Abstract
An association between late-stage hepatosplenic schistosomiasis and endomyocardial fibrosis (EMF) has been suggested but not proven. We present the case of a 12-year-old Ugandan boy with striking comorbidities, including advanced periportal fibrosis caused by Schistosoma mansoni infection and right ventricular EMF, and discuss the possible correlation between both diseases.
Introduction
In sub-Saharan Africa (SSA), infection with Schistosoma mansoni typically gives rise to intestinal and hepatosplenic disease. This disease originates from immunological lesions and fibrotic granulomata forming to egg toxins or around tissue-trapped eggs.1 With established periportal fibrosis, end-stage organ damage results in the gradual development of collateral circulation.1 It is this evolving portocaval circulation that permits dissemination of schistosome eggs, egg toxins, or activated eosinophils to other parts of the body that might otherwise become trapped in the periportal sinuses and in so doing, likely leads to right-side heart complications, such as fibrosis.2
Endomyocardial fibrosis (EMF) was first described in Uganda in 1948. It represents a significant local health burden, although its etiology remains enigmatic.3 EMF can feature right and/or left ventricular endocardial fibrosis extending to the inner third of the myocardium. It typically affects the apex and inflow regions,3 leading to restrictive cardiomyopathy and cardiac failure. Right ventricular EMF classically presents with elevated jugular venous pressure, ascites, and hepatomegaly, whereas left ventricular EMF presents with severe pulmonary hypertension.4 Prognosis is poor, with death commonly occurring within 2 years of diagnosis.3
The worldwide distribution of EMF is puzzling and includes countries like Brazil, China, India, Mozambique, and Nigeria, and there are imported cases reported in the United Kingdom, Europe, and the United States.4,5 Several theories of its etiology have been postulated3,5: associations have been found with eosinophilia (> 30% of patients5) possibly linked to helminth infections, such as lymphatic filariasis, poverty in general, and low-protein or imbalanced diets in particular.3,6,7
Although associations between hepatosplenic schistosomiasis and EMF have been suggested in Egypt and Brazil, a common pathogenic mechanism remains uncertain.2,8 We describe the case of a Ugandan child with EMF and advanced liver fibrosis and hypothesize that both diseases are unlikely to be coincidental. To our knowledge, this patient is the youngest child reported with these striking concomitant morbidities.
Case History
In November of 2012, while conducting an epidemiological survey of schistosomiasis around Lake Albert, Uganda, an 11-year-old boy was brought to the attention of the field medical team. He was from the Alur tribe and had been born and raised in the area. S. mansoni-related morbidities (intestinal and hepatosplenic disease) are hyperendemic in his village (Walukuba), with infection prevalence and intensity near the highest recorded in recent parasitological cross-sectional surveys in Uganda.9
On examination, the boy appeared chronically ill, with marked icterus and dry mucous membranes. His abdomen was grossly distended with massive hepatosplenomegaly, although he did not have peripheral edema. Cardiovascular examination revealed normal heart sounds with good peripheral pulses. No increased jugular venous pressure was noted, and his chest was clear. Parasitological investigations confirmed egg patent S. mansoni infection by Kato–Katz fecal smear with 5,000 eggs/1 g stool (epg) and a positive urine circulating cathodic antigen (CCA) rapid diagnostic test (Rapid Medical Diagnostics, South Africa). His rapid human immunodeficiency virus (HIV) test was negative (Determine HIV-1/2; World Health Organization [WHO] donation). No additional investigations were undertaken at that time because of a lack of resources. He was treated with a standard dose of praziquantel (40 mg/kg).
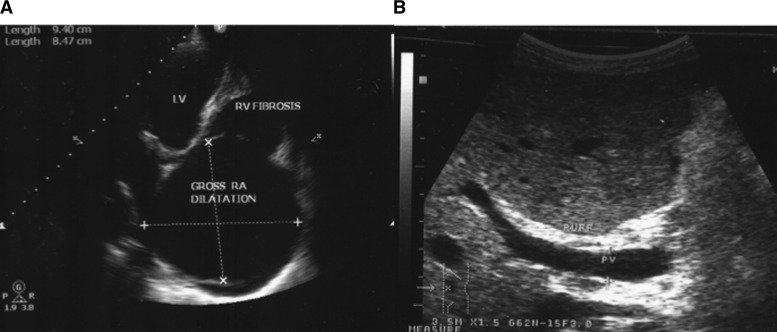
One year later, the team returned and found that there had been no clinical improvement in the boy's hepatosplenic disease. He was noted to be stunted for age: weight = 30 kg and height = 131 cm (−1 SD body mass index-for-age Z score; −2 SD height-for-age Z score). He had S. mansoni egg patent infection, with 24 epg and a strongly positive urine CCA. Finger-prick hemoglobin concentration was 11 g/dL by portable photometer (Hemocue, United Kingdom). A malaria rapid diagnostic test for Plasmodium spp. was negative. Portable abdominal ultrasound using a 3.5-MHz convex probe (Aloka 3500; Aloka, United Kingdom) showed hepatosplenomegaly, moderate ascites, portal vein dilatation, and advanced periportal fibrosis with ruff around the portal bifurcation. These findings represent stage D of the WHO classification10 for hepatosplenic schistosomiasis (Figure 1A).
Figure 1.
(A) Two-dimensional echocardiogram showing right ventricular (RV) fibrosis with gross right atrium (RA) dilatation and normal left ventricle (LV). (B) Abdominal ultrasound showing enlarged portal vein (PV) with periportal ruff and fibrosis stage D WHO classification.
Examination of the heart with the same ultrasound scanner showed a pericardial effusion of uncertain etiology, and the child was taken back to Kampala with the field team for additional investigations. At Mulago National Referral Hospital, a transthoracic echocardiogram revealed fibrosis of the right ventricular endocardium with a grossly dilated right atrium, a small anterior pericardial effusion of < 2.0 cm, and a normal left ventricle. The ejection fraction (E/F) was 52% (reference = 40–70%) with fractioning shortening (F/S) of 26% (reference = 25–45%). There was borderline normal systolic function, impaired diastolic function, and moderate functional tricuspic regurgitation caused by gross dilatation of right atrium (Figure 1B).
Extended laboratory investigations carried out in the same referral hospital revealed a total white blood cell (WBC) count of 9.1 × 103/μL (reference = 4.0–11.0 × 103/μL) with a raised eosinophil count of 3.58 × 103/μL (reference = 0.04–0.40 × 103/μL). Serum bilirubin was 1.2 mg/dL (reference = 0–1 mg/dL), albumin was 28 g/L (reference = 35–50 g/L), and total protein was 67.7 g/L (reference = 63–83 g/L). Hepatitis B surface antigen and rapid Hepatitis C antibody test were negative. Chest radiograph showed striking cardiomegaly.
The boy was treated again with a dose of praziquantel and started on daily propanolol (1 mg/kg). At follow-up 28 days later, he reported feeling better with more energy. There were no detectable S. mansoni eggs in his stool, although the urine CCA test remained weakly positive. A transthoracic echocardiogram 1 month later showed mildly improved cardiac function, with E/F of 64% and F/S of 34%. He remains on treatment with daily propanolol and will be reviewed again in November of 2014.
Discussion
Although this 12-year-old boy is young to present with advanced schistosomiasis, it is not uncommon around Lake Albert, where 17% of children under 9 years old are reported to have some degree of liver fibrosis (Bustinduy AL and others, unpublished data). Despite yearly praziquantel distribution to children in villages along this part of the shoreline, we learned through interview that this child did not receive his first dose of treatment until 2011. Children here are typically infected very soon after birth and can develop chronic schistosomiasis at an early age. It is, therefore, most likely that, in this boy's case, the hepatofibrotic disease preceded EMF. Furthermore, the boy's eosinophilia is unusual, because it is generally associated with early schistosomal infection rather than advanced periportal fibrotic disease. Eosinophils have the capacity to degranulate on contact with antibody-coated surfaces, such as tissues or parasites, and therefore, a raised eosinophil count may represent evidence of ongoing damage to the liver, heart, or both organs. Additional myocardial damage could be triggered by shunted schistosome eggs through the portocaval collateral circulation, which was postulated in adult Egyptian patients with schistosomiasis and EMF.2 Alternative etiologies for eosinophilia, such as Strongyloides, were not found.
Finding this child with advanced hepatosplenic disease and right ventricular EMF allows us to speculate more broadly on the etiology of EMF in Uganda. Both clinical entities are diseases of poverty and share a markedly profibrotic state with a strong relationship to eosinophilia. The high prevalence of EMF found in schistosomiasis-endemic countries, such as Uganda, Mozambique, and Nigeria, suggests that there may be an unidentified link.6,7,11 Therefore, cause or coincidence remains an open question in this impoverished part of Uganda, where hepatosplenic schistosomiasis is particularly rife and there is a dearth of cardiovascular disease surveillance.
Previously, only Brazil and Egypt had published cases with a suggested association between schistosomiasis and EMF. However, the patient selection may have been highly skewed, because all cases presented in cardiac failure. Little inference can, therefore, be made in earlier stages clinical interactions.2,8 Because EMF has a spectrum of disease (acute, subacute, and chronic), finding patients only in the burned out phase likely underreports those in less severe stages.12 In a population-based study in Brazil of 152 patients with schistosomiasis, no EMF was detected, but this study may have been underpowered to detect a disease that may not be that prevalent.13
If there is, indeed, an association between these diseases, it would be important to unveil it for several reasons. Understanding the interplay between schistosomiasis with EMF in SSA would change our perception of the burden of disease, bringing together new dialogue between cardiologists, infectious disease specialists, and epidemiologists. If this link is proven by subsequent epidemiological studies or experimental studies in laboratory models, it might lead to a future prevention strategy for EMF, which is presently lacking. For example, if regular praziquantel treatment could prevent portocaval circulation remodeling, lower eosinophilia levels, and reduce circulating egg toxins, the occurrence of EMF might be diminished or even averted. Because preventive chemotherapy campaigns delivering praziquantel en masse form the foundation of schistosomiasis control and because such programs are encouraged to document schistosomiasis-related morbidity, efforts should be mindful of a potential impact on cardiovascular disease.
In this scenario, we conclude that, although possible, it is unlikely that EMF and schistosomiasis are completely independent from one another, and this connection should be explored in greater detail. With this case report, our hope is to fuel interest in this very intriguing tandem of neglected diseases in SSA and encourage well-designed prospective studies addressing the combination of schistosomiasis and EMF. We, therefore, strongly advocate the use of future ultrasound and echocardiographic screening to not only quantify the burden of EMF but also, identify likely triggers, such as schistosomiasis.
ACKNOWLEDGMENTS
We are grateful to the Vector Control Division of the Ugandan Ministry of Health for allowing us to use their field station and Dr. Narcis Kabatereine and his team for help with logistics and technical support. We also thank Dr. Paul Neumann, Dr. Anthony Butterworth, and Dr. Aaron Bell for their valuable comments on the case.
Footnotes
Financial support: The study team received financial support from the London School of Hygiene and Tropical Medicine through the East African Diploma in Tropical Medicine and Hygiene.
Authors' addresses: Amaya L. Bustinduy, Department of Parasitology, Liverpool School of Tropical Medicine, Liverpool, United Kingdom, and Department of Pediatrics, Guy's and St. Thomas's NHS Trust, London, United Kingdom, E-mail: Amaya.Bustinduy@liverpool.ac.uk. Kenneth Luzinda, Department of Internal Medicine, Mulago Hospital, Kampala, Uganda, E-mail: luzindaken@gmail.com. Simon Mpoya, Department of Radiology, Mulago Hospital, Kampala, Uganda, E-mail: mposim2001@yahoo.co.uk. Philip Gothard and Stephen Wright, Hospital for Tropical Diseases, Mortimer Market Centre, London, United Kingdom, E-mails: philip.gothard@uclh.nhs.uk and stephenwright1@doctors.org.uk. Neil Stone, Department of Infection, Guy's and St. Thomas's NHS Trust, London, London, United Kingdom, E-mail: Neil.Stone@gstt.nhs.uk. J. Russell Stothard, Parasitology Department, Liverpool School of Tropical Medicine, Liverpool, United Kingdom, E-mail: R.Stothard@liverpool.ac.uk.
References
- 1.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Rashwan MA, Ayman M, Ashour S, Hassanin MM, Zeina AA. Endomyocardial fibrosis in Egypt: an illustrated review. Br Heart J. 1995;73:284–289. doi: 10.1136/hrt.73.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukhman G, Ziegler J, Parry E. Endomyocardial fibrosis: still a mystery after 60 years. PLoS Negl Trop Dis. 2008;2:e97. doi: 10.1371/journal.pntd.0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vijayaraghavan G, Sivasankaran S. Tropical endomyocardial fibrosis in India: a vanishing disease! Indian J Med Res. 2012;136:729–738. [PMC free article] [PubMed] [Google Scholar]
- 5.Mocumbi AO, Falase AO. Republished: recent advances in the epidemiology, diagnosis and treatment of endomyocardial fibrosis in Africa. Postgrad Med J. 2014;90:48–54. doi: 10.1136/postgradmedj-2012-303193rep. [DOI] [PubMed] [Google Scholar]
- 6.Rutakingirwa M, Ziegler JL, Newton R, Freers J. Poverty and eosinophilia are risk factors for endomyocardial fibrosis (EMF) in Uganda. Trop Med Int Health. 1999;4:229–235. doi: 10.1046/j.1365-3156.1999.43376.x. [DOI] [PubMed] [Google Scholar]
- 7.Andy JJ, Ogunowo PO, Akpan NA, Odigwe CO, Ekanem IA, Esin RA. Helminth associated hypereosinophilia and tropical endomyocardial fibrosis (EMF) in Nigeria. Acta Trop. 1998;69:127–140. doi: 10.1016/s0001-706x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- 8.Carneiro Rde C, Santos AL, Brant LC, Rabelo FT, Ligeiro CM, Barcelos IP, Silva VB, Silva VS, Nunes Mdo C. Endomyocardial fibrosis associated with mansoni schistosomiasis. Rev Soc Bras Med Trop. 2011;44:644–645. doi: 10.1590/s0037-86822011000500026. [DOI] [PubMed] [Google Scholar]
- 9.Sousa-Figueiredo JC, Pleasant J, Day M, Betson M, Rollinson D, Montresor A, Kazibwe F, Kabatereine NB, Stothard JR. Treatment of intestinal schistosomiasis in Ugandan preschool children: best diagnosis, treatment efficacy and side-effects, and an extended praziquantel dosing pole. In Health. 2010;2:103–113. doi: 10.1016/j.inhe.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter J, Hatz C, Campagne G, Bergquist NR, Jenkins JM. UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases . 2000. Ultrasound in schistosomiasis: a practical guide to the standard use of ultrasonography for assessment of schistosomiasis-related morbidity. Proceedings of the Second International Workshop, October 22–26, 1996; Niamey, Niger. [Google Scholar]
- 11.Mocumbi AO, Ferreira MB, Sidi D, Yacoub MH. A population study of endomyocardial fibrosis in a rural area of Mozambique. N Engl J Med. 2008;359:43–49. doi: 10.1056/NEJMoa0708629. [DOI] [PubMed] [Google Scholar]
- 12.Connor DH, Somers K, Hutt MS, Manion WC, D'Arbela PG. Endomyocardial fibrosis in Uganda (Davies' disease). 1. An epidemiologic, clinical, and pathologic study. Am Heart J. 1967;74:687–709. doi: 10.1016/0002-8703(67)90509-1. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa MM, Lamounier JA, Oliveira EC, Souza MV, Marques DS, Lambertucci JR. Short report: endomyocardial fibrosis and cardiomyopathy in an area endemic for schistosomiasis. Am J Trop Med Hyg. 1998;58:26–27. doi: 10.4269/ajtmh.1998.58.26. [DOI] [PubMed] [Google Scholar]