Abstract
Typhoid fever affects an estimated 22 million people annually and causes 216,000 deaths worldwide. We conducted an investigation in August and September 2010 to examine the acceptability of typhoid vaccine in Neno District, Malawi where a typhoid outbreak was ongoing. We used qualitative methods, including freelisting exercises, key informant and in-depth interviews, and group discussions. Respondents associated illness with exposure to “bad wind,” and transmission was believed to be airborne. Typhoid was considered extremely dangerous because of its rapid spread, the debilitating conditions it produced, the number of related fatalities, and the perception that it was highly contagious. Respondents were skeptical about the effectiveness of water, sanitation, and hygiene (WaSH) interventions. The perceived severity of typhoid and fear of exposure, uncertainty about the effectiveness of WaSH measures, and widespread belief in the efficacy of vaccines in preventing disease resulted in an overwhelming interest in receiving typhoid vaccine during an outbreak.
Introduction
Typhoid fever causes an estimated 22 million illnesses and 216,000 deaths annually worldwide, with the majority in resource-poor countries in south Asia and sub-Saharan Africa.1 Transmission of the etiologic agent, Salmonella Typhi, occurs through the fecal–oral route, most often by contaminated water or food. Over the past few years, several large outbreaks have occurred in sub-Saharan Africa where environmental conditions including suboptimal access to safe water and sanitation, poor hygiene, urbanization, and poverty place populations at risk for acquiring typhoid fever.2–10 Resistance to multiple antimicrobial agents has become increasingly common among S. Typhi strains in Africa and Asia, complicating treatment and outbreak control.10–16 Recent outbreak investigations in Uganda, the Democratic Republic of Congo, Malawi, and Mozambique reported widespread resistance to antimicrobials and life-threatening complications involving intestinal perforation and neurologic manifestations in people with confirmed typhoid fever.2–5,17 Despite this, control efforts in Africa have been limited, allowing an ongoing cycle of endemic disease and outbreaks to persist.10
Prevention and control of typhoid fever in endemic areas involves a comprehensive approach, including increasing access to safe water and sanitation, promotion of proper hygiene and food safety, and, in some settings, typhoid vaccination.6 Two typhoid fever vaccines have proven safety and efficacy, and one, the typhoid Vi polysaccharide vaccine, Typhim Vi (Sanofi Pasteur SA, Lyons, France), has been pre-qualified by the World Health Organization (WHO).12 Typhim Vi is administered by intramuscular injection as a single dose, providing 55–72% protection against typhoid fever to persons ≥ 2 years of age, with readministration required every 2–3 years. The Ty21a vaccine (Vivotif Typhoid Vaccine Live Oral, manufactured by Crucell, Leiden, The Netherlands) is a live attenuated oral vaccine administered in 3–4 alternate day doses; it provides 51–77% protection and is licensed for persons ≥ 5 years of age, with booster doses required every 5 years.11 The Ty21a vaccine is not WHO pre-qualified, and the requirement for multiple doses is impractical for mass campaigns. Typhoid Vi polysaccharide vaccines have been used in mass vaccination campaigns in several Asian countries where typhoid fever is endemic and its epidemiology is well understood.12,16,18,19 The success of vaccination campaigns has influenced WHO to recommend consideration of the use of these vaccines to target high-risk groups in countries where typhoid is endemic and to control epidemics.12
Social scientists have investigated behavioral, sociocultural, political, and economic factors that may influence vaccine acceptance.20–24 Many factors have been identified as relevant to individual decision-making processes, including perceptions of disease prevalence, characteristics and severity; subgroup vulnerability; availability and effectiveness of alternative preventive strategies; and expectations regarding treatment. Vaccine-related factors include understanding of the purpose of vaccines; the perceived efficacy; perceptions of risks and side effects; costs; vaccine characteristics; and prior experience with vaccine services. Socioeconomic factors including economic status, education and ethnicity also play a role in vaccine acceptance.25 Other studies have identified client–health worker communication, health care system infrastructure, and cultural meanings attached to vaccination by community leaders as important variables.26–28
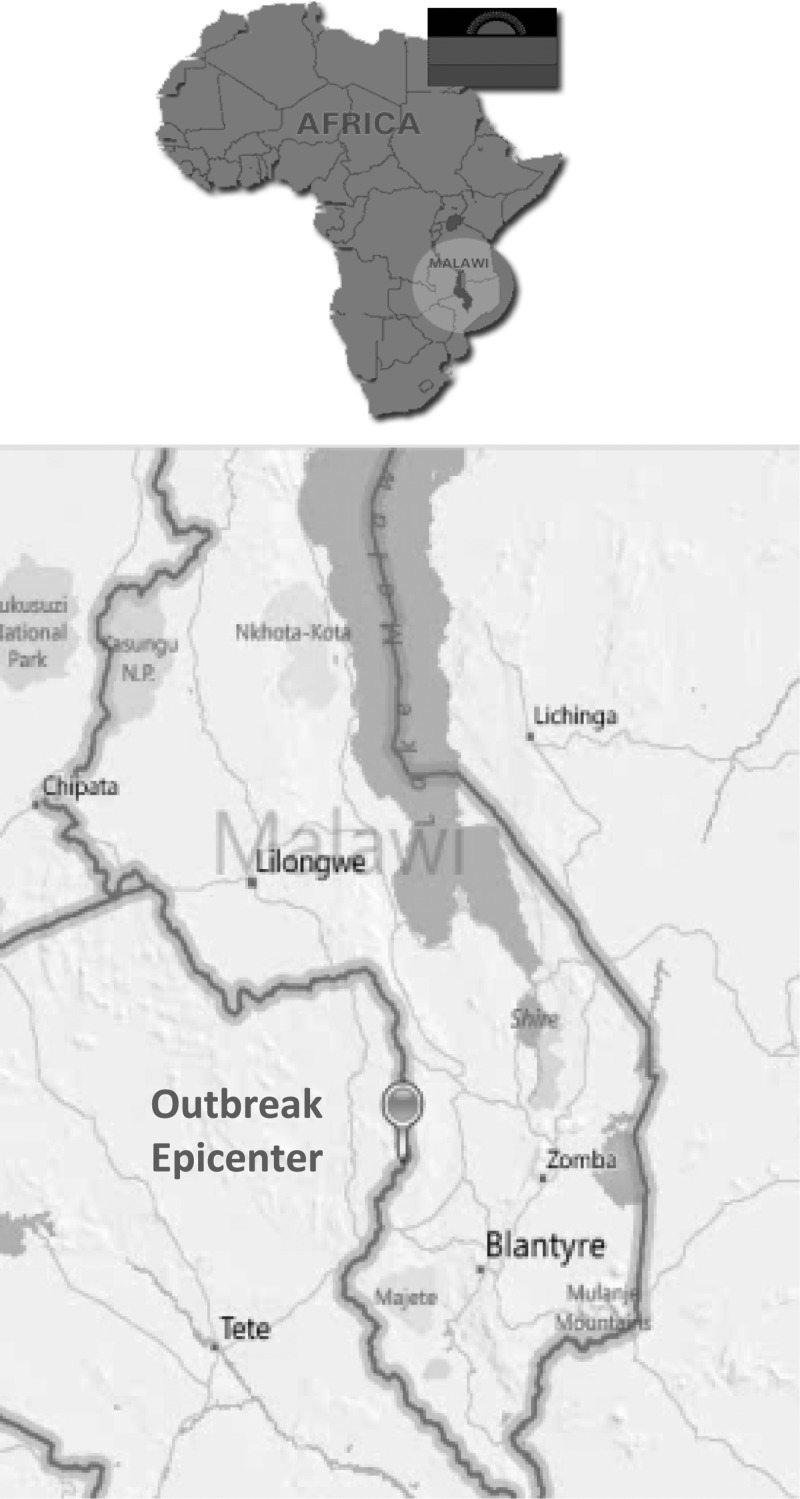
In June 2009, an outbreak of unexplained febrile illness was detected on the Malawi-Mozambique border in southwestern Malawi (Figure 1).3 Key features of those affected included headache, fever, confusion, inability to walk, inability to talk, and hyperreflexia. The unusual presentation, with neurological deficits as a prominent feature, initially generated a number of hypotheses regarding the etiology.17 In August, diagnostic tests confirmed the illness was typhoid fever. Subsequently, a team of health scientists from the Malawi Ministry of Health (MOH) and the Centers for Disease Control and Prevention (CDC) worked together to recommend control measures, including promotion of household water treatment and sanitation and hygiene (WaSH) interventions. However, implementation was hampered by rain and poor roads, and between March and November 2009, 303 cases occurred, including 46 culture-confirmed cases3; by September, 2010, 784 cases and 44 deaths had been reported in 17 villages.29 In response, vaccination was considered as an outbreak control measure.
Figure 1.
Map indicating the location of the typhoid fever outbreak area.
We investigated factors associated with the acceptability of typhoid vaccine in response to this ongoing typhoid outbreak. Qualitative methods were used to explore explanatory models of typhoid fever, local understandings of the purpose of vaccines, and the perceived need for and willingness to accept a typhoid vaccine, and sociocultural and political features that could influence vaccine acceptance. An assessment of the WaSH interventions implemented in the outbreak area was carried out shortly after the completion of this investigation.
Methods and Materials
Investigation site.
The investigation was conducted over a 3-week period during August and September 2010 in Neno District in southwestern Malawi bordering Mozambique. The area is rural and mountainous, with limited access, particularly during the rainy season. Residents live in villages of ∼350 to 1,050 inhabitants consisting of congregations of small houses. Villages are in close proximity to one another, within approximately a 14 km radius. Most residents are subsistence farmers with limited formal education. An inpatient government hospital is located in the district capital about 35 km from the outbreak area. A government health clinic is situated about an hour's walk from affected villages, and another health center is located in an outbreak village in Mozambique. Kiosks selling medications for common illnesses such as malaria, headache and fever are found in more populated village centers. Villages in the district had been targeted through outbreak control efforts for water, hygiene and sanitation (WaSH) interventions. Messages conveyed had also encouraged residents to seek health care for acute febrile illness. The investigation took place at the same time as a measles vaccination campaign conducted in Neno and other districts of Malawi.
Methods and populations.
We used a diverse set of complementary qualitative methods to identify relevant factors.
-
a.
Freelisting exercises were administered to respondents ≥ 18 years of age in 8 villages where typhoid cases had been confirmed to generate a preliminary inventory of the cultural domain of illnesses. Households were randomly selected in a systematic fashion and the first person ≥ 18 years of age encountered in those households was asked to list common illnesses and accompanying signs and symptoms. Items mentioned first and more often were considered to have greater significance in the local framework of illnesses.
-
b.
Key informant interviews were conducted with government officials, local religious and traditional leaders, community health workers (CHWs), and prominent residents in outbreak communities. We explored perceptions of typhoid fever and whether informants believed vaccine would be an acceptable control measure. Interviews with national and district level MOH officials were designed to examine experiences with vaccines and views on the introduction of typhoid vaccine to control the outbreak.
-
c.
In-depth interviews were conducted with residents living in nine villages where typhoid fever had been confirmed between August and November 2009. Purposive sampling was used, targeting heads of households or their spouses living with at least one child < 18 years of age. Interviews provided data on the local explanatory model of typhoid fever, perceptions of vaccines and vaccination services, and demand for typhoid vaccine.
-
d.
Severity rating exercises were conducted with the in-depth interview respondents to assess perceived severity of typhoid compared with other common illnesses. Respondents rated the illnesses on a bipolar three-step scale.
-
e.
Group discussions were conducted with separate groups of 18–29-year-old men and women, separate groups of male and female parents 30 years of age and older living with young children, and community leaders. Groups consisted of 8–10 participants and were designed to validate preliminary findings and assess programmatic issues related to introduction of typhoid vaccine.
Data collection.
After 3 days of training, three data collectors with experience in qualitative research administered the freelisting and severity rating exercises and conducted the in-depth interviews and group discussions in Chichewa, the local language. Whenever possible, data were collected in a private setting. In-depth interviews lasted ∼1 hour. Pairs of data collectors led group discussions, with one person moderating, and a second person taking notes. Interviews and group discussions were tape-recorded, transcribed, and translated into English by data collectors or a professional translator. Key informant interviews were conducted by a co-investigator and the lead investigator, with the assistance of a local interpreter.
Data analysis.
Freelisting and rating exercises were analyzed with Anthropac 4.983 software (Analytic Technologies, Lexington, KY). After completion of the in-depth interviews, a coding system capturing the main investigation themes was developed and interviews were coded using ATLAS.ti 6.0 software (Berlin, Germany). Key informant interviews and focus group discussions were coded manually. Content analysis was used to identify and compare trends of key concepts in the coded data. Triangulation was used to validate findings through a combination of data sources, such as multiple interviewees and group discussions.
Ethical considerations.
This investigation was initiated as a public health activity in response to an outbreak. The Malawi National Human Subjects Review Committee and CDC determined that the activities constituted public health response. Written informed consent was obtained from all investigation participants.
Results
Community perceptions of typhoid fever.
Freelisting exercises were administered to 31 respondents. The procedures elicited a core set of illnesses frequently mentioned by respondents, with malaria ranked first followed by cough, diarrhea, headache, and back pain. Typhoid fever ranked sixth among 72 illnesses listed. Although most illnesses were referred to in local terminology, typhoid fever was uniformly called typhoid.
In-depth interviews were carried out with 11 male and 9 female respondents. Virtually all respondents were farmers with minimal education (Table 1). Most resided in small mud huts, averaging 5.6 persons per household. Drinking water sources were primarily hand-dug wells or boreholes, and most households had a latrine. Review of vaccination histories of household members ≤ 20 years of age identified 80 eligible respondents, among whom 90% had a vaccination card and had received Bacille Calmette-Guérin (BCG) (71%), the third dose of diphtheria-tetanus-pertussis (DTP3) (83%), the third dose of polio (polio3) (83%), and measles (95%) vaccines.
Table 1.
Demographic characteristics of in-depth interview respondents, Neno District, Malawi
| Characteristic | Respondents (N = 20) |
|---|---|
| Sex | |
| Male | 11 |
| Female | 9 |
| Average age (years) | |
| Male | 32 |
| Female | 40 |
| Education | |
| Never attended school | 5 |
| Average years for those who attended school | 7 |
| Religious affiliation | |
| Catholic | 9 |
| Seventh Day Adventist | 6 |
| Zambezi Evangelical | 4 |
| Assemblies of God | 1 |
| Occupation | |
| Farmer | 19 |
| Housewife | 1 |
| Average number of family members | 5.6 |
| Animal ownership | |
| Goats | 9 |
| Cows | 2 |
| Pigs | 2 |
| Chickens | 8 |
| None | 8 |
| Household construction | |
| Mud and wood | 10 |
| Mud and bricks | 7 |
| Cement and bricks | 3 |
| Type of roof | |
| Thatched grass | 15 |
| Tin | 5 |
| Assets | |
| Bicycle | 9 |
| Radio | 17 |
| TV | 3 |
| Solar panel | 6 |
| Motorcycle | 0 |
| Primary drinking water source | |
| Well | 7 |
| Borehole | 7 |
| River/stream | 3 |
| Tap | 2 |
| No response | 1 |
| Household latrine | |
| Yes | 17 |
| No | 3 |
Eleven of the 20 in-depth interview respondents claimed to have experienced typhoid, with three reporting hospitalization. All but one of the other respondents reported that a family or community member had been diagnosed with typhoid, and 14 reported that a relative or community member had died of typhoid. Among five focus group discussions, most participants had known somebody with typhoid, with many claiming to have experienced the disease themselves.
Respondents mentioned headache, fever, body ache, and vomiting as initial signs and symptoms of typhoid, followed by a rapid 2- to 3-day deterioration consisting of severe headache, lack of appetite, difficulty swallowing, diarrhea and vomiting, rapid weight loss, and weakness. If left untreated, more severe conditions developed, including inability to walk, hear, or see; incoherent speech; paralysis of the neck, hands, and legs; memory loss, delirium or unconsciousness; and finally, death.
Respondents explained that the disease first appeared in a village bordering Mozambique, with many suggesting it came from Mozambique. Community residents and health workers stated that typhoid and malaria were at the outset indistinguishable, with many believing typhoid to be a more severe form of malaria not previously experienced. Some called the condition “strange” or “mystery” disease because it rapidly produced a range of rare and incapacitating conditions. There was no local term; over time, health workers informed villagers the illness was called typhoid fever.
Initially, the disease was attributed to a dispute over chieftainship between the chief of the village where most of the early cases occurred and his relatives in a neighboring community. People speculated that a curse had been enacted by family ancestors in response to the conflict and that the chief used witchcraft to maintain control of the village. The unusual disease symptoms, rapidly progressive and frequently fatal course, and failure of clinic-based health providers to identify the disease and provide effective treatment helped to substantiate this belief. Once the disease spread, causal explanations often alluded to “bad air” or wind that carried the illness from one village to another. Fewer respondents suggested that disease transmission was linked to poor hygiene or drinking unsafe water.
The majority of in-depth interview and focus group discussion respondents indicated that the disease was contagious and easily transmitted when in close contact with somebody who was sick, an explanation linked to the observation that the illness quickly spread to family members or visitors exposed to the contaminated air in the patient's surroundings. Respondent (P3) said,
“This disease spreads through sitting close to people suffering from the disease and breathing the same air. How people give others measles and this disease is the same, because it affects the whole house, especially caregivers staying close to the sick. That is when we realized that it was not because of charms or magic but due to mphepo (something bad in the air or wind or airborne). ”
When the outbreak first began, medications known to treat malaria and disease symptoms such as headache and fever were purchased in community kiosks and administered. The wide range of clinical manifestations, the ineffectiveness of locally available drugs, and reports that typhoid killed faster than malaria and infected many people simultaneously, led community members to assume that affected individuals had contracted different illnesses, or that several illnesses culminated into one new disease. Subsequent care seeking was more often at health centers where treatment was also ineffective, with many people dying. This raised alarm that even health officials were unable to recognize or treat the illness. Key informant and group discussion participants stated that some people consulted traditional practitioners. Respondents described a chaotic situation at the peak of the outbreak, with people accused of witchcraft and conflicts over the disease cause and treatment.
Respondents reported that as the disease reached epidemic levels, foreigners identified the disease and effective treatment. Medical personnel then worked with village chiefs and CHWs to ensure that suspected cases were transported to health facilities. Villagers observed that people who sought rapid medical care survived, whereas those who delayed seeking care suffered and eventually died; this convinced symptomatic persons to visit health centers. Over time, community members understood that treatment was only available in the district hospital where diagnosis could be made and appropriate medications provided. Some reported that hospital treatment was not always effective, with patients continuing to die.
Based on the freelist analysis and initial in-depth interview data, we selected 23 common illnesses to explore perceived disease severity. Eighteen of 20 in-depth interview respondents rated the illnesses; typhoid was considered the most serious (Table 2). All types of respondents underscored the severity and “power” of typhoid, highlighting the rapid spread and random nature with which it appeared to attack households, the rapid onset of a combination of diverse and severe symptoms, the absence of effect of local drugs, and, early in the outbreak, the inability of medical personnel to identify or treat it, and the high fatality rate, even among those who obtained medical care. Once treated, respondents emphasized that it could take months for the disease to leave the body, with some describing relapses or debilitating effects such as paralysis or reduced body strength, suggesting that the disease overpowered the medicine. Many described profound economic consequences because those afflicted were unable to farm. Because of its seriousness and the ongoing nature of the outbreak, respondents expressed grave concern that the number of cases would increase.
Table 2.
Perceptions elicited from in-depth interview respondents of severity of common illnesses
| Ranking | Illness name | Mean score* |
|---|---|---|
| 1 | Typhoid | 1.11 |
| 2 | HIV | 1.17 |
| 3 | Measles | 1.22 |
| 3 | Stroke | 1.22 |
| 4 | Dysentery | 1.33 |
| 5 | Malaria | 1.50 |
| 6 | Pneumonia | 1.56 |
| 7 | Asthma | 1.61 |
| 8 | Diarrhea | 1.67 |
| 9 | Heart disease | 1.76 |
| 10 | Chest pain | 1.94 |
| 11 | Stomach ache | 2.00 |
| 11 | Epilepsy | 2.00 |
| 12 | Neck pain | 2.12 |
| 12 | Vomiting | 2.12 |
| 13 | Reproductive tract illness in women | 2.24 |
| 14 | Back pain | 2.25 |
| 15 | Rheumatism | 2.28 |
| 16 | Toothache | 2.29 |
| 17 | Fever | 2.31 |
| 18 | Headache | 2.39 |
| 19 | Scabies | 2.44 |
| 20 | Ear infection | 2.53 |
| 21 | Conjunctivitis | 2.67 |
| 22 | Cough | 2.72 |
| 23 | Flu | 2.82 |
The mean scores were generated on Anthropac using 1 for most severe, 2 for moderately severe, and 3 for least severe.
Most respondents knew of preventive measures related to hygiene and consumption of safe water, with WaterGuard, the nationally produced chlorine-based point-of-use water treatment product distributed during the outbreak, generally mentioned as the best approach. Focus group discussion participants suggested that strategies involving improved hygiene and sanitation are ineffective, because the disease is airborne. Others mentioned that all water sources in the area are bad, and no measures can improve the water quality. Skepticism was also related to claims that because villagers all followed similar hygiene and sanitation practices, everyone should have become sick if poor sanitation and hygiene were to blame. In addition, residents stressed that they had historically been drinking the same water and living under conditions marked by poor sanitation without having experienced the disease. In-depth interview respondents noted that although hygiene and sanitation had improved, the disease was still prevalent, thus “confirming” airborne transmission. Respondents also suggested that preventive strategies are difficult to implement; we found that WaterGuard was not regularly distributed, and it was challenging to maintain an ongoing supply of water and soap for hand washing in households. Respondents concluded that improved hygiene strategies lacked widespread compliance, leaving residents susceptible to the disease. Young men were singled out as those who follow highly unhygienic practices and are unwilling to change their behavior.
Perceptions of vaccines and vaccination.
Vaccines were most commonly known to protect people from certain diseases, with some in-depth interview respondents specifying illnesses affecting children. Respondents showed a firm belief in the power of vaccines in preventing illness; fewer believed that if infected, vaccines could mitigate the illness. Focus group discussion participants suggested that vaccines can eradicate disease.
Vaccination was also believed to help the body remain strong and recover from illness quickly and protect people from dying. Some respondents explained that vaccinations control disease outbreaks by reducing the number of cases, and that receiving a vaccination can protect people from future outbreaks caused by the same disease. A few respondents stated that vaccinations are given for treatment in health facilities.
Respondents most commonly mentioned that vaccines are available to prevent measles; other conditions cited included polio, tetanus, cholera, malaria, diphtheria, pertussis, tuberculosis, intestinal worm, and vitamin A deficiency; older respondents stated that smallpox vaccine had been offered in the past. Vaccines recommended by health officials and supported by village chiefs signified the disease strength. Some respondents alluded to social pressure to be vaccinated, indicating that anybody who refuses and later gets sick will be blamed by villagers for introducing disease and placing others at risk. This respondent (P14) said,
“Anything about vaccination that comes from the chief cannot be refused. We know that the chief has been informed by hospital officials we rely on. If I refuse to receive a vaccination and later get sick, people will say I caused the disease, and because of me, they may lose their lives.”
Respondents differentiated between routine vaccination programs and vaccines given during disease outbreaks. They indicated that outbreak response campaigns occurred infrequently and lasted several days. Some respondents complained that the distance to emergency vaccine stations is far and that centers can become chaotic, attracting crowds jostling to receive vaccines, with many turned away. Respondents stated that vaccines are generally only offered to children.
Respondents indicated that all children residing in their respective households had been vaccinated during a recent measles campaign. Many expressed concern that adults were excluded and left vulnerable to disease. Respondent (P12) said,
“Vaccination is very important to everybody. Adults also need to receive vaccines because everybody can get sick from different illnesses. Vaccines should not only be for children.”
This was confirmed by the District Health Officer, who described the tremendous demand for measles vaccination, which targeted children < 15 years of age because they were most affected by the outbreak, and difficulties convincing people > 15 years of age that they were not eligible.
Many respondents were impressed by government efforts to protect people from measles through vaccination and the resultant public response, asserting that the campaign had encouraged them to receive other vaccinations to prevent outbreaks. Focus group respondents spontaneously mentioned that they were hoping a typhoid vaccine would be introduced, as they viewed vaccination as the most effective way to prevent disease.
The most common known immunization-related side effects included pain and swelling around the injection site, with fewer respondents mentioning fever, rash, and headache. Negative effects were described as brief and normal, and indicate that the vaccine is working, with the benefits far outweighing any adverse reactions. The majority of respondents indicated that there are no risks in getting vaccinated. Potential dangers mentioned included that the needle breaks or is inadvertently inserted in a vein, or that health providers misrepresent what is being administered and instead give poison, the wrong vaccine, or water, giving the false illusion of protection.
Problems related to getting vaccinated included: distance to the vaccination centers; overcrowding and long waits in the centers; inclement weather and poor roads, preventing vaccination teams from reaching stations; insufficient health staff to manage the workload and crowds during campaigns; inadequate vaccine supply; and husbands who prohibit their wives from attending. The majority of respondents indicated that they and their family members never experienced difficulties that could prevent their receiving vaccinations. Respondents insisted that there were no religious beliefs or groups in the area that prohibit vaccination, explaining that vaccines are associated with biomedicine and are therefore valued.
Perceptions about introduction of typhoid vaccine.
All in-depth interview respondents indicated a willingness to receive free typhoid vaccine, and local key informants, including village chiefs, categorically suggested that community members would be amenable to receiving such a vaccine. Only key informants were asked whether vaccine effectiveness of 60% would decrease acceptability; they stated that even that would be an improvement over the present situation. The most common explanation for desiring a vaccine was to provide protection from the potentially lethal or disabling disease. Respondents also highlighted concerns about contagion and rapid spread, adding that it affects people in their prime, with some mentioning that treatment was unavailable. This village chief said,
“We are ready to be vaccinated. If you have a vaccine, please bring it… We lost a lot of people and are scared of this disease.”
Respondents emphasized that the disease severity underscored the importance of making a vaccine available to people of all ages. A respondent (P14) explained,
“If a vaccine for typhoid comes and is only given to children, people will get worried. Now we do not believe in anything but vaccination. If a vaccine was available today you would see all of us rushing to get it because we know the dangers of this disease.”
Key informant government officials expressed interest in introducing typhoid vaccine as a control strategy, highlighting the urgency to eliminate the disease and emphasizing that improving water and sanitation systems requires a long-term commitment. Concerns included the costs and challenges faced in collaborating with Mozambican officials.
Suggested approaches for a successful typhoid vaccination program included offering vaccination in one village at a time; providing door-to-door vaccinations; increasing the number of people typically administering vaccines; and ensuring adequate supplies. Most respondents preferred injections to oral vaccines, explaining that injected vaccine goes into the blood stream and is therefore more effective and can “fight” other diseases at the same time, and that children often refuse tablets.
Respondents recommended that typhoid vaccine would be best introduced by village chiefs who command widespread respect and have supreme authority; people would discount a vaccine's importance if the chief were not involved. Some respondents stressed that district level government and health officials should provide information about the purpose and characteristics of the vaccine, to alleviate safety concerns. Essential information should include how the vaccine works in the body, its effectiveness, the duration of protection, potential side effects, eligibility to receive the vaccine, risks in refusing to accept the vaccine, and when and where it would be offered. Focus group discussion participants suggested imposing a fine on those who refuse the vaccine.
Discussion
This in-depth qualitative assessment allowed us to examine cultural concepts of typhoid fever in Neno District in southwestern Malawi, where a typhoid outbreak was ongoing and to explore factors that may influence acceptance of typhoid vaccination. The investigation illuminated a strong cultural consensus concerning the perceived seriousness of typhoid fever, widespread understanding of the purpose of vaccines and appreciation for their protective role, and trust in the medical system. The mountainous terrain, poor roads, and long distances limited access to health facilities for outbreak communities. Residents' perception of disease severity combined with limited access to effective medical treatment and the value they attributed to vaccines led to universal interest among respondents in receiving a vaccine for typhoid. Our investigation highlighted preferences for delivery strategies if a vaccine were introduced.
Our findings highlighted strong patterns regarding the local explanatory disease model, referring to culturally constructed concepts about etiology, symptom patterns, appropriate treatment, and expectation of outcomes of typhoid.30 Typhoid fever did not have a local name, and the previously unknown constellation of systemic and neurologic symptoms and ineffectiveness of locally available drugs, also suggested to respondents the disease was new, and serious.
Typhoid was universally viewed as prevalent and extremely dangerous as a result of the rapid onset and spread, the severity, the ineffectiveness of locally available medications, and the frequency and speed with which deaths occurred; this compelled ill persons to make the arduous trip to health facilities for care. Perceived severity was exacerbated by the peculiar combination of debilitating conditions it produced, the need for hospital treatment, and the uncertainty regarding treatment efficacy, signifying that the disease overpowered medicine. The local interpretation that the illness rendered medication ineffectively mirrors the biological finding that the involved typhoid strain was resistant to multiple drugs, and thus difficult to treat with the available medications.3 Concern was also heightened by the belief that the disease was highly contagious and transmitted through air, placing the general population at risk, including those responsible for economic livelihoods.
Common diseases, including malaria, were considered comparatively less serious because their sequelae were predictable and generally not debilitating, they could be treated with locally available medications, and they were not known to infect many people simultaneously or kill as rapidly. If the unusual neurologic manifestations had not been present, we suspect that typhoid may have gone unrecognized as a different disease entity for a longer period. Failure to detect typhoid is an ongoing problem in Africa where lack of laboratory diagnostic capacity limits the ability to distinguish the disease from other ubiquitous febrile illnesses.2,10,16
Respondents were skeptical about the role of improved water and hygiene in typhoid prevention. The incongruence between the local and medical causal interpretation of the disease and the established mode of typhoid transmission negatively influenced perceptions of recommended biomedical preventive measures. Doubts about prevention by WaSH interventions were strengthened by the occurrence of new cases even after the interventions were implemented. Respondents emphasized that difficulty maintaining adherence to WaSH interventions hindered universal compliance and undermined prevention efforts. Young men were identified as being less likely to alter their behavior, placing them at greater risk for exposure and spreading disease.
In Neno District, vaccines were appreciated for their ability to protect people from particular illnesses, to mitigate symptoms, and to enhance the health of recipients. This knowledge of the purpose and an appreciation for the efficacy of vaccines was consistent across all groups of respondents and may have been related in part to the coincidental occurrence of a nationwide measles immunization campaign, which included messaging about measles vaccine and resulted in reported coverage rates > 95%.31 Respondents also recognized the difference between routine and campaign vaccination, stating that government initiatives involving vaccines during disease outbreaks signify a serious public health threat. Our findings suggest that the value attached to vaccine induces social pressure to get vaccinated, with those refusing viewed as a liability to communities. Recent increases in vaccination coverage in Malawi affirm the significance attributed to vaccines: the 2010 Demographic and Health Survey indicated that 81% of children 12–23 months of age were fully vaccinated compared with only 64% in 2004.32,33
A combination of factors drove the overwhelming interest by community residents in receiving vaccine during a typhoid outbreak. The formative nature of this assessment did not permit a determination of whether expressed willingness to accept vaccination would correlate with vaccine uptake. Other studies have shown that intention to accept vaccination does not necessarily translate into behavior.34,35 Social scientists examining the intention–behavioral relationship have developed behavioral models to identify factors that play a crucial role in actual vaccination. Vaccine acceptance appears to be guided by perceived risk of and vulnerability to infection, an appreciation for the benefit of the vaccine, a strong history of vaccination, and social norms encouraging vaccine acceptance—all factors that would suggest widespread uptake in this setting. We did not identify risk factors that are known to outweigh benefits and dissuade vaccine acceptance, such as concerns about adverse effects or cultural prohibitions. The fact that this investigation was carried out during an outbreak undoubtedly increased the perceived threat of infection and likelihood of vaccine acceptance in a context where the local vaccination culture was positive, with some respondents spontaneously requesting that authorities provide vaccine. In addition, vaccination was viewed as easily accomplished, compared with improved water and hygiene measures, which respondents considered difficult because they require sustained behavior change across entire communities. National and local government officials and community leaders also expressed a keen interest in introducing typhoid vaccine as an immediate measure to curtail the outbreak. One concern related to the feasibility of collaboration with neighboring Mozambique, where government officials reportedly held different views about disease control. Because of the porosity of the border and the fact that the disease transgressed boundaries, the success of a typhoid vaccine aimed at containing disease was perceived as contingent on cross-border participation.
Our respondents highlighted some of the behavioral challenges related to introducing a WaSH intervention. Another study carried out in the outbreak area illuminated limited knowledge of the relationship between drinking unsafe water, poor hygiene, and typhoid fever; lack of regular access to household chlorination products; and poor sanitation infrastructures as major barriers to rapid implementation of WaSH interventions.29 The range of behavioral and structural factors involved in developing a robust water and sanitation program can take years to address. Although typhoid vaccine seems comparatively attractive, experience shows that limited supplies of the single-dose vaccine and its availability from only one WHO-prequalified manufacturer; the time required to get the vaccine released, delivered, and approved in the country; the need to coordinate with other expanded programs on immunization activities; and the cost for the vaccine and conducting the campaign, can cause critical delays in outbreak response.11 Because the vaccine is not 100% effective, and only provides protection for 2–3 years, the best approach is to combine vaccine and WaSH interventions.
A primary aim of this investigation was to identify information that would maximize participation if typhoid vaccine were introduced. Although community residents stressed the importance in knowing a vaccine is supported by government officials who can assure residents about the danger the disease poses and safety of the vaccine, more critical was the involvement of village chiefs. In the Malawian context, relations of power and dependency render extreme respect to the village chief, obliging villagers to obey the chief's requests. Another initiative showed that pre-vaccination information conveyed through trusted local leaders was a significant predictor of vaccine acceptance.35 Respondent recommendations regarding key pre-vaccination information were predictable and likely reflected educational messages disseminated during the measles campaign. Interestingly, residents requested advance information that would allow them to plan for vaccination in their schedule, a factor that has been shown to be critical to vaccine uptake.36 We found that there was a preference for injectable vaccines, because they are believed to be more powerful than oral vaccines.
The findings elucidated structural and logistical barriers in the measles vaccination campaign, which caused prolonged waits and deprived farmers of valuable work time. Suggestions to address these problems included providing door-to-door vaccinations or increasing the number of vaccination stations. We uncovered misunderstandings regarding vaccine eligibility, underscoring the need to specify the primary beneficiaries before the vaccine introduction and to explain why the target group is at greatest risk. In the case of typhoid vaccine, respondents stressed that all members of the population are vulnerable and that vaccine should be available to everyone.
Conclusion
This is the first in-depth assessment of typhoid vaccine acceptability during an outbreak in Africa, where multiple typhoid epidemics involving high mortality rates and long-term morbidity have recently been identified.2–10 From a theoretical standpoint, the findings illustrate a dynamic interaction of sociocultural, economic, and biomedical factors that guided perceptions of disease severity and vulnerability and the need for typhoid vaccine in a context with widespread trust in vaccine technology. Specifically, perceived personal risk related to the deadly or debilitating physical consequences of typhoid fever, and perceptions of social and economic vulnerability, shaped images about the gravity of the typhoid epidemic. This, along with popular views on vaccine potency and efficacy, guided a widespread social demand for vaccination, which has been recommended by the WHO for typhoid fever outbreak control. The challenges of introducing and maintaining WaSH interventions and changing behaviors, and the increase in antimicrobial resistance, heighten the potential benefits of targeted immunization programs in outbreak settings.1
The results of this investigation in conjunction with other considerations regarding cost-effectiveness, timing, and impact can contribute to policy and programmatic decisions on vaccine introduction, and may be relevant to other African contexts where typhoid outbreaks regularly occur and vaccination cultures are similar. Given that typhoid vaccines range in effectiveness from 51% to 77%, and that the protection period is < 5 years, public health authorities need to be careful not to raise expectations regarding disease protection that cannot be met. Typhoid vaccines should be introduced with WaSH programs to ensure a comprehensive and sustainable approach. The findings also illuminated misconceptions about the disease etiology, transmission, and treatment, underlining the need to increase awareness of typhoid fever in sub-Saharan Africa where emerging evidence suggests a high disease burden demanding attention to prevention and control measures.
ACKNOWLEDGMENTS
We are grateful to those who offered their time to participate in this study. We also thank the research assistants Christopher Ndembo, Josephine Chimoyo, and Watipatsa Mang'anda, and Ethel Mpagaja and Eustacia Chumbu in the CDC and USAID offices in Lilongwe for their support. We thank Tadala Khaila, the District Health Officer at the outset of the study, who helped make this investigation possible. We also thank Ben Nygren, CDC Atlanta, for his assistance on mapping the research sites, and Natalie Wilhelm, Travis Yates, and Daniele Lantagne of Tufts University for their review of the manuscript.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Financial support: The investigation was funded by the Division of Global Disease Detection and Emergency Response, CGH, CDC.
Authors' addresses: Lauren S. Blum, Dakar, Senegal, E-mail: laurensblum@yahoo.com. Holly Dentz, Center for Global Safe Water, Emory University, Atlanta, GA, E-mail: hollydentz@gmail.com. Felix Chingoli, Neno District Health Office, Neno, Malawi, E-mail: fchingoli@gmail.com. Benson Chilima, Ministry of Health, Lilongwe, Malawi, E-mail: bchilima2@yahoo.com. Thomas Warne, Centers for Disease Control and Prevention, Pretoria, South Africa, E-mail: warnet@sa.cdc.gov. Carla Lee, Terri Hyde, and Jacqueline Gindler, Global Immunization Division, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: cel1@cdc.gov, tkh4@cdc.gov, and jsg5@cdc.gov. James Sejvar and Eric D. Mintz, National Center for Emerging Infectious and Zoonotic Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: zea3@cdc.gov and edm1@cdc.gov.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;84:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Neil KP, Sodha SV, Lukwago L, O-Tipo S, Mikoleit M, Simington SD, Mukobi P, Balinandi S, Majalija S, Ayers J, Kagirita A, Wefula E, Asiimwe F, Kweyamba V, Talkington D, Shieh WJ, Adem P, Batten BC, Zaki SR, Mintz E. A large outbreak of typhoid fever associated with a high rate of intestinal perforation in Kasese District, Uganda, 2008–2009. Clin Infect Dis. 2012;54:1091–1099. doi: 10.1093/cid/cis025. [DOI] [PubMed] [Google Scholar]
- 3.Lutterloh E, Likaka A, Sejvar J, Manda R, Naiene J, Monroe SS, Khaila T, Chilima B, Mallewa M, Kampondeni SD, Lowther SA, Capewell L, Date K, Townes D, Redwood Y, Schier JG, Nygren B, Tippett Barr B, Demby A, Phiri A, Lungu R, Kaphiyo J, Humphrys M, Talkington D, Joyce K, Stockman LJ, Armstrong GL, Mintz E. Multidrug-resistant typhoid fever with neurologic findings on the Malawi-Mozambique border. Clin Infect Dis. 2012;54:1100–1106. doi: 10.1093/cid/cis012. [DOI] [PubMed] [Google Scholar]
- 4.Muyembe-Tamfum JJ, Veyi J, Kaswa M, Lunguya O, Verhaegen J, Boelaert M. An outbreak of peritonitis caused by multidrug-resistant Salmonella typhi in Kinshasa, Democratic Republic of Congo. Travel Med Infect Dis. 2009;7:40–43. doi: 10.1016/j.tmaid.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Walters MS, Routh J, Mikoleit M, Kadivane S, Ouma C, Mubiru D, Mbusa B, Murangi A, Ejoku E, Rwantangle A, Kule U, Lule J, Garrett N, Halpin J, Maxwell N, Kagirita A, Mulabya F, Makumbi I, Freeman M, Joyce K, Hill V, Downing R, Mintz E. Shifts in geographic distribution and antimicrobial resistance during a prolonged typhoid fever outbreak—Bundibugyo and Kasese Districts, Uganda, 2009–2011. PLoS Negl Trop Dis. 2014 doi: 10.1371/journal.pntd.0002726. http://www.plosntds.org/article/info3Adoi2F10.13712Fjournal.pntd.0002726 Available at. Accessed April 6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke KR, Kanyanga MK, Musenga E, Tambatamba B, Lukwesa C, Bopp C, Malama C, Routh J, Marx MA, Craig AS, Mintz E. Outbreak of multi-drug resistant Salmonella typhi, Lusaka, Zambia, 2011–2012. American Society of Tropical Medicine and Hygiene 61st Annual Meeting; 2012; Atlanta, GA. 2012. [Google Scholar]
- 7.Centers for Disease Control and Prevention Notes from the field: Salmonella typhi infections associated with contaminated water–Zimbabwe, October 2011–May 2012. MMWR 61. 2012:435. [PubMed] [Google Scholar]
- 8.Imanishi M, Kweza P, Slayton RB, Urayai T, Ziro O, Mushayi W, Chizororo M, Kuonza L, Ayers T, Freeman M, Govore E, Duri C, Chonzi P, Zinyowera S, Manangazira P, Kilmarx PH, Mintz E, Lantagne D. Zimbabwe Typhoid Fever Outbreak Working Group 2011–2012 Household water treatment uptake during a public health response to a large typhoid fever outbreak in Harare, Zimbabwe. Am J Trop Med Hyg. 2014;90:945–954. doi: 10.4269/ajtmh.13-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crump JA. Typhoid fever and the challenge of nonmalaria febrile illness in sub-saharan Africa. Clin Infect Dis. 2012;54:1107–1109. doi: 10.1093/cid/cis024. [DOI] [PubMed] [Google Scholar]
- 10.Nelson C. Controlling the typhoid epidemic plaguing sub-Saharan Africa. Atlantic. 2012;4 http://www.theatlantic.com/health/archive/2012/04/controlling-the-typhoid-epidemic-plaguing-sub-saharan-africa/255243/ Available at. Accessed December 2, 2013. [Google Scholar]
- 11.Slayton RB, Date KA, Mintz E. Vaccination for typhoid fever in sub-Saharan Africa. Hum Vaccin Immunother. 2013;9:1–4. doi: 10.4161/hv.23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Typhoid vaccines: WHO position paper. WER. 2008;83:49–60. doi: 10.1016/j.vaccine.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Lewis MD, Serichantalergs O, Pitarangsi C, Chuanak N, Mason CJ, Regmi LR, Pandey P, Laskar R, Shrestha CD, Malla S. Typhoid fever: a massive, single-point source, multidrug-resistant outbreak in Nepal. Clin Infect Dis. 2005;40:554–561. doi: 10.1086/427503. [DOI] [PubMed] [Google Scholar]
- 14.DeRoeck D, Jodar L, Clemens J. Putting typhoid vaccination on the global health agenda. N Engl J Med. 2007;357:1069–1071. doi: 10.1056/NEJMp078144. [DOI] [PubMed] [Google Scholar]
- 15.Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, Ochieng JB, Wamola N, Bigogo GM, Awiti G, Tabu CW, Burke H, Williamson J, Oundo JO, Mintz ED, Feikin DR. 2012. Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS ONE. 7:e29119. doi: 10.1371/journal.pone.0029119. Epub 2012 Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurice J. A first step in bringing typhoid fever out of the closet. Lancet. 2012;379:699–700. doi: 10.1016/s0140-6736(12)60294-3. [DOI] [PubMed] [Google Scholar]
- 17.Sejvar J, Lutterloh E, Naiene J, Likaka A, Manda R, Nygren B, Monroe S, Khaila T, Lowther SA, Capewell L, Date K, Townes D, Redwood Y, Schier J, Barr BT, Demby A, Mallewa M, Kampondeni S, Blount B, Humphrys M, Talkington D, Armstrong GL, Mintz E. Neurologic manifestations associated with an outbreak of typhoid fever, Malawi–Mozambique, 2009: an epidemiologic investigation. PLoS ONE. 2012;7:e46099. doi: 10.1371/journal.pone.0046099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang HH, Kilgore PE, Yang LH, Park JK, Pan YF, Kim Y, Lee YJ, Xu ZY, Clemens JD. An outbreak of typhoid fever, Xing-An County, People's Republic of China, 1999: estimation of the field effectiveness of Vi polysaccharide typhoid vaccine. J Infect Dis. 2001;183:1775–1780. doi: 10.1086/320729. [DOI] [PubMed] [Google Scholar]
- 19.Scobie HB, Nilles E, Kama M, Kool JL, Mintz E, Wannemuehler KA, Hyde TB, Dawainavesi A, Singh S, Korovou S, Jenkins K, Date K. Impact of a targeted typhoid vaccination campaign following cyclone Tomas, Republic of Fiji, 2010. Am J Trop Med Hyg. 2014 doi: 10.4269/ajtmh.13-0728. http://www.ajtmh.org/content/early/by/section Available at. Accessed June 2, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaljee LM, Pack R, Pach A, Nyamete A, Stanton B. Sociobehavioral research methods for the introduction of vaccines in the diseases of the most impoverished programme. J Health Popul Nutr. 2004;22:293–303. [PubMed] [Google Scholar]
- 21.Blum LS, Nahar N. Cultural and social context of dysentery: implications for the introduction of a new vaccine. J Health Popul Nutr. 2004;22:159–169. [PubMed] [Google Scholar]
- 22.Pack R, Wang Y, Singh A, Seidlein LV, Pach A, Kaljee L, Butraporn P, Youlong G, Blum L, Bhutta Z, Santoso SS, Trach DD, Waluyo I, Nyamete A, Clemens J, Stanton B. Willingness to be vaccinated against Shigella and other forms of dysentery: a comparison of three regions in Asia. Vaccine. 2006;24:485–494. doi: 10.1016/j.vaccine.2005.07.094. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Stanton B, Wang X, Nyamette A, Pach A, Kaljee L, Pack R, von Seidlein L, Clemens J, Gong Y, Mao R. Differences in perception of dysentery and enteric fever and willingness to receive vaccines among rural residents in China. Vaccine. 2006;24:561–571. doi: 10.1016/j.vaccine.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 24.Pach A, Tabbusam G, Khan MI, Suhag Z, Hussain I, Hussain E, Mumtaz U, Haq IU, Tahir R, Mirani A, Yousafzai A, Sahastrabuddhe S, Ochiai RL, Soofi S, Clemens JD, Favorov MO, Bhutta ZA. Formative research and development of an evidence-based communication strategy: the introduction of vi typhoid fever vaccine among school-aged children in Karachi, Pakistan. J Health Commun. 2013;18:306–324. doi: 10.1080/10810730.2012.727958. [DOI] [PubMed] [Google Scholar]
- 25.Kamal KM, Madhavan SS, Amonkar MM. Determinants of adult influenza and pneumonia immunization rates. J Am Pharm Assoc. 2003;43:403–411. doi: 10.1331/154434503321831120. [DOI] [PubMed] [Google Scholar]
- 26.Nichter M. Vaccinations in the Third World: a consideration of community demand. In: Nichter M, Nichter M, editors. Anthropology and International Health. Gordon and Breach Publishers; Amsterdam: 1996. pp. 329–366. [Google Scholar]
- 27.Streefland P, Chowdhury AM, Ramos-Jimenez P. Patterns of vaccination acceptance. Soc Sci Med. 1999;49:1705–1716. doi: 10.1016/s0277-9536(99)00239-7. [DOI] [PubMed] [Google Scholar]
- 28.Evans M, Stoddart H, Condon L, Freeman E, Grizzell M, Mullen R. Parents' perspectives on the MMR immunization: a focus group study. Br J Gen Pract. 2001;51:904–910. [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett SD, Lowther S, Chingoli F, Chilima B, Warne T, Ayers T, Capewell L, Taylor E, Nygren B, Mintz E. Responding to an outbreak of typhoid fever: assessment of water, sanitation and hygiene interventions in Neno District, Malawi. Am J Trop Med Hyg. 2011;85((Suppl 6)):106. [Google Scholar]
- 30.Kleinman AM. Patients and Healers in the Context of Culture: An Exploration of the Borderland between Anthropology, Medicine and Psychiatry. Berkeley, CA: University of California Press; 1980. [Google Scholar]
- 31.Minetti A, Kagoli M, Katsulukuta A, Huerga H, Featherstone A, Chiotcha H, Noel D, Bopp C, Sury L, Fricke R, Iscla M, Hurtado N, Ducomble T, Nicholas S, Kabuluzi S, Grais RF, Luquero FJ. Lessons and challenges for measles control from unexpected large outbreak, Malawi. Emerg Infect Dis. 2013;19:202–209. doi: 10.3201/eid1902.120301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Statistical Office (NSO) and ICF Macro . Malawi Demographic and Health Survey 2010. Zomba, Malawi and Calverton, MD: NSO and ICF Macro; 2011. [Google Scholar]
- 33.National Statistical Office (NSO) [Malawi], and ORC Macro . Malawi Demographic and Health Survey 2004. Calverton, MD: NSO and ORC Macro; 2005. [Google Scholar]
- 34.Harris KM, Maurer J, Lurie N. Do people who intend to get a flu shot actually get one? J Gen Intern Med. 2009;24:1311–1313. doi: 10.1007/s11606-009-1126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaljee LM, Pham V, Son ND, Hoa NT, Thiem VD, Canh do G, Thoa le TK, Ali M, Ochiai RL, Danovaro-Holliday MC, Acosta CJ, Stanton B, Clemens J. Trial participation and vaccine desirability for vi polysaccharide typhoid fever vaccine in Hue City, Viet Nam. Trop Med Int Health. 2007;12:25–36. doi: 10.1111/j.1365-3156.2006.01751.x. [DOI] [PubMed] [Google Scholar]
- 36.Liao Q, Cowling BJ, Lam WWT, Fielding R. Factors affecting intention to receive and self-reported receipt of 2009 pandemic (H1N1) vaccine in Hong Kong: a longitudinal study. PLoS ONE. 2011;6:e17713. doi: 10.1371/journal.pone.0017713. [DOI] [PMC free article] [PubMed] [Google Scholar]