Abstract
Objective
The molecular basis of failure to progress in labor is poorly understood. This study was undertaken to characterize the myometrial transcriptome of patients with an arrest of dilatation (AODIL).
Study design
Human myometrium was prospectively collected from women in the following groups: 1) spontaneous term labor (TL; n=29); and 2) arrest of dilatation (AODIL; n=14). Gene expression was characterized using Illumina® HumanHT-12 microarrays. A moderated student t-test and false discovery rate adjustment were used for analysis. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of selected genes was performed in an independent sample set. Pathway analysis was performed on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database using Pathway Analysis with Down-weighting of Overlapping Genes (PADOG). The Metacore knowledge base was also mined for pathway analysis.
Results
1) 42 genes differentially expressed were identified in women with an AODIL; 2) gene ontology analysis indicated enrichment of biological processes, which included: regulation of angiogenesis, response to hypoxia, inflammatory response, and chemokine-mediated signaling pathway. Enriched molecular functions included: transcription repressor activity, Heat shock protein (Hsp) 90 binding, and nitric oxide synthase (NOS) activity; 3) Metacore analysis identified immune response chemokine (C-C motif) ligand 2 (CCL2) signaling, muscle contraction regulation of eNOS activity in endothelial cells, and Triiodothyronine and Thyroxine signaling as significantly over-represented (FDR<0.05); 4) qRT-PCR confirmed overexpression of Nitric oxide synthase 3 NOS3; hypoxic ischemic factor (HIF1A), Chemokine (C-C motif) ligand 2 (CCL2); angiopoietin-like 4 (ANGPTL4), ADAM metallopeptidase with thrombospondin type 1, motif 9 (ADAMTS9), G protein-coupled receptor 4 (GPR4), metallothionein 1A (MT1A), MT2A, selectin E (SELE) in an AODIL.
Conclusion
The myometrium of women with arrest of dilatation have a stereotypic transcriptome profile. This disorder was associated with a pattern of gene expression involved in muscle contraction, an inflammatory response, and hypoxia. This is the first comprehensive and unbiased examination of the molecular basis of an AODIL.
Keywords: angiopoietin-like 4 (ANGPTL4), arrest disorders, chemokine (C-C motif) ligand 2 (CCL2), metallothionein (MT), myometrium, nitric oxide synthase (NOS), pregnancy, parturition, systems biology, TGF signaling pathway, transcriptomics
Introduction
Parturition, a key event for the survival of viviparous species, is a complex process involving myometrial activation, cervical ripening and membrane/decidual activation [6,25,30–32,36,74,94,107–109,111,129,134,137,139,148,150,157,177,184,189,190,194]. The myometrium is responsible for the contractile force required to propel the fetus through the birth canal. During pregnancy and active labor, extensive changes in the anatomy, physiology, and composition of the myometrium have been identified [28,29,40,43,46,48,54,55,62,67–72,76–78,92,112–114,117,118,120,136,142,164,166,168,169,176,183,185,186].
Dystocia, broadly defined as slow or abnormal progression of labor [1], is responsible for 18 percent of primary cesarean deliveries, 60 percent of all cesarean deliveries, and is considered one of the most common indications for intrapartum cesarean delivery [14,21,75,174]. Moreover, this condition is associated with 8% of maternal deaths worldwide [75,90,187]. Dystocia includes protraction disorders (slow dilatation) or arrest disorders (complete cessation of progress). The diagnosis of arrest of dilatation (AODIL) occurs when a patient in the active phase of labor does not have cervical dilatation for at least 2 hours [1,66]. The most common cause of an arrest of dilatation is thought to be cephalopelvic disproportion. However, the observation that patients with an arrest of dilatation in the first pregnancy often deliver a larger neonate vaginally in a subsequent pregnancy suggests that the problem is not one of disproportion [24]. Instead, a functional disorder in myometrial contractility has been postulated to be responsible for such disorder.
High-dimensional biology techniques (such as genomics, transcriptomics, and proteomics) have been used to gain insight into the molecular basis of parturition in the myometrium [4,27,34,60,88,124,135], the uterine cervix [85–87], and the chorioamniotic membranes [131]. Recently, the transcriptome of the myometrium in patients with an “arrest of descent” [123] and “labor dystocia” have been reported [23]. However, the transcriptome of human myometrium in AODIL has not yet been investigated. We undertook this study in order to characterize the transcriptome of the myometrium in patients with this condition.
Materials and Methods
A prospective study was performed in which human myometrium was obtained from women undergoing primary cesarean delivery at term (≥ 37 week of gestation) in the following groups: 1) spontaneous term labor (TL) (n=29); and 2) AODIL (n=14). Labor was diagnosed in the presence of spontaneous regular uterine contractions occurring at a minimum frequency of 2 every 10 minutes with cervical changes that led to progressive cervical dilatation. Women in the term labor group underwent cesarean delivery because of a non-reassuring fetal heart rate tracing (as determined by the physician) or fetal malpresentation. All patients presented in spontaneous labor and delivered an infant with a birth weight between the 10th and 90th percentiles [5]. Patients with clinical or histological chorioamnionitis, underlying medical or obstetrical complications, and those undergoing labor induction were excluded.
The diagnosis of AODIL was diagnosed in women in the active phase of labor who did not progress despite adequate contractions after 2 hours [1]. The placentas of all participating women were examined to exclude histological chorioamnionitis by experienced pathologists who were blinded to the clinical diagnosis.
All women provided written informed consent prior to the collection of myometrial samples. The collection and utilization of the samples for research purposes was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS, Bethesda, Maryland), and the Human Investigation Committees of Wayne State University (Detroit, Michigan) and the Sotero del Rio Hospital (Santiago, Chile).
Sample collection
Myometrial tissue samples were obtained from the lower uterine segment at the time of cesarean delivery, after placenta detachment. Biopsies were obtained from the midpoint of the superior aspect of the uterine incision using Metzenbaum scissors. Specimens measured approximately 1.0 × 1.0 × 1.0 cm. Tissue was ground under liquid nitrogen, placed in TRI Reagent® (Applied Biosystems, Foster City, CA) and kept at −80° Celsius until analysis.
Total RNA extraction
Total RNA was isolated from snap-frozen myometrium using TRI Reagent® combined with the Qiagen RNeasy Lipid Tissue kit protocol (Qiagen, Valencia, CA) following the manufacturers’ recommendations. RNA concentrations and the A260nm/A280nm ratio were assessed using a NanoDrop 1000 (Thermo Scientific, Wilmington, DE). RNA integrity numbers (RINs) were determined using the Bioanalyzer 2100 (Agilent Technologies, Wilmington, DE). An A260nm/A280nm ratio of 1.66, a 28S/18S ratio of 0.2, and a RIN of 3.8 were the minimum requirements for inclusion in the expression analysis.
Microarray experiments
The Illumina® HumanHT-12 version 3 expression microarray (Illumina, San Diego, CA) platform was used to determine the expression levels in each unpooled specimen per manufacturer’s instructions. In brief, after purification of RNA using an RNeasy Mini Kit (Qiagen), 500 ng of total RNA was amplified and biotin-labeled with the Illumina® TotalPrep RNA Amplification Kit (Ambion, Austin, TX). Labeled complementary RNAs were hybridized to the Illumina HumanHT-12 version 3 expression BeadChip and imaged using a BeadArray Reader. Raw data was obtained using the BeadStudio Software (Illumina).
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR)
A larger set of specimens for each group (spontaneous TL: n= 31; AODIL: n=18) were obtained for qRT-PCR assays of a selected group of genes found to be differentially expressed by microarray analysis. Total RNA (3 µg) was reverse transcribed using the SuperScript® III First-Strand Synthesis System and oligo(dT)20 primers (Invitrogen, Carlsbad, CA). qRT-PCR analyses were performed with TaqMan® Gene Expression Assays (HIF1A: Hs00936368_m1, GPR4: Hs00947870_m1, EXOG: Hs00270782_m1, ID1: Hs00357821_g1, ID3: Hs00171409_m1, NOS3: Hs01574659_m1, MT1A: Hs00831826_s1; MT2A: Hs02379661_g1; and SBNO2: Hs00209130_m1; Applied Biosystems, Foster City, CA, USA). Human 18S, GAPDH, and ACTIN were used as reference genes. The gene specific TaqMan® assays and the RPLPO housekeeping gene were run in triplicate (50 ng) for each case to allow for the assessment of technical variability.
Statistical analysis
Clinical data
Student's t tests and Fisher exact tests were used for comparisons of continuous and discrete demographic variables, respectively. The tests were conducted using the R statistical language and environment (R Development Core Team 2012). A p-value < 0.05 was considered significant.
Microarray analysis
The Illumina BeadStudio software suite (Illumina, Inc., San Diego, CA, USA) was used to extract gene expression values from the array images. The data quality was assessed based on Illumina’s positive and negative control probes on each array as well as by inspection of the distributions of probe intensities. Data was normalized using the quantile normalization method [18]. Probes that were considered present (detection p-value <0.1) in at least 5 samples were retained for further analysis. A moderated t-test implemented in the limma library of Bioconductor (Fred Hutchinson Cancer Research Center, Seattle, WA, USA) was applied to test differential expression, and a false discovery rate (FDR) adjustment of the p-values was performed to correct for multiple testing[173]. Probes were considered significant different if their adjusted p-value were < 0.25 and the fold change difference was at least 1.5.
Gene ontology analysis was performed using an over-representation approach implemented in the GO stats software package[61]. Pathway analysis was performed on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database using Pathway Analysis with Down-weighting of Overlapping Genes (PADOG) [181], which computes and enrichment score for each pathway from moderated t-scores of all genes in the pathway while giving more weight to genes that are pathway-specific. Metacore pathway analysis (Thomson, Reuters, NY, USA) was also performed using their proprietary pathways database and over-representation method.
qRT-PCR assays
Quantitative RT-PCR was performed for 9 genes selected among the top candidates from the microarray results and biological function. Data analysis was performed using an equal variance two-sample one-tailed t-test based on the hypothesis provided by the microarray data. Confirmation of microarray data with qRT-PCR was considered significant using a 0.05 threshold.
Results
Demographic, clinical and obstetrical characteristics are displayed in Table 1 according to study group: 1) pregnant women with spontaneous TL microarray (n=29); 2) patients with AODIL microarray (n=14); 3) spontaneous TL qRT-PCR (n=31); and 4) patients with AODIL qRT-PCR (n=18). Among samples used in microarray analysis, there was a significantly higher mean body mass index (BMI) in the spontaneous TL group than in the AODIL group (p = 0.0028).
Table 1.
Demographic and clinical characteristics of the study groups
| Microarray | qRT-PCR | |||||
|---|---|---|---|---|---|---|
| Term labor (n=29) |
Arrest of dilatation (n=14) |
p-value | Term labor (n=31) |
Arrest of dilatation (n=18) |
p-value* | |
| Maternal age (years) | 26.0 ± 6.5 | 24.4 ± 6.5 | 0.433 | 26.4 ± 6.6 | 24.1 ± 6.5 | 0.24 |
| BMI (kg/m2) | 32.6 ± 9.3 | 24.1 ± 2.8 | 0.0028 | 29.1 ± 7.3 | 32.2 ± 7.7 | 0.20 |
| Nulliparity, % | 55 (16/29) | 50 (7/14) | 1 | 38.7 (12/31) | 66.7 (12/18) | 0.06 |
| African-American, % | 55 (16/29) | 71 (10/14) | 0.82 | 64.5 (20/31) | 83.3 (15/18) | 0.25 |
| Smoking, % | 8 (2/26) | 14 (2/14) | 0.602 | 6.5 (2/31) | 22.22 (4/18) | 0.12 |
| Gestational age at delivery (weeks) | 39.3 ± 1.7 | 39.3 ± 1.3 | 0.956 | 39.4 ± 1.4 | 39.9 ± 1.2 | 0.21 |
| Birth weight (grams) | 3223 ± 440 | 3489 ± 371 | 0.06 | 3266 ± 441.3 | 3385.4 ± 456.7 | 0.37 |
Values are expressed as percentage (number) or mean ± standard deviation
BMI: body mass index.
P value: compare between term labor microarray and arrest of dilatation microarray
P value*: compare between term labor qRT-PCR and arrest of dilatation qRT-PCR
Microarray analysis
Table 2 lists 42 differentially expressed genes (43 probes) between (spontaneous TL and AODIL) ranked by P values. Differential expression results are depicted in Figure 1. The volcano plot (Figure 1) shows the magnitude (fold change) of the changes in the x-axis an the significance of gene expression changes in the y-axis.
Table 2.
List of all probes with differential expression between the arrest of dilatation and spontaneous term labor groups
| Illumina probe | SYMBOL | Name | p-value | q-value* | Fold Change |
Direction |
|---|---|---|---|---|---|---|
| ILMN_1708041 | PLEKHF1 | pleckstrin homology domain containing, family F (with FYVE domain) member 1 | 0.0000 | 0.1472 | 1.54 | Down |
| ILMN_1775224 | NOS3 | nitric oxide synthase 3 (endothelial cell) | 0.0000 | 0.1472 | 1.74 | Up |
| ILMN_1664861 | ID1 | inhibitor of DNA binding 1, dominant negative helix-loop-helix protein | 0.0000 | 0.1472 | 1.81 | Up |
| ILMN_2074477 | GPR4 | G protein-coupled receptor 4 | 0.0000 | 0.1480 | 1.68 | Up |
| ILMN_1794501 | HAS3 | hyaluronan synthase 3 | 0.0000 | 0.1480 | 1.52 | Up |
| ILMN_1737314 | BCL6 | B-cell CLL/lymphoma 6 | 0.0001 | 0.1924 | 1.57 | Up |
| ILMN_1710268 | ZNF385D | zinc finger protein 385D | 0.0001 | 0.1924 | 1.52 | Up |
| ILMN_1805543 | ADAMTS9 | ADAM metallopeptidase with thrombospondin type 1 motif, 9 | 0.0001 | 0.1924 | 2.19 | Up |
| ILMN_1660436 | HSPA1B | heat shock 70kDa protein 1B | 0.0001 | 0.1924 | 1.95 | Up |
| ILMN_1666503 | DENND2A | DENN/MADD domain containing 2A | 0.0001 | 0.1924 | 1.55 | Down |
| ILMN_1732296 | ID3 | inhibitor of DNA binding 3, dominant negative helix-loop-helix protein | 0.0002 | 0.1924 | 1.68 | Up |
| ILMN_1712888 | HSPH1 | heat shock 105kDa/110kDa protein 1 | 0.0002 | 0.1924 | 1.52 | Up |
| ILMN_1808811 | SBNO2 | strawberry notch homolog 2 (Drosophila) | 0.0002 | 0.1924 | 1.50 | Up |
| ILMN_1742461 | UAP1 | UDP-N-acteylglucosamine pyrophosphorylase 1 | 0.0003 | 0.1924 | 1.67 | Up |
| ILMN_1723684 | DARC | Duffy blood group, chemokine receptor | 0.0003 | 0.1924 | 1.85 | Up |
| ILMN_2226917 | KIAA0247 | KIAA0247 | 0.0003 | 0.1924 | 1.52 | Up |
| ILMN_1748538 | ALDH1A2 | aldehyde dehydrogenase 1 family, member A2 | 0.0004 | 0.1924 | 1.72 | Down |
| ILMN_1709747 | EXOG | endo/exonuclease (5'-3'), endonuclease G-like | 0.0005 | 0.1924 | 2.43 | Up |
| ILMN_2316386 | GPBAR1 | G protein-coupled bile acid receptor 1 | 0.0005 | 0.1924 | 1.51 | Down |
| ILMN_1710514 | BCL3 | B-cell CLL/lymphoma 3 | 0.0005 | 0.1935 | 1.68 | Up |
| ILMN_1656920 | CRIP1 | cysteine-rich protein 1 (intestinal) | 0.0005 | 0.1935 | 1.73 | Down |
| ILMN_2136089 | MT1IP | metallothionein 1I, pseudogene | 0.0005 | 0.1935 | 2.38 | Up |
| ILMN_1691156 | MT1A | metallothionein 1A | 0.0005 | 0.1935 | 2.19 | Up |
| ILMN_2379788 | HIF1A | hypoxia inducible factor 1, alpha subunit (basic helix-loop-helix transcription factor) | 0.0006 | 0.1935 | 1.60 | Up |
| ILMN_1686664 | MT2A | metallothionein 2A | 0.0006 | 0.1935 | 2.11 | Up |
| ILMN_2116299 | FAM110D | family with sequence similarity 110, member D | 0.0007 | 0.1935 | 1.53 | Up |
| ILMN_1719170 | WBSCR27 | Williams Beuren syndrome chromosome region 27 | 0.0008 | 0.1935 | 1.55 | Down |
| ILMN_2329914 | SPRY1 | sprouty homolog 1, antagonist of FGF signaling (Drosophila) | 0.0009 | 0.2026 | 1.55 | Up |
| ILMN_1674243 | TFRC | transferrin receptor (p90, CD71) | 0.0010 | 0.2026 | 1.83 | Up |
| ILMN_1704730 | CD93 | CD93 molecule | 0.0013 | 0.2047 | 1.61 | Up |
| ILMN_1776998 | DNAJA4 | DnaJ (Hsp40) homolog, subfamily A, member 4 | 0.0014 | 0.2080 | 1.62 | Up |
| ILMN_1789074 | HSPA1A | heat shock 70kDa protein 1A | 0.0017 | 0.2156 | 1.73 | Up |
| ILMN_2086105 | SPRY4 | sprouty homolog 4 (Drosophila) | 0.0018 | 0.2170 | 1.50 | Up |
| ILMN_1659631 | LOC440905 | uncharacterized LOC440905 | 0.0019 | 0.2254 | 2.12 | Down |
| ILMN_1763852 | ACACB | acetyl-CoA carboxylase beta | 0.0019 | 0.2254 | 1.53 | Down |
| ILMN_2053103 | SLC40A1 | solute carrier family 40 (iron-regulated transporter), member 1 | 0.0020 | 0.2265 | 1.77 | Down |
| ILMN_1720048 | CCL2 | chemokine (C-C motif) ligand 2 | 0.0020 | 0.2265 | 2.32 | Up |
| ILMN_1797744 | TPPP3 | tubulin polymerization-promoting protein family member 3 | 0.0021 | 0.2265 | 1.64 | Down |
| ILMN_1801504 | RUNX1 | runt-related transcription factor 1 | 0.0021 | 0.2265 | 1.65 | Up |
| ILMN_1707727 | ANGPTL4 | angiopoietin-like 4 | 0.0022 | 0.2267 | 2.07 | Up |
| ILMN_1739393 | SELE | selectin E | 0.0022 | 0.2267 | 2.13 | Up |
| ILMN_1761281 | MT2A | metallothionein 2A | 0.0025 | 0.2382 | 2.10 | Up |
| ILMN_1685540 | SHROOM3 | shroom family member 3 | 0.0027 | 0.2482 | 1.89 | Up |
P-values adjusted for multiple comparisons using the False Discovery Rate method
Figure 1. Microarray analysis of the gene expression profiles of myometrium in spontaneous term labor (TL) and arrest of dilatation (AODIL).
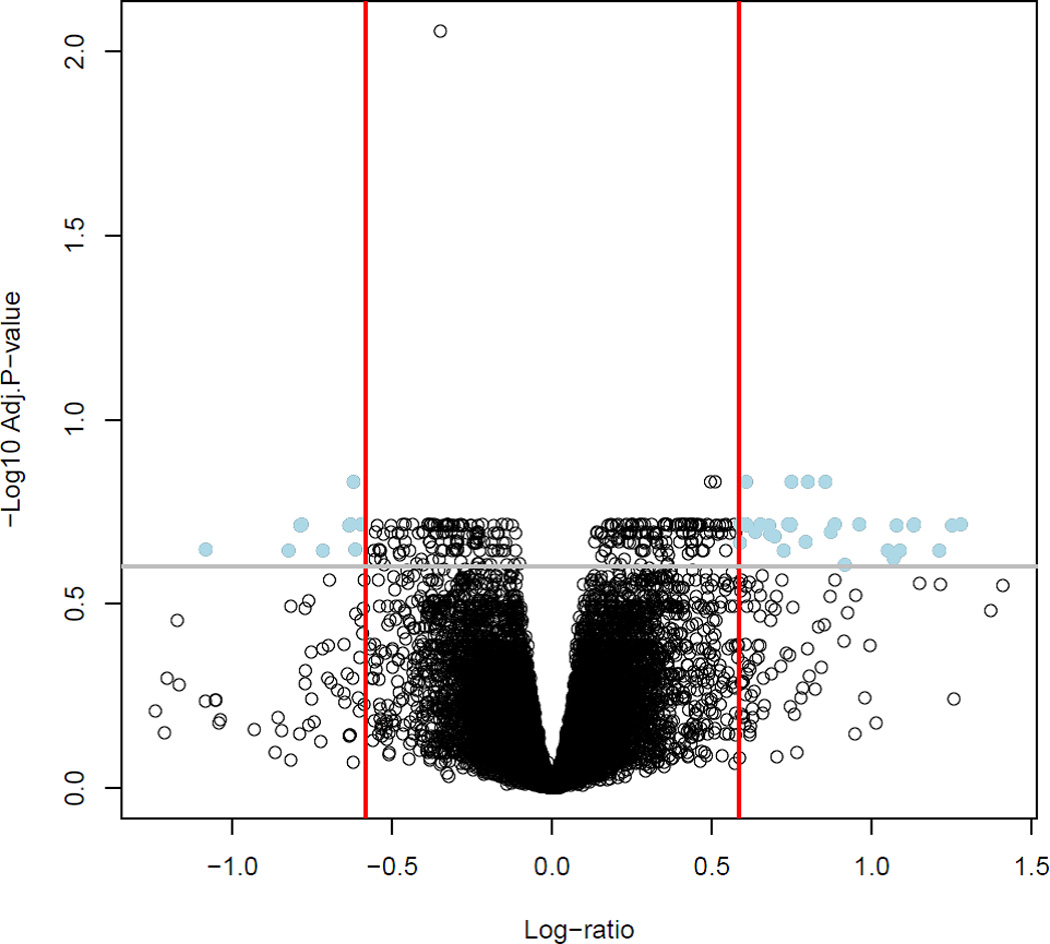
A Volcano plot shows differentially gene expression between AODIL and TL. Dots in the upper right and left quadrants represent genes with a fold change greater than 1.5 and a false discovery rate corrected p-value < 0.25. With these criteria, 42 genes were differentially expressed between the myometrial transcriptome of the two groups.
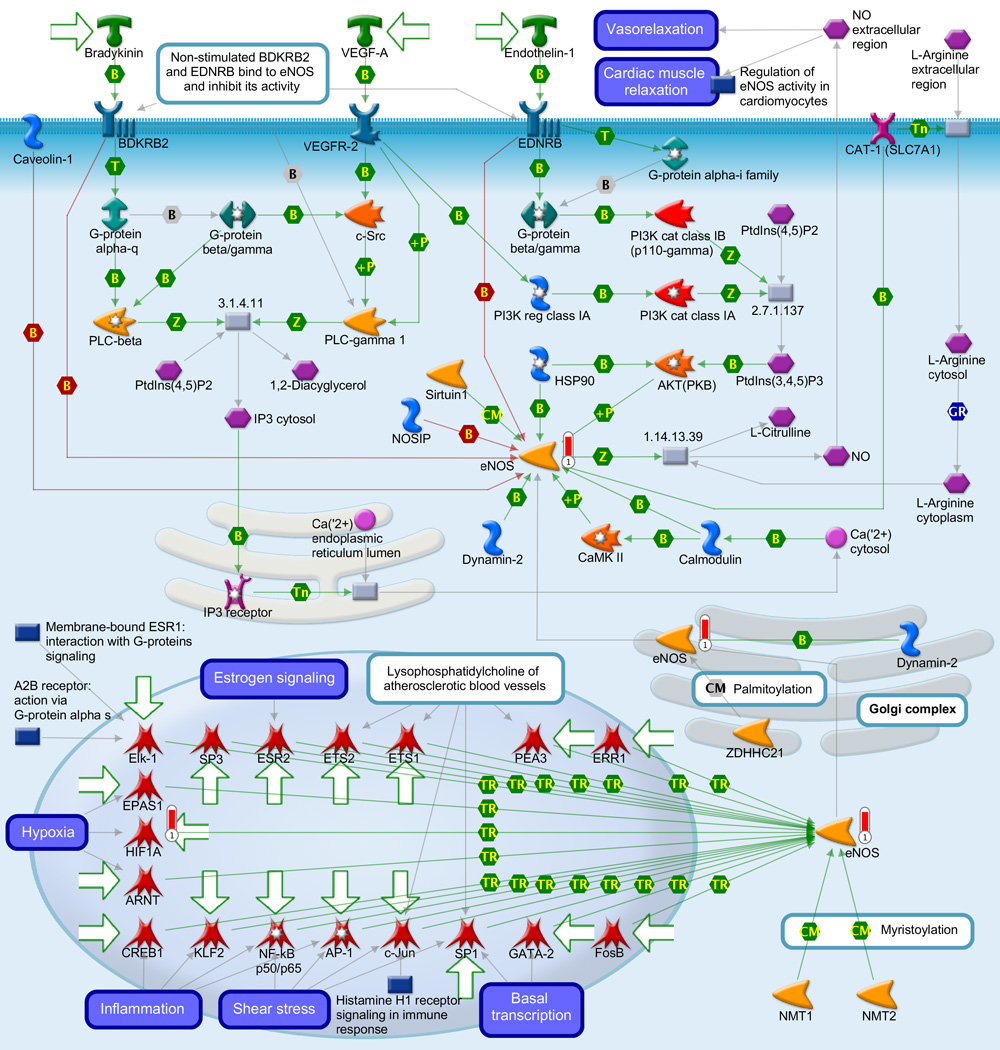
Gene ontology (GO) meta-analysis was used to gain insight into the biology related to the stereotypic differences between the myometrial transcriptome of AODIL and spontaneous TL. Significant enrichment of 106 distinct biological processes includes regulation of angiogenesis, response to hypoxia, inflammatory response and regulation of nitric-oxide synthase activity (Table 3). Pathway Analysis with PADOG ranked the TGF-β signaling pathway and Protein processing in endoplasmic reticulum pathways with a nominal p-value <0.01 (non-significant after adjusting for the 227 pathways tested). The MetaCore database was also mined (Table 4). The three pathways ranked at the top by Metacore were Regulation of metabolism were: 1) Immune response chemokine (C-C motif) ligand 2 (CCL2) signaling (p=0.001); 2) Muscle Contraction - Regulation of eNOS Activity in Endothelial Cells (p=0.01); and 3) Triiodothyronine and Thyroxine signaling (p=0.009). Figure 2A depicts immune response CCL2 signaling with CCL2 regulating the transcription of HIF1A. Figure 2B demonstrates the Muscle Contraction - Regulation of eNOS Activity in Endothelial Cells pathway with the eNOS regulating the transcription of HIF1A. CCL2, eNOS, and HIF1A were upregulated in the myometrium of patients with AODIL.
Table 3.
Gene Ontology analysis: top 40 biological processes associated with differentially expressed genes between arrest of dilatation and term labor
| GO ID | GO Name | DE genes |
GO Size |
Odds Ratio |
p- value |
q-value |
|---|---|---|---|---|---|---|
| GO:0045765 | regulation of angiogenesis | 6 | 129 | 22.3 | 0.0000 | 0.001 |
| GO:0006915 | apoptotic process | 13 | 1380 | 5.2 | 0.0000 | 0.007 |
| GO:0012501 | programmed cell death | 13 | 1391 | 5.2 | 0.0000 | 0.007 |
| GO:0022603 | regulation of anatomical structure morphogenesis | 8 | 480 | 8.1 | 0.0000 | 0.007 |
| GO:0045766 | positive regulation of angiogenesis | 4 | 69 | 26.4 | 0.0000 | 0.007 |
| GO:0072358 | cardiovascular system development | 9 | 653 | 6.9 | 0.0000 | 0.007 |
| GO:0072359 | circulatory system development | 9 | 653 | 6.9 | 0.0000 | 0.007 |
| GO:0048646 | anatomical structure formation involved in morphogenesis | 9 | 666 | 6.7 | 0.0000 | 0.007 |
| GO:0002376 | immune system process | 13 | 1492 | 4.8 | 0.0001 | 0.008 |
| GO:0008219 | cell death | 13 | 1529 | 4.7 | 0.0001 | 0.008 |
| GO:0016265 | death | 13 | 1531 | 4.7 | 0.0001 | 0.008 |
| GO:0045926 | negative regulation of growth | 5 | 169 | 13.4 | 0.0001 | 0.008 |
| GO:0001568 | blood vessel development | 7 | 426 | 7.7 | 0.0001 | 0.010 |
| GO:0050793 | regulation of developmental process | 11 | 1143 | 5.0 | 0.0001 | 0.010 |
| GO:0010043 | response to zinc ion | 3 | 37 | 36.7 | 0.0001 | 0.011 |
| GO:0046677 | response to antibiotic | 3 | 37 | 36.7 | 0.0001 | 0.011 |
| GO:0001944 | vasculature development | 7 | 447 | 7.4 | 0.0001 | 0.011 |
| GO:0001525 | angiogenesis | 6 | 311 | 8.9 | 0.0001 | 0.011 |
| GO:0001666 | response to hypoxia | 5 | 197 | 11.5 | 0.0001 | 0.011 |
| GO:0009887 | organ morphogenesis | 8 | 631 | 6.1 | 0.0002 | 0.012 |
| GO:0070482 | response to oxygen levels | 5 | 212 | 10.6 | 0.0002 | 0.014 |
| GO:0009653 | anatomical structure morphogenesis | 13 | 1711 | 4.1 | 0.0002 | 0.014 |
| GO:0010038 | response to metal ion | 5 | 223 | 10.1 | 0.0003 | 0.016 |
| GO:0045064 | T-helper 2 cell differentiation | 2 | 10 | 101.1 | 0.0003 | 0.016 |
| GO:0071294 | cellular response to zinc ion | 2 | 10 | 101.1 | 0.0003 | 0.016 |
| GO:0035295 | tube development | 6 | 359 | 7.6 | 0.0003 | 0.016 |
| GO:0048514 | blood vessel morphogenesis | 6 | 374 | 7.3 | 0.0004 | 0.019 |
| GO:0043066 | negative regulation of apoptotic process | 7 | 535 | 6.1 | 0.0004 | 0.020 |
| GO:0043069 | negative regulation of programmed cell death | 7 | 539 | 6.0 | 0.0004 | 0.020 |
| GO:0006952 | defense response | 9 | 911 | 4.8 | 0.0004 | 0.020 |
| GO:0070098 | chemokine-mediated signaling pathway | 2 | 12 | 80.8 | 0.0004 | 0.020 |
| GO:0051093 | negative regulation of developmental process | 6 | 394 | 6.9 | 0.0005 | 0.021 |
| GO:0006954 | inflammatory response | 6 | 396 | 6.9 | 0.0005 | 0.021 |
| GO:0070555 | response to interleukin-1 | 3 | 60 | 21.9 | 0.0005 | 0.021 |
| GO:0060548 | negative regulation of cell death | 7 | 560 | 5.8 | 0.0005 | 0.021 |
| GO:0006879 | cellular iron ion homeostasis | 3 | 63 | 20.8 | 0.0006 | 0.023 |
| GO:0001936 | regulation of endothelial cell proliferation | 3 | 66 | 19.8 | 0.0007 | 0.024 |
| GO:0009408 | response to heat | 3 | 66 | 19.8 | 0.0007 | 0.024 |
| GO:0046631 | alpha-beta T cell activation | 3 | 66 | 19.8 | 0.0007 | 0.024 |
| GO:0002467 | germinal center formation | 2 | 15 | 62.2 | 0.0007 | 0.024 |
Table 4.
Pathways enriched in an arrest of dilatation as determined by Metacore pathway
| Pathway | Ratio | p-value | q-value | |
|---|---|---|---|---|
| Immune response-CCL2 signaling | 3 | 38 | 0.0003 | 0.001 |
| Regulation of metabolism_Triiodothyronine and Thyroxine signaling | 2 | 28 | 0.005 | 0.009 |
| Muscle contraction-Regulation of eNOS activity in endothelial cells | 2 | 36 | 0.0078 | 0.01 |
| Development VEGF signaling via VEGFR2 - generic cascades | 2 | 51 | 0.015 | 0.015 |
CCL2 = chemokine (C-C motif) ligand 2;
eNOS = endothelial nitric oxide synthase;
VEGF=Vascular endothelial growth factor;
VEGFR2 = Vascular endothelial growth factor receptor 2
Figure 2.
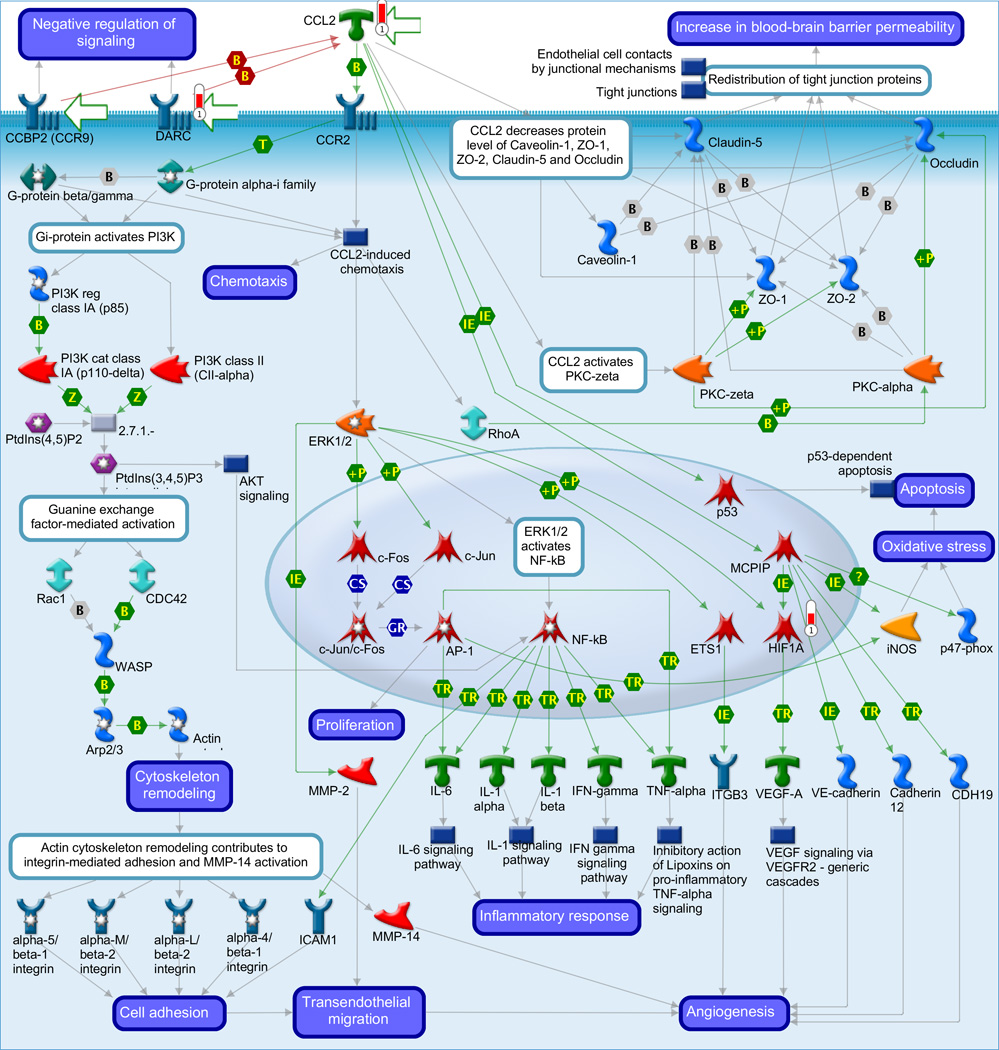
A: Display of differentially expressed genes in the arrest of dilatation group on the most significantly over-represented MetaCore pathway: chemokine (C-C motif) ligand 2 (CCL2) signaling
B: Display of differentially expressed genes in the arrest of dilatation group on the most significantly over-represented MetaCore pathway: Muscle Contraction Regulation of eNOS activity in endothelial cells
Quantitative RT-PCR (qRT-PCR)
Confirmation of microarray results was performed using qRT-PCR. An extended set of myometrium samples was used to perform qRT-PCR assays on 9 selected genes based upon the microarray data and biological significance.
qRT-PCR confirmed differential expression of all 9 genes identify with microarray analysis. Significantly overexpressed genes in AODIL included nitric oxide synthase 3 (NOS3), Angiopoietin-like 4 (ANGPTL4), ADAM metallopeptidase with thrombospondin type 1 motif, 9 (ADAMTS9), chemokine (C-C motif) ligand 2 (CCL2), G protein-coupled receptor 4 (GPR4), metallothionein (MT1A), MT2A, selectin E (SELE), hypoxia inducible factor 1 alpha subunit (HIF1A) (Figure 3). A comparison of the PCR results with the microarray data of the selected genes is shown in Table 5.
Figure 3.
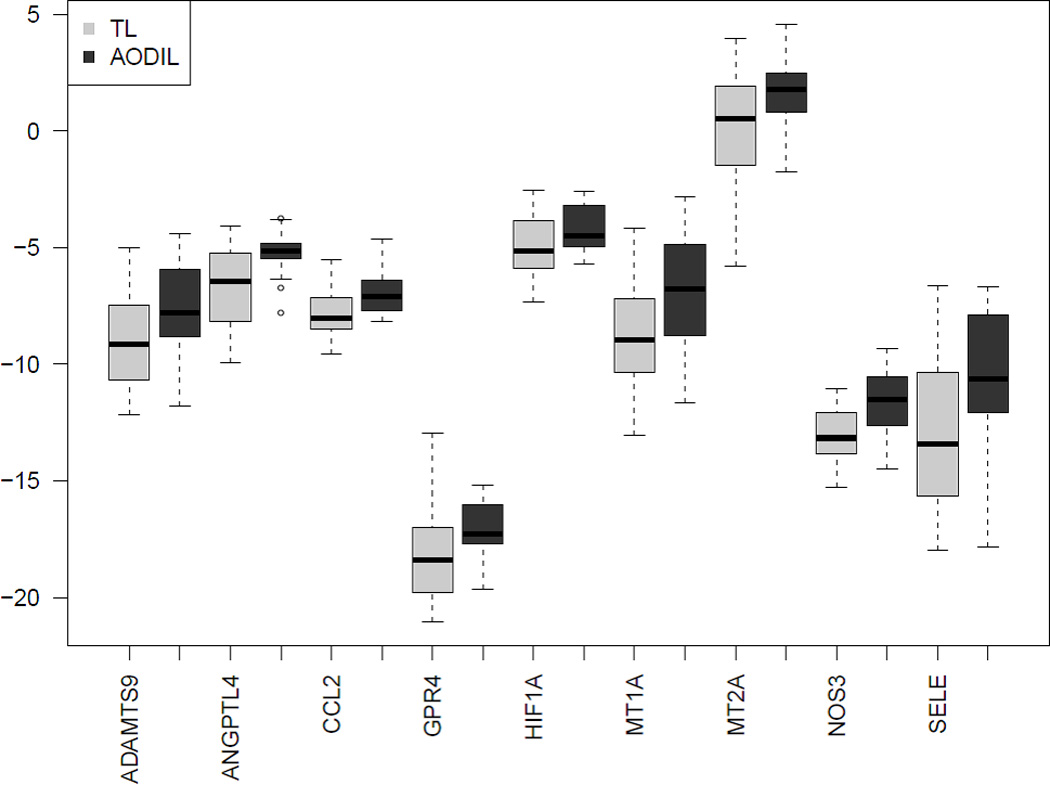
Boxplot of qRT-PCR data for all 9 tested genes in an expanded sample set including those used in the microarray experiment. The data is presented as the −ΔCt values (surrogate of log2 expression). The boxes encompass 50% of the data from the 1st quartile to the 3rd quartile. The middle line represents the median value (50%) quartile. The whiskers extend to the most extreme data point, but do not exceed values >1.5 times the interquartile range from the box. The circles represent outliers. TL=spontaneous term labor; AODIL= arrest of dilatation.
Table 5.
Comparison of qRT-PCR and microarray analysis of select genes*
| Microarray | qRT-PCR | |||||
|---|---|---|---|---|---|---|
| SYMBOL | p-value | Fold Change | Direction | P value | Fold Change | Direction |
| NOS3 | 0.0000 | 1.74 | Up | 0.000317 | 2.70 | Up |
| ANGPTL4 | 0.0022 | 2.07 | Up | 0.000701 | 3.02 | Up |
| ADAMTS9 | 0.0001 | 2.19 | Up | 0.003901 | 3.02 | Up |
| CCL2 | 0.0020 | 2.32 | Up | 0.005847 | 1.82 | Up |
| GPR4 | 0.0000 | 1.68 | Up | 0.006322 | 2.34 | Up |
| MT1A | 0.0005 | 2.19 | Up | 0.006342 | 3.66 | Up |
| MT2A | 0.0006 | 2.11 | Up | 0.008314 | 3.01 | Up |
| SELE | 0.0022 | 2.13 | Up | 0.011038 | 4.66 | Up |
| HIF1A | 0.0006 | 1.60 | Up | 0.019147 | 1.72 | Up |
Genes with significant results by microarray analysis and confirmed differential expression by qRT-PCR
Direction of change denotes changes of gene expression in arrest of dilatation compared to the term labor group
Discussion
Principal findings of the study
1) the myometrial transcriptome of patients with an AODIL has been characterized; differential expression of 42 genes was identified; 2) Gene Ontology analysis revealed enrichment of multiple biological processes and molecular functions impacting regulation of angiogenesis, response to hypoxia, inflammatory response, chemokine-mediated signaling pathway, apoptosis, stress response, and muscle contraction in an AODIL; 3) using Metacore pathway analysis, we identified enrichment of the immune response CCL2 signaling pathway, muscle contraction - regulation of eNOS Activity in the Endothelial Cells pathway, and Triiodothyronine and Thyroxine signaling; and 4) qRT-PCR performed in an independent sample group confirmed overexpression of NOS3, ANGPTL4, ADAMTS9, CCL2, GPR4, MT1A, MT2A, SELE, HIF1A in an AODIL. This is the first study to describe the transcriptome of myometrium in patients with AODIL.
Differentially expressed genes related to muscle contraction
Although action potentials in myometrium are initiated by a cellular influx of Ca2+ ions, repolarization depends on K+ ion efflux combined with inhibition of Ca2+ ion channels. A growing body of evidence supports the essential role of ion channels in uterine contractility [9,13,16,19,20,35,37,53,68,79,101,103,105,126,132,140,144,160,166,172,193,200,202]. Microarray analysis showed significant overexpression of NOS3 and HIF1A in patients with an AODIL (P<0.001); confirmatory qRT-PCR assays were also significant (P<0.05). A role for these 2 genes in the regulation of myometrial contractility has been proposed. During pregnancy, the placenta [42,50,147,161,182,201], myometrium [57,127,147,182], and chorioamniotic membranes [49,50] produce nitric oxide (NO) to maintain uterine quiescence [38,106,130,133,170,171,195–198] by stimulation of guanylate cyclase, leading to the production of cGMP and a reduction in intracellular Ca2+ [26,51,52,104,110,125,133,170,197]. Previous studies have demonstrated that uterine NO production has been up-regulated during pregnancy and down-regulated during term and preterm labor [130,171,196–198]. Overexpression of NO synthase in AODIL may change the availability of NO in parturition.
Hypoxia is associated with the reduction of blood flow to the uterus during contractions [22,80,96]. Moreover, hypoxia/ischemia of the myometrium has been linked to decreased uterine contractility, a potential cause for dysfunctional labor [84,141,145,178,179,191,192]. HIF1A is an oxygen-sensitive transcription factor that allows adaptation to hypoxic environments and response to metabolic, hypoxic or inflammatory stress [165]. In human pregnancy, HIF1A plays a role in the survival of hematopoietic precursors, embryonic vasculature [146] and trophoblast differentiation [3,44,119] during development [2]. Mittal et al [123] demonstrated a higher expression of HIF1A in the myometrium of patients with an arrest of descent (AOD) than in those with spontaneous term labor. Our findings suggest that during the course of failure to progress in labor (AODIL or AOD), a change in the expression of factors responsible for sensing tissue hypoxia occurs.
Differentially expressed genes related to inflammatory and immune response signaling in arrest of dilatation
CCL-2, also known as monocyte chemoattractant protein 1 (MCP1), is a member of a large chemokine family of soluble chemoattractant cytokines, which locally mediate leukocyte migration into various tissues [82,115]. CCL-2 is produced by endothelial cells, fibroblasts, monocytes, lymphocytes, and smooth muscle cells [47,89]. Recent evidence suggests that CCL-2 is up-regulated in human myometrium and amniotic fluid [58,91] as well as rat myometrium during labor [167]. Esplin et al. demonstrated that the amniotic fluid concentration of CCL-2 increased in women with preterm labor [59]. Moreover, mechanical strain caused by the growing fetus and experimental mechanical stretch of rat myometrial smooth muscle cells induces the release of CCL-2, and promotes chemotaxis of rat monocytes [167,168]. Hua et al [91] also reported that stretch and inflammatory cytokines (IL-1β and TNF α) induce marked increases in the expression of CCL-2 and CXCL-8 (IL8) in human term myometrium. We have previously reported that IL-8 [154] and IL-1 [155,156] were increased in the amniotic fluid of women in spontaneous labor at term. Our findings indicate that patients with an AODIL have overexpression of CCL-2 in myometrium. qRT-PCR confirmed a significant increase in CCL-2 expression in AODIL.
Biological functions of a select group of differentially expressed genes
Angiopoietin-like protein 4 (ANPTL4) is a multifunctional signal protein and expressed in the liver [102], adipose tissue [56], intestine [12] and placenta [100,188,199,203]. ANGPTL4 has been recognized as a central molecule in energy homeostasis. One of its actions is to inhibit lipoprotein lipase (LPL). This molecule also activates specific integrins to facilitate wound healing, modulate vascular permeability, and regulates reactive oxygen species (ROS) to promote tumorigenesis [81,204]. ANPTL4 is considered a positive acute phase protein since its expression in liver, heart, muscle and adipose tissue, and plasma are up regulated after LPS, TNF alpha, IL-1β, and interferon gamma treatment in mice [116]. We found a significantly higher expression of ANPTL4 in the myometrium of pregnant women with an AODIL than in those with spontaneous TL, and this finding was confirmed by qRT-PCR data.
We also found a significantly higher expression of metallothionein (MT1A) and MT2A in the myometrial of patients with an AODIL than in those with spontaneous TL. Metallothioneins have been recognized to protect against oxidative stress [93,97,162,163]. Prior studies have demonstrated increase oxidative stress in the human placenta during labor [39]. Moreover, there is an increase in pro-inflammatory cytokines/chemokines [11,17,33,45,83,99,128,138,158,175,180] and eicosanoids [73,98,121,122] and lipooxygenase arachidonate products [143,151–153] during labor. These changes accompany the increase in PMN migration and increased production of ROS in the myometrium [41,95,149]. Overexpression of MT1A and MT2A in the myometrium of patients with AODIL is consistent with previous studies demonstrating that Metallothionein genes are transcriptionally activated in cells and tissues in response to oxidative stress [7,8,159]. This overexpression in AODIL may be attributed to an increase in ROS production.
Pathway analysis
Most gene set analysis methods treat genes equally, regardless of how specific they are to a given gene set. PADOG is a new gene set analysis method that computes a gene set score as the mean of absolute values of weighted moderated gene T-scores. The gene weights are designed to emphasize the genes specific to a given pathway [181]. PADOG significantly improves gene set ranking and boosts sensitivity of analysis compared to methods that treat genes equally [181]. Using PADOG we identified enrichment of the TGF- Beta signaling Pathway and Protein processing in endoplasmic reticulum having unadjusted p-values <0.01. However, the adjusted p value was not significant (q-value=0.81 for both pathways). The Metacore database demonstrated a significantly enriched pathway; “immune response CCL2 signaling” and “muscle contraction-regulation of eNOS activity in endothelial cells” in the myometrium from patients with AODIL.
Muscle contraction-Regulation of eNOS activity in endothelial cells
Endothelial nitric oxide synthase (eNOS), one of the three distinct isoforms of nitric oxide synthases, is expressed in endothelial cells, cardiac myocytes, platelets, neurons of the brain, syncytiotrophoblasts of human placenta and kidney tubular epithelial cells [63,65]. eNOS plays a role in blood vessel vasodilatation, blood pressure regulation, platelet and leukocyte adhesion to endothelium and myometrial contractility [10,15,63]. Batlett et al reported eNOS expression (immunoblot, mRNA and protein) in the vascular endothelium of human pregnant myometrium, but not in cultured myometrial smooth muscle cells [15]. The diffusion of NO generated within the endothelium of the myometrial vasculature has implicated in uterine relaxation [15]. eNOS activity is regulated by mechanical forces such as shear stress [63] and humoral factors including estrogen, vascular endothelial growth factor, insulin and bradykinin [63]. eNOS is calcium-dependent; therefore, a higher concentration of intracellular calcium leads to eNOS activation [64].
Comparison with previous reports of functional genomics in labor dystocia
Our findings indicate that AODIL is associated with changes in the genes involved in inflammatory processes. These findings are consistent with prior reports from our group that focus on arrest of descent. [123]. Mittal et al. [123] first characterized the myometrial transcriptome of patients with an arrest of descent; 400 genes were differentially expressed. Impacted pathways included inflammation and muscle function. qRT-PCR confirmed the overexpression of HIF1A, IL-6 and prostaglandin-endoperoxide synthase 2 or cycloozygenase-2(COX2) in patients with an arrest of descent. However, our results are different from those of Brennan et al [23] who reported that 70 genes were differentially expressed in nulliparous women with dystocia (n=4) and women in spontaneous TL (n=4). Enrichment was observed in genes involved in immune response, transcription, and DNA replication [23]. Technical validation with qRT-PCR confirmed overexpression for endoplasmic reticulum aminopeptidase 2 (ERAP2), major histocompatibility complex, class II, DQ beta 1; (HLADQB1), cluster of differentiation (CD) 28, and leukocyte immunoglobulin-like receptor, subfamily A, member 3 (LILRA3) [23]. Potential explanations for the apparent differences include: 1) the criteria to diagnose labor disorders were different between our study and that of Brennan et al. We focused on AODIL, while Brennan et al studied “labor dystocia”. Yet, the two conditions are different; and 2) the sample sizes between the two studies were also different [23].
Strengths and Limitations
Strengths of this study include its prospective design, the use of a large sample set, stringent inclusion and exclusion criteria, and qRT-PCR confirmation in an independent set of specimens (biological replication).
A potential limitation of the study is that the frequency of nulliparous women was higher in the group with spontaneous labor at term than in patients with an AODIL. Yet, there was no effect modification of parity on gene expression by clinical groups (AODIL vs. TL), with one exception: KIF5C: kinesin family member 5C. Another limitation was that women in the AODIL group had lower BMI and received intrapartum oxytocin more frequently than women in spontaneous term labor [71.4% (10/14) vs. 27.6% (8/29), p=0.006]. However, we did not detect a significant interaction between AODIL and BMI or oxytocin treatment in myometrium gene expression. Lastly, the myometrial samples in the current study were taken from the lower uterine segment. It has been reported that gene expression of the lower uterine segment may be different from that of upper uterine segment [27].
Conclusion
The myometrial transcriptome of patients with an arrest of dilatation has been characterized. This disorder has been associated with a pattern of gene expression involved in muscle contraction, an inflammatory response, and hypoxia. The findings reported herein provide insight into the molecular basis, biological processes, and pathway associated with an arrest of dilatation.
Acknowledgment
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C. We wish to thank Dr. Susan Land and Dan Lott at the Applied Genomics Technology Center of Wayne State University for performing the microarrays and acknowledge the contributions of the nursing staff of the Perinatology Research Branch and Hutzel Women’s Hospital.
References
- 1.ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445–1454. doi: 10.1016/j.obstetgynecol.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Adelman DM, Maltepe E, Simon MC. HIF-1 is essential for multilineage hematopoiesis in the embryo. Adv Exp Med Biol. 2000;475:275–284. doi: 10.1007/0-306-46825-5_26. [DOI] [PubMed] [Google Scholar]
- 3.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguan K, Carvajal JA, Thompson LP, Weiner CP. Application of a functional genomics approach to identify differentially expressed genes in human myometrium during pregnancy and labour. Mol Hum Reprod. 2000;6:1141–1145. doi: 10.1093/molehr/6.12.1141. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 6.Alfaidy N, Sun M, Challis JR, Gibb W. Expression of membrane prostaglandin E synthase in human placenta and fetal membranes and effect of labor. Endocrine. 2003;20:219–225. doi: 10.1385/ENDO:20:3:219. [DOI] [PubMed] [Google Scholar]
- 7.Andrews GK. Regulation of metallothionein gene expression. Prog Food Nutr Sci. 1990;14:193–258. [PubMed] [Google Scholar]
- 8.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 9.Anwer K, Oberti C, Perez GJ, Perez-Reyes N, McDougall JK, Monga M, et al. Calcium-activated K+ channels as modulators of human myometrial contractile activity. Am J Physiol. 1993;265:C976–C985. doi: 10.1152/ajpcell.1993.265.4.C976. [DOI] [PubMed] [Google Scholar]
- 10.Arnal JF, Dinh-Xuan AT, Pueyo M, Darblade B, Rami J. Endothelium-derived nitric oxide and vascular physiology and pathology. Cell Mol Life Sci. 1999;55:1078–1087. doi: 10.1007/s000180050358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999;181:989–994. doi: 10.1016/s0002-9378(99)70337-6. [DOI] [PubMed] [Google Scholar]
- 12.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai X, Bugg GJ, Greenwood SL, Glazier JD, Sibley CP, Baker PN, et al. Expression of TASK and TREK, two-pore domain K+ channels, in human myometrium. Reproduction. 2005;129:525–530. doi: 10.1530/rep.1.00442. [DOI] [PubMed] [Google Scholar]
- 14.Barber EL, Lundsberg LS, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol. 2011;118:29–38. doi: 10.1097/AOG.0b013e31821e5f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett SR, Bennett PR, Campa JS, Dennes WJ, Slater DM, Mann GE, et al. Expression of nitric oxide synthase isoforms in pregnant human myometrium. J Physiol. 1999;521(Pt 3):705–716. doi: 10.1111/j.1469-7793.1999.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanks AM, Zhao ZH, Shmygol A, Bru-Mercier G, Astle S, Thornton S. Characterization of the molecular and electrophysiological properties of the T-type calcium channel in human myometrium. J Physiol. 2007;581:915–926. doi: 10.1113/jphysiol.2007.132126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogavac MA, Brkic S. Serum proinflammatory cytokine - interleukin-8 as possible infection site marker in preterm deliveries. J Perinat Med. 2009;37:707–708. doi: 10.1515/JPM.2009.115. [DOI] [PubMed] [Google Scholar]
- 18.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 19.Boyle MB, Heslip LA. Voltage-dependent Na+ channel mRNA expression in pregnant myometrium. Receptors Channels. 1994;2:249–253. [PubMed] [Google Scholar]
- 20.Brainard AM, Korovkina VP, England SK. Potassium channels and uterine function. Semin Cell Dev Biol. 2007;18:332–339. doi: 10.1016/j.semcdb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branch DW, Silver RM. Managing the primary cesarean delivery rate. Clin Obstet Gynecol. 2012;55:946–960. doi: 10.1097/GRF.0b013e318263c547. [DOI] [PubMed] [Google Scholar]
- 22.Brar HS, Platt LD, DeVore GR, Horenstein J, Medearis AL. Qualitative assessment of maternal uterine and fetal umbilical artery blood flow and resistance in laboring patients by Doppler velocimetry. Am J Obstet Gynecol. 1988;158:952–956. doi: 10.1016/0002-9378(88)90100-7. [DOI] [PubMed] [Google Scholar]
- 23.Brennan DJ, McGee SF, Rexhepaj E, O'Connor DP, Robson M, O'Herlihy C. Identification of a myometrial molecular profile for dystocic labor. BMC Pregnancy Childbirth. 2011;11:74. doi: 10.1186/1471-2393-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brill Y, Windrim R. Vaginal birth after Caesarean section: review of antenatal predictors of success. J Obstet Gynaecol Can. 2003;25:275–286. doi: 10.1016/s1701-2163(16)31030-1. [DOI] [PubMed] [Google Scholar]
- 25.Brown AG, Leite RS, Engler AJ, Discher DE, Strauss JF., 3rd A hemoglobin fragment found in cervicovaginal fluid from women in labor potentiates the action of agents that promote contraction of smooth muscle cells. Peptides. 2006;27:1794–1800. doi: 10.1016/j.peptides.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Buhimschi I, Yallampalli C, Dong YL, Garfield RE. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. Am J Obstet Gynecol. 1995;172:1577–1584. doi: 10.1016/0002-9378(95)90500-6. [DOI] [PubMed] [Google Scholar]
- 27.Bukowski R, Hankins GD, Saade GR, Anderson GD, Thornton S. Labor-associated gene expression in the human uterine fundus, lower segment, and cervix. PLoS Med. 2006;3:e169. doi: 10.1371/journal.pmed.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bytautiene E, Vedernikov YP, Saade GR, Romero R, Garfield RE. Degranulation of uterine mast cell modifies contractility of isolated myometrium from pregnant women. Am J Obstet Gynecol. 2004;191:1705–1710. doi: 10.1016/j.ajog.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Carbillon L, Seince N, Uzan M. Myometrial maturation and labour. Ann Med. 2001;33:571–578. [PubMed] [Google Scholar]
- 30.Carvajal JA, Vidal RJ, Cuello MA, Poblete JA, Weiner CP. Mechanisms of paracrine regulation by fetal membranes of human uterine quiescence. J Soc Gynecol Investig. 2006;13:343–349. doi: 10.1016/j.jsgi.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Challis JR, Patel FA, Pomini F. Prostaglandin dehydrogenase and the initiation of labor. J Perinat Med. 1999;27:26–34. doi: 10.1515/JPM.1999.003. [DOI] [PubMed] [Google Scholar]
- 32.Challis JRG. Mechanism of parturition and preterm labor. Obstet Gynecol Surv. 2000;55:650–660. doi: 10.1097/00006254-200010000-00025. [DOI] [PubMed] [Google Scholar]
- 33.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 34.Chan EC, Fraser S, Yin S, Yeo G, Kwek K, Fairclough RJ, et al. Human myometrial genes are differentially expressed in labor: a suppression subtractive hybridization study. J Clin Endocrinol Metab. 2002;87:2435–2441. doi: 10.1210/jcem.87.6.8439. [DOI] [PubMed] [Google Scholar]
- 35.Chanrachakul B, Matharoo-Ball B, Turner A, Robinson G, Broughton-Pipkin F, Arulkumaran S, et al. Immunolocalization and protein expression of the alpha subunit of the large-conductance calcium-activated potassium channel in human myometrium. Reproduction. 2003;126:43–48. doi: 10.1530/rep.0.1260043. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhari BP, Plunkett J, Ratajczak CK, Shen TT, DeFranco EA, Muglia LJ. The genetics of birth timing: insights into a fundamental component of human development. Clin Genet. 2008;74:493–501. doi: 10.1111/j.1399-0004.2008.01124.x. [DOI] [PubMed] [Google Scholar]
- 37.Chung D, Kim YS, Phillips JN, Ulloa A, Ku CY, Galan HL, et al. Attenuation of canonical transient receptor potential-like channel 6 expression specifically reduces the diacylglycerol-mediated increase in intracellular calcium in human myometrial cells. Endocrinology. 2010;151:406–416. doi: 10.1210/en.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chwalisz K, Garfield RE. Role of nitric oxide in the uterus and cervix: implications for the management of labor. J Perinat Med. 1998;26:448–457. doi: 10.1515/jpme.1998.26.6.448. [DOI] [PubMed] [Google Scholar]
- 39.Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, et al. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am J Pathol. 2007;171:1168–1179. doi: 10.2353/ajpath.2007.070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cong B, Zhang L, Gao L, Ni X. Reduced expression of CRH receptor type 1 in upper segment human myometrium during labour. Reprod Biol Endocrinol. 2009;7:43. doi: 10.1186/1477-7827-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–277. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 42.Conrad KP, Vill M, McGuire PG, Dail WG, Davis AK. Expression of nitric oxide synthase by syncytiotrophoblast in human placental villi. FASEB J. 1993;7:1269–1276. doi: 10.1096/fasebj.7.13.7691671. [DOI] [PubMed] [Google Scholar]
- 43.Cordeaux Y, Pasupathy D, Bacon J, Charnock-Jones DS, Smith GC. Characterization of serotonin receptors in pregnant human myometrium. J Pharmacol Exp Ther. 2009;328:682–691. doi: 10.1124/jpet.108.143040. [DOI] [PubMed] [Google Scholar]
- 44.Cowden Dahl KD, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM, et al. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol Cell Biol. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox SM, King MR, Casey ML, MacDonald PC. Interleukin-1 beta-1 alpha, and-6 and prostaglandins in vaginal/cervical fluids of pregnant women before and during labor. J Clin Endocrinol Metab. 1993;77:805–815. doi: 10.1210/jcem.77.3.8370702. [DOI] [PubMed] [Google Scholar]
- 46.Dalrymple A, Mahn K, Poston L, Songu-Mize E, Tribe RM. Mechanical stretch regulates TRPC expression and calcium entry in human myometrial smooth muscle cells. Mol Hum Reprod. 2007;13:171–179. doi: 10.1093/molehr/gal110. [DOI] [PubMed] [Google Scholar]
- 47.De Rossi M, Bernasconi P, Baggi F, de Waal Malefyt R, Mantegazza R. Cytokines and chemokines are both expressed by human myoblasts: possible relevance for the immune pathogenesis of muscle inflammation. Int Immunol. 2000;12:1329–1335. doi: 10.1093/intimm/12.9.1329. [DOI] [PubMed] [Google Scholar]
- 48.de Wit NC, Heck AJ, Thornton S. The effect of oxytocin and an oxytocin antagonist on the human myometrial proteome. Reprod Sci. 2010;17:40–46. doi: 10.1177/1933719109345287. [DOI] [PubMed] [Google Scholar]
- 49.Dennes WJ, Slater DM, Bennett PR. Nitric oxide synthase mRNA expression in human fetal membranes: a possible role in parturition. Biochem Biophys Res Commun. 1997;233:276–278. doi: 10.1006/bbrc.1997.6439. [DOI] [PubMed] [Google Scholar]
- 50.Di Iulio JL, Gude NM, King RG, Brennecke SP. Human placental and fetal membrane nitric oxide synthase activity before, during and after labour at term. Reprod Fertil Dev. 1995;7:1505–1508. doi: 10.1071/rd9951505. [DOI] [PubMed] [Google Scholar]
- 51.Diamond J. Lack of correlation between cyclic GMP elevation and relaxation of nonvascular smooth muscle by nitroglycerin, nitroprusside, hydroxylamine and sodium azide. J Pharmacol Exp Ther. 1983;225:422–426. [PubMed] [Google Scholar]
- 52.Diamond J, Holmes TG. Effects of potassium chloride and smooth muscle relaxants on tension and cyclic nucleotide levels in rat myometrium. Can J Physiol Pharmacol. 1975;53:1099–1107. doi: 10.1139/y75-153. [DOI] [PubMed] [Google Scholar]
- 53.Doheny HC, Lynch CM, Smith TJ, Morrison JJ. Functional coupling of beta3-adrenoceptors and large conductance calcium-activated potassium channels in human uterine myocytes. J Clin Endocrinol Metab. 2005;90:5786–5796. doi: 10.1210/jc.2005-0574. [DOI] [PubMed] [Google Scholar]
- 54.Dong YL, Fang L, Kondapaka S, Gangula PR, Wimalawansa SJ, Yallampalli C. Involvement of calcitonin gene-related peptide in the modulation of human myometrial contractility during pregnancy. J Clin Invest. 1999;104:559–565. doi: 10.1172/JCI6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doring B, Shynlova O, Tsui P, Eckardt D, Janssen-Bienhold U, Hofmann F, et al. Ablation of connexin43 in uterine smooth muscle cells of the mouse causes delayed parturition. J Cell Sci. 2006;119:1715–1722. doi: 10.1242/jcs.02892. [DOI] [PubMed] [Google Scholar]
- 56.Dutton S, Trayhurn P. Regulation of angiopoietin-like protein 4/fasting-induced adipose factor (Angptl4/FIAF) expression in mouse white adipose tissue and 3T3-L1 adipocytes. Br J Nutr. 2008;100:18–26. doi: 10.1017/S0007114507882961. [DOI] [PubMed] [Google Scholar]
- 57.Eis AL, Brockman DE, Pollock JS, Myatt L. Immunohistochemical localization of endothelial nitric oxide synthase in human villous and extravillous trophoblast populations and expression during syncytiotrophoblast formation in vitro. Placenta. 1995;16:113–126. doi: 10.1016/0143-4004(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 58.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J Matern Fetal Neonatal Med. 2003;14:51–56. doi: 10.1080/jmf.14.1.51.56. [DOI] [PubMed] [Google Scholar]
- 59.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–373. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 60.Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26:661–671. doi: 10.1016/j.placenta.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 61.Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics. 2007;23:257–258. doi: 10.1093/bioinformatics/btl567. [DOI] [PubMed] [Google Scholar]
- 62.Fischer DP, Hutchinson JA, Farrar D, O'Donovan PJ, Woodward DF, Marshall KM. Loss of prostaglandin F2alpha, but not thromboxane, responsiveness in pregnant human myometrium during labour. J Endocrinol. 2008;197:171–179. doi: 10.1677/JOE-07-0494. [DOI] [PubMed] [Google Scholar]
- 63.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. 37a–37d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forstermann U, Pollock JS, Schmidt HH, Heller M, Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88:1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, et al. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.hyp.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 66.Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567–589. doi: 10.1016/s0029-7844(02)02398-0. [DOI] [PubMed] [Google Scholar]
- 67.Friel AM, Curley M, Ravikumar N, Smith TJ, Morrison JJ. Rho A/Rho kinase mRNA and protein levels in human myometrium during pregnancy and labor. J Soc Gynecol Investig. 2005;12:20–27. doi: 10.1016/j.jsgi.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Gao L, Cong B, Zhang L, Ni X. Expression of the calcium-activated potassium channel in upper and lower segment human myometrium during pregnancy and parturition. Reprod Biol Endocrinol. 2009;7:27. doi: 10.1186/1477-7827-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garfield RE. Control of myometrial function in preterm versus term labor. Clin Obstet Gynecol. 1984;27:572–591. doi: 10.1097/00003081-198409000-00007. [DOI] [PubMed] [Google Scholar]
- 70.Garfield RE, Ali M, Yallampalli C, Izumi H. Role of gap junctions and nitric oxide in control of myometrial contractility. Semin Perinatol. 1995;19:41–51. doi: 10.1016/s0146-0005(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 71.Giannoulias D, Patel FA, Holloway AC, Lye SJ, Tai HH, Challis JR. Differential changes in 15-hydroxyprostaglandin dehydrogenase and prostaglandin H synthase (types I and II) in human pregnant myometrium. J Clin Endocrinol Metab. 2002;87:1345–1352. doi: 10.1210/jcem.87.3.8317. [DOI] [PubMed] [Google Scholar]
- 72.Giannoulias D, Alfaidy N, Holloway AC, Gibb W, Sun M, Lye SJ, et al. Expression of prostaglandin I(2) synthase, but not prostaglandin E synthase, changes in myometrium of women at term pregnancy. J Clin Endocrinol Metab. 2002;87:5274–5282. doi: 10.1210/jc.2002-020521. [DOI] [PubMed] [Google Scholar]
- 73.Gibb W. The role of prostaglandins in human parturition. Ann Med. 1998;30:235–241. doi: 10.3109/07853899809005850. [DOI] [PubMed] [Google Scholar]
- 74.Gibb W, Challis JR. Mechanisms of term and preterm birth. J Obstet Gynaecol Can. 2002;24:874–883. doi: 10.1016/s1701-2163(16)31044-1. [DOI] [PubMed] [Google Scholar]
- 75.Gifford DS, Morton SC, Fiske M, Keesey J, Keeler E, Kahn KL. Lack of progress in labor as a reason for cesarean. Obstet Gynecol. 2000;95:589–595. doi: 10.1016/s0029-7844(99)00575-x. [DOI] [PubMed] [Google Scholar]
- 76.Grammatopoulos DK. The role of CRH receptors and their agonists in myometrial contractility and quiescence during pregnancy and labour. Front Biosci. 2007;12:561–571. doi: 10.2741/2082. [DOI] [PubMed] [Google Scholar]
- 77.Grammatopoulos DK. Placental corticotrophin-releasing hormone and its receptors in human pregnancy and labour: still a scientific enigma. J Neuroendocrinol. 2008;20:432–438. doi: 10.1111/j.1365-2826.2008.01660.x. [DOI] [PubMed] [Google Scholar]
- 78.Grammatopoulos DK, Hillhouse EW. Role of corticotropin-releasing hormone in onset of labour. Lancet. 1999;354:1546–1549. doi: 10.1016/S0140-6736(99)03418-2. [DOI] [PubMed] [Google Scholar]
- 79.Greenwood IA, Yeung SY, Tribe RM, Ohya S. Loss of functional K+ channels encoded by ether-a-go-go-related genes in mouse myometrium prior to labour onset. J Physiol. 2009;587:2313–2326. doi: 10.1113/jphysiol.2009.171272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greiss FC., Jr Effect of labor on uterine blood flow. Observations on gravid ewes. Am J Obstet Gynecol. 1965;93:917–923. doi: 10.1016/0002-9378(65)90150-x. [DOI] [PubMed] [Google Scholar]
- 81.Grootaert C, Van de Wiele T, Verstraete W, Bracke M, Vanhoecke B. Angiopoietin-like protein 4: health effects, modulating agents and structure-function relationships. Expert Rev Proteomics. 2012;9:181–199. doi: 10.1586/epr.12.12. [DOI] [PubMed] [Google Scholar]
- 82.Gruden G, Setti G, Hayward A, Sugden D, Duggan S, Burt D, et al. Mechanical stretch induces monocyte chemoattractant activity via an NF-kappaB-dependent monocyte chemoattractant protein-1-mediated pathway in human mesangial cells: inhibition by rosiglitazone. J Am Soc Nephrol. 2005;16:688–696. doi: 10.1681/ASN.2004030251. [DOI] [PubMed] [Google Scholar]
- 83.Hamill N, Romero R, Gotsch F, Kusanovic JP, Edwin S, Erez O, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med. 2008;36:217–227. doi: 10.1515/JPM.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harrison N, Larcombe-McDouall JB, Earley L, Wray S. An in vivo study of the effects of ischaemia on uterine contraction, intracellular pH and metabolites in the rat. J Physiol. 1994;476:349–354. doi: 10.1113/jphysiol.1994.sp020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195:778–786. doi: 10.1016/j.ajog.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 86.Hassan SS, Romero R, Tarca AL, Draghici S, Pineles B, Bugrim A, et al. Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2007;197:250 e1–250 e7. doi: 10.1016/j.ajog.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Vaisbuch E, Erez O, et al. The transcriptome of cervical ripening in human pregnancy before the onset of labor at term: identification of novel molecular functions involved in this process. J Matern Fetal Neonatal Med. 2009;22:1183–1193. doi: 10.3109/14767050903353216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Havelock JC, Keller P, Muleba N, Mayhew BA, Casey BM, Rainey WE, et al. Human myometrial gene expression before and during parturition. Biol Reprod. 2005;72:707–719. doi: 10.1095/biolreprod.104.032979. [DOI] [PubMed] [Google Scholar]
- 89.He YY, Du MR, Guo PF, He XJ, Zhou WH, Zhu XY, et al. Regulation of C-C motif chemokine ligand 2 and its receptor in human decidual stromal cells by pregnancy-associated hormones in early gestation. Hum Reprod. 2007;22:2733–2742. doi: 10.1093/humrep/dem208. [DOI] [PubMed] [Google Scholar]
- 90.Hofmeyr GJ. Obstructed labor: using better technologies to reduce mortality. Int J Gynaecol Obstet. 2004;85(Suppl 1):S62–S72. doi: 10.1016/j.ijgo.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 91.Hua R, Pease JE, Sooranna SR, Viney JM, Nelson SM, Myatt L, et al. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-kappaB activation. Endocrinology. 2012;153:481–491. doi: 10.1210/en.2011-1506. [DOI] [PubMed] [Google Scholar]
- 92.Hurd WW, Gibbs SG, Rudinsky KA. Differential regulation of myometrial prostaglandin production by changes in length. Am J Obstet Gynecol. 2008;198:225 e1–225 e4. doi: 10.1016/j.ajog.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 93.Hussain S, Slikker W, Jr, Ali SF. Role of metallothionein and other antioxidants in scavenging superoxide radicals and their possible role in neuroprotection. Neurochem Int. 1996;29:145–152. doi: 10.1016/0197-0186(95)00114-x. [DOI] [PubMed] [Google Scholar]
- 94.Imamura T, Luedke CE, Vogt SK, Muglia LJ. Oxytocin modulates the onset of murine parturition by competing ovarian and uterine effects. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1061–R1067. doi: 10.1152/ajpregu.2000.279.3.R1061. [DOI] [PubMed] [Google Scholar]
- 95.Jenkin G, Young IR. Mechanisms responsible for parturition; the use of experimental models. Anim Reprod Sci. 2004;82–83:567–581. doi: 10.1016/j.anireprosci.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 96.Jones NW, Raine-Fenning NJ, Jayaprakasan K, Mousa HA, Taggart MJ, Bugg GJ. Changes in myometrial 'perfusion' during normal labor as visualized by three-dimensional power Doppler angiography. Ultrasound Obstet Gynecol. 2009;33:307–312. doi: 10.1002/uog.6303. [DOI] [PubMed] [Google Scholar]
- 97.Kang YJ, Li G, Saari JT. Metallothionein inhibits ischemia-reperfusion injury in mouse heart. Am J Physiol. 1999;276:H993–H997. doi: 10.1152/ajpheart.1999.276.3.H993. [DOI] [PubMed] [Google Scholar]
- 98.Keelan JA, Sato TA, Gupta DK, Marvin KW, Mitchell MD. Prostanoid stimulation of cytokine production in an amnion-derived cell line: evidence of a feed-forward mechanism with implications for term and preterm labor. J Soc Gynecol Investig. 2000;7:37–44. doi: 10.1016/s1071-5576(99)00065-9. [DOI] [PubMed] [Google Scholar]
- 99.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol. 1999;181:1530–1536. doi: 10.1016/s0002-9378(99)70400-x. [DOI] [PubMed] [Google Scholar]
- 100.Kersten S. Regulation of lipid metabolism via angiopoietin-like proteins. Biochem Soc Trans. 2005;33:1059–1062. doi: 10.1042/BST20051059. [DOI] [PubMed] [Google Scholar]
- 101.Khan RN, Smith SK, Morrison JJ, Ashford ML. Properties of large-conductance K+ channels in human myometrium during pregnancy and labour. Proc Biol Sci. 1993;251:9–15. doi: 10.1098/rspb.1993.0002. [DOI] [PubMed] [Google Scholar]
- 102.Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, et al. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J. 2000;346(Pt 3):603–610. [PMC free article] [PubMed] [Google Scholar]
- 103.Korovkina VP, Brainard AM, England SK. Translocation of an endoproteolytically cleaved maxi-K channel isoform: mechanisms to induce human myometrial cell repolarization. J Physiol. 2006;573:329–341. doi: 10.1113/jphysiol.2006.106922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krall JF, Morin A. The role of cyclic GMP in cells with the properties of smooth muscle cultured from the rat myometrium. J Cell Physiol. 1986;129:250–256. doi: 10.1002/jcp.1041290217. [DOI] [PubMed] [Google Scholar]
- 105.Ku CY, Babich L, Word RA, Zhong M, Ulloa A, Monga M, et al. Expression of transient receptor channel proteins in human fundal myometrium in pregnancy. J Soc Gynecol Investig. 2006;13:217–225. doi: 10.1016/j.jsgi.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 106.Ledingham MA, Thomson AJ, Greer IA, Norman JE. Nitric oxide in parturition. BJOG. 2000;107:581–593. doi: 10.1111/j.1471-0528.2000.tb13297.x. [DOI] [PubMed] [Google Scholar]
- 107.Lei H, Vadillo-Ortega F, Paavola LG, Strauss JF., 3rd 92-kDa gelatinase (matrix metalloproteinase-9) is induced in rat amnion immediately prior to parturition. Biol Reprod. 1995;53:339–344. doi: 10.1095/biolreprod53.2.339. [DOI] [PubMed] [Google Scholar]
- 108.Lei H, Kalluri R, Furth EE, Baker AH, Strauss JF., 3rd Rat amnion type IV collagen composition and metabolism: implications for membrane breakdown. Biol Reprod. 1999;60:176–182. doi: 10.1095/biolreprod60.1.176. [DOI] [PubMed] [Google Scholar]
- 109.Lei H, Furth EE, Kalluri R, Chiou T, Tilly KI, Tilly JL, et al. A program of cell death and extracellular matrix degradation is activated in the amnion before the onset of labor. J Clin Invest. 1996;98:1971–1978. doi: 10.1172/JCI119001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leiber D, Vesin MF, Harbon S. Regulation of guanosine 3',5'-cyclic monophosphate levels and contractility in rat myometrium. FEBS Lett. 1978;86:183–187. doi: 10.1016/0014-5793(78)80559-6. [DOI] [PubMed] [Google Scholar]
- 111.Li W, Alfaidy N, Challis JR. Expression of extracellular matrix metalloproteinase inducer in human placenta and fetal membranes at term labor. J Clin Endocrinol Metab. 2004;89:2897–2904. doi: 10.1210/jc.2003-032048. [DOI] [PubMed] [Google Scholar]
- 112.Li Y, Reznichenko M, Tribe RM, Hess PE, Taggart M, Kim H, et al. Stretch activates human myometrium via ERK, caldesmon and focal adhesion signaling. PLoS One. 2009;4:e7489. doi: 10.1371/journal.pone.0007489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liggins GC. Fetal influences on myometrial contractility. Clin Obstet Gynecol. 1973;16:148–165. doi: 10.1097/00003081-197309000-00010. [DOI] [PubMed] [Google Scholar]
- 114.Linton EA, Woodman JR, Asboth G, Glynn BP, Plested CP, Bernal AL. Corticotrophin releasing hormone: its potential for a role in human myometrium. Exp Physiol. 2001;86:273–281. doi: 10.1113/eph8602183. [DOI] [PubMed] [Google Scholar]
- 115.Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, et al. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu B, Moser A, Shigenaga JK, Grunfeld C, Feingold KR. The acute phase response stimulates the expression of angiopoietin like protein 4. Biochem Biophys Res Commun. 2010;391:1737–1741. doi: 10.1016/j.bbrc.2009.12.145. [DOI] [PubMed] [Google Scholar]
- 117.Maggi M, Del Carlo P, Fantoni G, Giannini S, Torrisi C, Casparis D, et al. Human myometrium during pregnancy contains and responds to V1 vasopressin receptors as well as oxytocin receptors. J Clin Endocrinol Metab. 1990;70:1142–1154. doi: 10.1210/jcem-70-4-1142. [DOI] [PubMed] [Google Scholar]
- 118.Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13:981–992. doi: 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- 119.Maltepe E, Krampitz GW, Okazaki KM, Red-Horse K, Mak W, Simon MC, et al. Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development. 2005;132:3393–3403. doi: 10.1242/dev.01923. [DOI] [PubMed] [Google Scholar]
- 120.Maul H, Longo M, Saade GR, Garfield RE. Nitric oxide and its role during pregnancy: from ovulation to delivery. Curr Pharm Des. 2003;9:359–380. doi: 10.2174/1381612033391784. [DOI] [PubMed] [Google Scholar]
- 121.Mitchell MD, Edwin S, Romero RJ. Prostaglandin biosynthesis by human decidual cells: effects of inflammatory mediators. Prostaglandins Leukot Essent Fatty Acids. 1990;41:35–38. doi: 10.1016/0952-3278(90)90128-8. [DOI] [PubMed] [Google Scholar]
- 122.Mitchell MD, Romero RJ, Edwin SS, Trautman MS. Prostaglandins and parturition. Reprod Fertil Dev. 1995;7:623–632. doi: 10.1071/rd9950623. [DOI] [PubMed] [Google Scholar]
- 123.Mittal P, Romero R, Tarca AL, Draghici S, Nhan-Chang CL, Chaiworapongsa T, et al. A molecular signature of an arrest of descent in human parturition. Am J Obstet Gynecol. 2011;204:177 e15–177 e33. doi: 10.1016/j.ajog.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med. 2010;38:617–643. doi: 10.1515/JPM.2010.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 126.Morrison JJ, Ashford ML, Khan RN, Smith SK. The effects of potassium channel openers on isolated pregnant human myometrium before and after the onset of labor: potential for tocolysis. Am J Obstet Gynecol. 1993;169:1277–1285. doi: 10.1016/0002-9378(93)90294-s. [DOI] [PubMed] [Google Scholar]
- 127.Myatt L, Brockman DE, Eis AL, Pollock JS. Immunohistochemical localization of nitric oxide synthase in the human placenta. Placenta. 1993;14:487–495. doi: 10.1016/s0143-4004(05)80202-4. [DOI] [PubMed] [Google Scholar]
- 128.Nace J, Fortunato SJ, Maul H, Menon R. The expression pattern of two novel cytokines (IL-24 and IL-29) in human fetal membranes. J Perinat Med. 2010;38:665–670. doi: 10.1515/jpm.2010.093. [DOI] [PubMed] [Google Scholar]
- 129.Nakla S, Skinner K, Mitchell BF, Challis JR. Changes in prostaglandin transfer across human fetal membranes obtained after spontaneous labor. Am J Obstet Gynecol. 1986;155:1337–1341. doi: 10.1016/0002-9378(86)90170-5. [DOI] [PubMed] [Google Scholar]
- 130.Natuzzi ES, Ursell PC, Harrison M, Buscher C, Riemer RK. Nitric oxide synthase activity in the pregnant uterus decreases at parturition. Biochem Biophys Res Commun. 1993;194:1–8. doi: 10.1006/bbrc.1993.1776. [DOI] [PubMed] [Google Scholar]
- 131.Nhan-Chang CL, Romero R, Tarca AL, Mittal P, Kusanovic JP, Erez O, et al. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. Am J Obstet Gynecol. 2010;202:462 e1–462 e41. doi: 10.1016/j.ajog.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Noble K, Matthew A, Burdyga T, Wray S. A review of recent insights into the role of the sarcoplasmic reticulum and Ca entry in uterine smooth muscle. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S11–S19. doi: 10.1016/j.ejogrb.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 133.Norman JE, Cameron IT. Nitric oxide in the human uterus. Rev Reprod. 1996;1:61–68. doi: 10.1530/ror.0.0010061. [DOI] [PubMed] [Google Scholar]
- 134.Norwitz ER, Robinson JN, Challis JR. The control of labor. N Engl J Med. 1999;341:660–666. doi: 10.1056/NEJM199908263410906. [DOI] [PubMed] [Google Scholar]
- 135.O'Brien M, Morrison JJ, Smith TJ. Upregulation of PSCDBP, TLR2, TWIST1, FLJ35382, EDNRB, and RGS12 gene expression in human myometrium at labor. Reprod Sci. 2008;15:382–393. doi: 10.1177/1933719108316179. [DOI] [PubMed] [Google Scholar]
- 136.Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol. 2003;17:717–730. doi: 10.1016/s1521-6934(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 137.Olson DM, Mijovic JE, Sadowsky DW. Control of human parturition. Semin Perinatol. 1995;19:52–63. doi: 10.1016/s0146-0005(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 138.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–229. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- 139.Paavola LG, Furth EE, Delgado V, Boyd CO, Jacobs CC, Lei H, et al. Striking changes in the structure and organization of rat fetal membranes precede parturition. Biol Reprod. 1995;53:321–338. doi: 10.1095/biolreprod53.2.321. [DOI] [PubMed] [Google Scholar]
- 140.Parkington HC, Tonta MA, Davies NK, Brennecke SP, Coleman HA. Hyperpolarization and slowing of the rate of contraction in human uterus in pregnancy by prostaglandins E2 and f2alpha: involvement of the Na+ pump. J Physiol. 1999;514(Pt 1):229–243. doi: 10.1111/j.1469-7793.1999.229af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parratt J, Taggart M, Wray S. Abolition of contractions in the myometrium by acidification in vitro. Lancet. 1994;344:717–718. doi: 10.1016/s0140-6736(94)92209-8. [DOI] [PubMed] [Google Scholar]
- 142.Patel FA, Challis JR. Prostaglandins and uterine activity. Front Horm Res. 2001;27:31–56. doi: 10.1159/000061040. [DOI] [PubMed] [Google Scholar]
- 143.Petersen G. Study of the results of a complementary course. Sygeplejersken. 1975;75:18–19. [PubMed] [Google Scholar]
- 144.Phillippe M, Basa A. Effects of sodium and calcium channel blockade on cytosolic calcium oscillations and phasic contractions of myometrial tissue. J Soc Gynecol Investig. 1997;4:72–77. [PubMed] [Google Scholar]
- 145.Phoenix J, Wray S. Changes in frequency and force production of the human myometrium with alteration of pH and metabolism. J Reprod Fertil. 1993;97:507–512. doi: 10.1530/jrf.0.0970507. [DOI] [PubMed] [Google Scholar]
- 146.Ramirez-Bergeron DL, Runge A, Adelman DM, Gohil M, Simon MC. HIF-dependent hematopoietic factors regulate the development of the embryonic vasculature. Dev Cell. 2006;11:81–92. doi: 10.1016/j.devcel.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ramsay B, Sooranna SR, Johnson MR. Nitric oxide synthase activities in human myometrium and villous trophoblast throughout pregnancy. Obstet Gynecol. 1996;87:249–253. doi: 10.1016/0029-7844(95)00391-6. [DOI] [PubMed] [Google Scholar]
- 148.Ratajczak CK, Muglia LJ. Insights into parturition biology from genetically altered mice. Pediatr Res. 2008;64:581–589. doi: 10.1203/PDR.0b013e31818718d2. [DOI] [PubMed] [Google Scholar]
- 149.Rizzo A, Roscino MT, Binetti F, Sciorsci RL. Roles of reactive oxygen species in female reproduction. Reprod Domest Anim. 2012;47:344–352. doi: 10.1111/j.1439-0531.2011.01891.x. [DOI] [PubMed] [Google Scholar]
- 150.Roizen J, Luedke CE, Herzog ED, Muglia LJ. Oxytocin in the circadian timing of birth. PLoS One. 2007;2:e922. doi: 10.1371/journal.pone.0000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Romero R, Wu YK, Mazor M, Hobbins JC, Mitchell MD. Increased amniotic fluid leukotriene C4 concentration in term human parturition. Am J Obstet Gynecol. 1988;159:655–657. doi: 10.1016/s0002-9378(88)80028-0. [DOI] [PubMed] [Google Scholar]
- 152.Romero R, Wu YK, Mazor M, Hobbins JC, Mitchell MD. Amniotic fluid concentration of 5-hydroxyeicosatetraenoic acid is increased in human parturition at term. Prostaglandins Leukot Essent Fatty Acids. 1989;35:81–83. doi: 10.1016/0952-3278(89)90169-5. [DOI] [PubMed] [Google Scholar]
- 153.Romero R, Emamian M, Wan M, Grzyboski C, Hobbins JC, Mitchell MD. Increased concentrations of arachidonic acid lipoxygenase metabolites in amniotic fluid during parturition. Obstet Gynecol. 1987;70:849–851. [PubMed] [Google Scholar]
- 154.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 155.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 156.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35:235–238. [PubMed] [Google Scholar]
- 157.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine. 1993;5:81–88. doi: 10.1016/1043-4666(93)90027-3. [DOI] [PubMed] [Google Scholar]
- 159.Samson SL, Gedamu L. Molecular analyses of metallothionein gene regulation. Prog Nucleic Acid Res Mol Biol. 1998;59:257–288. doi: 10.1016/s0079-6603(08)61034-x. [DOI] [PubMed] [Google Scholar]
- 160.Sanborn BM. Hormonal signaling and signal pathway crosstalk in the control of myometrial calcium dynamics. Semin Cell Dev Biol. 2007;18:305–314. doi: 10.1016/j.semcdb.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schonfelder G, John M, Hopp H, Fuhr N, van Der Giet M, Paul M. Expression of inducible nitric oxide synthase in placenta of women with gestational diabetes. FASEB J. 1996;10:777–784. doi: 10.1096/fasebj.10.7.8635695. [DOI] [PubMed] [Google Scholar]
- 162.Schwarz MA, Lazo JS, Yalowich JC, Reynolds I, Kagan VE, Tyurin V, et al. Cytoplasmic metallothionein overexpression protects NIH 3T3 cells from tert-butyl hydroperoxide toxicity. J Biol Chem. 1994;269:15238–15243. [PubMed] [Google Scholar]
- 163.Schwarz MA, Lazo JS, Yalowich JC, Allen WP, Whitmore M, Bergonia HA, et al. Metallothionein protects against the cytotoxic and DNA-damaging effects of nitric oxide. Proc Natl Acad Sci U S A. 1995;92:4452–4456. doi: 10.1073/pnas.92.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sharkey JT, Puttaramu R, Word RA, Olcese J. Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J Clin Endocrinol Metab. 2009;94:421–427. doi: 10.1210/jc.2008-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shay JE, Celeste Simon M. Hypoxia-inducible factors: crosstalk between inflammation and metabolism. Semin Cell Dev Biol. 2012;23:389–394. doi: 10.1016/j.semcdb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 166.Shmygol A, Blanks AM, Bru-Mercier G, Gullam JE, Thornton S. Control of uterine Ca2+ by membrane voltage: toward understanding the excitation-contraction coupling in human myometrium. Ann N Y Acad Sci. 2007;1101:97–109. doi: 10.1196/annals.1389.031. [DOI] [PubMed] [Google Scholar]
- 167.Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181:1470–1479. doi: 10.4049/jimmunol.181.2.1470. [DOI] [PubMed] [Google Scholar]
- 168.Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S2–S10. doi: 10.1016/j.ejogrb.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 169.Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman N, et al. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod. 2006;74:839–849. doi: 10.1095/biolreprod.105.048124. [DOI] [PubMed] [Google Scholar]
- 170.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–R463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- 171.Sladek SM, Regenstein AC, Lykins D, Roberts JM. Nitric oxide synthase activity in pregnant rabbit uterus decreases on the last day of pregnancy. Am J Obstet Gynecol. 1993;169:1285–1291. doi: 10.1016/0002-9378(93)90295-t. [DOI] [PubMed] [Google Scholar]
- 172.Smith RC, McClure MC, Smith MA, Abel PW, Bradley ME. The role of voltage-gated potassium channels in the regulation of mouse uterine contractility. Reprod Biol Endocrinol. 2007;5:41. doi: 10.1186/1477-7827-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Smyth GK, Yang YH, Speed T. Statistical issues in cDNA microarray data analysis. Methods Mol Biol. 2003;224:111–136. doi: 10.1385/1-59259-364-X:111. [DOI] [PubMed] [Google Scholar]
- 174.Soni S, Chivan N, Cohen WR. Effect of maternal body mass index on oxytocin treatment for arrest of dilatation. J Perinat Med. 2013:1–5. doi: 10.1515/jpm-2013-0024. [DOI] [PubMed] [Google Scholar]
- 175.Steinborn A, Kuhnert M, Halberstadt E. Immunmodulating cytokines induce term and preterm parturition. J Perinat Med. 1996;24:381–390. doi: 10.1515/jpme.1996.24.4.381. [DOI] [PubMed] [Google Scholar]
- 176.Stevens MY, Challis JR, Lye SJ. Corticotropin-releasing hormone receptor subtype 1 is significantly up-regulated at the time of labor in the human myometrium. J Clin Endocrinol Metab. 1998;83:4107–4115. doi: 10.1210/jcem.83.11.5272. [DOI] [PubMed] [Google Scholar]
- 177.Strauss JF, 3rd, Sokoloski J, Caploe P, Duffy P, Mintz G, Stambaugh RL. On the role of prostaglandins in parturition in the rat. Endocrinology. 1975;96:1040–1043. doi: 10.1210/endo-96-4-1040. [DOI] [PubMed] [Google Scholar]
- 178.Taggart M, Wray S. Simultaneous measurement of intracellular pH and contraction in uterine smooth muscle. Pflugers Arch. 1993;423:527–529. doi: 10.1007/BF00374951. [DOI] [PubMed] [Google Scholar]
- 179.Taggart MJ, Wray S. Occurrence of intracellular pH transients during spontaneous contractions in rat uterine smooth muscle. J Physiol. 1993;472:23–31. doi: 10.1113/jphysiol.1993.sp019933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tanaka Y, Narahara H, Takai N, Yoshimatsu J, Anai T, Miyakawa I. Interleukin-1beta and interleukin-8 in cervicovaginal fluid during pregnancy. Am J Obstet Gynecol. 1998;179:644–649. doi: 10.1016/s0002-9378(98)70058-4. [DOI] [PubMed] [Google Scholar]
- 181.Tarca AL, Draghici S, Bhatti G, Romero R. Down-weighting overlapping genes improves gene set analysis. BMC Bioinformatics. 2012;13:136. doi: 10.1186/1471-2105-13-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thomson AJ, Telfer JF, Kohnen G, Young A, Cameron IT, Greer IA, et al. Nitric oxide synthase activity and localization do not change in uterus and placenta during human parturition. Hum Reprod. 1997;12:2546–2552. doi: 10.1093/humrep/12.11.2546. [DOI] [PubMed] [Google Scholar]
- 183.Ticconi C, Belmonte A, Piccione E, Rao CH. Feto-placental communication system with the myometrium inpregnancy and parturition: the role of hormones, neurohormones, inflammatory mediators, and locally active factors. J Matern Fetal Neonatal Med. 2006;19:125–133. doi: 10.1080/14767050600555808. [DOI] [PubMed] [Google Scholar]
- 184.Tornblom SA, Patel FA, Bystrom B, Giannoulias D, Malmstrom A, Sennstrom M, et al. 15-hydroxyprostaglandin dehydrogenase and cyclooxygenase 2 messenger ribonucleic acid expression and immunohistochemical localization in human cervical tissue during term and preterm labor. J Clin Endocrinol Metab. 2004;89:2909–2915. doi: 10.1210/jc.2003-031149. [DOI] [PubMed] [Google Scholar]
- 185.Tyson EK, Smith R, Read M. Evidence that corticotropin-releasing hormone modulates myometrial contractility during human pregnancy. Endocrinology. 2009;150:5617–5625. doi: 10.1210/en.2009-0348. [DOI] [PubMed] [Google Scholar]
- 186.Van Meir CA, Ramirez MM, Matthews SG, Calder AA, Keirse MJ, Challis JR. Chorionic prostaglandin catabolism is decreased in the lower uterine segment with term labour. Placenta. 1997;18:109–114. doi: 10.1016/s0143-4004(97)90081-3. [DOI] [PubMed] [Google Scholar]
- 187.WHO. World Health Organization. Maternal mortality. World health day-safe motherhood. 1998:1–3.
- 188.Wiesner G, Morash BA, Ur E, Wilkinson M. Food restriction regulates adipose-specific cytokines in pituitary gland but not in hypothalamus. J Endocrinol. 2004;180:R1–R6. doi: 10.1677/joe.0.180r001. [DOI] [PubMed] [Google Scholar]
- 189.Winchester SK, Imamura T, Gross GA, Muglia LM, Vogt SK, Wright J, et al. Coordinate regulation of prostaglandin metabolism for induction of parturition in mice. Endocrinology. 2002;143:2593–2598. doi: 10.1210/endo.143.7.8926. [DOI] [PubMed] [Google Scholar]
- 190.Winkler M, Rath W. Changes in the cervical extracellular matrix during pregnancy and parturition. J Perinat Med. 1999;27:45–60. doi: 10.1515/JPM.1999.006. [DOI] [PubMed] [Google Scholar]
- 191.Wray S. Uterine contraction and physiological mechanisms of modulation. Am J Physiol. 1993;264:C1–C18. doi: 10.1152/ajpcell.1993.264.1.C1. [DOI] [PubMed] [Google Scholar]
- 192.Wray S, Duggins K, Iles R, Nyman L, Osman V. The effects of metabolic inhibition and acidification on force production in the rat uterus. Exp Physiol. 1992;77:307–319. doi: 10.1113/expphysiol.1992.sp003590. [DOI] [PubMed] [Google Scholar]
- 193.Wray S, Jones K, Kupittayanant S, Li Y, Matthew A, Monir-Bishty E, et al. Calcium signaling and uterine contractility. J Soc Gynecol Investig. 2003;10:252–264. doi: 10.1016/s1071-5576(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 194.Xu P, Alfaidy N, Challis JR. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab. 2002;87:1353–1361. doi: 10.1210/jcem.87.3.8320. [DOI] [PubMed] [Google Scholar]
- 195.Yallampalli C, Garfield RE, Byam-Smith M. Nitric oxide inhibits uterine contractility during pregnancy but not during delivery. Endocrinology. 1993;133:1899–1902. doi: 10.1210/endo.133.4.8404632. [DOI] [PubMed] [Google Scholar]
- 196.Yallampalli C, Izumi H, Byam-Smith M, Garfield RE. An L-arginine-nitric oxide-cyclic guanosine monophosphate system exists in the uterus and inhibits contractility during pregnancy. Am J Obstet Gynecol. 1994;170:175–185. doi: 10.1016/s0002-9378(94)70405-8. [DOI] [PubMed] [Google Scholar]
- 197.Yallampalli C, Byam-Smith M, Nelson SO, Garfield RE. Steroid hormones modulate the production of nitric oxide and cGMP in the rat uterus. Endocrinology. 1994;134:1971–1974. doi: 10.1210/endo.134.4.8137765. [DOI] [PubMed] [Google Scholar]
- 198.Yallampalli C, Dong YL, Gangula PR, Fang L. Role and regulation of nitric oxide in the uterus during pregnancy and parturition. J Soc Gynecol Investig. 1998;5:58–67. doi: 10.1016/S1071-5576(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 199.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, et al. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol. 2000;20:5343–5349. doi: 10.1128/mcb.20.14.5343-5349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Young RC, Bemis A. Calcium-activated chloride currents prolongs the duration of contractions in pregnant rat myometrial tissue. Reprod Sci. 2009;16:734–739. doi: 10.1177/1933719109334965. [DOI] [PubMed] [Google Scholar]
- 201.Zarlingo TJ, Eis AL, Brockman DE, Kossenjans W, Myatt L. Comparative localization of endothelial and inducible nitric oxide synthase isoforms in haemochorial and epitheliochorial placentae. Placenta. 1997;18:511–520. doi: 10.1016/0143-4004(77)90004-2. [DOI] [PubMed] [Google Scholar]
- 202.Zhou XB, Wang GX, Ruth P, Huneke B, Korth M. BK(Ca) channel activation by membrane-associated cGMP kinase may contribute to uterine quiescence in pregnancy. Am J Physiol Cell Physiol. 2000;279:C1751–C1759. doi: 10.1152/ajpcell.2000.279.6.C1751. [DOI] [PubMed] [Google Scholar]
- 203.Zhu H, Li J, Qin W, Yang Y, He X, Wan D, et al. Cloning of a novel gene, ANGPTL4 and the functional study in angiogenesis. Zhonghua Yi Xue Za Zhi. 2002;82:94–99. [PubMed] [Google Scholar]
- 204.Zhu P, Goh YY, Chin HF, Kersten S, Tan NS. Angiopoietin-like 4: a decade of research. Biosci Rep. 2012;32:211–219. doi: 10.1042/BSR20110102. [DOI] [PubMed] [Google Scholar]


