Abstract
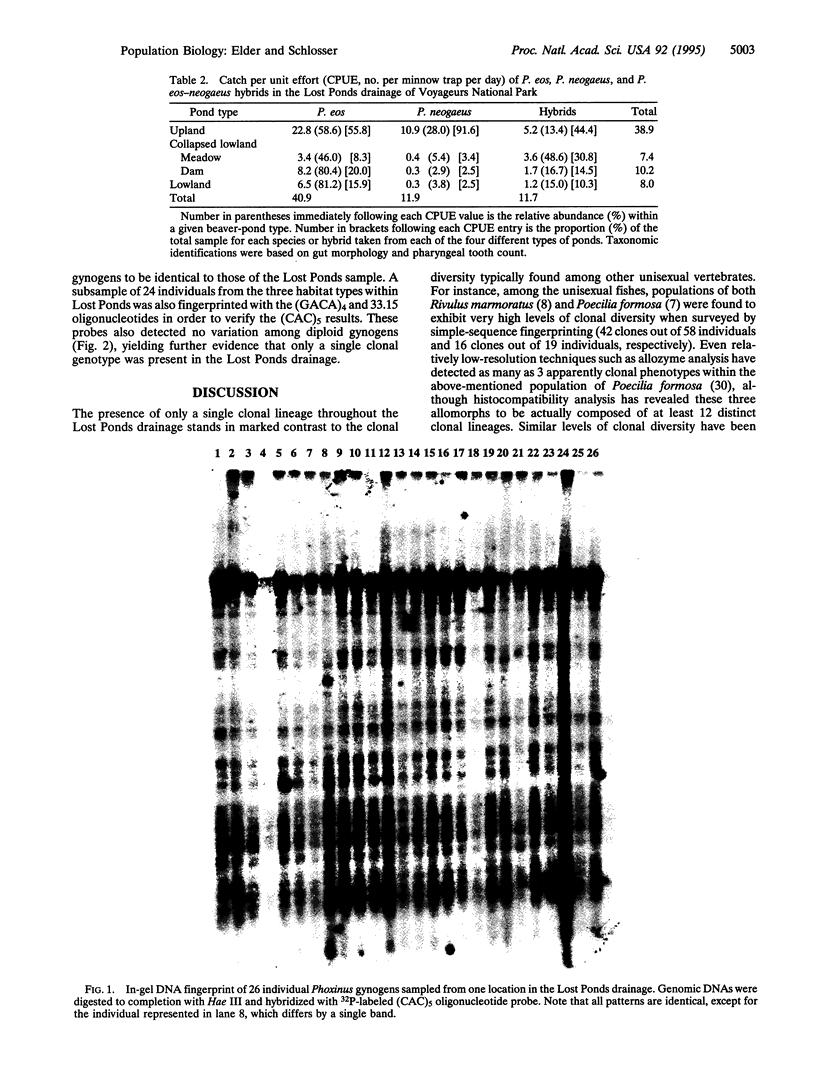
Genetic surveys of parthenogenetic vertebrate populations have demonstrated a common pattern of relatively high degrees of clonal variation and the coexistence of numerous clones. In striking contrast, the Phoxinus eos/Phoxinus neogaeus/hybrid gynogen complex of cyprinid fishes exhibits no clonal variation within a northern Minnesota drainage characterized by successional beaver ponds. Gynogens were sampled from three habitats in each of four different pond types in a single drainage in Voyageurs National Park, Minnesota. The abundance of gynogens relative to sexual dace varied with pond type, being least common in deep upland ponds and most common in shallow, collapsed, lowland ponds (13.4% and 48.6%, respectively). Simple-sequence multilocus DNA fingerprinting of 464 individual gynogens detected one, and only one, clone. DNA fingerprints, generated sequentially by using three oligonucleotide probes, (CAC)5, (GACA)4, and the Jeffreys' 33.15 probe, all revealed the same unprecedented lack of variation. The extreme lack of clonal diversity in these gynogens across a range of habitat types does not fit the general pattern of high clonal diversity found within populations of other vertebrate parthenogens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hedges S. B., Bogart J. P., Maxson L. R. Ancestry of unisexual salamanders. Nature. 1992 Apr 23;356(6371):708–710. doi: 10.1038/356708a0. [DOI] [PubMed] [Google Scholar]
- Hubbs C. L., Hubbs L. C. APPARENT PARTHENOGENESIS IN NATURE, IN A FORM OF FISH OF HYBRID ORIGIN. Science. 1932 Dec 30;76(1983):628–630. doi: 10.1126/science.76.1983.628. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Schäfer R., Zischler H., Epplen J. T. (CAC)5, a very informative oligonucleotide probe for DNA fingerprinting. Nucleic Acids Res. 1988 Jun 10;16(11):5196–5196. doi: 10.1093/nar/16.11.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolsky C. M., Phillips C. A., Uzzell T. Antiquity of clonal salamander lineages revealed by mitochondrial DNA. Nature. 1992 Apr 23;356(6371):706–708. doi: 10.1038/356706a0. [DOI] [PubMed] [Google Scholar]
- Tautz D. Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res. 1989 Aug 25;17(16):6463–6471. doi: 10.1093/nar/17.16.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. J., Elder J. F., Jr, Laughlin T. F., Davis W. P. Genetic variation in clonal vertebrates detected by simple-sequence DNA fingerprinting. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5653–5657. doi: 10.1073/pnas.87.15.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. J., Elder J. F., Jr, Laughlin T. F., Davis W. P., Taylor D. S. Extreme clonal diversity and divergence in populations of a selfing hermaphroditic fish. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10643–10647. doi: 10.1073/pnas.89.22.10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijenhoek R. C. Coexistence of clones in a heterogeneous environment. Science. 1978 Feb 3;199(4328):549–552. doi: 10.1126/science.199.4328.549. [DOI] [PubMed] [Google Scholar]