treatment of multi-drug resistant tuberculosis: case control study. PLoS ONE. 2008;3:e3173. doi: 10.1371/journal.pone.0003173. [DOI] [PMC free article] [PubMed] [Google Scholar]