Abstract
Coxiella burnetii, the causative agent of Q fever, is present worldwide. Recent studies have shown that this bacterium is an emerging pathogen in French Guiana and has a high prevalence (24% of community-acquired pneumonia). In this review, we focus on the peculiar epidemiology of Q fever in French Guiana. We place it in the context of the epidemiology of the disease in the surrounding countries of South America. We also review the clinical features of Q fever in this region, which has severe initial presentation but low mortality rates. These characteristics seem to be linked to a unique genotype (genotype 17). Finally, we discuss the issue of the animal reservoir of C. burnetii in French Guiana, which is still unknown. Further studies are necessary to identify this reservoir. Identification of this reservoir will improve the understanding of the Q fever epidemic in French Guiana and will provide new tools to control this public health problem.
Introduction
Q fever is a zoonosis caused by Coxiella burnetii. This intracellular bacterium can cause different clinical manifestations, such as influenza-like syndromes and pneumonia, as well as endocarditis and vascular infections in patients with underlying cardiovascular abnormalities, vascular prosthesis, or immunosuppression.1 The main global reservoirs of infection are farm animals and, sometimes, pets.2 Humans are mainly infected through inhaling contaminated aerosols.2 Q fever was first described in 1935 in Australia,3 and it was later reported to be present nearly worldwide.2 It can be a public health threat, especially when persons are in close contact with domestic animals, cattle, sheep, or goats. The potential impact of Q fever on public health was recently demonstrated by an important outbreak in the Netherlands.4 However, in most developing countries, the prevalence of Q fever is most likely underestimated. This finding may be caused by a lack of laboratory techniques to diagnose this fastidious intracellular bacterium or to insufficient awareness on the part of clinicians.
In South America and Central America, Q fever cases have been reported in some countries, but global epidemiologic data are scarce. Conversely, in French Guiana, Q fever is a common agent of community-acquired pneumonia (CAP), and the prevalence of Q fever is the highest ever reported (the cause of 24.4% CAP cases).5 French Guiana is a French overseas region located on the northeastern coast of South America. Its geography consists of a coastal plain representing 10% of its surface, where 90% of the population lives. The rest of the country is covered by Amazonian rain forest. The city of Cayenne and its suburbs of Remire-Montjoly and Matoury contain half of the 215,000 inhabitants, where most of the cases of Q fever have been described.
In this review, we focus on the epidemiology of Q fever in French Guiana compared with that for other countries in South America and Central America. We also review studies that have increased our knowledge about clinical presentation and microbiologic characteristics of Q fever in this region. Finally, we discuss the still unanswered question concerning the animal reservoir of this zoonosis in this region.
Peculiar Epidemiologic Profile In South America And Central America
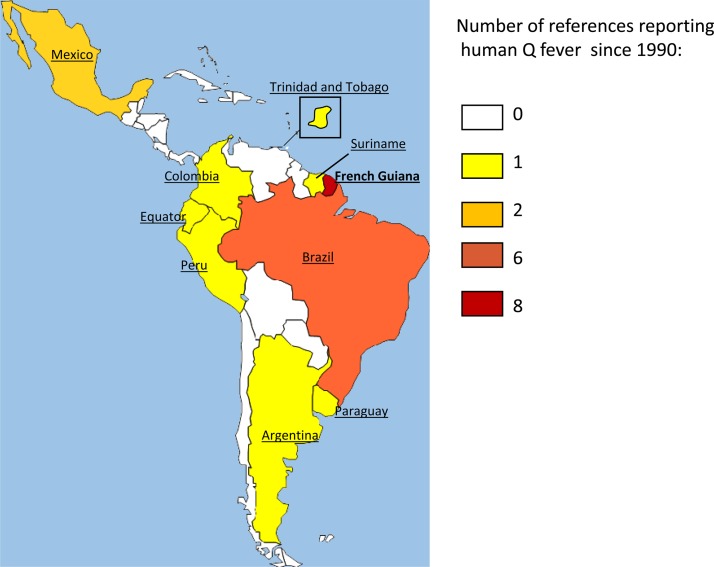
Q fever epidemiology is not well known in South and Central America (Figure 1). Most cases have been described in Brazil. In 1962, a pioneering study was conducted in the state of Sao Paulo and showed a seroprevalence rate of 8.5% among 200 dairy farm workers.6 Ten years later, other authors observed seropositivity rates of 22% and 29% among 219 veterinary personnel and 144 slaughterhouse employees in Minas Gerais. Brazil.7,8 Another serosurvey conducted in Minas Gerais in 2005 found a 3.9% seropositivity rate among the healthy adult population.9 In 2006, the first case series of 16 patients with Q fever among 726 febrile patients during 2001–2004 in the state of Minas Gerais10 was published. Molecular detection of C. burnetii in Brazil was first achieved in 2011.11 In recent years, cases of C. burnetii pneumonia12 and endocarditis,13–15 and serologic evidence of Q fever in patients infected with human immunodeficiency virus in Brazil have also been reported.16 Published data are scarce for the remaining countries in South America and Central America; often only one reference is found in the published literature.
Figure 1.
Number of references regarding human cases of Q fever in countries in South America and Central America since 1990.
In Peru, an investigation of a cluster of febrile illness in the subtropical Andean region found a C. burnetii antibody rate of 1–15% in different villages.17 In the Amazon Basin of Ecuador, a longitudinal observational study of 533 patients with acute undifferentiated febrile illness over a three-year period found a Q fever seroprevalence of 4.9%.18 These data suggest a significant presence of the pathogen in rural Amazonian areas. In Argentina, the first case of Q fever was described in 1959,19 and no other case was published until 1997, when a study on the etiology of CAP in a Buenos Aires hospital found only one case of Q fever among 343 patients.20 Two outbreaks of Q fever were described in Colombia and Uruguay, which occurred exclusively among workers in the meat industry.21,22 In Mexico, 17 cases of Q fever were reported in the state of Hidalgo in persons with known risk factors for infection by this bacterium.23 One case of C. burnetii endocarditis24 and one case of granulomatous hepatitis have also been reported in this country.25 A seroprevalence study of Q fever was conducted in 1980 in Panama26 after the identification of C. burnetii in this country27,28 and found a rate of 9% among 1,059 workers in contact with livestock. A total of 4.4% of livestock and slaughterhouse workers were seropositive for C. burnetii in Trinidad.29 Only one seroprevalence study has been found for Jamaica, also among workers in the meat industry; two positive cases were reported.30 One case of Q fever myocarditis in an infant was suspected to be linked with a stay in Surinam.31 We found no data in the published literature regarding human cases or serologic profiles of Q fever in Guyana, Chile, Bolivia, Paraguay, Venezuela, Salvador, Guatemala, Nicaragua, Costa Rica, or Honduras.
In contrast to this lack of information about Q fever in South America and Central America, several publications are available for French Guiana. The first case was described in 1955 by in a slaughterhouse worker in Cayenne.32 Sporadic cases were then described until 1998, when a significant increase of Q fever incidence was described.33 The authors screened serum samples of febrile patients who were negative for dengue fever during 1992–1996. They showed that the seropositivity rate of Q fever increased from 1.9% in 1992 to 23.9% in 1996. Three patients with Q fever CAP were hospitalized in an intensive care unit in Cayenne, and one of them died of distress respiratory syndrome in 1996.33 The reason for this increase in incidence remains unexplained. During 1996–2000, the annual incidence of Q fever in French Guiana was high (37 cases/100,000 persons).34 In 2005, it increased to 150 cases/100,000 persons35 (Table 1). Recently, a retrospective case–control study found a prevalence of 24.4% of Q fever5 among inpatients admitted to Cayenne Hospital for CAP, which is the highest prevalence ever reported in the literature. This work confirmed that this infection has become a major public health problem in French Guiana.
Table 1.
Q fever incidence in French Guiana compared with other countries in Oceania, Asia, and Europe
| Country | Incidence per 100,000 population |
|---|---|
| South Korea | 0.02 |
| France | 2.5 |
| Taiwan | 0.38 |
| Australia (New South Wales) | 2.8 |
| French Guiana | 37–150 |
The other peculiarity of Q fever in French Guiana is its spatial repartition. Most of the cases identified during 1996– 2000 occurred in persons who lived in Cayenne and its suburbs, which is unusual for a zoonosis that is often described in outbreaks in rural areas of countries in Europe.34 Only 19% of the cases were diagnosed from patients outside the urbanized Cayenne Island. This phenomenon may also result from a lack of diagnostic procedures in rural areas of French Guiana. However, a study that used remotely censed data identified a strong heterogeneity in disease incidence on Cayenne Island. The authors identified seven areas of high incidence rates for Q fever, all of which were located in the outskirts of Cayenne.36 These areas were characterized by the presence of hills and clusters of rainforest near houses. This unusual epidemiologic profile remains to be elucidated and is linked to the question of the unknown animal reservoir of Q fever in this region.
Clinical Features
Most Q fever cases reported in French Guiana since 1998 showed pneumonia.33 In 2010, a description of the clinical and biologic profile of these pneumonia cases was reported.5 The authors compared clinical and biological features of 32 Q fever patients with those of 99 patients who had CAP caused by other etiologies and were hospitalized in Cayenne during 2004–2007.5 Of the patients with Q fever, 87.5% were men. This result was quite predictable because male sex predominance in symptomatic cases of Q fever had already been described.37 The mean age of patients with Q fever pneumonia was 46.5 years. Patients with Q fever had significantly less comorbid conditions than patients with other etiologies of CAP. For example, no patients with diabetes, alcoholism, or infection with human immunodeficiency were found among the 32 Q fever patients. These patients with Q fever also had a significantly greater severe initial presentation, characterized by more marked pain syndrome, chills, and night sweats than patients with other etiologies of CAP. However, the outcome was favorable for all patients, except for one patient who had respiratory distress syndrome but survived. Pulmonary auscultation and chest radiographic signs were similar for the two groups.
The diagnosis of Q fever pneumonia in French Guiana is still based on seroconversion. Thus, the diagnosis is often obtained late after the onset of clinical symptoms. For this reason, empiric antibiotic therapy of community-acquired pneumonia at Cayenne Hospital combines amoxicillin and doxycycline to treat infection with C. burnetii. To better guide the choice of first-line antibiotic therapy for CAP in Cayenne, the authors constructed a predictive score to identify patients with Q fever earlier.5 Multivariate logistic regression identified several clinical and biological risk factors for Q fever pneumonia: male sex, middle age, headaches, a leukocyte count < 10 × 109 cells/L, and C-reactive protein level > 185 mg/L were independently associated with Q fever.5 Combining these factors led to elaboration of a score ranging from 0 to 9. Patients with a score < 3 were at low risk of Q fever (negative predictive value = 97%). Nevertheless, given the small number of cases in this study, further investigation is needed to validate this score.
Recently, we compared the clinical and biologic features of 115 Q fever patients from French Guiana with those of 182 Q fever patients from metropolitan France identified during 2008–2011.38 This study showed that age and sex of patients did not significantly differ between the two areas. Patients from Cayenne had a significantly higher prevalence of fever (97%) and pneumonia (83%) than did patients from metropolitan France (81% and 8%, respectively). However, patients from Cayenne had less endocarditis (7% versus 17%) and hepatitis (32% versus 54%). No vascular infection was reported in patients from French Guiana. This study also showed that the serologic profile of Q fever patients from Cayenne was unusual; significantly higher phase I IgG titers were observed.38
The main clinical presentation of Q fever in French Guiana is pneumonia, and it has an exceptionally high prevalence. Patients with pneumonia have a good outcome despite a more severe initial clinical presentation. Pneumonia occurs in middle-age men without any remarkable comorbidity, and a rapid diagnosis can be made because of the predictive score, although the score needs to be validated with a larger sample of cases.
Microbiologic Features
Serologic profile.
Because C. burnetii is a fastidious bacterium, serologic analysis is a key factor for diagnosis of Q fever.39 Coxiella burnetii has two phases depending on the length of its lipopolysaccharide. The phase variation exhibited by C. burnetii correlates with the shift from virulent phase I to avirulent phase II. The serologic response to this phase variation is a keystone in the differentiation of the clinical forms of Q fever, distinguishing acute Q fever from chronic Q fever, which in practice entails endocarditis or vascular infection. A better cutoff value to predict chronic Q fever was first fixed at 800 on the basis of a limited sample size.40 A subsequent study in our laboratory reevaluated the positive predictive value of serologic analysis in predicting Q fever endocarditis in France and proposed an IgG I cutoff titer > 1,600.41 However, a high phase I antibody titer is not sufficient to define the presence of endocarditis or vascular infection, and this is particularly true regarding Q fever in French Guiana. We showed that patients from Cayenne had significantly higher levels of phase I immunoglobulin than patients from Marseille38 (57% versus 44%, IgG1 titers > 800; P = 0.024) (Table 2); the median value of the IgG1 titers was also higher for these patients. Nevertheless, diagnosed patients from the Cayenne area had endocarditis less frequently in this study than did patients from Marseille (7% versus 17%; P = 0.017). As a consequence, the positive predictive values used to diagnose cardiovascular infection in patients from Cayenne who had IgG1 titers > 6,400 was only 50%, but it was 92% in patients from the Marseille area with the same titers. This difference illustrates that the clinicobiologic forms of Q fever are multiple and may be dependent on the strain of C. burnetii implicated; the strains are geographically variable, as described.42–44 As a consequence of these unique clinicobiologic features, there is a need to define adapted criteria to differentiate acute Q fever from endocarditis in French Guiana.
Table 2.
Serologic profiles of Q fever patients from Cayenne, French Guiana, and Marseille, France*
| Profile | Cayenne area, n = 115 | Marseille area, n = 182 | P |
|---|---|---|---|
| IgG phase I titer < 800, no. (%) | 49 (43) | 102 (56) | 0.024 |
| IgG phase I titer ≥ 800, no. (%) | 66 (57) | 80 (44) | 0.024 |
| IgG phase I titer ≥1,600, no. (%) | 42 (36) | 39 (21) | 0.03 |
| IgG phase I titer ≥ 3,200, no. (%) | 21 (18) | 21 (11) | 0.1 |
| IgG phase I titer ≥ 6,400, no. (%) | 10 (9) | 14 (8) | 0.9 |
Adapted from Edouard and others.38
Unique genotype.
For many years, no strain causing Q fever has been isolated in French Guiana by culture. This finding can be explained by the absence of a local laboratory able to culture C. burnetii. We finally isolated five C. burnetii strains from biological samples of patients from Cayenne that were obtained during 2000–2012.45 Two strains were isolated from patients with endocarditis, and the three other strains were isolated from patients with pneumonia. Genotyping of these strains was performed and identified all of them as genotype 17. A comparison with all other isolates of the National French Reference Center for Q fever showed that all of the genotype 17 strains identified in the laboratory were obtained from patients who lived or had lived in Cayenne. Thus, this clone is unique to this area to date (Table 3). Moreover, genotype 17 harbors the QpH1 plasmid, which is present in clones that cause severe clinical forms of Q fever in animal models.45 This finding result suggests that the severe initial presentation for patients with of Q fever pneumonia in this region is linked to this particular strain. All genotype 17 strains are susceptible to antibiotics, particularly doxycycline, suggesting that this drug remains a good first-line treatment for Q fever pneumonia in French Guiana.45
Table 3.
Genotypes of Coxiella burnetii described worldwide*
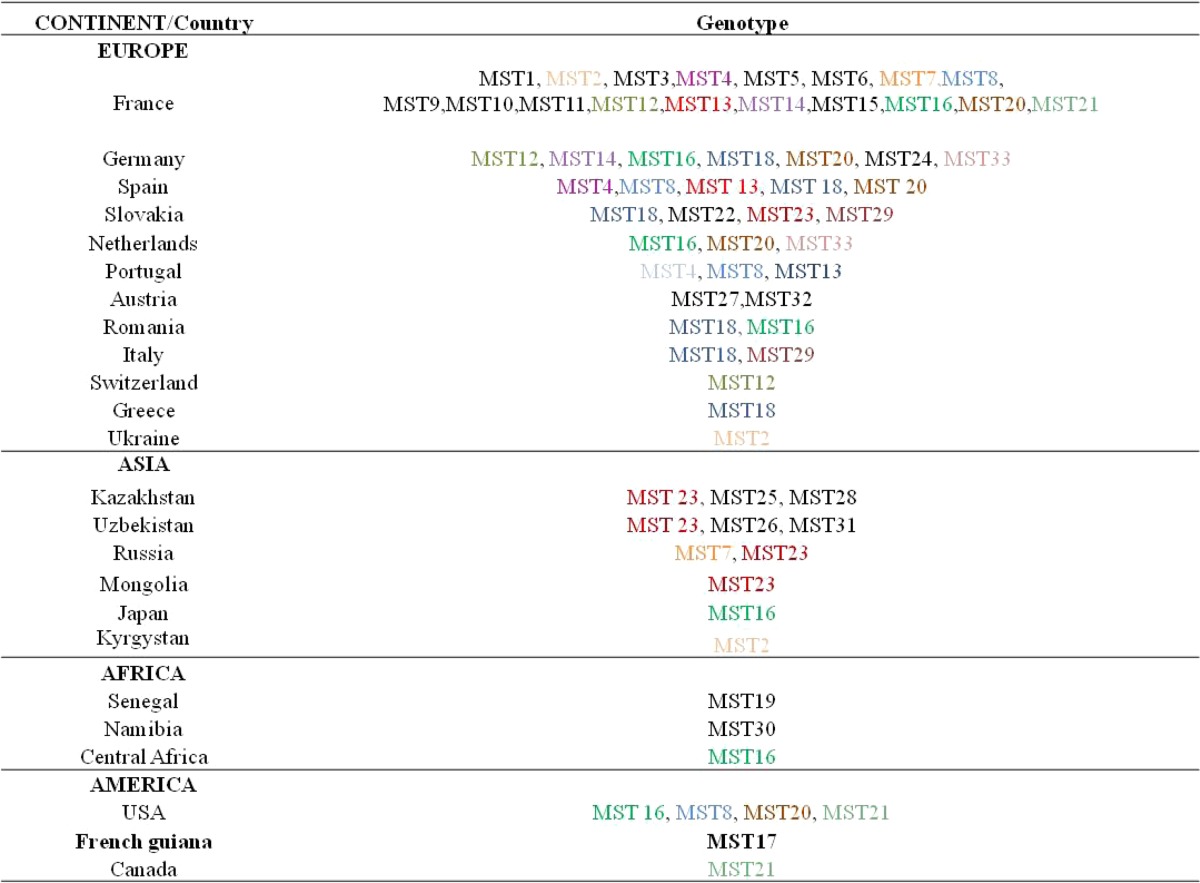
Genotypes that have been found in two or more countries are in bold. MST = multispacer sequencing type.
Animal Reservoir Issue
The main animal reservoir of Q fever in French Guiana remains unknown. The first published case of Q fever in Cayenne was found in persons who had classical risk factors of contamination (i.e., persons who worked in close contact with farm animals, in this case, slaughterhouse workers). However, despite the increase of incidence in 1996, no classical risk factor was found in persons with Q fever, and testing animals that were classically implicated in the transmission remained unsuccessful. In 1998, a seroprevalence survey was conducted for military dogs, and 1 of the 19 dogs from French Guiana was positive for Q fever.46 After the first outbreak in 1996, a study was conducted to identify risk factors for Q fever on the island of Cayenne.34 A case–control study was conducted during 1996–2000 among 60 Q fever patients and 105 controls from the arbovirus laboratory register. The authors also tested serum samples of various animals (domestic cattle, sheep, pigs, goats, pets, wild rodents, marsupials, and bats) for C. burnetii antibodies and C. burnetii DNA in an attempt to identify potential reservoirs. Of 471 cattle, sheep, and goats tested, only 6 cows (1.7%) had antibodies against C. burnetii, suggesting a negligible role of these animals in transmission of Q fever on Cayenne Island. Seven dogs had a positive serologic results, but only two of them belonged to patients. All cats were seronegative. Of the 361 wild animals tested, four rodents, five marsupials, and one bird had C. burnetii antibodies. All batrachians and chiropters were seronegative. No arthropods were tested, but increased tick bites were not found in patients. Coxiella burnetii DNA was not detected in the lung, liver, or blood of any wild animals tested. However, this study found a strong correlation between the incidence of Q fever and the precipitation rate, with a lag of 1–3 months, and an interruption of contamination during the dry season. The correlation between incidence of Q fever and monthly rainfall was statistically significant. Living near the forest was found to be a risk factor, as was frequently seeing bats, marsupials, or wild mammals near the house. Owning an air-conditioned vehicle and performing terracing work near the house were also identified as statistically significant risk factors. The activities of gardening and working in the building trade or public work were others risk factors. Since this study, no other study has been found in the published literature regarding the risk factors and reservoirs of Q fever in French Guiana. As a consequence, identifying the animal reservoir remains critical for our understanding of Q fever epidemiology and for organizing the future prevention of this disease in French Guiana.
Conclusions
We have reviewed the main aspects of Q fever in French Guiana. These different elements illustrate that Q fever in this country shows an atypical profile in terms of its epidemiology, clinical presentation, and environmental source. The outbreak of 1996 appears to be ongoing because the incidence has continued to increase to the highest rate ever reported in the literature. Moreover, the atypical immune response of Q fever patients in this country, including higher levels of phase I immunoglobulin, obliged us to reanalyze the accuracy of current criteria for the definition of Q fever endocarditis and vascular infections. These particular characteristics seem to be linked to the presence of a single clone (genotype 17) circulating in Cayenne, French Guiana, which has not been found elsewhere. The genomic study of this strain remains to be performed, which would help identify virulence determinants. Genomic study would also enable development of a specific quantitative polymerase chain reaction, which would provide a good diagnostic tool for clinicians in this region.
Identification of the animal reservoir of Q fever in French Guiana is also a crucial point that remains to be elucidated. Previous epidemiologic studies have helped define some risk factors, leading to the hypothesis of a wild reservoir, but new surveys among wild animals living in the surrounding hills of Cayenne have yet to be performed. Identifying the environmental source of Q fever will increase understanding of the wild lifecycle of C. burnetii and enable development of efficient prevention tools to fight this emerging disease in French Guiana.
Note: During the revision process of this paper, C. burnetii has been detected by qPCR in the spleen and feces samples of a dead three-toed-sloth for the first time. Further studies are needed to determine if this animal is the wild reservoir of the disease. Reference: Bernard Davoust, Jean-Louis Marié, Vincent Pommier de Santi, Jean-Michel Berenger, Sophie Edouard, and Didier Raoult. The three-toed sloth, a putative reservoir of Q fever, Cayenne, French Guiana. Emerg Inf Dis In press
Footnotes
Authors' addresses: Carole Eldin and Didier Raoult, Unité de Recherche sur Les Maladies Infectieuses et Ttropicales Émergentes, Unités Mxtes de Rcherche, Centre National de la Recherche Scientifique 7278, Institut de Recherche pour le Développement 198, Institut National de la Santé et de la Recherche Médicale Unite 1095, Faculté de Médecine, Aix Marseille Université, 27 Bd Jean Moulin, Marseille 13005, France, E-mails: carole.eldin@gmail.com and didier.raoult@gmail.com. Aba Mahamat, Magalie Demar, Philippe Abboud, and Félix Djossou, Unité de Maladies Infectieuses et Tropicales, Centre Hospitalier Andrée Rosemon, Cayenne, French Guiana, E-mails: mahamataba@gmail.com, mdemar@yahoo.com, philippe.abboud@gmail.com, and felix.djossou@ch-cayenne.fr.
References
- 1.Million M, Lepidi H, Raoult D. Q fever: current diagnosis and treatment options [in French] Med Mal Infect. 2009;39:82–94. doi: 10.1016/j.medmal.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Angelakis E, Raoult D. 2009. Q fever. Vet Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Derrick EH. Q fever, a new fever entity: clinical features, diagnosis and laboratory investigation. Rev Infect Dis. 1983;5:790–800. doi: 10.1093/clinids/5.4.790. [DOI] [PubMed] [Google Scholar]
- 4.Roest HI, Tilburg JJ, van der Hoek W, Vellema P, van Zijderveld FG, Klaassen CH, Raoult D. The Q fever epidemic in The Netherlands: history, onset, response and reflection. Epidemiol Infect. 2011;139:1–12. doi: 10.1017/S0950268810002268. [DOI] [PubMed] [Google Scholar]
- 5.Epelboin L, Chesnais C, Boullé C, Drogoul A-S, Raoult D, Djossou F, Mahamat A. Q fever pneumonia in French Guiana: prevalence, risk factors, and prognostic score. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;55:67–74. doi: 10.1093/cid/cis288. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro-Netto A, Nikitin T, Ribeiro IF. Q fever study in Sao Paulo. 3. Prevalence among milkers and dairy farm workers [in Portuguese] Rev Inst Med Trop Sao Paulo. 1964;6:255–257. [PubMed] [Google Scholar]
- 7.Riemann HP, Brant PC, Franti CE, Reis R, Buchanan AM, Stormont C, Behymer DE. Antibodies to Toxoplasma gondii and Coxiella burnetii among students and other personnel in veterinary colleges in California and Brazil. Am J Epidemiol. 1974;100:197–208. doi: 10.1093/oxfordjournals.aje.a112028. [DOI] [PubMed] [Google Scholar]
- 8.Riemann HP, Brant PC, Behymer DE, Franti CE. Toxoplasma gondii and Coxiella burnetii antibodies among Brazilian slaughterhouse employees. Am J Epidemiol. 1975;102:386–393. doi: 10.1093/oxfordjournals.aje.a112177. [DOI] [PubMed] [Google Scholar]
- 9.Da Costa PS, Brigatte ME, Greco DB. Antibodies to Rickettsia rickettsii, Rickettsia typhi, Coxiella burnetii, Bartonella henselae, Bartonella quintana, and Ehrlichia chaffeensis among healthy population in Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2005;100:853–859. doi: 10.1590/s0074-02762005000800006. [DOI] [PubMed] [Google Scholar]
- 10.da Costa PS, Brigatte ME, Greco DB. Questing one Brazilian query: reporting 16 cases of Q fever from Minas Gerais, Brazil. Rev Inst Med Trop Sao Paulo. 2006;48:5–9. doi: 10.1590/s0036-46652006000100002. [DOI] [PubMed] [Google Scholar]
- 11.Lemos ER, Rozental T, Mares-Guia MA, Almeida DN, Moreira N, Silva RG, Barreira JD, Lamas CC, Favacho AR, Damasco PV. Q fever as a cause of fever of unknown origin and thrombocytosis: first molecular evidence of Coxiella burnetii in Brazil. Vector Borne Zoonotic Dis. 2011;11:85–87. doi: 10.1089/vbz.2009.0261. [DOI] [PubMed] [Google Scholar]
- 12.Rozental T, Mascarenhas LF, Rozenbaum R, Gomes R, Mattos GS, Magno CC, Almeida DN, Rossi MI, Favacho AR, de Lemos ER. Coxiella burnetii, the agent of Q fever in Brazil: its hidden role in seronegative arthritis and the importance of molecular diagnosis based on the repetitive element IS1111 associated with the transposase gene. Mem Inst Oswaldo Cruz. 2012;107:695–697. doi: 10.1590/s0074-02762012000500021. [DOI] [PubMed] [Google Scholar]
- 13.Lamas C da C, Ramos RG, Lopes GQ, Santos MS, Golebiovski WF, Weksler C, Ferraiuoli GI, Fournier P-E, Lepidi H, Raoult D. Bartonella and Coxiella infective endocarditis in Brazil: molecular evidence from excised valves from a cardiac surgery referral center in Rio de Janeiro, Brazil, 1998 to 2009. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2013;17:e65–e66. doi: 10.1016/j.ijid.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Siciliano RF, Ribeiro HB, Furtado RH de M, Castelli JB, Sampaio RO, dos Santos FCP, Colombo S, Grinberg M, Strabelli TM. Endocarditis due to Coxiella burnetii (Q fever): a rare or underdiagnosed disease? Case report [in Portuguese] Rev Soc Bras Med Trop. 2008;41:409–412. doi: 10.1590/s0037-86822008000400017. [DOI] [PubMed] [Google Scholar]
- 15.Siciliano RF, Strabelli TM, Zeigler R, Rodrigues C, Castelli JB, Grinberg M, Colombo S, da Silva LJ, Mendes do Nascimento EM, Pereira dos Santos FC, Uip DE. Infective endocarditis due to Bartonella spp. and Coxiella burnetii: experience at a cardiology hospital in Sao Paulo, Brazil. Ann N Y Acad Sci. 2006;1078:215–222. doi: 10.1196/annals.1374.123. [DOI] [PubMed] [Google Scholar]
- 16.Lamas CC, Rozental T, Bóia MN, Favacho AR, Kirsten AH, da Silva AP, de Lemos ER. Seroprevalence of Coxiella burnetii antibodies in human immunodeficiency virus-positive patients in Jacarepaguá, Rio de Janeiro, Brazil. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2009;15((Suppl 2)):140–141. doi: 10.1111/j.1469-0691.2008.02144.x. [DOI] [PubMed] [Google Scholar]
- 17.Blair PJ, Schoeler GB, Moron C, Anaya E, Caceda R, Cespedes M, Cruz C, Felices V, Guevara C, Huaman A, Luckett R, Mendoza L, Richards AL, Rios Z, Sumner JW, Villaseca P, Olson JG. Evidence of rickettsial and Leptospira infections in Andean northern Peru. Am J Trop Med Hyg. 2004;70:357–363. [PubMed] [Google Scholar]
- 18.Manock SR, Jacobsen KH, de Bravo NB, Russell KL, Negrete M, Olson JG, Sanchez JL, Blair PJ, Smalligan RD, Quist BK, Espín JF, Espinoza WR, MacCormick F, Fleming LC, Kochel T. Etiology of acute undifferentiated febrile illness in the Amazon basin of Ecuador. Am J Trop Med Hyg. 2009;81:146–151. [PubMed] [Google Scholar]
- 19.Romana C, Roldan L, Torrico R, Mayer H. First acute case of Q fever diagnosed in Argentina (preliminary note) [in Spanish] Sem Med. 1959;115:506–507. [PubMed] [Google Scholar]
- 20.Luna CM, Famiglietti A, Absi R, Videla AJ, Nogueira FJ, Fuenzalida AD, Gené RJ. Community-acquired pneumonia: etiology, epidemiology, and outcome at a teaching hospital in Argentina. Chest. 2000;118:1344–1354. doi: 10.1378/chest.118.5.1344. [DOI] [PubMed] [Google Scholar]
- 21.Somma-Moreira RE, Caffarena RM, Somma S, Pérez G, Monteiro M. Analysis of Q fever in Uruguay. Rev Infect Dis. 1987;9:386–387. doi: 10.1093/clinids/9.2.386. [DOI] [PubMed] [Google Scholar]
- 22.de Ruiz HL de. Q fever in Colombia, S.A. A serological survey of human and bovine populations. Zentralbl Veterinarmed B. 1977;24:287–292. doi: 10.1111/j.1439-0450.1977.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 23.Araujo-Meléndez J, Sifuentes-Osornio J, Bobadilla-Del-valle JM, Aguilar-Cruz A, Torres-Angeles O, Ramírez-González JL, Ponce-de-León A, Ruiz-Palacios GM, Guerrero-Almeida ML. What do we know about Q fever in Mexico? Rev Investig Clínica Organo Hosp Enfermedades Nutr. 2012;64:541–545. [PubMed] [Google Scholar]
- 24.Sahagún Sánchez G, Cotter Lemus L, Zamora González C, Reyes PA, Ramírez S, Buendía A. Coxiella burnetii endocarditis. A report of the first case diagnosed in Mexico [in Spanish] Arch Inst Cardiol Mex. 1998;68:322–327. [PubMed] [Google Scholar]
- 25.Aguilar-Olivos N, del Carmen Manzano-Robleda M, Gutiérrez-Grobe Y, Chablé-Montero F, Albores-Saavedra J, López-Méndez E. Granulomatous hepatitis caused by Q fever: a differential diagnosis of fever of unknown origin. Ann Hepatol. 2013;12:138–141. [PubMed] [Google Scholar]
- 26.Kourany M, Johnson KM. A survey of Q fever antibodies in a high risk population in Panama. Am J Trop Med Hyg. 1980;29:1007–1011. doi: 10.4269/ajtmh.1980.29.1007. [DOI] [PubMed] [Google Scholar]
- 27.Cheney G, Geib WA. The identification of Q fever in Panama. Am J Hyg. 1946;44:158–172. doi: 10.1093/oxfordjournals.aje.a119080. [DOI] [PubMed] [Google Scholar]
- 28.De Rodaniche EC, Rodaniche A. Studies on Q fever in Panama. Am J Hyg. 1949;49:67–75. doi: 10.1093/oxfordjournals.aje.a119260. [DOI] [PubMed] [Google Scholar]
- 29.Adesiyun A, Dookeran S, Stewart-Johnson A, Rahaman S, Bissessar S. Frequency of seropositivity for Coxiella burnetii immunoglobulins in livestock and abattoir workers in Trinidad. New Microbiol. 2011;34:219–224. [PubMed] [Google Scholar]
- 30.Grant LS. A serological survey for Q. fever antibodies in man and animals in Jamaica, West Indies. West Indian Med J. 1961;10:234–239. [PubMed] [Google Scholar]
- 31.Drexhage VR, Dumas AM, Sukhai RN, Witsenburg M. Q-fever as a cause of myocarditis in childhood [in Dutch] Ned Tijdschr Geneeskd. 1989;133:2517–2519. [PubMed] [Google Scholar]
- 32.Floch H. Q fever in French Guiana [in French] Publ Cayenne Fr Guiana Inst Pasteur Guyane Fr Inini. 1957;18:1–5. [PubMed] [Google Scholar]
- 33.Pfaff F, François A, Hommel D, Jeanne I, Margery J, Guillot G, Couratte-Arnaude Y, Hulin A, Talarmin A. Q fever in French Guiana: new trends. Emerg Infect Dis. 1998;4:131–132. doi: 10.3201/eid0401.980124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardon J, Héraud JM, Laventure S, Ladam A, Capot P, Fouquet E, Favre J, Weber S, Hommel D, Hulin A, Couratte Y, Talarmin A. Suburban transmission of Q fever in French Guiana: evidence of a wild reservoir. J Infect Dis. 2001;184:278–284. doi: 10.1086/322034. [DOI] [PubMed] [Google Scholar]
- 35.Grangier C, Debin M, Ardillon V, Mahamat A, Fournier P, Simmonnet C. Epidemiology of Q fever in in Guyana, 1990–2006. Bull Veille Sanit 2009 [Google Scholar]
- 36.Tran A, Gardon J, Weber S, Polidori L. Mapping disease incidence in suburban areas using remotely sensed data. Am J Epidemiol. 2002;156:662–668. doi: 10.1093/aje/kwf091. [DOI] [PubMed] [Google Scholar]
- 37.Leone M, Honstettre A, Lepidi H, Capo C, Bayard F, Raoult D, Mege J-L. Effect of sex on Coxiella burnetii infection: protective role of 17β-estradiol. J Infect Dis. 2004;189:339–345. doi: 10.1086/380798. [DOI] [PubMed] [Google Scholar]
- 38.Edouard S, Mahamat A, Demar M, Abboud P, Djossou F, Raoult D. Comparison between emerging Q fever in French Guiana and endemic Q fever in Marseille, France. Am J Trop Med Hyg. 2014;90:915–919. doi: 10.4269/ajtmh.13-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fournier PE, Marrie TJ, Raoult D. Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834. doi: 10.1128/jcm.36.7.1823-1834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dupont HT, Thirion X, Raoult D. Q fever serology: cutoff determination for microimmunofluorescence. Clin Diagn Lab Immunol. 1994;1:189–196. doi: 10.1128/cdli.1.2.189-196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frankel D, Richet H, Renvoisé A, Raoult D. Q fever in France, 1985–2009. Emerg Infect Dis. 2011;17:350–356. doi: 10.3201/eid1703.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glazunova O, Roux V, Freylikman O, Sekeyova Z, Fournous G, Tyczka J, Tokarevich N, Kovacava E, Marrie TJ, Raoult D. Coxiella burnetii genotyping. Emerg Infect Dis. 2005;11:1211–1217. doi: 10.3201/eid1108.041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein A, Louveau C, Lepidi H, Ricci F, Baylac P, Davoust B, Raoult D. Q fever pneumonia: virulence of Coxiella burnetii pathovars in a murine model of aerosol infection. Infect Immun. 2005;73:2469–2477. doi: 10.1128/IAI.73.4.2469-2477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell-Lodrigue KE, Andoh M, Poels MW, Shive HR, Weeks BR, Zhang GQ, Tersteeg C, Masegi T, Hotta A, Yamaguchi T, Fukushi H, Hirai K, McMurray DN, Samuel JE. Coxiella burnetii isolates cause genogroup-specific virulence in mouse and guinea pig models of acute Q fever. Infect Immun. 2009;77:5640–5650. doi: 10.1128/IAI.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahamat A, Edouard S, Demar M, Abboud P, Patrice J-Y, La Scola B, Okandze A, Djossou F, Raoult D. Unique clone of Coxiella burnetii causing severe Q fever, French Guiana. Emerg Infect Dis. 2013;19:1102–1104. doi: 10.3201/eid1907.130044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boni M, Davoust B, Tissot-Dupont H, Raoult D. Survey of seroprevalence of Q fever in dogs in the southeast of France, French Guyana, Martinique, Senegal and the Ivory Coast. Vet Microbiol. 1998;64:1–5. doi: 10.1016/s0378-1135(98)00247-8. [DOI] [PubMed] [Google Scholar]