Abstract
Seventy-one asymptomatic human immunodeficiency virus-1 (HIV-1) -infected individuals who underwent colonoscopy for detection of diseases other than amebiasis were included in this study. Ulcerative lesions caused by Entamoeba histolytica were identified by colonoscopy and biopsy in 11.3% (8 of 71) of individuals. Stool microscopic examination hardly identified Entamoeba, whereas serum antibody against E. histolytica was often elevated in patients with subclinical intestinal amebiasis. Human leukocyte antigen (HLA) class II allele against E. histolytica infection (DQB1*06:01) was frequently identified in these patients. This study emphasizes the endemic nature of E. histolytica infection in our cohort and the difficulties in epidemiological control.
Introduction
Invasive amebiasis caused by Entamoeba histolytica is the second most common cause of parasite infection-related mortality worldwide, accounting for 40,600 to 73,800 deaths annually.1 Recent studies indicated that invasive amebiasis is prevalent in not only developing countries, where food or water is contaminated with stool, but also, East Asian developed countries, including Japan, as a sexually transmitted infection.2–5 We reported previously high seropositivity for E. histolytica among asymptomatic human immunodeficiency virus-1 (HIV-1)-infected individuals in Japan and showed relatively high incidence of invasive amebiasis in that population, probably because of exacerbation of subclinical infection.6 Other groups also reported that serum antibody against E. histolytica can be elevated, even in asymptomatic-infected individuals, and that seroconversion was seen in the absence of any symptoms in longitudinal follow-up in endemic areas.7 These results indicate that subclinical infection of E. histolytica is frequent in high-risk populations, making it difficult to control E. histolytica endemicity.
Evidence suggests that human leukocyte antigen (HLA) type plays a role in amebiasis. For example, Duggal and others8 reported previously that HLA DQB1*0601 seemed to provide protection against E. histolytica infection in Bangladeshi children.
This cross-sectional study was designed to determine the prevalence of ulcerative lesions associated with E. histolytica infection in asymptomatic HIV-1–infected individuals in Japan. We also examined the pathogenesis of subclinical intestinal amebiasis and the role of HLA genotypes.
Materials and Methods
Ethics statement.
The study was approved by the Human Research Ethics Committee of our hospital, the National Center for Global Health and Medicine in Tokyo. The study was conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all participants. No children were included in the study.
Study design and participants.
This cross-sectional study included HIV-infected patients who underwent colonoscopy between June of 2010 and June of 2013. One week before colonoscopy, each patient filled out a questionnaire about lower gastrointestinal symptoms based on the Gastrointestinal Symptom Rating Scale (GSRS) rating on a seven-graded Likert scale.9 Asymptomate for lower gastrointestinal diseases was defined as GSRS scores of one or two for three questions on the diarrhea syndrome domain (diarrhea, loose stools, and urgent need to defecate) and one question on bloody stool.10 Serum antibody testing against E. histolytica was performed in all participants on the day of colonoscopy. Serum antibody was tested by indirect fluorescent antibody assay using whole E. histolytica antigen according to the protocol described in the instruction sheet of the approved kit(bioMerieux, SA). Seropositivity was defined as positive response in a serum sample diluted at 1:100 (×100), and anti-Eh titer was determined by the highest dilution for the positive response. HLA type was determined by standard sequence-based genotyping (HLA Laboratory, Kyoto, Japan). The diagnosis of subclinical intestinal infection of E. histolytica was established on confirmation of one or two of the following two criteria: (1) identification of amebic trophozoites in biopsy specimens from gross ulcerative lesions obtained during colonoscopy and/or (2) no pathogens identified in biopsy specimens of gross ulcerative lesion, which were compatible with amebic ulcer,11 but ulcerative lesion resolved completely after metronidazole monotherapy as confirmed by colonoscopy.
Statistical analysis.
The patients' characteristics and serum positivities for anti-E. histolytica antibody were compared using χ2 or Mann–Whitney U test for qualitative or quantitative variables, respectively. Statistical significance was defined as two-sided P value < 0.05. All statistical analyses were performed using The Statistical Package for Social Sciences (SPSS Inc., Chicago, IL).
Results
Study population.
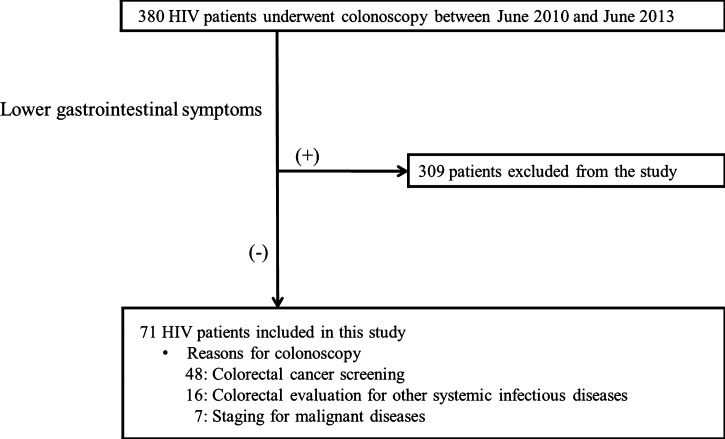
In total, 380 HIV-1–infected individuals were enrolled during the study period, and 71 patients met the criteria of no symptoms for lower gastrointestinal diseases according to the GSRS. The most common reason for colonoscopy was colorectal cancer screening (N = 48), whereas the other 23 patients underwent colonoscopy for evaluation of progression of malignancies or infections (e.g., malignant lymphoma, Kaposi's sarcoma, tuberculosis, and cytomegalovirus) (Figure 1).
Figure 1.
Flow diagram of the patient recruitment process. Lower abdominal symptoms were collected based on the GSRS rating on a seven-graded Likert scale at 1 week before colonoscopy.
Frequency of intestinal amebic infection among asymptomatic HIV-1–infected individuals.
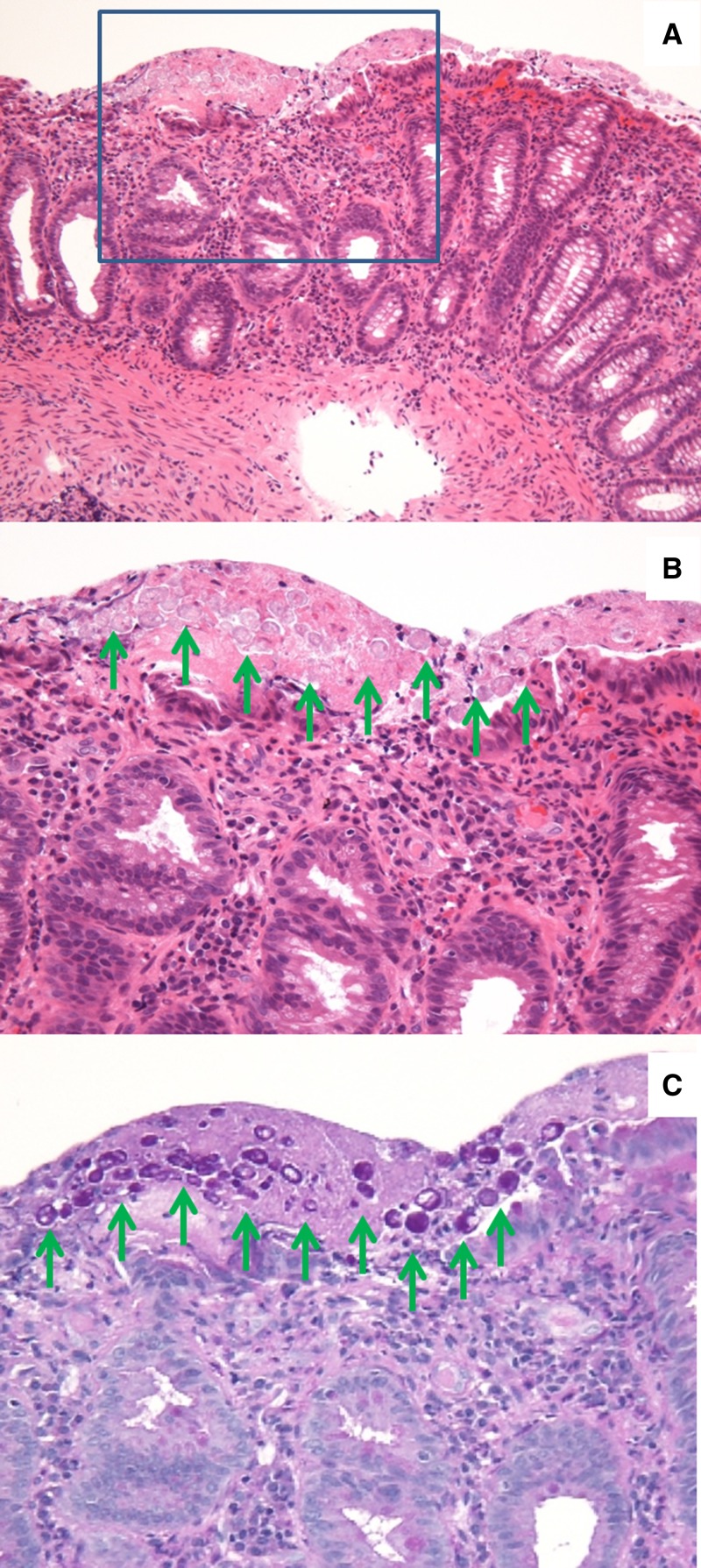
Amebic colitis was confirmed in eight (11.3%) cases. Gross ulcerative lesions were identified by colonoscopy in all eight cases. Amebic trophozoite was identified in the biopsy specimens of five cases (Figure 2). Although amebic trophozoites were not identified in the biopsy specimens of the other three cases, their sera were positive for antibody against E. histolytica. In all patients, the ulcerative lesions resolved completely after metronidazole monotherapy.
Figure 2.
Histopathological findings in subclinical intestinal amebiasis. Colonic tissue section was obtained during colonoscopy from a representative asymptomatic patient. E. histolytica on the surface of large-intestinal mucosa was clearly stained with periodic acid-Schiff (PAS) staining (green arrows). (A) Hematoxylin-eosin staining, ×100. (B) Higher magnification of the boxed area in A. Hematoxylin-eosin staining, ×400. (C) PAS staining, ×400.
Clinical features and presentation of patients with and without intestinal amebic infection.
As shown in Table 1, patients with amebic intestinal ulcerative lesions tended to be younger, be male homosexuals, have low CD4 counts, and have high HIV-RNA levels, although these differences were not statistically significant. Multiple ulcerative lesions were found in four cases (50%), and the most frequently involved location was the cecum (five cases; 62.5%). Serum antibody against E. histolytica was positive in 7 of 8 (87.5%) patients with amebic intestinal ulcerative lesions compared with positivity in only 11 of 63 (17.5%) patients without amebic ulcerative lesions (Table 2).
Table 1.
Characteristics of patients with and without subclinical intestinal amebiasis
| Amebiasis | No amebiasis | P value | |
|---|---|---|---|
| n | 8 | 63 | |
| Age (years), median (range) | 39 (27–62) | 51 (26–81) | 0.07 |
| Male sex (%) | 8/8 (100%) | 56/63 (88.9%) | 1.00 |
| Men who have sex with men (%) | 8/8 (100%) | 44/63 (69.8%) | 0.10 |
| Past history of amebiasis (%) | 0/8 (0%) | 7/63 (11.1%) | 1.00 |
| CD4/μL, median (range) | 301 (70–584) | 436 (21–1,697) | 0.28 |
| HIV-RNA (LC/mL), median (range) | 4.02 (UD–5.41) | UD (UD–5.85) | 0.09 |
LC/mL = log 10 copies per milliliter; UD = undetectable.
Table 2.
Clinical presentation of patients with and without subclinical intestinal amebiasis
| Amebiasis | No amebiasis | P value | |
|---|---|---|---|
| n | 8 | 63 | |
| Serum positivity for anti-E. histolytica antibody (%) | 7/8 (87.5%) | 11/63 (17.5%) | < 0.001 |
| < ×100 | 1 | 52 | |
| ×100 | 1 | 5 | |
| ×200 | 0 | 3 | |
| ×400 | 3 | 2 | |
| ×800 | 1 | 1 | |
| ×1,600 | 2 | 0 | |
| Site of intestinal amebiasis | |||
| Cecum | 5 | ||
| Ascending | 3 | ||
| Transverse | 1 | ||
| Descending | 0 | ||
| Sigmoid | 1 | ||
| Rectum | 4 | ||
From the limited data on fecal occult blood testing (FOB) and stool microscopic examination before treatment in cases with amebic ulcerative lesions, FOB was positive in two of three cases (66.7%), and the cyst form, not trophozoite form, Entoamoeba was found in only one of four cases (25%).
HLA class II allele frequencies in patients with and without subclinical intestinal amebiasis.
HLA data were available for 57 patients (7 of 8 patients with amebiasis and 50 of 63 patients without amebiasis) in our study. We investigated the relation between HLA alleles identified in more than five patients (frequency > 10%) and subclinical intestinal amebiasis. HLA DQB1*06:01 allele was significantly more frequent in patients with subclinical intestinal amebiasis than those without it (Table 3). All the HLA DQB1*06:01 holders were heterozygotes. The frequency of the HLA DRB1*15:02 allele was also significantly higher in patients with subclinical intestinal amebiasis (P = 0.05); 7 of 10 patients with HLA DQB1*06:01 also held HLA DRB1*15:02. No colonic amebic ulceration was detected in DQB1*06:01 (−)/DRB1*15:02 (+) patients. Thus, DQB1*06:01 seemed to be the primary HLA allele associated with subclinical intestinal amebiasis in the study population.
Table 3.
Frequencies of HLA class II alleles in patients with and without amebiasis
| Patients with amebiasis (N = 7) | Patients without amebiasis (N = 50) | P value | |
|---|---|---|---|
| DRB1 | |||
| *04:03 | 1 (14.3%) | 5 (10.0%) | 0.56 |
| *04:05 | 3 (42.9%) | 16 (32.0%) | 0.68 |
| *04:06 | 1 (14.3%) | 5 (10.0%) | 0.56 |
| *09:01 | 1 (14.3%) | 17 (34.0%) | 0.41 |
| *11:01 | 0 (0.0%) | 6 (12.0%) | 1.00 |
| *13:02 | 0 (0.0%) | 7 (14.0%) | 0.58 |
| *15:01 | 1 (14.3%) | 7 (14.0%) | 1.00 |
| *15:02 | 3 (42.9%) | 5 (10.0%) | 0.050 |
| DQB1 | |||
| *03:01 | 1 (14.3%) | 11 (22.0%) | 1.00 |
| *03:02 | 2 (28.6%) | 12 (24.0%) | 1.00 |
| *03:03 | 1 (14.3%) | 20 (40.0%) | 0.24 |
| *04:01 | 3 (42.9%) | 16 (32.0%) | 0.68 |
| *05:02 | 1 (14.3%) | 3 (6.0%) | 0.42 |
| *05:03 | 0 (0.0%) | 6 (12.0%) | 1.00 |
| *06:01 | 5 (71.4%) | 5 (10.0%) | 0.001 |
| *06:02 | 1 (14.3%) | 7 (14.0%) | 1.00 |
| *06:04 | 0 (0.0%) | 7 (14.0%) | 0.58 |
Data are numbers and frequencies of patients harboring each HLA allele. HLA data were available in 57 patients. HLA alleles identified in more than five patients (> 10%) were considered.
Discussion
The pathogenesis of amebiasis remains unclear, including the incubation period after cyst ingestion and the mechanism of spontaneous remission. We reported previously high seroprevalence of E. histolytica (21.3%) in HIV-1–infected individuals and that the majority of these patients (78.3%) had no history of invasive amebiasis. In that study, the patients were considered to be at high risk for developing symptomatic amebic infection in longitudinal follow-up (about 20% within the first 1 year of the follow-up period).6 Based on those results, we speculated the presence of subclinical intestinal amebiasis in patients positive for antibody against E. histolytica in the serum resulting in high frequency of symptomatic amebic diseases thereafter, although we did not identify the lesions of E. histolytica in these individuals in that study. However, Okamoto and others12 reported that intestinal ulcerative lesions of E. histolytica were rare based on colonoscopic examination in the general population in Japan with positive FOB (0.1%; 4 of 5,193). Our group reported previously that patients with cecal amebic ulcers were sometimes asymptomatic.11 In this regard, however, the clinical significance of E. histolytica infection in asymptomatic individuals had not been fully assessed. In this study, we identified gross amebic ulcers by colonoscopy in 11.2% of asymptomatic HIV-1–infected individuals.
Detection of intestinal amebiasis in asymptomatic individuals is important for not only treatment but also, epidemiological control, especially in endemic areas, because individuals with intestinal amebic ulcers can act as a reservoir for E. histolytica. However, it is sometimes difficult to identify amebiasis in these individuals, because they lack typical abdominal symptoms related to amebiasis, such as tenesmus, diarrhea, and dysentery. Moreover, our results showed that stool microscopic examination hardly identified amebiasis in these individuals. FOB is more sensitive than stool microscopic examination. However, FOB was positive in 72.7% (16 of 22) of patients free of amebic ulceration. Serum antibody against E. histolytica might be a sensitive marker of amebic ulcer in asymptomatic individuals. However, low titers of serum antibody were frequently found in individuals without amebic ulcer. The optimal cutoff value of antibody titer for amebic ulcer is still unclear (for cutoff titer of ×100, sensitivity is 87.5%, and specificity is 82.5%, whereas for cutoff titer ×400, sensitivity is 75.0%, and specificity is 95.2%) (Table 2).
Interestingly, our analysis showed high frequency of HLA DQB1*06:01 heterozygote in patients with subclinical intestinal amebiasis. This allele was reported previously to provide protection against E. histolytica infection in Bangladeshi patients.8 One possible explanation is that ulcerative lesions could occur asymptomatically in patients with HLA DQB1*06:01 and that their immune system could prevent the development of invasive disease from E. histolytica, resulting in the high frequency of subclinical intestinal amebiasis observed in our cross-sectional analysis. Genetic differences between Bangladeshi and Japanese patients should also be considered. HLA DQB1*06:01 and DRB1*15:01 were the most common haplotypes in Bangladesh, although they were not identified in our patients. Additional studies are needed to examine the effects of host genetic factors on E. histolytica infection and the development of invasive disease. Interestingly, not only HLA but also, mutation of the leptin receptor were reported to be associated with amebic infection.13
In conclusion, intestinal amebic ulcerative lesions were frequently found in asymptomatic HIV-1–infected Japanese individuals who could otherwise act as reservoirs for new infection in other high-risk populations. Additional studies of subclinical infection are needed to control the E. histolytica endemicity.
ACKNOWLEDGMENTS
We thank all the clinical staff at The AIDS Clinical Center and the Department of Gastroenterology and Hepatology for their help in the completion of this study.
Footnotes
Financial support: This study was supported by a grant for from the Ministry of Health, Labor, and Welfare of Japan (H25-promotion-general-014).
Authors' addresses: Koji Watanabe, Junko Tanuma, Yoshimi Kikuchi, Shinichi Oka, and Hiroyuki Gatanaga, AIDS Clinical Center, National Center for Global Health and Medicine, Tokyo, Japan, E-mails: kwatanab@acc.ncgm.go.jp, jtanuma@acc.ncgm.go.jp, yoshik@acc.ncgm.go.jp, oka@acc.ncgm.go.jp, and higatana@acc.ncgm.go.jp. Naoyoshi Nagata, Katsunori Sekine, and Kazuhiro Watanabe, Department of Gastroenterology and Hepatology, National Center for Global Health and Medicine, Tokyo, Japan, E-mails: nnagata_ncgm@yahoo.co.jp, hawaiiantuberider@gmail.com, and 12pagani@yahoo.co.jp. Toru Igari, Pathology Division of Clinical Laboratory, National Center for Global Health and Medicine, National Center for Global Health and Medicine, Tokyo, Japan, E-mail: tigari@hosp.ncgm.go.jp.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;15:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hung CC, Chang SY, Ji DD. Entamoeba histolytica infection in men who have sex with men. Lancet Infect Dis. 2012;12:729–736. doi: 10.1016/S1473-3099(12)70147-0. [DOI] [PubMed] [Google Scholar]
- 3.Park WB, Choe PG, Jo JH, Kim SH, Bang JH, Kim HB, Kim NJ, Oh MD. Amebic liver abscess in HIV-infected patients, Republic of Korea. Emerg Infect Dis. 2007;13:516–517. doi: 10.3201/eid1303.060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagata N, Shimbo T, Akiyama J, Nakashima R, Nishimura S, Yada T, Watanabe K, Oka S, Uemura N. Risk factors for intestinal invasive amebiasis in Japan, 2003–2009. Emerg Infect Dis. 2012;18:717–724. doi: 10.3201/eid1805.111275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe K, Gatanaga H, Escueta-de Cadiz A, Tanuma J, Nozaki T, Oka S. Amebiasis in HIV-1-infected Japanese men: clinical features and response to therapy. PLoS Negl Trop Dis. 2011;5:e1318. doi: 10.1371/journal.pntd.0001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe K, Aoki T, Nagata N, Tanuma J, Kikuchi Y, Oka S, Gatanaga H. Clinical significance of high anti-entamoeba histolytica antibody titer in asymptomatic HIV-1-infected individuals. J Infect Dis. 2014;209:1801–1807. doi: 10.1093/infdis/jit815. [DOI] [PubMed] [Google Scholar]
- 7.Hung CC, Ji DD, Sun HY, Lee YT, Hsu SY, Chang SY, Wu CH, Chan YH, Hsiao CF, Liu WC, Colebunders R. Increased risk for Entamoeba histolytica infection and invasive amebiasis in HIV seropositive men who have sex with men in Taiwan. PLoS Negl Trop Dis. 2008;2:e175. doi: 10.1371/journal.pntd.0000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duggal P, Haque R, Roy S, Mondal D, Sack RB, Farr BM, Beaty TH, Petri WA., Jr Influence of human leukocyte antigen class II alleles on susceptibility to Entamoeba histolytica infection in Bangladeshi children. J Infect Dis. 2004;189:520–526. doi: 10.1086/381272. [DOI] [PubMed] [Google Scholar]
- 9.Svedlund J, Sjodin I, Dotevall G. GSRS–a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–134. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 10.Hamada Y, Nagata N, Nishijima T, Shimbo T, Asayama N, Kishida Y, Sekine K, Tanaka S, Aoki T, Watanabe K, Akiyama J, Igari T, Mizokami M, Uemura N, Oka S. Impact of HIV infection on colorectal tumors: a prospective colonoscopic study of Asian patients. J Acquir Immune Defic Syndr. 2014;65:312–317. doi: 10.1097/QAI.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 11.Nagata N, Shimbo T, Akiyama J, Nakashima R, Niikura R, Nishimura S, Yada T, Watanabe K, Oka S, Uemura N. Predictive value of endoscopic findings in the diagnosis of active intestinal amebiasis. Endoscopy. 2012;44:425–428. doi: 10.1055/s-0031-1291631. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto M, Kawabe T, Ohata K, Togo G, Hada T, Katamoto T, Tanno M, Matsumura M, Yamaji Y, Watabe H, Ikenoue T, Yoshida H, Omata M. Amebic colitis in asymptomatic subjects with positive fecal occult blood test results: clinical features different from symptomatic cases. Am J Trop Med Hyg. 2005;73:934–935. [PubMed] [Google Scholar]
- 13.Duggal P, Guo X, Haque R, Peterson KM, Ricklefs S, Mondal D, Alam F, Noor Z, Verkerke HP, Marie C, Leduc CA, Chua SC, Jr, Myers MG, Jr, Leibel RL, Houpt E, Gilchrist CA, Sher A, Porcella SF, Petri WA., Jr A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest. 2011;121:1191–1198. doi: 10.1172/JCI45294. [DOI] [PMC free article] [PubMed] [Google Scholar]