Abstract
Objective
To determine if there is an association between cervical strain, evaluated using ultrasound elastography, and spontaneous preterm delivery (sPTD) <37 weeks of gestation.
Methods
One hundred and eighty nine (189) women at 16–24 weeks of gestation were evaluated. Ultrasound elastography was used to estimate cervical strain in three anatomical planes: one mid-sagittal in the same plane used for cervical length measurement, and two cross sectional images: one at the level of the internal cervical os, and the other at the level of the external cervical os. In each plane, two regions of interest (endocervix and entire cervix) were examined; a total of six regions of interest were evaluated.
Results
The prevalence of sPTD was 11% (21/189). Strain values from each of the six cervical regions correlated weakly with cervical length (r= −0.24, p<0.001 to r= −0.03, p=0.69). Strain measurements obtained in a cross sectional view of the internal cervical os were significantly associated with sPTD. Women with strain values ≤25th centile in the endocervical canal (0.19) and in the entire cervix (0.14) were 80% less likely to have a sPTD than women with strain values >25th centile (endocervical: odds ratio [OR] 0.2; 95% confidence interval [CI], 0.03–0.96; entire cervix: OR 0.17; 95% CI, 0.03–0.9). Additional adjustment for gestational age, race, smoking status, parity, maternal age, pre-pregnancy body mass index and previous preterm delivery did not appreciably alter the magnitude or statistical significance of these associations. Strain values obtained from the external cervical os and from the sagittal view were not associated with sPTD.
Conclusion
Low strain values in the internal cervical os were associated with a significantly lower risk of spontaneous preterm delivery <37 weeks of gestation.
Keywords: Cervical elasticity, short cervix, stiffness, cervical length, prematurity
Introduction
Cervical length evaluated by transvaginal ultrasound is currently the most powerful method for identifying women at risk of preterm delivery (1–18). The risk increases as the cervical length shortens (19–26). While 58% of women who deliver before 32 weeks of gestation have a cervical length ≤15 mm at 23 weeks of gestation, a large number might reach term or near term pregnancy (27,28). Similarly, of all women having a cervical length <25mm in the mid-trimester approximately 38% will have a preterm delivery (28). Moreover, in women with short cervix, there is evidence that vaginal progesterone, cervical cerclage, and perhaps a cervical pessary prevent preterm delivery (29–47). Vaginal progesterone has been shown to reduce the rate of preterm birth in women with a short cervix with or without a history of previous preterm birth (34, 48–50). Progesterone is as effective as cervical cerclage in women with a prior history of preterm birth and a short cervix (51, 52). Accordingly, additional parameters to cervical length are needed to improve the identification of women at risk of preterm delivery. Of particular interest is whether ultrasound elastography can provide relevant information about the risk of preterm delivery (53).
Biochemical and biophysical changes associated with cervical ripening have been described in different studies during the last decades (54–73). Changes in collagen organization, water content, as well as concentration of proteoglycans in the extracellular matrix are considered to be the basis for the modifications in biomechanical properties that make a cervix soft or hard (74–79). Elastography measures the percentage of tissue deformation that occurs when oscillatory compression is applied (80–83). The degree of tissue deformation can be expressed as strain (84–87). Increased strain reflects increased deformation (therefore, softer tissue), while decreasing strain reflects reduced deformation (therefore, stiffer tissue) (84, 88). Our team previously reported a standardized protocol for obtaining cervical strain measurements in pregnancy, and also demonstrated that estimated strain values differed across anatomical planes and regions of interest. Cervical strain values differed even further by patient characteristics including parity and prior preterm delivery, gestational age at examination, and cervical length (89). In this study, we explore whether cervical strain evaluated by ultrasound elastography is associated with spontaneous preterm delivery (sPTD).
Methods
Study Design & Participants
This was a cross-sectional study performed at the Center for Advanced Obstetrical Care and Research [Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, Wayne State University School of Medicine, Hutzel Women’s Hospital, Detroit MI]. Women with singleton pregnancies and without structural or chromosomal abnormalities were invited to participate. Patients with a short cervix or previous preterm delivery treated with vaginal progesterone or cerclage, as indicated by the treating physician, were excluded from the analysis. All patients provided written informed consent for ultrasound examination and were enrolled in research protocols approved by the Human Investigation Committee of Wayne State University and the Institutional Review Board of the NICHD. Spontaneous preterm delivery was considered as that resulting from the spontaneous onset of labor, or spontaneous rupture of membranes at <37 weeks of gestation.
Ultrasound Examination
All patients were enrolled before 11 weeks of gestation when the first ultrasound was performed, and gestational age was consistent with the results of crown-rump length measurement. The cervix was evaluated at 16–24 weeks of gestation using transvaginal ultrasound (Hitachi 8–4 MHz, HI Vision 900, Hitachi Medical Corporation, Tokyo, Japan). Cervical length was measured in a sagittal view of the cervix with a clear image of the endocervical canal, the internal and external cervical os, and with a similar size of the anterior and posterior cervical lips (90). For elastography quantification, three cervical projections were analyzed: mid-sagittal at the same level of the cervical length measurement, and two cross-sectional: one at the level of the internal os, and the other at the level of the external cervical os (89) (Figure 1). The proposed regions of interest were selected based on the feasibility of obtaining the ultrasound images and to delineate the regions of interest. The sagittal plane was the same used for cervical length measurement, and the two cross-sectional views are obtained with a 90° rotation of the ultrasound probe. Each plane was examined in two regions of interest (ROI), the endocervical canal and entire cervix, delineated as previously described (89).
Figure 1.
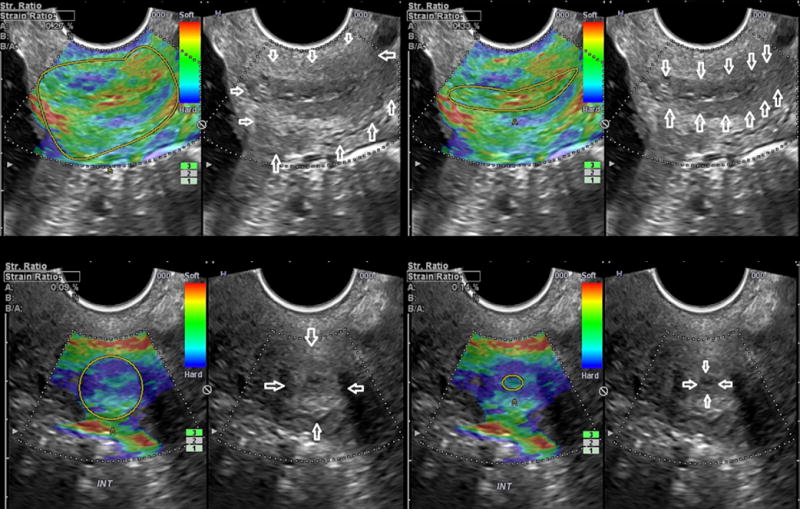
Cervical elastography and strain rate calculation in sagittal and cross sectional projections of the cervix. The boundaries of the endocervical canal, and of the entire cervix, are highlighted in the corresponding gray scale image.
Six operators, each having more than three years of experience in obstetrical ultrasound, acquired the elastography images. All operators were trained prior to the beginning of the study by evaluating at least 20 patients and analyzing a minimum of 120 elastography images. The reproducibility analysis showed a substantial overall agreement for considering soft/stiff estimates (kappa=0.75), matching on 82% of these classifications (89).
Measurements were performed while adjusting the region of interest to include the entire cervix and by manually applying continuous oscillatory pressure to the cervix using the ultrasound probe. The elastography equipment used in this study included a press indicator that displays the average strain in the region of interest to evaluate the condition of compression from minimal to high (levels 1 to 7). All measurements were performed while keeping the press indicator at a value of three (level 3). Additionally, to further standardize the method of measurement, we ensured that: 1) the posterior and anterior cervical lips in the ultrasound image had similar dimensions; 2) the endocervical canal was completely visualized, and 3) lateral areas of the cervix were equidistant relative to the ultrasound probe.
The measured strain values represented the percentage (%) of displacement or deformation of tissues within the cervical area averaged among consecutive ultrasound frames during manual application of oscillatory pressure. Dichotomous variables were constructed to describe whether each patient’s measurement was in the bottom quartile (≤25th centile) for each cervical region determined among the entire study population. Strain values in the bottom quartile can be viewed as representing ‘stiff’ or less tensile tissue compared to values in higher quartiles.
Statistical Analysis
Strain measurements obtained at each patient’s first visit during the study period were used to perform cross-sectional analyses. Binomial proportions with 95% confidence intervals (CI) and medians with interquartile ranges were calculated for categorical and arithmetic variables. Logistic regression models were fit to examine the magnitude of association between cervical tissue strain classification (+/−25th centile) and sPTD. Covariables considered as potential confounders in multivariable models included: the following: previous preterm delivery, gestational age at examination, nulliparity, smoking status, pre-pregnancy body mass index (BMI), maternal age at study enrollment, prior preterm delivery and race. Firth’s penalized likelihood estimation (91) was performed to resolve separation issues (i.e., small cell counts in 2 × 2 contingency tables representing a separation of outcomes among patients with and without a factor of interest). Statistical significance was defined as a p value <0.05. Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
The characteristics of women who subsequently had a sPTD (n=21) and those who delivered at term (n=168) are shown in Table 1. Women who subsequently had a sPTD had a significantly higher prevalence of cervical length <25 mm than those who delivered at term. The frequency of women with a history of a previous preterm delivery, or identified as smokers was also non-significantly higher in women presenting with sPTD.
Table 1.
Characteristics of patients presenting with spontaneous preterm delivery and patients delivering at term.
| Spontaneous preterm delivery <37 weeks (n=21) |
Delivery ≥37 weeks (n=168) |
|||
|---|---|---|---|---|
| Maternal Age, years (median, range) | 23 | 18–37 | 23 | 16–41 |
| African-American (n, %) | 21 | 100% | 151 | 90% |
| Smoker (n,%) | 9 | 43% | 30 | 18% |
| Nulliparous (n,%) | 6 | 29% | 73 | 43% |
| Body Mass Index (median, range) | 27 | 17–48 | 27 | 17–48 |
| Cervical Length <25mm (n,%)* | 7 | 33% | 10 | 6% |
| Prior PTD (n,%) | 7 | 33% | 31 | 18% |
| Gestational weeks at scan (median, range) | 19 | 17–24 | 19 | 16–24 |
| Gestational weeks at delivery (median, range) | 36 | 23–37 | 39 | 20–42 |
P<0.05
Table 2 shows the demographic characteristics of pregnant women according to the strain values from the entire cervix of the internal cervical os. Only one patient out of 51 women (2%) with strain values ≤25th centile presented with sPTD; in contrast, twenty of the 135 women (15%) whose cervical strain values were >25th centile subsequently had a sPTD.
Table 2.
Patient characteristics according to strain values obtained in the entire cervix of the internal cervical os
| Strain values ≤25th centile (n=51) |
Strain value >25th centile (n=138) |
|||
|---|---|---|---|---|
| Maternal Age, years (median, range) | 23 | 16–41 | 23 | 18–37 |
| African-American (n, %) | 47 | 92% | 123 | 89% |
| Smoker (n,%) | 11 | 22% | 27 | 20% |
| Nulliparous (n,%) | 25 | 49% | 52 | 38% |
| Body Mass Index (median, range) | 27 | 19–48 | 27 | 17–48 |
| Cervical Length <25mm (n,%) | 3 | 6% | 14 | 10% |
| Spontaneous preterm delivery* | 1 | 2% | 20 | 15% |
| Prior PTD (n,%) | 11 | 22% | 26 | 19% |
| Gestational weeks at scan (median, range) | 20 | 16–24 | 19 | 16–24 |
| Gestational weeks at delivery (median, range) | 39 | 35–42 | 39 | 20–42 |
P<0.05
Table 3 shows the magnitudes of association among strain values obtained in each cervical region and sPTD. Strain measurements obtained in a cross sectional view of the internal cervical os were significantly associated with sPTD. The strain values corresponding to the 25th centile in the internal cervical os were 0.19 for the endocervical canal, and 0.14 for the entire cervix. Women with strain values ≤25th centile were approximately 80% less likely to subsequently deliver preterm than those whose values were >25th centile (entire cervix; odds ratio [OR] 0.17; 95%CI 0.03–0.9; endocervical canal OR 0.2; 95% CI, 0.03–0.96). Additional adjustment for gestational age at examination, maternal age, race, smoking status, parity, pre-pregnancy body mass index (BMI) and previous preterm delivery did not change the magnitude of this association (entire cervix, OR 0.17; 95% CI 0.03–0.9; endocervical, OR 0.2; 95% CI 0.04–0.9). In contrast, strain values from the external cervical os and from the sagittal view of the cervix were not associated with sPTD.
Table 3.
Magnitudes of association among strain values ≤25th centile in each cervical region and spontaneous preterm delivery (<37 weeks)
| Cervical region Strain | Model I | Model II | |||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| Internal os endocervical | |||||
| ≤ 0.19 | 0.2 | 0.03–0.96 | 0.2 | 0.04–0.9 | |
| > 0.19 | 1 | Reference | 1 | Reference | |
| Internal os entire cervix | |||||
| ≤ 0.14 | 0.17 | 0.03–0.9 | 0.17 | 0.03–0.9 | |
| > 0.14 | 1 | Reference | 1 | Reference | |
| Sagittal endocervical | |||||
| ≤ 0.25 | 0.8 | 0.3–2.2 | 0.9 | 0.3–2.5 | |
| > 0.25 | 1 | Reference | 1 | Reference | |
| Sagittal entire cervix | |||||
| ≤ 0.2 | 0.7 | 0.2–2.0 | 0.8 | 0.3–2.4 | |
| > 0.2 | 1 | Reference | 1 | Reference | |
| External os endocervical | |||||
| ≤ 0.35 | 1.2 | 0.4–3.3 | 1.3 | 0.5–4.0 | |
| > 0.35 | 1 | Reference | 1 | Reference | |
| External os entire cervix | |||||
| ≤ 0.26 | 1.6 | 0.6–4.2 | 1.7 | 0.6–4.5 | |
| > 0.26 | 1 | Reference | 1 | Reference | |
Model I adjusted for gestational age at examination; Model II adjusted for gestational age at examination, race, smoking status, parity, maternal age, pre-pregnancy body mass index, and prior preterm delivery.
Secondary analyses revealed that women with cervical strain values ≤25th centile from the internal cervical os were 86%–88% less likely to subsequently have a sPTD at <34 weeks of gestation than women with strain values >25th centile (entire cervix: OR 0.14; 95%CI, 0.01–1.6; and endocervical canal: OR 0.12; 95%CI 0.01,–1.6); however, these associations did not reach statistical significance, most likely due to the limited number of cases available for analysis (n=9). In contrast, none of the strain estimations performed in sagittal or external views was significantly associated with sPTD at <34 weeks.
Table 4 shows the characteristics of women who subsequently presented with sPTD. Of these 21 patients, 33% (n=7) had a cervical length <25 mm, and all but one (95%) had cervical strain values above the 25th centile.
Table 4.
Cervical length and strain values ≤25th centile of the entire cervix from the internal cervical os in women with spontaneous preterm delivery.
| Patient | Gestational weeks at scan | Cervical length (mm) |
Strain ≤ 0.14 (Yes/No) |
Gestational weeks at delivery |
|---|---|---|---|---|
| 1 | 18 | 18 | No | 22 |
| 2 | 16 | 27 | No | 23 |
| 3 | 17 | 36 | No | 24 |
| 4 | 19 | 16 | No | 25 |
| 5 | 22 | 6 | No | 25 |
| 6 | 18 | 29 | No | 27 |
| 7 | 18 | 36 | No | 33 |
| 8 | 21 | 32 | No | 33 |
| 9 | 21 | 5 | No | 33 |
| 10 | 18 | 35 | No | 34 |
| 11 | 16 | 39 | No | 35 |
| 12 | 16 | 42 | Yes | 35 |
| 13 | 21 | 22 | No | 35 |
| 14 | 21 | 28 | No | 35 |
| 15 | 23 | 36 | No | 35 |
| 16 | 24 | 22 | No | 35 |
| 17 | 18 | 32 | No | 36 |
| 18 | 18 | 35 | No | 36 |
| 19 | 19 | 16 | No | 36 |
| 20 | 19 | 37 | No | 36 |
| 21 | 20 | 44 | No | 36 |
Note: Strain was estimated using a cross sectional projection of the entire cervix at the level of the internal os
Table 5 shows the correlations between cervical length (mm) and strain values from each of the six cervical regions of interest. Strain values from the internal cervical os showed a weak negative correlation with cervical length, whereas strain values from the external cervical os and the sagittal plane were not correlated with the cervical length.
Table 5.
Correlation between strain estimations obtained in the different cervical regions and cervical length.
| Variable | Correlation | 95% Confidence intervals | p value | |
|---|---|---|---|---|
| Internal Endocervical | −0.24 | −0.37 | −0.10 | <0.001 |
| Internal Complete | −0.22 | −0.36 | −0.08 | 0.002 |
| Sagittal Endocervical | −0.13 | −0.27 | 0.01 | 0.08 |
| Sagittal Complete | −0.07 | −0.21 | 0.07 | 0.34 |
| External Endocervical | −0.03 | −0.17 | 0.12 | 0.69 |
| External Complete | −0.06 | −0.20 | 0.09 | 0.43 |
Discussion
Principal findings of the study
Cervical elastography performed at 16–24 weeks of gestation showed that: 1) low strain values estimated in a cross sectional view of the internal cervical os were significantly associated with a lower risk of spontaneous delivery <37 weeks of gestation. Women with values ≤25th centile, representing stiff or less tensile tissue, were 80% less likely to subsequently have a sPTD than women with strain values >25th centile; 2) only one patient with a strain value ≤25th centile subsequently had a spontaneous preterm delivery, whereas 15% of patients with strain values >25th centile subsequently had a sPTD; and 3) the association between strain values ≤25th centile in the internal cervical os and sPTD remained significant when adjusting for potential confounders.
Elastography of the uterine cervix and spontaneous preterm delivery
The relationship of cervical strain evaluated by elastography to the risk of preterm delivery is of considerable interest. Prior reports of elastography of the uterine cervix have focused on measurement standardization or assessment of reproducibility (92–97). Only two articles have reported the relationship between cervical elastography and clinical outcome. Swiatkowska-Freund et al. (94) used ultrasound elastography in the evaluation of women with post-term pregnancies, and reported that a soft endocervical canal was associated with successful induction of labor. Additionally, Khalil et al. (97) suggested that ultrasound elastography might have potential value in predicting the risk of preterm delivery, although few patients (n=12) were examined. Our results show that strain values in the internal cervical os are associated with a risk of sPTD. When strain values in the internal cervical os were ≤25th centile, only one woman had a spontaneous preterm delivery. Despite the finding that strain values >25th centile were significantly associated with a risk of sPTD, a clear separation between those who will and who will not deliver preterm is still a goal. There was a modest correlation between cervical length and strain values in the internal cervical os. This is consistent with our previous report in uncomplicated women delivering at term (89). Cervical strain seems to provide different information about cervical characteristics from cervical length. The association with cervical length, as well as biochemical markers in a larger population might improve the discrimination of women with a higher/lower risk of preterm delivery.
The importance of evaluating cervical characteristics besides cervical length, such as deformation, elasticity, ultrasound attenuation, strain, and collagen composition, is to provide information on the tensile properties of the cervix during pregnancy and before the onset of preterm or term labor (98–112). Changes in such cervical characteristics can be related to the content and organization of collagen and proteoglycans in the extracellular matrix (56, 72, 73, 75, 113–117). In particular, there is a relationship between collagen fiber cross links and the degree of tissue stiffness. In experimental models, changes in tissue stiffness induced by increasing the number of collagen cross links have been demonstrated with ultrasound techniques (118, 119).
Regional differences in cervical tissue characteristics have been previously described. Feltovich et al. (120) suggested that the lack of homogeneity of cervical tissue can be related to differences between the alignment and organization of the collagen network. Carlson et al. (121) reported a higher shear wave speed in the proximal (internal os) cervical segment when compared with the distal (external os) segment of the cervix in studied specimens obtained after hysterectomy. McFarlin et al. (112) reported that the lack of homogeneity of the cervix might be a limitation when studying ultrasound properties such as attenuation. Using elastography, Molina et al. (95) also reported an apparent lack of homogeneity in the measurable stiffness of the cervix. Therefore, inherent strain differences in cervical regions are not surprising, and might be related to differences in tissue composition; whereas the internal cervical os is mainly formed by simple columnar epithelium, the external os has non-keratinized stratified squamous epithelium (122). It may be possible that different tissue characteristics are affected in different cervical regions in relation to sPTD.
Technical issues
By following a standard protocol for image acquisition and strain calculation, we were able to reduce the variability of elastography estimations (89). Reproducibility is facilitated by the relatively easy identification of the proposed planes for elastography evaluation. The sagittal projection is obtained in the same image as that used to measure the cervical length; then by rotating the ultrasound probe 90º, the transverse planes for the internal and external cervical os are obtained. These transverse planes are located immediately below the transducer, assuring a more homogenous pressure distribution. Nevertheless, two technical aspects should be considered when manual compression is applied for ultrasound elastography; 1) ultrasound cannot directly measure pressure; it can evaluate changes in frequency or amplitude of the backscattered radiofrequency signals. Therefore, strain measurements and color elastograms are directly related to the movement or displacement of the structures (123), and 2) the variability of compression applied by the operator can lead to differences in the displacement of structures; slight compression may create a mild displacement in stiff areas; strong compression can produce large displacements in soft areas. Therefore, a standardized method, independent of the operator, is desirable. The ultrasound equipment used for elastography herein has a press indicator, displaying the average displacement of the structures ranging from mild to high (levels 1 to 7, respectively). We used an intermediate level (bar level 3) for all examinations; this value represents a moderate compression applied to the region of interest. In addition, we ensured that the anterior and posterior cervical lips had similar dimensions; we obtained adequate visualization of the endocervical canal, and located the lateral parts of the cervix equidistant from the ultrasound probe.
The current study was not designed to assess test performance in a large number of patients. Rather, this is the first step to evaluate the association between cervical strain estimated using elastography and sPTD. Our findings suggest that elastography may have value as a tool in clinical risk assessment of sPTD. Further investigation is required to examine whether it improves sPTD risk assessment when performed in combination with cervical length and/or other biochemical parameters, particularly for early preterm delivery (109, 124–132).
Conclusion
The risk of spontaneous preterm delivery is significantly lower in women with low strain values obtained in the internal cervical os. Ultrasound elastography may have a role in the assessment of risk for preterm delivery.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C.
References
- 1.Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–86. doi: 10.1002/uog.5174. [DOI] [PubMed] [Google Scholar]
- 2.Welsh A, Nicolaides K. Cervical screening for preterm delivery. Curr Opin Obstet Gynecol. 2002;14:195–202. doi: 10.1097/00001703-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–67. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 4.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa I, Tanaka K, Takahashi K, Tanaka T, Aoki K, Torii Y, et al. Transvaginal ultrasonographic cervical assessment for the prediction of preterm delivery. J Matern Fetal Med. 1996;5:305–9. doi: 10.1002/(SICI)1520-6661(199611/12)5:6<305::AID-MFM2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 6.Okitsu O, Mimura T, Nakayama T, Aono T. Early prediction of preterm delivery by transvaginal ultrasonography. Ultrasound Obstet Gynecol. 1992;2:402–9. doi: 10.1046/j.1469-0705.1992.02060402.x. [DOI] [PubMed] [Google Scholar]
- 7.Watson WJ, Stevens D, Welter S, Day D. Observations on the sonographic measurement of cervical length and the risk of premature birth. J Matern Fetal Med. 1999;8:17–9. doi: 10.1002/(SICI)1520-6661(199901/02)8:1<17::AID-MFM4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Hibbard JU, Tart M, Moawad AH. Cervical length at 16–22 weeks’ gestation and risk for preterm delivery. Obstet Gynecol. 2000;96:972–8. doi: 10.1016/s0029-7844(00)01074-7. [DOI] [PubMed] [Google Scholar]
- 9.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–67. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 10.Cook CM, Ellwood DA. The cervix as a predictor of preterm delivery in ’at-risk’ women. Ultrasound Obstet Gynecol. 2000;15:109–13. doi: 10.1046/j.1469-0705.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 11.Berghella V, Berghella M. Cervical length assessment by ultrasound. Acta Obstet Gynecol Scand. 2005;84:543–4. doi: 10.1111/j.0001-6349.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 12.Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193:1170–4. doi: 10.1016/j.ajog.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 13.Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol. 2008;31:579–87. doi: 10.1002/uog.5323. [DOI] [PubMed] [Google Scholar]
- 14.Celik E, To M, Gajewska K, Smith GC, Nicolaides KH. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol. 2008;31:549–54. doi: 10.1002/uog.5333. [DOI] [PubMed] [Google Scholar]
- 15.Mella MT, Berghella V. Prediction of preterm birth: cervical sonography. Semin Pernatol. 2009;33:317–24. doi: 10.1053/j.semperi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Lim K, Butt K, Crane JM. Ultrasonographic cervical length assessment in predicting preterm birth in singleton pregnancies. J Obstet Gynaecol Can. 2011;33:486–99. doi: 10.1016/j.jogc.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Di Renzo GC, Roura LC, Facchinetti F, Antsaklis A, Breborowicz G, Gratacos E, et al. Guidelines for the management of spontaneous preterm labor: identification of spontaneous preterm labor, diagnosis of preterm premature rupture of membranes, and preventive tools for preterm birth. J Matern Fetal Neonatal Med. 2011;24:659–67. doi: 10.3109/14767058.2011.553694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Andrade E, Romero R, Ahn H, Hussein Y, Yeo L, Korzeniewski SJ, et al. Transabdominal evaluation of uterine cervical length during pregnancy fails to identify a substantial number of women with a short cervix. J Matern Fetal Neonatal Med. 2012;25:1682–9. doi: 10.3109/14767058.2012.657278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:104–6. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 20.Bergelin I, Valentin L. Normal cervical changes in parous women during the second half of pregnancy–a prospective, longitudinal ultrasound study. Acta Obstet Gynecol Scand. 2002;81:31–8. doi: 10.1046/j.0001-6349.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- 21.To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362–7. doi: 10.1002/uog.2773. [DOI] [PubMed] [Google Scholar]
- 22.Meijer-Hoogeveen M, Stoutenbeek P, Visser GH. Methods of sonographic cervical length measurement in pregnancy: a review of the literature. J Matern Fetal Neonatal Med. 2006;19:755–62. doi: 10.1080/14767050600852601. [DOI] [PubMed] [Google Scholar]
- 23.Berghella V, Roman A, Daskalakis C, Ness A, Baxter JK. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110:311–7. doi: 10.1097/01.AOG.0000270112.05025.1d. [DOI] [PubMed] [Google Scholar]
- 24.Vaisbuch E, Romero R, Erez O, Kusanovic JP, Mazaki-Tovi S, Gotsch F, et al. Clinical significance of early (< 20 weeks) vs. late (20–24 weeks) detection of sonographic short cervix in asymptomatic women in the mid-trimester. Ultrasound Obstet Gynecol. 2010;36:471–81. doi: 10.1002/uog.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva SV, Damiao R, Fonseca EB, Garcia S, Lippi UG. Reference ranges for cervical length by transvaginal scan in singleton pregnancies. J Matern Fetal Neonatal Med. 2010;23:379–82. doi: 10.3109/14767050903177169. [DOI] [PubMed] [Google Scholar]
- 26.Moroz LA, Simhan HN. Rate of sonographic cervical shortening and the risk of spontaneous preterm birth. Am J Obstet Gynecol. 2012;206:234 e1–5. doi: 10.1016/j.ajog.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–7. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 28.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks’ gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902–7. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 29.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–24. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–9. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 31.Dodd JM, Ashwood P, Flenady V, Jenkins-Manning S, Cincotta R, Crowther CA. A survey of clinician and patient attitudes towards the use of progesterone for women at risk of preterm birth. Aust N Z J Obstet Gynaecol. 2007;47:106–9. doi: 10.1111/j.1479-828X.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 32.Berghella V. Novel developments on cervical length screening and progesterone for preventing preterm birth. BJOG. 2009;116:182–7. doi: 10.1111/j.1471-0528.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 33.Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero R, Nicolaides K, Conde-Agudelo A, Tabor A, O’Brien JM, Cetingoz E, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124 e1–19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Combs CA. Vaginal progesterone or cerclage to prevent recurrent preterm birth? Am J Obstet Gynecol. 2013;208:1–2. doi: 10.1016/j.ajog.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Lewis DF, Baker SL, Stauffer R. Short cervix and vaginal progesterone: a model on how to tackle the problem of idiopathic preterm labor. J Reprod Med. 2013;58:434–7. [PubMed] [Google Scholar]
- 37.O’Brien JM, Defranco EA, Adair CD, Lewis DF, Hall DR, How H, et al. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2009;34:653–9. doi: 10.1002/uog.7338. [DOI] [PubMed] [Google Scholar]
- 38.Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185:1106–12. doi: 10.1067/mob.2001.118655. [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keeler SM, Kiefer D, Rochon M, Quinones JN, Novetsky AP, Rust O. A randomized trial of cerclage vs. 17 alpha-hydroxyprogesterone caproate for treatment of short cervix. J Perinat Med. 2009;37:473–9. doi: 10.1515/JPM.2009.083. [DOI] [PubMed] [Google Scholar]
- 41.Berghella V, Mackeen AD. Cervical length screening with ultrasound-indicated cerclage compared with history-indicated cerclage for prevention of preterm birth: a meta-analysis. Obstet Gynecol. 2011;118:148–55. doi: 10.1097/AOG.0b013e31821fd5b0. [DOI] [PubMed] [Google Scholar]
- 42.Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2012;4:CD008991. doi: 10.1002/14651858.CD008991.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Kanninen TT, Herway C, Skupski DW, Eglinton GS, Witkin SS. Endocervical immune mediator production following successful rescue or ultrasound indicated cerclage placement. J Perinat Med. 2012;40:159–63. doi: 10.1515/JPM.2011.132. [DOI] [PubMed] [Google Scholar]
- 44.Dharan VB, Ludmir J. Alternative treatment for a short cervix: the cervical pessary. Semin Perinatol. 2009;33:338–42. doi: 10.1053/j.semperi.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Kimber-Trojnar Z, Patro-Malysza J, Leszczynska-Gorzelak B, Marciniak B, Oleszczuk J. Pessary use for the treatment of cervical incompetence and prevention of preterm labour. J Matern Fetal Neonatal Med. 2010;23:1493–9. doi: 10.3109/14767051003678093. [DOI] [PubMed] [Google Scholar]
- 46.Goya M, Pratcorona L, Merced C, Rodo C, Valle L, Romero A, et al. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomised controlled trial. Lancet. 2012;379:1800–6. doi: 10.1016/S0140-6736(12)60030-0. [DOI] [PubMed] [Google Scholar]
- 47.Alfirevic Z, Owen J, Carreras Moratonas E, Sharp AN, Szychowski JM, et al. Vaginal progesterone, cerclage or cervical pessary for preventing preterm birth in asymptomatic singleton pregnant women with a history of preterm birth and a sonographic short cervix. Ultrasound Obstet Gynecol. 2013;41:146–51. doi: 10.1002/uog.12300. [DOI] [PubMed] [Google Scholar]
- 48.Rode L, Langhoff-Roos J, Andersson C, Dinesen J, Hammerum MS, Mohapeloa H, et al. Systematic review of progesterone for the prevention of preterm birth in singleton pregnancies. Acta Obstet Gynecol Scand. 2009;88:1180–9. doi: 10.3109/00016340903280982. [DOI] [PubMed] [Google Scholar]
- 49.DeFranco EA, O’Brien JM, Adair CD, Lewis DF, Hall DR, Fusey S, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:697–705. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
- 50.Campbell S. Universal cervical-length screening and vaginal progesterone prevents early preterm births, reduces neonatal morbidity and is cost saving: doing nothing is no longer an option. Ultrasound Obstet Gynecol. 2011;38:1–9. doi: 10.1002/uog.9073. [DOI] [PubMed] [Google Scholar]
- 51.Conde-Agudelo A, Romero R, Nicolaides K, Chaiworapongsa T, O’Brien JM, Cetingoz E, et al. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol. 2013;208:42 e1–e18. doi: 10.1016/j.ajog.2012.10.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owen J, Hankins G, Iams JD, Berghella V, Sheffield JS, Perez-Delboy A, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375 e1–8. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feltovich H, Hall TJ, Berghella V. Beyond cervical length: emerging technologies for assessing the pregnant cervix. Am J Obstet Gynecol. 2012;207:345–54. doi: 10.1016/j.ajog.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danforth DN, Buckingham JC, Roddick JW., Jr Connective tissue changes incident to cervical effacement. Am J Obstet Gynecol. 1960;80:939–45. doi: 10.1016/0002-9378(60)90472-5. [DOI] [PubMed] [Google Scholar]
- 55.Danforth DN, Veis A, Breen M, Weinstein HG, Buckingham JC, Manalo P. The effect of pregnancy and labor on the human cervix: changes in collagen, glycoproteins, and glycosaminoglycans. Am J Obstet Gynecol. 1974;120:641–51. doi: 10.1016/0002-9378(74)90608-5. [DOI] [PubMed] [Google Scholar]
- 56.Uldbjerg N, Ekman G, Malmstrom A, Olsson K, Ulmsten U. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am J Obstet Gynecol. 1983;147:662–6. doi: 10.1016/0002-9378(83)90446-5. [DOI] [PubMed] [Google Scholar]
- 57.Granstrom LM, Ekman GE, Malmstrom A, Ulmsten U, Woessner JF., Jr Serum collagenase levels in relation to the state of the human cervix during pregnancy and labor. Am J Obstet Gynecol. 1992;167:1284–8. doi: 10.1016/s0002-9378(11)91701-3. [DOI] [PubMed] [Google Scholar]
- 58.El Maradny E, Kanayama N, Kobayashi H, Hossain B, Khatun S, Liping S, et al. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum Reprod. 1997;12:1080–8. doi: 10.1093/humrep/12.5.1080. [DOI] [PubMed] [Google Scholar]
- 59.Shi L, Shi SQ, Saade GR, Chwalisz K, Garfield RE. Changes in cervical resistance and collagen fluorescence during gestation in rats. J Perinat Med. 1999;27:188–94. doi: 10.1515/JPM.1999.026. [DOI] [PubMed] [Google Scholar]
- 60.Straach KJ, Shelton JM, Richardson JA, Hascall VC, Mahendroo MS. Regulation of hyaluronan expression during cervical ripening. Glycobiology. 2005;15:55–65. doi: 10.1093/glycob/cwh137. [DOI] [PubMed] [Google Scholar]
- 61.Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol. 2005;66:161–73. doi: 10.1016/j.jri.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Maul H, Mackay L, Garfield RE. Cervical ripening: biochemical, molecular, and clinical considerations. Clin Obstet Gynecol. 2006;49:551–63. doi: 10.1097/00003081-200609000-00015. [DOI] [PubMed] [Google Scholar]
- 63.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 64.Ruscheinsky M, De la Motte C, Mahendroo M. Hyaluronan and its binding proteins during cervical ripening and parturition: dynamic changes in size, distribution and temporal sequence. Matrix Biol. 2008;27:487–97. doi: 10.1016/j.matbio.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Vaisbuch E, Erez O, et al. The transcriptome of cervical ripening in human pregnancy before the onset of labor at term: identification of novel molecular functions involved in this process. J Matern Fetal Neonatal Med. 2009;22:1183–93. doi: 10.3109/14767050903353216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schlembach D, Mackay L, Shi L, Maner WL, Garfield RE, Maul H. Cervical ripening and insufficiency: from biochemical and molecular studies to in vivo clinical examination. Eur J Obstet Gynecol Reprod Biol. 2009;144:S70–6. doi: 10.1016/j.ejogrb.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 67.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Mittal P, Vaisbuch E, et al. The molecular basis for sonographic cervical shortening at term: identification of differentially expressed genes and the epithelial-mesenchymal transition as a function of cervical length. Am J Obstet Gynecol. 2010;203:472 e1–e14. doi: 10.1016/j.ajog.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 68.Gonzalez JM, Dong Z, Romero R, Girardi G. Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PloS one. 2011;6:e26877. doi: 10.1371/journal.pone.0026877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol. 2011;179:838–49. doi: 10.1016/j.ajpath.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holt R, Timmons BC, Akgul Y, Akins ML, Mahendroo M. The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology. 2011;152:1036–46. doi: 10.1210/en.2010-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sundtoft I, Sommer S, Uldbjerg N. Cervical collagen concentration within 15 months after delivery. Am J Obstet Gynecol. 2011;205:59. doi: 10.1016/j.ajog.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 72.Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction. 2012;143:429–38. doi: 10.1530/REP-11-0466. [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez JM, Romero R, Girardi G. Comparison of the mechanisms responsible for cervical remodeling in preterm and term labor. J Reprod Immunol. 2013;97:112–9. doi: 10.1016/j.jri.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winkler M, Rath W. Changes in the cervical extracellular matrix during pregnancy and parturition. J Perinat Med. 1999;27:45–60. doi: 10.1515/JPM.1999.006. [DOI] [PubMed] [Google Scholar]
- 75.House M, Kaplan DL, Socrate S. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin Perinatol. 2009;33:300–7. doi: 10.1053/j.semperi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Myers K, Socrate S, Tzeranis D, House M. Changes in the biochemical constituents and morphologic appearance of the human cervical stroma during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009;144:S82–9. doi: 10.1016/j.ejogrb.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 77.Xu X, Akgul Y, Mahendroo M, Jerschow A. Ex vivo assessment of mouse cervical remodeling through pregnancy via 23Na MRS. NMR Biomed. 2010;23:907–12. doi: 10.1002/nbm.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akgul Y, Holt R, Mummert M, Word A, Mahendroo M. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology. 2012;153:3493–503. doi: 10.1210/en.2011-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Souza GN, Camano L, Araujo Junior E, Nader HB, Medeiros V, Martins JR, et al. The expression of glycosaminoglycans and proteoglycans in the uterine cervix of albino rats after local hyaluronidase infusion. J Matern Fetal Neonatal Med. 2013 doi: 10.3109/14767058.2013.845159. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 80.Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrasonic imaging. 1991;13:111–34. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 81.Cespedes I, Ophir J, Ponnekanti H, Maklad N. Elastography: elasticity imaging using ultrasound with application to muscle and breast in vivo. Ultrasonic imaging. 1993;15:73–88. doi: 10.1177/016173469301500201. [DOI] [PubMed] [Google Scholar]
- 82.Ophir J, Alam SK, Garra B, Kallel F, Konofagou E, Krouskop T, et al. Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. Proch Inst Mech Eng H. 1999;213:203–33. doi: 10.1243/0954411991534933. [DOI] [PubMed] [Google Scholar]
- 83.Parker KJ, Doyley MM, Rubens DJ. Imaging the elastic properties of tissue: the 20 year perspective. Phus Med Biol. 2011;56:R1–R29. doi: 10.1088/0031-9155/56/1/R01. [DOI] [PubMed] [Google Scholar]
- 84.Greenleaf JF, Fatemi M, Insana M. Selected methods for imaging elastic properties of biological tissues. Annu Rev Biiomed Eng. 2003;5:57–78. doi: 10.1146/annurev.bioeng.5.040202.121623. [DOI] [PubMed] [Google Scholar]
- 85.Gilman G, Khandheria BK, Hagen ME, Abraham TP, Seward JB, Belohlavek M. Strain rate and strain: a step-by-step approach to image and data acquisition. J Am Soc Echocardiogr. 2004;17:1011–20. doi: 10.1016/j.echo.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 86.Di Salvo G, Pacileo G, Faillace D, Gala S, Iacomino M, Fratta F, et al. Strain rate imaging: data acquisition and postrocessing. Minerva Cardioangiol. 2006;54:451–9. [PubMed] [Google Scholar]
- 87.Garra BS. Elastography: current status, future prospects, and making it work for you. Ultrasound Q. 2011;27:177–86. doi: 10.1097/RUQ.0b013e31822a2138. [DOI] [PubMed] [Google Scholar]
- 88.Hall TJ. AAPM/RSNA physics tutorial for residents: topics in US: beyond the basics: elasticity imaging with US. Radiographics. 2003;23:1657–71. doi: 10.1148/rg.236035163. [DOI] [PubMed] [Google Scholar]
- 89.Hernandez-Andrade E, Hassan SS, Ahn H, Korzeniewski SJ, Yeo L, Chaiworapongsa T, et al. Evaluation of cervical stiffness during pregnancy using semiquantitative ultrasound elastography. Ultrasound Obstet Gynecol. 2013;41:152–61. doi: 10.1002/uog.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Romero R, Yeo L, Miranda J, Hassan SS, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41:27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.FIRTH D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 92.Thomas A. Imaging of the cervix using sonoelastography. Ultrasound Obstet Gynecol. 2006;28 :356–7. doi: 10.1002/uog.3813. [DOI] [PubMed] [Google Scholar]
- 93.Thomas A, Kummel S, Gemeinhardt O, Fischer T. Real-time sonoelastography of the cervix: tissue elasticity of the normal and abnormal cervix. Acad Radiol. 2007;14:193–200. doi: 10.1016/j.acra.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 94.Swiatkowska-Freund M, Preis K. Elastography of the uterine cervix: implications for success of induction of labor. Ultrasound Obstet Gynecol. 2011;38:52–6. doi: 10.1002/uog.9021. [DOI] [PubMed] [Google Scholar]
- 95.Molina F, Gomez L, Florido J, Padilla M, Nicolaides K. Quantification of cervical elastography. A reproducibility study. Ultrasound Obstet Gynecol. 2012;39:685–9. doi: 10.1002/uog.11067. [DOI] [PubMed] [Google Scholar]
- 96.Fruscalzo A, Steinhard J, Londero AP, Frohlich C, Bijnens B, Klockenbusch W, et al. Reliability of quantitative elastography of the uterine cervix in at-term pregnancies. J Perinat Med. 2013:1–7. doi: 10.1515/jpm-2012-0180. [DOI] [PubMed] [Google Scholar]
- 97.Khalil MR, Thorsen P, Uldbjerg N. Cervical ultrasound elastography may hold potential to predict risk of preterm birth. Dan Med J. 2013;60:A4570. [PubMed] [Google Scholar]
- 98.Garfield RE, Chwalisz K, Shi L, Olson G, Saade GR. Instrumentation for the diagnosis of term and preterm labour. J Perinat Med. 1998;26:413–36. doi: 10.1515/jpme.1998.26.6.413. [DOI] [PubMed] [Google Scholar]
- 99.Fittkow CT, Shi SQ, Bytautiene E, Olson G, Saade GR, Garfield RE. Changes in light–induced fluorescence of cervical collagen in guinea pigs during gestation and after sodium nitroprusside treatment. J Perinat Med. 2001;29:535–43. doi: 10.1515/JPM.2001.074. [DOI] [PubMed] [Google Scholar]
- 100.Maul H, Olson G, Fittkow CT, Saade GR, Garfield RE. Cervical light-induced fluorescence in humans decreases throughout gestation and before delivery: Preliminary observations. Am J Obstet Gynecol. 2003;188:537–41. doi: 10.1067/mob.2003.94. [DOI] [PubMed] [Google Scholar]
- 101.Tekesin I, Wallwiener D, Schmidt S. The value of quantitative ultrasound tissue characterization of the cervix and rapid fetal fibronectin in predicting preterm delivery. J Perinat Med. 2005;33:383–91. doi: 10.1515/JPM.2005.070. [DOI] [PubMed] [Google Scholar]
- 102.Basgul A, Kavak ZN, Bakirci N, Gokaslan H. Three-dimensional ultrasound power Doppler assessment of the cervix: comparison between nulliparas and multiparas. J Perinat Med. 2007;35:48–50. doi: 10.1515/JPM.2007.007. [DOI] [PubMed] [Google Scholar]
- 103.Kuwata T, Matsubara S, Taniguchi N, Ohkuchi A, Ohkusa T, Suzuki M. A novel method for evaluating uterine cervical consistency using vaginal ultrasound gray-level histogram. J Perinat Med. 2010;38:491–4. doi: 10.1515/jpm.2010.079. [DOI] [PubMed] [Google Scholar]
- 104.Parra-Saavedra M, Gomez L, Barrero A, Parra G, Vergara F, Navarro E. Prediction of preterm birth using the cervical consistency index. Ultrasound Obstet Gynecol. 2011;38:44–51. doi: 10.1002/uog.9010. [DOI] [PubMed] [Google Scholar]
- 105.Yilmaz NC, Yigiter AB, Kavak ZN, Durukan B, Gokaslan H. Longitudinal examination of cervical volume and vascularization changes during the antepartum and postpartum period using three-dimensional and power Doppler ultrasound. J Perinat Med. 2010;38:461–5. doi: 10.1515/jpm.2010.087. [DOI] [PubMed] [Google Scholar]
- 106.Kuon RJ, Shi SQ, Maul H, Sohn C, Balducci J, Shi L, et al. A novel optical method to assess cervical changes during pregnancy and use to evaluate the effects of progestins on term and preterm labor. Am J Obstet Gynecol. 2011;205:82 e15–20. doi: 10.1016/j.ajog.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Badir S, Bajka M, Mazza E. A novel procedure for the mechanical characterization of the uterine cervix during pregnancy. J Mech Behav Biomed Mater. 2013;27:143–53. doi: 10.1016/j.jmbbm.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 108.Badir S, Mazza E, Zimmermann R, Bajka M. Cervical softening occurs early in pregnancy: characterization of cervical stiffness in 100 healthy women using the aspiration technique. Prenat Diagn. 2013;33:737–41. doi: 10.1002/pd.4116. [DOI] [PubMed] [Google Scholar]
- 109.Reusch LM, Feltovich H, Carlson LC, Hall G, Campagnola PJ, Eliceiri KW, et al. Nonlinear optical microscopy and ultrasound imaging of human cervical structure. J Biomed Opt. 2013;18:031110. doi: 10.1117/1.JBO.18.3.031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Poellmann MJ, Chien EK, McFarlin BL, Wagoner Johnson AJ. Mechanical and structural changes of the rat cervix in late-stage pregnancy. Journal of the mechanical behavior of biomedical materials. J Mech Behav Biomed Mater. 2013;17:66–75. doi: 10.1016/j.jmbbm.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kahyaoglu S, Kahyaoglu I, Kaymak O, Sagnic S, Mollamahmutoglu L, Danisman N. Can transvaginal ultrasonographic evaluation of the endocervical glandular area predict preterm labor among patients who received tocolytic therapy for threatened labor: a cross-sectional study. J Matern Fetal Neonatal Med. 2013;26:920–5. doi: 10.3109/14767058.2013.766703. [DOI] [PubMed] [Google Scholar]
- 112.McFarlin BL, Bigelow TA, Laybed Y, O’Brien WD, Oelze ML, Abramowicz JS. Ultrasonic attenuation estimation of the pregnant cervix: a preliminary report. Ultrasound Obstet Gynecol. 2010;36:218–25. doi: 10.1002/uog.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rechberger T, Uldbjerg N, Oxlund H. Connective tissue changes in the cervix during normal pregnancy and pregnancy complicated by cervical incompetence. Obstet Gynecol. 1988;71:563–7. [PubMed] [Google Scholar]
- 114.Osmers R, Rath W, Pflanz MA, Kuhn W, Stuhlsatz HW, Szeverenyi M. Glycosaminoglycans in cervical connective tissue during pregnancy and parturition. Obstet Gynecol. 1993;81:88–92. [PubMed] [Google Scholar]
- 115.Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol. 1995;38:267–79. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 116.Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction. 2007;134:327–40. doi: 10.1530/REP-07-0032. [DOI] [PubMed] [Google Scholar]
- 117.Akerud A, Dubicke A, Sennstrom M, Ekman-Ordeberg G, Malmstrom A. Differences in heparan sulfate production in cervical fibroblast cultures from women undergoing term and preterm delivery. Acta Obstet Gynecol Scand. 2008;87:1220–8. doi: 10.1080/00016340802460313. [DOI] [PubMed] [Google Scholar]
- 118.Hall CS, Dent CL, Scott MJ, Wickline SA. High-frequency ultrasound detection of the temporal evolution of protein cross linking in myocardial tissue. IEEE Trans Ultrason Ferroelectr Freq Control. 2000;47:1051–8. doi: 10.1109/58.852089. [DOI] [PubMed] [Google Scholar]
- 119.Akins ML, Luby-Phelps K, Bank RA, Mahendroo M. Cervical softening during pregnancy: regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse. Biol Reprod. 2011;84:1053–62. doi: 10.1095/biolreprod.110.089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feltovich H, Hall TJ. Quantitative imaging of the cervix: setting the bar. Ultrasound Obstet Gynecol. 2013;41:121–8. doi: 10.1002/uog.12383. [DOI] [PubMed] [Google Scholar]
- 121.Carlson LC, Feltovich H, Palmeri ML, Dahl JJ, Munoz Del, Rio A, Hall TJ. Shear wave speed estimation in the human uterine cervix. Ultrasound Obstet Gynecol. 2013 Jul 8; doi: 10.1002/uog.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ross MW. P Female Reproductive System. In: Pawlina RMW, editor. Histology: A Text and Atlas China. Lippincott Williams & Wilkins; 2010. pp. 830–92. [Google Scholar]
- 123.Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169–84. doi: 10.1055/s-0033-1335205. [DOI] [PubMed] [Google Scholar]
- 124.Mazza E, Nava A, Bauer M, Winter R, Bajka M, Holzapfel GA. Mechanical properties of the human uterine cervix: an in vivo study. Med Image Anal. 2006;10:125–36. doi: 10.1016/j.media.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 125.Feltovich H, Nam K, Hall TJ. Quantitative ultrasound assessment of cervical microstructure. Ultrasonic imaging. 2010;32:131–42. doi: 10.1177/016173461003200302. [DOI] [PubMed] [Google Scholar]
- 126.McLaughlin JR, Zhang N, Manduca A. Calculating tissue shear modulus and pressure by 2D Log-Elastographic methods. Inverse Probl. 2010;26 doi: 10.1088/0266-5611/26/8/085007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Akins ML, Luby-Phelps K, Mahendroo M. Second harmonic generation imaging as a potential tool for staging pregnancy and predicting preterm birth. J Biomed Opt. 2010;15:026020. doi: 10.1117/1.3381184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ierullo AM, Fernandez S, Palacio M, Gratacos E, Hernandez-Andrade E. Cervical blood perfusion assessed using power Doppler-derived estimation of fractional moving blood volume: a reproducibility study. Ultrasound Obstet Gynecol. 2011;38:57–61. doi: 10.1002/uog.8974. [DOI] [PubMed] [Google Scholar]
- 129.Hee L. Likelihood ratios for the prediction of preterm delivery with biomarkers. Acta Obstet Gynecol Scand. 2011;90:1189–99. doi: 10.1111/j.1600-0412.2011.01187.x. [DOI] [PubMed] [Google Scholar]
- 130.Brik M, Antonio P, Perales-Puchalt A, Diago V, Perales A. Cervical interleukin-6 as a predictive test for preterm delivery in symptomatic women: preliminary results. Eur J Obstet Gynecol Reprod Biol. 2011;155:14–8. doi: 10.1016/j.ejogrb.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y, Akins ML, Murari K, Xi J, Li MJ, Luby-Phelps K, et al. A compact fiber-optic SHG scanning endomicroscope and its application to visualize cervical remodeling during pregnancy. Proc Natl Acad Sci U S A. 2012;109:12878–83. doi: 10.1073/pnas.1121495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vargis E, Brown N, Williams K, Al-Hendy A, Paria BC, Reese J, et al. Detecting biochemical changes in the rodent cervix during pregnancy using Raman spectroscopy. Ann Biomed Eng. 2012;40:1814–24. doi: 10.1007/s10439-012-0541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


