Abstract
Co-infection with pathogens that cause acute febrile illness creates a diagnostic challenge as a result of overlapping clinical manifestations. Here, we describe four fatal cases of Leptospira species/dengue virus co-infection in Puerto Rico. Although all patients sought care early, antibiotic administration was delayed for most. Steroids were administered to all patients, in most cases before antibiotics. These cases show the need for clinicians evaluating patients in or recently returned from the tropics with acute febrile illness to consider both dengue and leptospirosis. Furthermore, they illustrate the need for nucleic acid- or antigen-based rapid diagnostic tests to enable timely patient diagnosis and management. In particular, antibiotic therapy should be initiated early for patients with suspected leptospirosis, and steroids should not be administered to patients with suspected dengue.
Introduction
Leptospirosis and dengue are acute febrile illnesses (AFI) that are endemic throughout the tropics,1 including the Caribbean.2,3 Leptospirosis is a zoonosis caused by infection with Leptospira species bacteria that are transmitted through direct or indirect contact with animal urine.4 Dengue is an arboviral disease caused by infection with any of four dengue virus-types (DENV-1–4).1 Although most patients infected with DENV or Leptospira spp. will experience either no symptoms of disease or a self-limited AFI, a small percentage will develop severe disease.1,4 Of patients hospitalized with leptospirosis, 5–15% die typically as a result of organ (e.g., kidney, liver) failure, pulmonary hemorrhage, and/or septic shock.4,5 Severe dengue has a case fatality rate of < 0.1–10%, most frequently caused by plasma leakage evidenced by hypovolemic shock with or without severe hemorrhage.1,6
Puerto Rico is a United States territory located in the Caribbean, and in 2010 had a population of 3.7 million residents.7 Although dengue is a common cause of AFI in Puerto Rico,8,9 the incidence of leptospirosis10 is unclear because of underreporting and lack of routine diagnostic testing.11 Identification of leptospirosis cases through diagnostic testing of suspected dengue cases12 has enabled detection of concurrent epidemics.13 The incidence of reported dengue and leptospirosis cases is typically highest during and soon after the rainy season (i.e., May–November).
The overlapping clinical manifestations of leptospirosis and dengue create a diagnostic challenge,14,15 as even fatal cases cannot always be differentiated based solely upon the patients' clinical course, illustrating the need for diagnostic testing using blood or tissue specimens. Furthermore, Leptospira spp./DENV co-infections have been reported from Mexico,16 Barbados,17 India,18–22 Pakistan,23 and Jamaica,24 although it is unclear if co-infection is more likely to result in death. Here, we report four fatal cases of Leptospira spp./DENV co-infection in Puerto Rico, of which one (Case 1) was previously described.25
Materials and Methods
In 2010, the Centers for Disease Control and Prevention (CDC) initiated enhanced surveillance for fatal AFI in Puerto Rico, including collection of tissue specimens during autopsies conducted at the Institute of Forensic Sciences of Puerto Rico; routinely querying hospital infection control nurses for recent deaths; systematic review of all death certificates at the Vital Registry of Puerto Rico; and diagnostic testing of specimens for evidence of dengue, leptospirosis, and other AFI. Medical records from all health care visits of each patient were reviewed using a standardized chart abstraction form. All blood and tissue specimens were tested for molecular and serologic evidence of DENV infection as previously described.9 Polymerase chain reaction (PCR) with primers specific for Leptospira spp.26 and microscopic agglutination test (MAT)27 were performed on all blood specimens. Immunohistochemistry (IHC) with a mixture of 16 reference rabbit polyclonal anti-Leptospira spp. antibodies28 was performed on all tissue specimens. Co-infection was defined as detection of two pathogens by molecular or antigen-based diagnostics in the same individual during a single illness episode. This investigation underwent CDC human subjects review and was determined not to be research involving human subjects; as such, Institutional Review Board approval was not required.
Case Reports
Case 2.
In July 2010, an unemployed 22-year-old male smoker from eastern Puerto Rico with a history of asthma, obesity, and alcohol and marijuana abuse presented to the emergency department (ED) due to a 3-day history of fever, malaise, vomiting, and diarrhea (Table 1). The patient had been taking acetaminophen and ibuprofen at home. Potential sources of animal, mosquito, or environmental exposure were not reported. Upon arrival at the ED, the patient was febrile (temperature [T] = 39.5°C), tachycardic (heart rate [HR] = 135 beats per minute [bpm]), and had leukocytosis and thrombocytopenia (Table 2). He was admitted with a diagnosis of viral syndrome, given methylprednisolone and acetaminophen, and dengue diagnostic testing was ordered.
Table 1.
Demographic and clinical characteristics of four individuals that died following co-infection with Leptospira species and dengue virus, Puerto Rico, 2010–2012
| Case ID | Age (years) | Sex | Risk factors and exposure history | DPO first presentation | DPO first received antibiotics | DPO first received steroids | DPO of death | DPO dengue/leptospirosis diagnostic test ordered | Cause of death† |
|---|---|---|---|---|---|---|---|---|---|
| 1* | 42 | M | Hypertension; recently incarcerated | 4 | 6 | 4 | 15 | NA/4 | Leptospirosis |
| 2 | 22 | M | Asthma, smoker, alcohol abuse | 3 | 5 | 3 | 11 | 3/7 | Cardiorespiratory arrest |
| 3 | 64 | M | Smoker; exposure to animals, cleaned roof gutters with skin abrasion on finger | 2 | 2 | 3 | 3 | 2/2 | Septic shock |
| 4 | 67 | M | Diabetes, hypertension;farmer, exposure to animals | 4 | 5 | 4 | 7 | 5/5 | Multiple organ failure; acute viral syndrome |
A detailed description of the clinical course of Case 1 can be found in Reference 25.
As listed on the death certificate; if a death certificate was not available, discharge diagnosis was used instead
DPO = day post-illness onset; M = male; NA = not applicable.
Table 2.
Laboratory values of four individuals that died following co-infection with Leptospira species and dengue virus, Puerto Rico, 2010–2012
| Case ID | WBC | Platelets | Hematocrit | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First | Highest | First | Lowest | First | Lowest | |||||||||||
| DPO | Value | DPO | Value | DPO | Value | DPO | Value | DPO | Value | DPO | Value | |||||
| 1* | 4 | 19.1 | 6 | 21.7 | 4 | 111 | 7 | 70 | 4 | 40.4 | 7 | 26.10 | ||||
| 2 | 3 | 9.6 | 10 | 38.3 | 3 | 58 | 5 | 34 | 3 | 37.1 | 8 | 23.3 | ||||
| 3 | 2 | 8.2 | 3 | 18.5 | 2 | 29 | 2 | 20 | 2 | 36.1 | 3 | 24.3 | ||||
| 4 | 4 | 7.7 | 6 | 13.9 | 4 | 25 | 5 | 15 | 4 | 37.9 | 5 | 33.0 | ||||
| Case ID | Creatinine | Total bilirubin | AST | ALT | ||||||||||||
| First | Highest | First | Highest | First | Highest | First | Highest | |||||||||
| DPO | Value | DPO | Value | DPO | Value | DPO | Value | DPO | Value | DPO | Value | DPO | Value | DPO | Value | |
| 1* | 6 | 2.86 | 6 | 2.86 | 7 | 4.77 | 7 | 4.77 | 6 | 285 | 6 | 285 | 7 | 140 | 7 | 140 |
| 2 | 3 | 1.25 | 6 | 1.55 | 5 | 0.51 | 10 | 6.9 | 5 | 29 | 10 | 389 | 5 | 68 | 5 | 68 |
| 3 | 2 | 5.8 | 3 | 7.4 | 2 | 4.04 | 3 | 5.19 | 2 | 48 | 3 | 1,117 | 2 | 23 | 3 | 351 |
| 4 | 4 | 4.6 | 5 | 6.2 | 5 | 4.01 | 6 | 7.28 | 5 | 94 | 6 | 196 | 5 | 93 | 5 | 93 |
A detailed description of the clinical course of Case 1 can be found in Reference 25.
Abbreviations: WBC = white blood cell count; DPO = day post-illness onset; AST = aspartate aminotransferase; ALT = alanine aminotransferase
Units: WBC and Platelets = × 103/mm3; Hematocrit = %; AST and ALT = international units/L; Cre and Total bilirubin = mg/dL.
On hospital Day 2, oseltamivir, amikacin, ceftazidime, and a 1 L bolus of half normal saline were administered. Laboratory results showed worsening thrombocytopenia and bloody stool. Blood and urine cultures from admission were negative. Methicillin-sensitive Staphylococcus aureus was cultured from a bronchial aspirate, and influenza A/B virus antibody was detected by complement fixation; it was unclear if this antibody was the result of recent or historic infection. Chest radiograph (CXR) revealed peribronchial thickening and alveolar opacities consistent with bronchitis.
On hospital Day 3, the differential diagnoses following an infectious disease consultation included dengue, influenza, bronchopneumonia, and sepsis. The patient was transferred to the intensive care unit (ICU) at a tertiary care hospital with a diagnosis of severe bilateral pneumonia, and upon arrival he was afebrile and had hemoptysis. Azithromycin was administered, and Candida albicans was cultured in a bronchial aspirate.
On hospital Day 4, the patient developed severe respiratory difficulty, arterial blood gases (ABG) showed low oxygen partial pressure (PaO2) (49.7 mm of Hg), and mechanical ventilation was initiated. CXR showed extensive bilateral lung opacities consistent with extensive pulmonary edema or acute respiratory distress syndrome, pneumopericardium and pneumomediastinum, and subcutaneous air in the left supraclavicular space. He was given vancomycin, co-trimoxazole, and norepinephrine. The patient developed jaundice and soon after became combative, extubated himself, and in so doing lacerated his trachea. The patient was then sedated. Following an infectious disease consultation, suspected leptospirosis was added to the differential diagnosis, cefepime and penicillin were added to his antibiotic regimen, and leptospirosis diagnostic testing was ordered.
On hospital Day 5, he was transfused with two units each of fresh-frozen plasma (FFP) and packed red blood cells (PRBCs). His hematocrit continued to drop and hemoptysis was again reported in addition to new onset hematuria. CXR revealed worsening pneumomediastinum.
On hospital Day 6, two units each of FFP and PRBCs were transfused. The patient continued to have hemoptysis, hematuria, and jaundice. On hospital Day 7, he was transfused with another unit of PRBCs and platelet apheresis was performed. CXR showed subcutaneous emphysema and atelectasis in the left lower lobe. On hospital Day 8, platelet apheresis and one unit of PRBCs were administered. Repeat ABG revealed a drop in PaO2 (41.4 mm of Hg). CXR revealed bilateral ground-glass opacities and hypoaerated lungs. It became increasingly difficult to adequately ventilate the patient and he was pronounced dead on hospital Day 9.
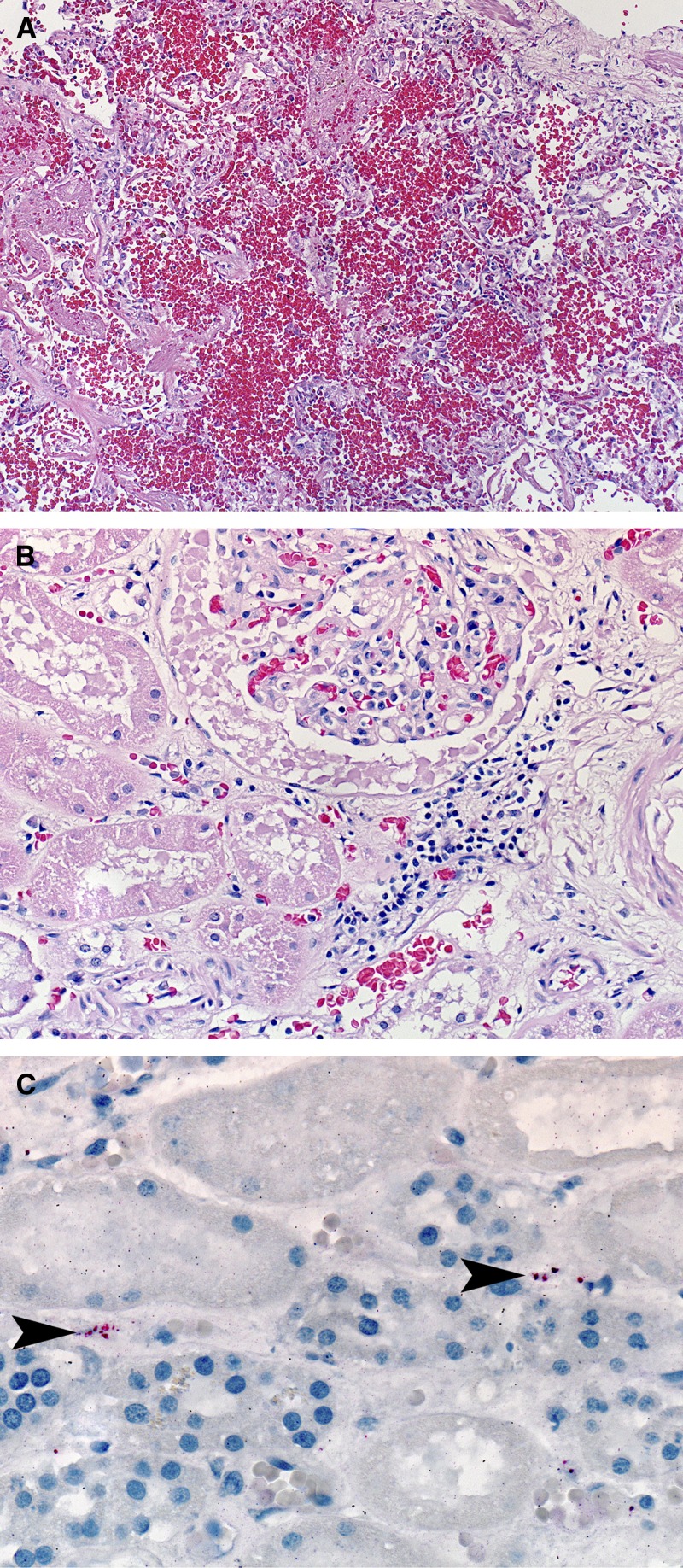
Autopsy revealed ascites, bilateral pleural effusions, swelling of the right hand and arm, and two ecchymoses in the left upper arm. Microscopic examination of tissue specimens revealed centrolobular necrosis of the liver, extensive intraalveolar hemorrhage mixed with fibrin and edema (Figure 1A), interstitial nephritis (Figure 1B), and type II pneumocyte hyperplasia in the lung. DENV-4 was detected by reverse transcription-PCR (RT-PCR) of nucleic acid extracted from kidney, liver, lung, and spleen specimens; DENV-specific IHC was negative in the same tissues. Leptospira spp. antigen was detected by IHC in the kidney (Figure 1C), liver, and lung. Detection of anti-Leptospira spp. immunoglobulin M (IgM) by enzyme-linked immunosorbent assay (ELISA) in the pre-mortem specimen was reported after the patient died; neither DENV nor Leptospira spp. nucleic acid were detected by PCR. A MAT titer of 1:800 was detected in a post-mortem whole blood specimen; highest reactivity was to serovar Icterohemorrhagiae.
Figure 1.
Histopathologic evaluation of tissue specimens collected post-mortem from an individual (Case 2) that died in Puerto Rico in 2010 following co-infection with Leptospira species and dengue virus-type 4. Tissue specimens were taken from the lung (A) and kidney (B and C) and stained with hemotoxylin-eosin (A and B; original magnification 10× and 20×, respectively) or probed with polyclonal anti-Leptospira antibody for immunohistochemical detection of Leptospira antigen (C; arrowheads indicate antigen; original magnification 63×).
Case 3.
In December 2011, an obese 64-year-old retired construction worker from the San Juan metropolitan area with a history of skin cancer presented to the ED due to a 2-day history of fever, icteric sclera, chills, myalgia, and malaise. He denied shortness of breath, hematuria, vomiting, diarrhea, cough, bleeding, and recent travel. He had been taking acetaminophen, glucosamine, and simvastatin at home. The patient reported that 1 week before admission he had cleaned out the roof gutters of his house with bare hands while he had an abrasion on his finger.
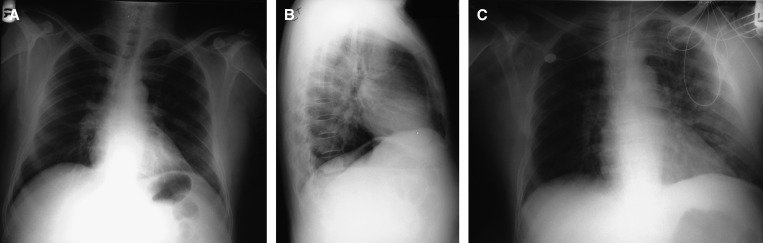
Upon evaluation, the patient was afebrile, tachycardic (HR = 110 bpm), hypotensive (blood pressure = 88/55), and had thrombocytopenia. Leptospirosis was included in the differential diagnosis on the same day, and leptospirosis and dengue diagnostic testing were ordered. Later that day, the patient was admitted to the ICU with a diagnosis of suspected leptospirosis, acute renal failure, and severe thrombocytopenia. Laboratory results showed hyperglycemia (glucose = 237 mg/dL) and metabolic acidosis (pH = 7.18; PaO2 = 41.4 mm of Hg). Blood and urine cultures were negative. Methicillin-sensitive S. aureus was cultured from a bronchial aspirate. CXR revealed normal heart size and no pleural effusion (Figure 2A and 2B), suggesting that the patient did not have appreciable plasma leakage. The patient was given ceftriaxone, mannitol, bicarbonate drip, docusate, and ranitidine.
Figure 2.
Chest radiographs from an individual (Case 3) that died in Puerto Rico in 2011 following co-infection with Leptospira species and dengue virus-type 1. Posteroanterior (A and C) and lateral (B) chest radiographs were taken on Day 1 (A and B) and Day 2 (C) of hospitalization. The patient died on hospital Day 2.
On hospital Day 2, ABG showed worsening metabolic and respiratory acidosis. Methylprednisone was administered after mechanical ventilation was initiated, and the patient became combative and was restrained. CXR suggested congestive heart failure in the absence of pleural effusions (Figure 2C). Laboratory results revealed a 33% drop in hematocrit, suggestive of occult hemorrhage. The patient was transfused with one unit of platelets and hemodialysis was initiated. He became severely hypotensive and died on hospital Day 2. Autopsy was not performed.
Dengue and leptospirosis diagnostic test results were received after the patient died. DENV-1 and Leptospira spp. were detected by PCR of nucleic acid extracted from a single serum specimen. Anti-Leptospira spp. antibody was not detectable in serum by IgM ELISA or MAT. Anti-DENV IgG antibody was detected by ELISA.
Case 4.
A 67-year-old obese male from central Puerto Rico with a medical history of diabetes, hypertension, and gout presented to the ED in July 2012 due to a 4-day history of fever, headache, malaise, anorexia, and vomiting. The patient reported a history of unspecified animal exposure while working on a farm. Laboratory values revealed thrombocytopenia and acute renal injury. He was admitted with a presumptive diagnosis of acute viral syndrome, suspected dengue, suspected leptospirosis, and renal failure. He was given a 1 L bolus of half normal saline and methylprednisone.
On hospital Day 2 he developed dyspnea, tachypnea, and bibasilar crackles. CXR revealed elevated right hemidiaphragm and mild pulmonary congestive changes. Abdominal ultrasound revealed hepatomegaly and thickened gallbladder wall suggestive of acalculous cholecystitis. Urinalysis revealed gross hematuria, ABG showed metabolic acidosis, and he developed respiratory failure. The patient was transferred to the ICU for mechanical ventilation and upon arrival was given azithromycin and ceftriaxone. Repeat laboratory values revealed worsening thrombocytopenia and continued decline in renal function consistent with intrarenal acute tubular necrosis. Additional laboratory results showed elevated serial troponins (1.39 ng/mL). Serum was collected for dengue and leptospirosis diagnostic testing.
Although blood cultures were negative on hospital Day 3, vancomycin and levofloxacin were added to the patient's antibiotic regimen, and oseltemavir and 25% albumin were given. The patient's condition continued to deteriorate, culminating in cardiac arrest and death on hospital Day 4. Autopsy was not performed.
Results from laboratory testing were received after the patient expired. DENV-1 and Leptospira spp. nucleic acid were amplified by PCR from the serum specimen, in which anti-DENV IgG was detected by ELISA and a MAT titer of 1:100 was detected; highest reactivity was against serovar Icterohemorrhagiae.
Discussion
Clinicians in Puerto Rico and throughout the tropics should be aware of both dengue and leptospirosis, including the possibility of co-infection. Molecular and serologic diagnostic tests for both illnesses should be ordered to properly diagnose and manage patients with AFI. The cases described in this report show the need for nucleic acid- or antigen-based rapid diagnostic tests to identify patients with dengue and leptospirosis in a timely manner. Because most currently available leptospirosis rapid diagnostic tests reliably detect anti-Leptospira IgM antibody 7 days after illness onset,29 such tests would have had minimal clinical use for the cases described herein, all of which presented within four days of illness onset.
Because antibiotics may reduce the morbidity and mortality caused by leptospirosis if administered early in the illness,30,31 accurate and rapid diagnosis is critical to enable potentially life-saving antibiotic therapy. As such, clinicians should initiate early antibiotic therapy if leptospirosis is suspected. Equally important is the need for patients to seek care early in their illness to receive appropriate anticipatory guidance and treatment. Targeted community outreach programs should be conducted in Puerto Rico to improve awareness of leptospirosis, especially in at-risk individuals (e.g., agricultural workers4), and during the rainy season13 and after floods12 when environmental conditions are favorable for human exposure to Leptospira spp.4
Steroids were administered to all four of the fatal cases described herein, and in three cases were administered before antibiotics, which may have contributed to the patients' fatal outcome. Although the practice of administering steroids for suspected dengue patients is not uncommon in Puerto Rico32 and other regions with endemic dengue,33,34 studies have shown no benefit and potential detriment (e.g., immunosuppression, gastrointestinal bleeding) to patient outcome from using steroids to treat dengue.35–37 As such, the World Health Organization (WHO) does not recommend steroids to treat dengue patients.1 Similarly, controlled trial of steroids to treat leptospirosis showed no benefit to patient outcome.38 Steroids should be reserved for patients that have a clinical condition for which they have reproducibly been shown to be beneficial.
This investigation was subject to several limitations. Because all data were collected retrospectively through medical chart review, behaviors that may have exposed patients to Leptospira spp. were not elucidated for all case-patients. Similarly, nasopharyngeal swabs were not routinely collected from all patients, and thus, unless otherwise noted, diagnostic testing for respiratory pathogens was not performed. Finally, because dengue and leptospirosis diagnostic test results were infrequently available when death certificates were completed, the case-patients' listed causes of death may be incomplete. Although all patients had evidence of severe leptospirosis (e.g., renal failure, hemoptysis), two (Cases 1 and 2) also had signs and symptoms associated with severe dengue (e.g., evidence of plasma leakage, hypovolemic shock). Nonetheless, it is difficult to confidently assess the relative contributions of dengue, leptospirosis and nosocomial infections to the patients' fatal outcome.
Improved leptospirosis surveillance is needed in Puerto Rico to better understand the epidemiology of leptospirosis, detect outbreaks, and identify population-specific risk factors for infection. Future studies should evaluate DENV/Leptospira spp. co-infection, delayed administration of antibiotics, and administration of steroids as risk factors for death.
ACKNOWLEDGMENTS
We thank Mahesh Swaminathan and D. Fermín Argüello for assistance with medical chart review and helpful discussions.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Nicole M. Pérez Rodríguez, Aidsa Rivera, David Noyd, Kay M. Tomashek, and Tyler M. Sharp, Dengue Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, San Juan, PR, E-mails: xhp4@cdc.gov, erj2@cdc.gov, wyf1@cdc.gov, kct9@cdc.gov, and tsharp@cdc.gov. Renee Galloway, Rita Traxler, and William A. Bower, Bacterial Special Pathogens Branch, Division of High Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, GA., E-mails: zul0@cdc.gov, gna9@cdc.gov, and wab4@cdc.gov. Dianna M. Blau, Julu Bhatnagar, and Sherif R. Zaki, Infectious Diseases Pathology Branch, Division of High Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, GA., E-mails: bvv1@cdc.gov, zrn1@cdc.gov, and sxz1@cdc.gov. Xavier E. Santiago-Albizu, San Lucas Episcopal Hospital, Ponce, PR., E-mail: xsantiagoal@yahoo.com. Jose V. Torres, Medicolegal and Toxicological Investigation Division, Puerto Rico Institute of Forensic Sciences, Carparra Heights Station, San Juan, PR., E-mail: JVTorres@icf.gobierno.pr. Brenda Rivera-García, Epidemiology and Research Office, Puerto Rico Department of Health, Medical Center Area, San Juan, PR., E-mail: brendarivera@salud.pr.gov.
References
- 1.World Health Organization . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: WHO; 2009. [PubMed] [Google Scholar]
- 2.Brandling-Bennet D, Pinheiro F. Infectious diseases in Latin America and the Caribbean: are they really emerging and increasing? Emerg Infect Dis. 1996;2:3. doi: 10.3201/eid0201.960109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.San Martin JL, Brathwaite O, Zambrano B, Solorzano JO, Bouckenooghe A, Dayan GH, Guzman MG. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg. 2010;82:128–135. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 5.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam PK, Tam DT, Diet TV, Tam CT, Tien NT, Kieu NT, Simmons C, Farrar J, Nga NT, Qui PT, Dung NM, Wolbers M, Wills B. Clinical characteristics of dengue shock syndrome in Vietnamese children: a 10-year prospective study in a single hospital. Clin Infect Dis. 2013;57:1577–1586. doi: 10.1093/cid/cit594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United States Census Bureau . American FactFinder. 2011. http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml Available at. Accessed January 9, 2012. [Google Scholar]
- 8.Neff JM, Morris L, Gonzalez-Alcover R, Coleman PH, Lyss SB, Negron H. Dengue fever in a Puerto Rican community. Am J Epidemiol. 1967;86:162–184. doi: 10.1093/oxfordjournals.aje.a120722. [DOI] [PubMed] [Google Scholar]
- 9.Sharp TM, Hunsperger E, Santiago GA, Munoz-Jordan JL, Santiago LM, Rivera A, Rodriguez-Acosta RL, Gonzalez Feliciano L, Margolis HS, Tomashek KM. Virus-specific differences in rates of disease during the 2010 Dengue epidemic in Puerto Rico. PLoS Negl Trop Dis. 2013;7:e2159. doi: 10.1371/journal.pntd.0002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koppisch E, Suarez RM, Kohlschūter E, Hernandez-Morales F. Weil's disease in Puerto Rico: report of five cases, one of them with post-mortem findings. PR J Public Health Trop Med. 1942;17:36. [Google Scholar]
- 11.Centers for Disease Control and Prevention Investigation of leptospirosis underreporting- Puerto Rico, 2010. Morbidity and Mortality Weekly Report. 2012;61:421. [PubMed] [Google Scholar]
- 12.Sanders EJ, Rigau-Perez JG, Smits HL, Deseda CC, Vorndam VA, Aye T, Spiegel RA, Weyant RS, Bragg SL. Increase of leptospirosis in dengue-negative patients after a hurricane in Puerto Rico in 1996 [correction of 1966] Am J Trop Med Hyg. 1999;61:399–404. doi: 10.4269/ajtmh.1999.61.399. [DOI] [PubMed] [Google Scholar]
- 13.Bruce MG, Sanders EJ, Leake JA, Zaidel O, Bragg SL, Aye T, Shutt KA, Deseda CC, Rigau-Perez JG, Tappero JW, Perkins BA, Spiegel RA, Ashford DA. Leptospirosis among patients presenting with dengue-like illness in Puerto Rico. Acta Trop. 2005;96:36–46. doi: 10.1016/j.actatropica.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Karande S, Gandhi D, Kulkarni M, Bharadwaj R, Pol S, Thakare J, De A. Concurrent outbreak of leptospirosis and dengue in Mumbai, India, 2002. J Trop Pediatr. 2005;51:174–181. doi: 10.1093/tropej/fmh100. [DOI] [PubMed] [Google Scholar]
- 15.Flannery B, Pereira MM, Velloso Ld F, Carvalho Cd C, De Codes LG, Orrico Gd S, Dourado CM, Riley LW, Reis MG, Ko AI. Referral pattern of leptospirosis cases during a large urban epidemic of dengue. Am J Trop Med Hyg. 2001;65:657–663. doi: 10.4269/ajtmh.2001.65.657. [DOI] [PubMed] [Google Scholar]
- 16.Dircio Montes Sergio A, Gonzalez Figueroa E, Maria Saadia VG, Elizabeth SH, Beatriz RS, Altuzar Aguilar Victor M, Navarrete Espinosa J. Leptospirosis prevalence in patients with initial diagnosis of dengue. J Trop Med. 2012;2012(519701) doi: 10.1155/2012/519701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levett PN, Branch SL, Edwards CN. Detection of dengue infection in patients investigated for leptospirosis in Barbados. Am J Trop Med Hyg. 2000;62:112–114. doi: 10.4269/ajtmh.2000.62.112. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhry R, Pandey A, Das A, Broor S. Infection potpourri: are we watching? Indian J Pathol Microbiol. 2009;52:125. doi: 10.4103/0377-4929.44990. [DOI] [PubMed] [Google Scholar]
- 19.Kaur H, John M. Mixed infection due to Leptospira and dengue. Indian J Gastroenterol. 2002;21:206. [PubMed] [Google Scholar]
- 20.Rele MC, Rasal A, Despande SD, Koppikar GV, Lahiri KR. Mixed infection due to Leptospira and dengue in a patient with pyrexia. Indian J Med Microbiol. 2001;19:206–207. [PubMed] [Google Scholar]
- 21.Behera B, Chaudhry R, Pandey A, Mohan A, Dar L, Premlatha MM, Gupta E, Broor S, Aggarwal P. Co-infections due to Leptospira, dengue and hepatitis E: a diagnostic challenge. J Infect Dev Ctries. 2009;4:48–50. doi: 10.3855/jidc.535. [DOI] [PubMed] [Google Scholar]
- 22.Singh RK, Ghatak T, Baronia AK, Garg P. Intracranial hemorrhage in a patient coinfected with dengue and leptospirosis. J Neurosci Rural Pract. 2013;4:366–367. doi: 10.4103/0976-3147.118761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yong LS, Koh KC. A case of mixed infections in a patient presenting with acute febrile illness in the tropics. Case Rep Infect Dis. 2013;2013(562175) doi: 10.1155/2013/562175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindo J, Brown PD, Vickers I, Brown M, Jackson ST, Lewis-Fuller E. Leptospirosis and malaria as causes of febrile illness during a dengue epidemic in Jamaica. Pathog Glob Health. 2013;107:329–334. doi: 10.1179/2047773213Y.0000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharp TM, Bracero J, Rivera A, Shieh WJ, Bhatnagar J, Rivera-Diez I, Hunsperger E, Munoz-Jordan J, Zaki SR, Tomashek KM. Fatal human co-infection with Leptospira spp. and dengue virus, Puerto Rico, 2010. Emerg Infect Dis. 2012;18:878–880. doi: 10.3201/eid1805.111555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoddard RA, Gee JE, Wilkins PP, McCaustland K, Hoffmaster AR. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn Microbiol Infect Dis. 2009;64:247–255. doi: 10.1016/j.diagmicrobio.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Dikken H, Kmety E. Serological typing methods of leptospires. In: Bergan T, Norris J, editors. Methods in Microbiology. London: Academic Press; 1978. pp. 259–307. [Google Scholar]
- 28.Zaki SR, Shieh WJ. Leptospirosis associated with outbreak of acute febrile illness and pulmonary hemorrhage, Nicaragua, 1995. The Epidemic Working Group at Ministry of Health in Nicaragua. Lancet. 1996;347:535–536. doi: 10.1016/s0140-6736(96)91167-8. [DOI] [PubMed] [Google Scholar]
- 29.Musso D, Scola BL. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46:8. doi: 10.1016/j.jmii.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Brett-Major DM, Coldren R. Antibiotics for leptospirosis. Cochrane Database Syst Rev. 2012;2:CD008264. doi: 10.1002/14651858.CD008264.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vinetz JM. A mountain out of a molehill: do we treat acute leptospirosis, and if so, with what? Clin Infect Dis. 2003;36:1514–1515. doi: 10.1086/375275. [DOI] [PubMed] [Google Scholar]
- 32.Tomashek KM, Gregory CJ, Rivera Sanchez A, Bartek MA, Garcia Rivera EJ, Hunsperger E, Munoz-Jordan JL, Sun W. Dengue deaths in Puerto Rico: lessons learned from the 2007 epidemic. PLoS Negl Trop Dis. 2012;6:e1614. doi: 10.1371/journal.pntd.0001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kularatne SA. Survey on the management of dengue infection in Sri Lanka: opinions of physicians and pediatricians. Southeast Asian J Trop Med Public Health. 2005;36:1198–1200. [PubMed] [Google Scholar]
- 34.Rajapakse S, Ranasinghe C, Rodrigo C. Corticosteroid therapy in dengue infection—opinions of junior doctors. J Glob Infect Dis. 2010;2:199–200. doi: 10.4103/0974-777X.62861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panpanich R, Sornchai P, Kanjanaratanakorn K. Corticosteroids for treating dengue shock syndrome. Cochrane Database Syst Rev. 2006;(3):CD003488. doi: 10.1002/14651858.CD003488.pub2. [DOI] [PubMed] [Google Scholar]
- 36.Tam DT, Ngoc TV, Tien NT, Kieu NT, Thuy TT, Thanh LT, Tam CT, Truong NT, Dung NT, Qui PT, Hien TT, Farrar JJ, Simmons CP, Wolbers M, Wills BA. Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo-controlled trial. Clin Infect Dis. 2012;55:1216–1224. doi: 10.1093/cid/cis655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tassniyom S, Vasanawathana S, Chirawatkul A, Rojanasuphot S. Failure of high-dose methylprednisolone in established dengue shock syndrome: a placebo-controlled, double-blind study. Pediatrics. 1993;92:111–115. [PubMed] [Google Scholar]
- 38.Niwattayakul K, Kaewtasi S, Chueasuwanchai S, Hoontrakul S, Chareonwat S, Suttinont C, Phimda K, Chierakul W, Silpasakorn S, Suputtamongkol Y. An open randomized controlled trial of desmopressin and pulse dexamethasone as adjunct therapy in patients with pulmonary involvement associated with severe leptospirosis. Clin Microbiol Infect. 2010;16:1207–1212. doi: 10.1111/j.1469-0691.2009.03037.x. [DOI] [PubMed] [Google Scholar]