Abstract
Background
Chagas disease remains a serious medical and social problem in Latin America and is an emerging concern in nonendemic countries as a result of population movement, transfusion of infected blood or organs and congenital transmission. The current treatment of infected patients is unsatisfactory due to strain-specific drug resistance and the side effects of the current medications. For this reason, the discovery of safer and more effective chemotherapy is mandatory for the successful treatment and future eradication of Chagas disease.
Methods and Findings
We investigated the effect of a ruthenium complex with benznidazole and nitric oxide (RuBzNO2) against Trypanosoma cruzi both in vitro and in vivo. Our results demonstrated that RuBzNO2 was more effective than the same concentrations of benznidazole (Bz) in eliminating both the extracellular trypomastigote and the intracellular amastigote forms of the parasite, with no cytotoxic effect in mouse cells. In vivo treatment with the compound improved the survival of infected mice, inhibiting heart damage more efficiently than Bz alone. Accordingly, tissue inflammation and parasitism was significantly diminished after treatment with RuBzNO2 in a more effective manner than that with the same concentrations of Bz.
Conclusions
The complexation of Bz with ruthenium and nitric oxide (RuBzNO2) increases its effectiveness against T. cruzi and enables treatment with lower concentrations of the compound, which may reduce the side effects of Bz. Our findings provide a new potential candidate for the treatment of Chagas disease.
Author Summary
Chagas disease, caused by the parasite Trypanosoma cruzi, is a serious medical and social problem in Latin America and is also a worldwide concern due to widespread immigration. The current treatment with benznidazole is effective in the acute phase of the disease but has several limitations and many side effects. We showed that ruthenium complex with benznidazole and nitric oxide (RuBzNO2) is very effective in killing the extracellular and intracellular forms of T. cruzi in vitro. In addition, low concentrations of this compound are able to substantially ameliorate the survival of infected mice by decreasing the amount of parasites in the heart and, consequently, reducing heart inflammation and lesions. Low concentrations of RuBzNO2 acted in a more effective manner than the same concentrations of benznidazole, indicating a synergistic effect due to NO and benznidazole. The substantial efficacy of treatment with benznidazole at a lower concentration than that used currently in clinical practice is very promising for avoiding the side effects that occur. Thus, our data provide a new potential candidate for the treatment of Chagas disease.
Introduction
Chagas disease, caused by the protozoan Trypanosoma cruzi, is still a major public health problem in Latin America despite more than a century of research. Most infected individuals remain asymptomatic for several years. However, approximately 30% of these individuals progress to the cardiac or digestive forms of the disease, manifesting symptoms such as arrhythmias, heart insufficiency, megacolon and megaesophagus [1]. The disease affects more than 10 million people worldwide, and 100 million are at risk of infection. Although the control of natural transmission by insect vectors has been achieved in several countries, natural transmission persists in certain regions of Latin America [2]. Moreover, transmission of T. cruzi by other routes, e.g., blood transfusion, organ transplantation and congenitally, have also been detected in nonendemic regions such as USA and Europe [3], [4], [5].
Despite the intense efforts to achieve an improved method of chemotherapy for the treatment of acute Chagas disease, nifurtimox and benznidazole (Bz) remain the only available drugs. These drugs have been used for more than 50 years. Treatment with Bz during the acute phase of the disease is able to cure approximately 60% of the patients. However, the cure rates do not exceed 20% when Bz is administered in the chronic phase, even in patients treated for more than 10 years [6]. Additionally, the treatment is lengthy and is relatively inefficient against certain strains of T. cruzi. Moreover, it has many side effects, from anorexia, headaches and fatigue to digestive intolerance, dermatitis, toxic hepatitis, depression of bone marrow and polyneuritis [7]. These problems encourage researchers to study new potential targets to develop better and definitive methods of chemotherapy.
The trypanocidal effect of Bz has been reported to involve the reduction of a nitro group to a nitro radical anion that is able to covalently bind to several macromolecules of the parasite, such as its DNA, inhibiting their functions [8]. With the purpose of enhancing the hydrosolubility of Bz and hence increasing its activity and reducing toxicity, we previously described a ruthenium complex coordinated to Bz (trans-[Ru(Bz)(NH3)4SO2](CF3SO3)2) that exhibits a more active trypanocidal effect than Bz both in vitro and in vivo [9]. Additionally, we have previously shown the efficacy of ruthenium as a nitric oxide (NO) donor against T. cruzi. NO donors show high trypanocidal activity at very low concentrations, enhancing the survival of treated mice after T. cruzi infection [10].
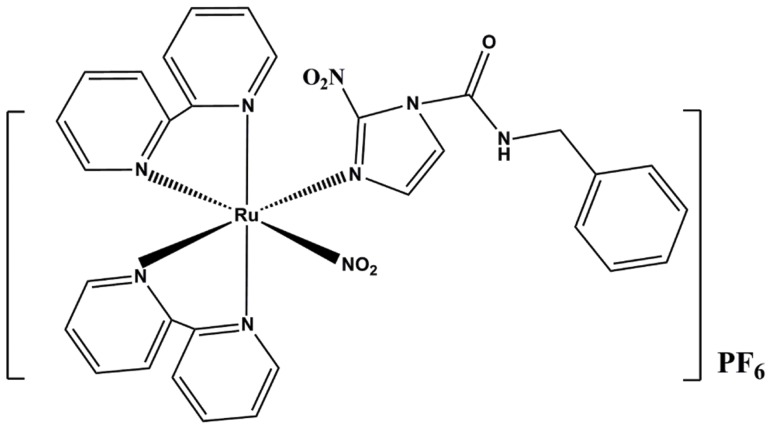
In the present study, we tested the in vitro and in vivo effects of a ruthenium complex that combined the two compounds described above, Bz and NO. The representative molecular structure of cis-[Ru(NO2)(bpy)2(Bz)](PF6), here denoted RuBzNO2, is shown in Figure 1. We found that low concentrations of RuBzNO2 had high trypanocidal activity in vitro against both trypomastigotes and amastigotes but did not show cytotoxicity in mouse cells. The compound was able to enhance the survival of treated mice after T. cruzi infection. The improvement provided by treatment was related to decreased heart damage, which was followed by reduced inflammation and parasitism in the myocardium. Importantly, the effects of RuBzNO2 were more favorable than those of the same concentrations of Bz.
Figure 1. Chemical structure of RuBzNO2.
Methods
Synthesis of cis-[Ru(NO2)(bpy)2(Bz)](PF6)
The cis-[Ru(NO2)(bpy)2(NO)](PF6)2 (I) complex was synthesized and characterized following published procedures [11]. The complex (I) (0.230 g, 0.29 mmol) was dissolved in acetone (15 mL). NaN3 (0,02 g) was then dissolved in methanol (5 mL) and added dropwise to the above solution. After 10 min, Bz (0.260 g, 0.99 mmol) previously dissolved in acetone was added to the solution and the reaction mixture was stirred at 50.0°C for 24 h. The resulting solution was concentrated by rotary evaporation until reduction to a third of initial volume and then NH4PF6 (0.300 g) dissolved in water (1.0 mL) was added. The cis-[Ru(NO2)(bpy2)(Bz)](PF6) ((RuBzNO2) complex salt was obtained as a brown precipitate, which was collected by filtration and washed with ethanol (10 mL) (yield: 70%). Elemental analysis (%) for RuCHN Expt. (Calc.) C, 39.2 (38.8), H, 3.1 (3.2), N, 12.5 (12.3).
Parasite and trypanocidal assays
Trypomastigotes of Y strain of T. cruzi were obtained from infected LLC-MK2 cell culture and suspended at a concentration of 6.5×106 parasites/ml in RPMI 1640 medium (Gibco-BRL LifeTechnologies, Grand Island, NY, USA) containing 10% fetal bovine serum (Life Technologies Inc., Bethesda, MD, USA) and antibiotics (Sigma Chemical Co., St. Louis, MO, USA) and cultured in flat-bottom 96-well plates with various concentrations of benznidazole (Bz) or RuBzNO2 at 37°C for 24 h. The viability of the parasites was determined by counting the motile parasites in a Neubauer chamber as previously described [12]. The concentration of the compound corresponding to 50% of trypanocidal activity in trypomastigotes was expressed as the IC50.
To evaluate the trypanocidal activity of the compound in amastigote forms of T. cruzi, Vero cells (ATCC) were suspended in RPMI medium at 5×104 cells/well and were cultured in 8-well chamber slides and infected with trypomastigote forms of T. cruzi Y strain at 1×105/well for 24 h. The cells were washed to remove parasites in the supernatant and incubated with Bz or RuBzNO2 for an additional 24 h at 37°C. Slides were stained with Giemsa dye and evaluated by optical microscopy [13]. Trypanocidal activity was determined by counting the parasites/cell in at least 200 cells.
Cytotoxicity assay
Mouse spleen cells were isolated and incubated for 5 min with red blood cell lysis buffer (one part of 0.17 M 700 Tris–HCl [pH 7.5] and nine parts of 0.16 M ammonium chloride). The cells were suspended in RPMI 1640 medium and cultured in flat-bottom 96-well plates at 5×105 cells/well with various concentrations of Bz or RuBzNO2 at 37°C. Tween 20 at 0.5% was used as a positive control for cell death. After 24 h of culture, cells were incubated with 10 µg/mL propidium iodide (Sigma) and acquired by flow cytometry using a FACSCantoII (Becton-Dickinson Immunocytometry System Inc., San Jose, CA, USA). Propidium-iodide positive cells were quantified using FlowJo software (Ashland, Oregon, USA).
Intracellular release of nitric oxide
The intracellular release of NO by RuBzNO2 was evaluated as previously described [12]. Briefly, Vero cells were cultured on slides at 1×106 cell/ml in RPMI medium for 24 h at 37°C. The NO fluorescent dye 4,5-diaminofluorescein diacetate (DAF-2 DA) (10 mM) and the reducing agent phenylephrine (0.1 µM) were added to the culture and incubated for 30 min at room temperature. In the cytoplasm, DAF-2 DA is hydrolyzed by cytosolic esterases to DAF-2, which cannot leave the cell. In the presence of NO and oxygen, DAF-2 forms the fluorescent product DAF-2 triazole (DAF-2T). Thus, RuBzNO2 was added to the culture, and the cytosolic NO concentration was assessed by fluorescence microscopy.
Mice, infection and treatment
Female Swiss mice (6–8 weeks old) were intraperitoneally infected with 2000 bloodstream trypomastigotes of T. cruzi Y strain. The mice were treated orally from the first day of patent parasitemia (day 5 of infection) for 10 consecutive days with 4 µmol/kg or 0.4 µmol/kg of Bz (1.24 mg/kg and 0.124 mg/kg, respectively) or RuBzNO2 (5.2 mg/kg and 0.52 mg/kg, respectively). The survival was monitored and parasitemia was evaluated in 5 µl of blood from the tail vein by counting parasites through a optical microscope [14].
Ethics
All the procedures and animal protocols were conducted in accordance with the National Brazilian College of Animal Experimentation (COBEA) and approved by the Ethical Commission of Ethics in Animal Research of the University of São Paulo, Medical School of Ribeirão Preto (CETEA) - Protocol number 076/2010.
Tissue assessment
Histopathological analyses of the heart were performed by collecting tissues from mice on day 20 of infection. The tissues were fixed, dehydrated and embedded in paraffin. Slides were prepared with 5 µm sections and stained with hematoxylin and eosin (H&E). Photomicrography at 400× magnification of 20 randomly chosen fields was performed with a microcamera (Zeiss Co., Oberkochen, Germany) coupled with an Olympus BHS microscope (Olympus, Miami, FL, USA). Inflammatory infiltrate was quantified using ImageJ software. Heart lesions were evaluated by quantifying serum creatine kinase MB (CK-MB) according to the manufacturer's instructions (Labtest, Lagoa Santa, MG, Brazil).
Tissue parasitism
Tissue parasitism was evaluated by real-time PCR as previously described [15]. Briefly, DNA was extracted from tissue using a Wizard SV Genomic DNA Purification System (Promega, Madison, WI, USA), and 1 µg DNA was incubated with 25 pmol of primers S35 (5′AAATAATGTACGGG(T/G)GAGATGCATGA3′) and S36 (5′GGGTTCGATTGGGGTTGGTGT3′) (Sigma) and GoTaq qPCR master Mix (Promega) according to the manufacturer's instructions. Samples were amplified for 40 cycles in a StepOnePlus (Applied Biosystems, Foster City, CA, USA).
Statistical analysis
An analysis of variance (ANOVA) followed by a Tukey-Kramer test was used to determine differences among the experimental groups. The results were evaluated at a statistical significance level of p<0.05. A Kaplan-Meyer test was used to evaluate the survival rate.
Results
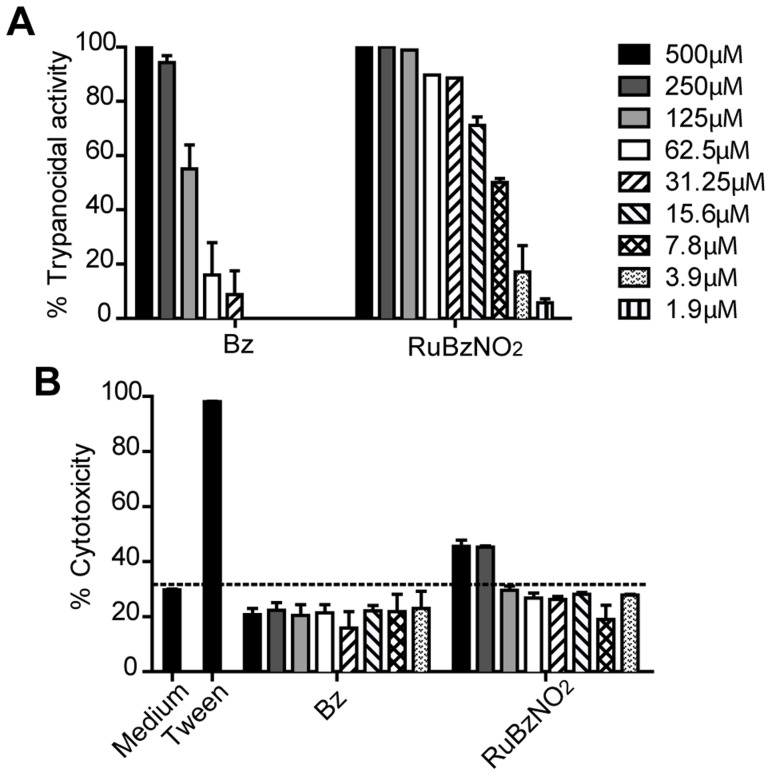
To assess the trypanocidal activity of RuBzNO2, trypomastigotes of T. cruzi Y strain, a strain partially resistant to Bz treatment, were incubated with serial dilutions of RuBzNO2 or Bz for 24 h, and live trypomastigotes were counted. The trypanocidal activity of RuBzNO2 was substantially higher and significantly greater than that of Bz, reaching an IC50 (concentration able to kill 50% of the parasites) of 7.28 µM, whereas the IC50 of Bz was 110.48 µM. In addition, at concentrations as low as 3.9 µM, RuBzNO2 still showed considerable activity against trypomastigotes (Figure 2A). The toxicity of RuBzNO2 was specific to T. cruzi, as incubation with mouse spleen cells at the same concentrations revealed no cytotoxicity, although toxic effects were observed at higher concentrations, 250–500 µM (Figure 2B).
Figure 2. RuBzNO2 is highly effective in killing trypomastigotes and does not show cytotoxicity.
Trypomastigote forms of T. cruzi (A) or spleen cells of mice (B) were cultured with serial dilutions of RuBzNO2 or Bz for 24 h at 37°C. A. Live parasites were counted by microscopy to determine the trypanocidal activity of the compounds. B. Dead cells were stained with propidium iodide and quantified by flow cytometry. The bars indicate means+SEM of duplicates and are representative of three independent experiments.
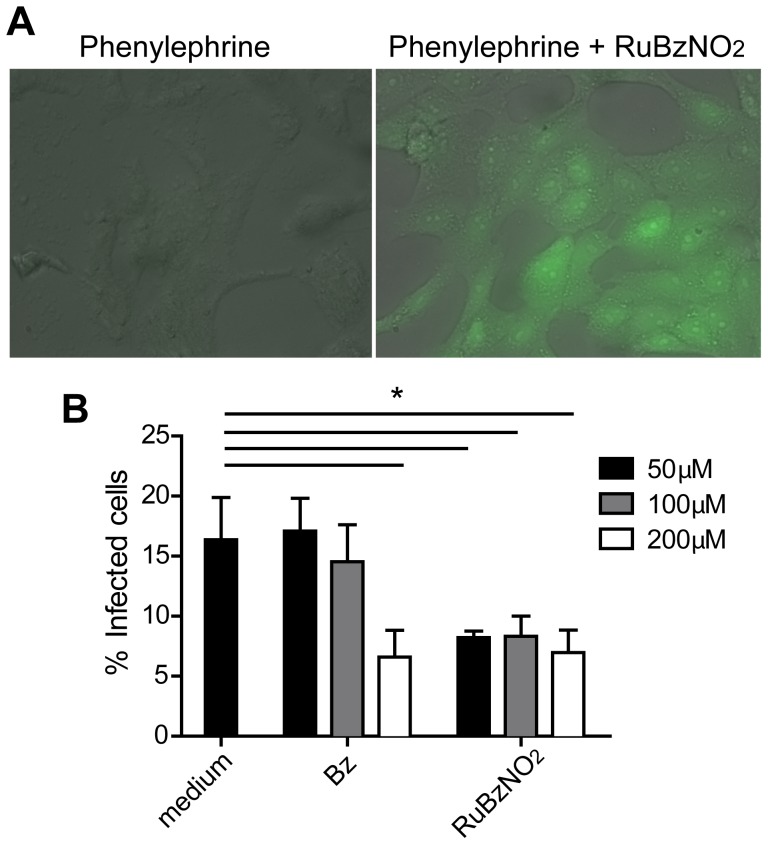
We next investigated whether RuBzNO2 is able to enter the cell and affect the amastigotes, the intracellular forms of T. cruzi. Treatment of Vero cells with RuBzNO2 increased the intracellular concentration of NO, as observed from the conversion of the NO fluorescent dye DAF-2 DA to the fluorescent product DAF-2T, which occurs in the presence of NO (Figure 3A). In addition, Vero cells were infected with T. cruzi, and parasites were removed from the culture after 24 h of incubation. Cells were treated with RuBzNO2 for an additional 24 h. Through the quantification of the intracellular amastigotes, we found that RuBzNO2 was able to inhibit the replication or survival of the amastigotes more efficiently than Bz because RuBzNO2 at 50 µM decreased the percentage of infected cells to the same extent that Bz did at 200 µM (Figure 3B). Overall, these data show that RuBzNO2 is able to enter the cell and is very efficient in eliminating extracellular and intracellular parasites.
Figure 3. RuBzNO2 is able to release NO inside the cell and kills the amastigotes.
A. Vero cells were incubated with DAF-2 DA and the reducing agent phenylephrine in the presence or absence of RuBzNO2. The formation of the fluorescent compound (DAF-2T), which occurs in the presence of NO in the cytoplasm, was assessed by fluorescence microscopy. B. Cells were incubated with trypomastigote forms of T. cruzi for 24 h at 37°C. The remaining parasites were washed from the culture, and the cells were incubated at 37°C for an additional 24 h. Cells were stained with Giemsa dye, and the percentage of infected cells was determined by optical microscopy. The bars indicate means+SEM of triplicates and are representative of two independent experiments. * represents p<0.05.
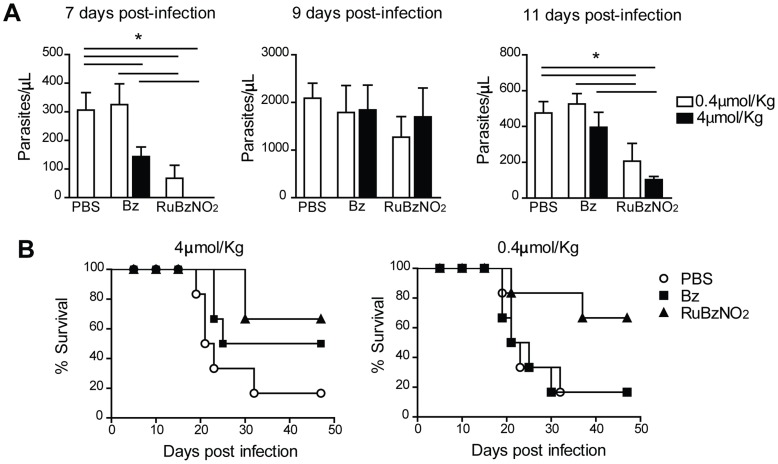
To assess the efficiency of RuBzNO2 in vivo, mice were infected with 2000 blood-derived Y strain trypomastigotes and orally treated with RuBzNO2 or Bz at the same concentrations for 10 days from the first day of patent parasitemia (day 5 of infection). The doses used in the treatment were about 100 (4 µmol/Kg) to 1000 (0.4 µmol/Kg) times lower than the considered optimal dose of Bz (385 µmol/Kg) [16], [17]. Although neither Bz nor RuBzNO2 were able to change the peak of parasitemia, which occurred after 9 days of infection, RuBzNO2 significantly reduced the parasites in the blood before and after the peak (7 and 11 days post-infection) more efficiently than the same concentrations of Bz (Figure 4A). The treatment with RuBzNO2 at 4 µmol/Kg improved the survival of infected mice more efficiently than the treatment with Bz at the same concentration. Moreover, RuBzNO2 still had the same efficacy in the survival of the mice when used at a concentration that was 10 times lower (0.4 µmol/Kg), whereas Bz had no effect at this concentration (Figure 4 B).
Figure 4. Treatment with RuBzNO2 reduces parasitemia and improves the survival of T. cruzi-infected mice.
Mice were infected with 2000 trypomastigotes of T. cruzi and treated orally for 10 days from the first day of patent parasitemia (5 days post-infection) with 4 µmol/kg or 0.4 µmol/kg of Bz or RuBzNO2. A. Parasitemia was evaluated in the blood by counting parasites with an optical microscope. B. Survival was monitored daily. The bars indicate means+SEM of 6–7 mice/experiment and are representative of three independent experiments. * represents p<0.05.
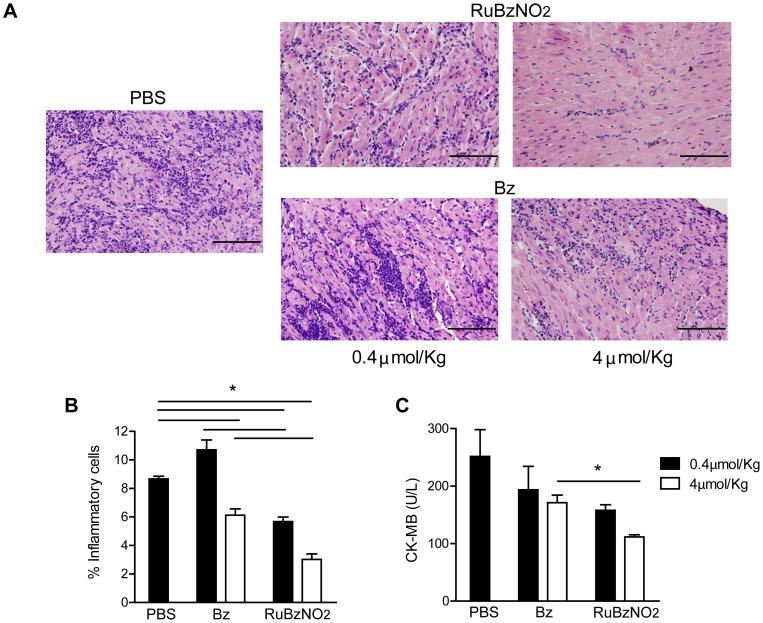
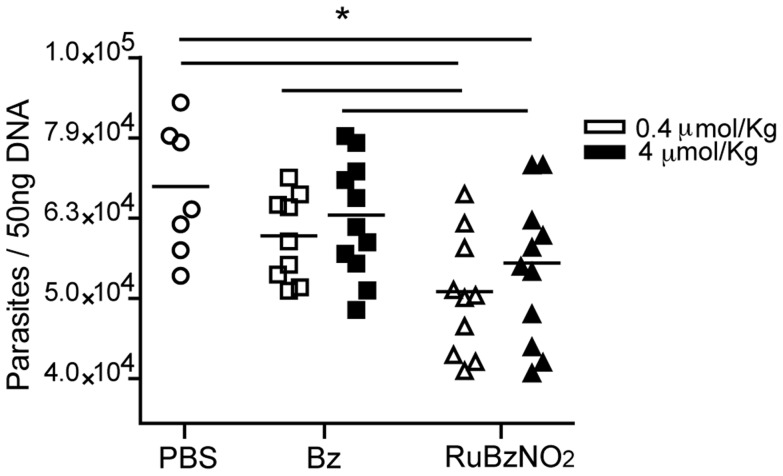
An analysis of heart tissue performed at day 20 of infection showed that RuBzNO2 significantly reduced the inflammatory infiltrate in the tissue in a dose-dependent manner. RuBzNO2 was as efficient in reducing inflammation as Bz when used at a concentration 10 times lower (Figure 5 A, B). Accordingly, the serum concentration of creatine kinase MB (CK-MB), which indicates heart lesions, was decreased after treatment with RuBzNO2 more efficiently than the same concentration of Bz (Figure 5C). These data strongly suggest that RuBzNO2 is able to improve the survival of mice after T. cruzi infection by decreasing tissue lesions. Tissue inflammation is induced by the presence of parasites in the tissue. We found that although the treatment with RuBzNO2 at these concentrations was not able to eliminate the parasites, it significantly reduced the parasitism of the heart, whereas treatment with Bz at the same concentrations had no effect (Figure 6). Both concentrations of RuBzNO2 had the same effect on tissue parasitism. These data indicate that low concentrations of RuBzNO2 are more efficient than Bz in decreasing the amount of parasites in the blood and, consequently, in the tissue. The lower level of heart parasitism and inflammation observed in the mice treated with RuBzNO2 may explain the increased survival of these animals.
Figure 5. Treatment with RuBzNO2 ameliorates heart inflammation and lesions induced by T. cruzi infection.
Mice were infected with 2000 trypomastigotes of T. cruzi and treated from day 5 for 10 days with various concentrations of Bz or RuBzNO2. Twenty days after infection, the heart histology (A–B) was evaluated, and the CK-MB level (C) was measured in the serum. A. Representative photomicrograph of heart stained with H&E. B. Quantification of the inflammatory cells shown in A. The bars indicate means+SEM of 4–6 mice/experiment and are representative of two independent experiments. * represents p<0.05.
Figure 6. Heart parasitism is reduced after treatment with RuBzNO2.
Mice were infected with 2000 trypomastigotes of T. cruzi and treated with various concentrations of Bz or RuBzNO2. After 20 dpi, the DNA from the heart was isolated, and T. cruzi was quantified with real-time PCR. Each dot indicates an individual mouse, and the graph is representative of two independent experiments. * represents p<0.05.
Discussion
The current chemotherapy available for the treatment of Chagas disease, Bz, has good effectiveness when administered in the acute phase of the disease; however, it has limitations, such as the side effects exhibited [18]. Accordingly, a way to enhance the effectiveness of Bz and to decrease its undesired effects is very desirable for generating potential new candidates for the treatment of a disease that has previously been incurable. With this aim, we previously synthesized a ruthenium complex with Bz and nitric oxide, RuBzNO2. With this compound, we aimed to improve the action of Bz and to provide additional trypanocidal activity produced by NO because cis-[Ru(NO2)(bpy)2(Bz)] (PF6) could be a NO donor agent due to high stability in physiological solution and the ability to catalyze the conversion of nitrite to nitrosyl.
Substantial effort has been dedicated to the synthesis and evaluation of ruthenium complexes as antiparasitic agents. New series of ruthenium complexes have furnished improvements, as they show lower toxicity and higher hydrosolubility than the first metal derivatives synthesized [19], [20], [21], [22]. In a previous study, we showed that the complexation of Bz with ruthenium improved its solubility and efficacy against T. cruzi both in vitro and in vivo when infected mice were treated for 15 consecutive days [9]. Ruthenium complexes used as a NO carrier also showed high trypanocidal activity at very low concentrations [10], [23].
The mechanism of action of Bz involves the generation of free radicals, in particular, a nitro anion radical (R-NO2 −). Instead of stimulating redox cycling, these agents covalently bind to macromolecules of the parasite, modifying or inhibiting their functions [8]. In contrast, NO is a major mediator produced by infected cardiac myocytes and macrophages in response to IFN-γ and TNF-α, with pronounced trypanocidal activity via an oxidative stress-dependent mechanism [24]. In addition, NO is a negative regulator of chemokine production, decreasing the recruitment of inflammatory infiltrate that is partially responsible for the myocarditis induced by T. cruzi infection [10], [12], [25]. Thus, based on the possible synergistic action of two different compounds in one complex that show different mechanisms of action, we aimed to improve the effects occurring individually. RuBzNO2 was very effective in killing free trypomastigotes in culture. Moreover, as expected, it was more active than Bz because a lower concentration was necessary to kill 50% of the parasites (lower IC50). Notably, the toxicity of the compound was specific to the parasites because no toxicity was observed in mouse cells. RuBzNO2 showed an ability to enter the cell and release NO within the cytoplasm. This characteristic is very important for enhancing the effectiveness of the compound by enabling it to kill the intracellular amastigotes. In fact, RuBzNO2 was more effective than Bz in eliminating amastigote forms of T. cruzi, decreasing the frequency of infected cells.
The results of the treatment of T. cruzi-infected mice with RuBzNO2 were very promising because low concentrations of the compound used in a short-term treatment were able to significantly improve the survival of the animals. Treatment with the same concentrations of Bz was not able to produce such improvement. This finding shows that the complexation of Bz with ruthenium and nitric oxide generated a compound with enhanced power against experimental Chagas disease compared to Bz alone. The treatment was effective when it was initiated after parasites were found in the blood. This treatment protocol, unlike the ones initiated when the animal is infected, is more likely to be administered in human patients with the acute form of the disease, whose symptoms appear after parasite proliferation and spread in the blood. The treatment with a concentration as low as 0.4 µmol/Kg of RuBzNO2 was able to decrease the parasite loads in the heart, whereas Bz did not affect parasitism of the heart when administered at the same concentration. The lower level of parasitism in RuBzNO2-treated mice was followed by reduced inflammatory cell infiltration and, consequently, reduced tissue lesions.
Overall, these data suggest that Bz, when complexed with ruthenium and NO, may be used for treatment at lower concentrations to kill parasites and reduce cardiac lesions. Treatment at lower concentrations, or even for shorter times, with Bz may reduce the discontinuation of treatment by patients resulting from the side effects of the drug. Therefore, our findings provide a new candidate for the treatment of Chagas disease.
Future studies are necessary to determine whether treatment with RuBzNO2 is effective in achieve the parasitological cure of infected mice. It is also worth to pay special attention to the treatment during the chronic phase of experimental Chagas disease and against other strains of T. cruzi. Moreover, additional adjustments to the dose and time of treatment may permit the treatment to achieve greater effects.
Acknowledgments
We thank Wander C. R. da Silva for excellent technical support.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, (CAPES) Proc PNPD 02883/09-0. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Clayton J (2010) Chagas disease 101. Nature 465: S4–5. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (2010). Chagas Disease (American trypanosomiasis) June 2010
- 3. Rassi A Jr, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 4. Sesti-Costa R, Silva JS, Gutierrez FR (2012) Congenital Chagas disease: time to screen pregnant women? Expert Rev Anti Infect Ther 10: 1279–1282. [DOI] [PubMed] [Google Scholar]
- 5. Kransdorf EP, Zakowski PC, Kobashigawa JA (2014) Chagas disease in solid organ and heart transplantation. Curr Opin Infect Dis 418–424. [DOI] [PubMed] [Google Scholar]
- 6. Docampo R (2001) Recent developments in the chemotherapy of Chagas disease. Curr Pharm Des 7: 1157–1164. [DOI] [PubMed] [Google Scholar]
- 7. Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, et al. (2009) Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther 7: 157–163. [DOI] [PubMed] [Google Scholar]
- 8. Maya JD, Cassels BK, Iturriaga-Vasquez P, Ferreira J, Faundez M, et al. (2007) Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp Biochem Physiol A Mol Integr Physiol 146: 601–620. [DOI] [PubMed] [Google Scholar]
- 9. Nogueira Silva JJ, Pavanelli WR, Gutierrez FR, Alves Lima FC, Ferreira da Silva AB, et al. (2008) Complexation of the anti-Trypanosoma cruzi drug benznidazole improves solubility and efficacy. J Med Chem 51: 4104–4114. [DOI] [PubMed] [Google Scholar]
- 10. Silva JJ, Guedes PM, Zottis A, Balliano TL, Nascimento Silva FO, et al. (2010) Novel ruthenium complexes as potential drugs for Chagas's disease: enzyme inhibition and in vitro/in vivo trypanocidal activity. Br J Pharmacol 160: 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Godwin JB, Meyer TJ (1971) Preparation of ruthenium nitrosyl complexes containig 2.2′-bipyridine and 1.10-phenanthroline. Inorg Chem 4. [Google Scholar]
- 12. Guedes PM, Oliveira FS, Gutierrez FR, da Silva GK, Rodrigues GJ, et al. (2010) Nitric oxide donor trans-[RuCl([15]aneN)NO] as a possible therapeutic approach for Chagas' disease. Br J Pharmacol 160: 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martins-Teixeira MB, Campo VL, Biondo M, Sesti-Costa R, Carneiro ZA, et al. (2013) alpha-Selective glycosylation affords mucin-related GalNAc amino acids and diketopiperazines active on Trypanosoma cruzi. Bioorg Med Chem 21: 1978–1987. [DOI] [PubMed] [Google Scholar]
- 14. Brener Z (1962) Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo 4: 389–396. [PubMed] [Google Scholar]
- 15. Gomes ML, Macedo AM, Vago AR, Pena SD, Galvao LM, et al. (1998) Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp Parasitol 88: 28–33. [DOI] [PubMed] [Google Scholar]
- 16. Molina J, Martins-Filho O, Brener Z, Romanha AJ, Loebenberg D, et al. (2000) Activities of the triazole derivative SCH 56592 (posaconazole) against drug-resistant strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrob Agents Chemother 44: 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferraz ML, Gazzinelli RT, Alves RO, Urbina JA, Romanha AJ (2007) The Anti-Trypanosoma cruzi activity of posaconazole in a murine model of acute Chagas' disease is less dependent on gamma interferon than that of benznidazole. Antimicrob Agents Chemother 51: 1359–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Castro JA, de Mecca MM, Bartel LC (2006) Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis). Hum Exp Toxicol 25: 471–479. [DOI] [PubMed] [Google Scholar]
- 19. Sanchez-Delgado RA, Anzellotti A (2004) Metal complexes as chemotherapeutic agents against tropical diseases: trypanosomiasis, malaria and leishmaniasis. Mini Rev Med Chem 4: 23–30. [DOI] [PubMed] [Google Scholar]
- 20. Sánchez-Delgado R, Navarro M, Lazardi K, Atencio R, Capparelli M, et al. (1998) Toward a novel metal based chemotherapy against tropical diseases 4. Synthesis and characterization of new metal-clotrimazole complexes and evaluation of their activity against Trypanosoma cruzi Inorganica Chimica Acta 275: 528–540. [Google Scholar]
- 21. Caulton K, Davies G, Holt E (1990) Synthesis, molecular structures, properties and reactions of halo- and carbonyl(amine)copper(I) complexes. Polyhedron 9: 2319–2351. [Google Scholar]
- 22. Iniguez E, Sanchez A, Vasquez MA, Martinez A, Olivas J, et al. (2013) Metal-drug synergy: new ruthenium(II) complexes of ketoconazole are highly active against Leishmania major and Trypanosoma cruzi and nontoxic to human or murine normal cells. J Biol Inorg Chem 18: 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silva JJ, Pavanelli WR, Pereira JC, Silva JS, Franco DW (2009) Experimental chemotherapy against Trypanosoma cruzi infection using ruthenium nitric oxide donors. Antimicrob Agents Chemother 53: 4414–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gutierrez FR, Mineo TW, Pavanelli WR, Guedes PM, Silva JS (2009) The effects of nitric oxide on the immune system during Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz 104 Suppl 1: 236–245. [DOI] [PubMed] [Google Scholar]
- 25. Machado FS, Souto JT, Rossi MA, Esper L, Tanowitz HB, et al. (2008) Nitric oxide synthase-2 modulates chemokine production by Trypanosoma cruzi-infected cardiac myocytes. Microbes Infect 10: 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.