Abstract
Drug transporters such as P-glycoprotein (ABCB1) have been associated with chemotherapy resistance and are considered unfavorable prognostic factors for survival of cancer patients. Analyzing mRNA expression levels of a subset of drug transporters by quantitative reverse transcription polymerase chain reaction (qRT-PCR) or protein expression by tissue microarray (TMA) in tumor samples of therapy naïve stage IV head and neck squamous cell carcinoma (HNSCC) (qRT-PCR, n = 40; TMA, n = 61), this in situ study re-examined the significance of transporter expression for progression-free survival (PFS) and overall survival (OS). Data from The Cancer Genome Atlas database was used to externally validate the respective findings (n = 317). In general, HNSCC tended to lower expression of drug transporters compared to normal epithelium. High ABCB1 mRNA tumor expression was associated with both favorable progression-free survival (PFS, p = 0.0357) and overall survival (OS, p = 0.0535). Similar results were obtained for the mRNA of ABCC1 (MRP1, multidrug resistance-associated protein 1; PFS, p = 0.0183; OS, p = 0.038). In contrast, protein expression of ATP7b (copper transporter ATP7b), mRNA expression of ABCG2 (BCRP, breast cancer resistance protein), ABCC2 (MRP2), and SLC31A1 (hCTR1, human copper transporter 1) did not correlate with survival. Cluster analysis however revealed that simultaneous high expression of SLC31A1, ABCC2, and ABCG2 indicates poor survival of HNSCC patients. In conclusion, this study militates against the intuitive dogma where high expression of drug efflux transporters indicates poor survival, but demonstrates that expression of single drug transporters might indicate even improved survival. Prospectively, combined analysis of the ‘transportome’ should rather be performed as it likely unravels meaningful data on the impact of drug transporters on survival of patients with HNSCC.
Introduction
Chemotherapy with classical cytostatics such as antimetabolites, platinum drugs, or taxanes remains a cornerstone in the therapy of head and neck squamous cell carcinoma (HNSCC) [1]. Unresponsiveness to antineoplastic agents is frequently due to a phenomenon called multidrug-resistance (MDR) [2]. The classical MDR phenotype is mediated by ATP-binding cassette (ABC)-transporters such as P-glycoprotein (Pgp, ABCB1), breast cancer resistance protein (BCRP, ABCG2), or several multidrug-resistance-associated proteins (MRPs, ABCC family). These membrane-located proteins extrude anticancer agents or their metabolites from cells mediating drug resistance [2]. Paclitaxel, cisplatin, and 5-fluorouracil (5-FU) are standard anti-HNSCC drugs [1], the efficacies of which are limited by several ABC-transporters at least in vitro [3]–[8]. In contrast to experimental studies, clinical data on the role of these proteins is less clear, although some studies for other tumor entities indeed indicated that ABC-transporters negatively influence clinical response or survival of patients suffering from tumors of the lung [9]–[10], the breast [11]–[12], the liver [13], or the kidney [14]. For HNSCC, the significance of ABC-transporters is even more uncertain. First, expression levels have been reported to range from very low [15]–[16] to high expression [17]. Second, impact on chemotherapy response and survival is also inconsistent. MRP1 expression in nasopharyngeal carcinomas was reported to predispose for recurrence and metastasis and to indicate poor 5-year-survival [18]. On the other hand, MRP1 was also documented not to correlate with drug sensitivity or lymph node metastasis [19]. MRP2 and Pgp expression even indicated favorable local tumor control and improved overall survival, respectively [20]. In addition to ABC-transporters such as MRP2, efficacy of cisplatin is also influenced by transporters physiologically involved in copper homeostasis. Human copper transporter 1 (hCTR1/SLC31A1) mediates the cellular uptake of copper, cisplatin, and oxaliplatin [21]. The P-type ATPase ATP7b (Wilson disease protein) is also associated with transport of and resistance to cisplatin in vitro, inferior clinical response to cisplatin chemotherapy, and poor survival of HNSCC patients [22]–[23].
We therefore determined expression levels of important drug transporters in tumor specimens of therapy-naïve patients with stage IV HNSCC using quantitative reverse transcription real-time polymerase chain reaction, tissue microarray approach, and external validation controls in order to re-examine contradictory findings by others [20]. The aim of this study was to gain a concluding overview on the significance of all these different drug transporters for progression-free and overall survival times of HNSCC patients.
Materials and Methods
Patients
Samples from HNSCC tumor patients and normal mucosa samples from non-cancer patients who underwent tonsillectomy were obtained from the Tissue bank of the National Center for Tumor Diseases (NCT, Heidelberg, Germany) (project no. 374). The study was approved by the institutional ethics committee and written informed consent was obtained from each patient. Clinical data of patients were assessed in an MS Access database (Microsoft, Redmond, USA). Clinical staging and follow-up data were obtained by reviewing the medical records, radiographic images and either by telephone or written correspondence. Patients included did not receive chemo- or radiotherapy prior to surgery. Patients were followed from date of first diagnosis to the end of study, whereas patients who were still alive were censored. Before further use, a vital tumor cell content ≥70% was confirmed on hematoxylin and eosin stained sections by an experienced pathologist of the National Cancer for Tumor Diseases (NCT) Tissuebank.
Quantification of mRNA expressions by quantitative real time reverse transcription polymerase chain reaction (qRT-PCR)
To exclude possible prognostic confounders and to analyze a rather homogeneous qRT-PCR study sample in the univariate survival analysis all included patients were in clinical stage IVa (Table 1). Drug transporter gene expression was quantified by qRT-PCR. RNA was isolated from tumor specimen using RNeasy-Kit (Qiagen, Hilden, Germany) and cDNA was synthesized with the Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science, Mannheim, Germany) according to the manufacturers' instructions. Expression of mRNA was quantified by qRT-PCR with a LightCycler 480 (Roche Applied Science, Mannheim, Germany) using the SYBR Green format with the Absolute QPCR SYBR Green Mix. Primer sequences were published previously [24]. The following genes were quantified: ABCB1, ABCC1, ABCC2, ABCG2, SLC31A1, and ATP7b. The most suitable housekeeping gene for normalization was identified using geNorm (version 3.4, Center for Medical Genetics, Ghent, Belgium) [25]. Among the housekeeping genes tested (β2-microglobulin; glucose-6-phosphate dehydrogenase, G6PDH; glucuronidase β, GU; ribosomal protein L13, RPL13; hypoxanthine-phosphoribosyltransferase 1, HPRT; 60S human acidic ribosomal protein P1, HUPO, GU proved to be the most stable one for this data set. Data were evaluated by calibrator-normalized relative quantification with efficiency correction using LightCycler 480 software as published previously [26]. Results are expressed as the ratio target gene/housekeeping genes divided by the corresponding ratio of the calibrator (equivolumetric mixture of all samples). All samples were amplified in duplicate. Patient characteristics are shown in Table 1.
Table 1. Clinical characteristics of qPCR including n = 40 HNSCC patients used for correlation of protein expression with survival.
| Mean ± SD | Range | |
| Characteristics | ||
| Age (years) | 59±9 | 46–85 |
| OS (weeks) | 186±164 | 15–886 |
| PFS (weeks) | 167±150 | 6–670 |
| Frequency | Percent | |
| Gender | ||
| M | 35 | 87.5 |
| F | 5 | 12.5 |
| Localisation | ||
| Oropharynx | 21 | 52.5 |
| Hypopharynx | 11 | 27.5 |
| Larynx | 3 | 7.5 |
| Oral Cavity | 5 | 12.5 |
| Therapy | ||
| Radiation | 5 | 12.5 |
| Chemotherapy | 11 | 27.5 |
| Radio/Chemotherapy | 24 | 60.0 |
| Surgery | 40 | 100.0 |
| Clinical stage | ||
| I–II | 0 | 0.0 |
| III–IV | 40 | 100.0 |
Quantification of ATP7b expression by tissue microarray (TMA)
Formalin-fixed, paraffin-embedded tissue samples from 87 patients were used for TMA design (Table 2). Representative tumor regions were identified by an experienced pathologist on H&E-stained tissue sections. From all selected regions, tissue cylinders with a diameter of 0.6 mm were obtained and arrayed into a recipient block as described earlier [27]. The recipient block was subsequently cut into 5 µm sections on precleaned microscope slides (Superfrost Plus, Thermo Scientific, Braunschweig, Germany).
Table 2. Clinical characteristics of TMA including n = 61 HNSCC patients used for correlation of protein expression with survival.
| Mean ± SD | Range | |
| Characteristics | ||
| Age (years) | 59±10 | 35–87 |
| OS (weeks) | 191±140 | 5–544 |
| PFS (weeks) | 162±143 | 5–520 |
| Frequency | Percent | |
| Gender | ||
| M | 51 | 85.0 |
| F | 9 | 15.0 |
| Localisation | ||
| Oropharynx | 29 | 47.5 |
| Hypopharynx | 15 | 24.6 |
| Larynx | 14 | 23.0 |
| Oral Cavity | 3 | 4.9 |
| Therapy | ||
| Radiation | 12 | 19.7 |
| Chemotherapy | 24 | 39.3 |
| Radio/Chemotherapy | 25 | 41.0 |
| Surgery | 61 | 100.0 |
| Clinical stage | ||
| I–II | 8 | 13.1 |
| III–IV | 53 | 86.9 |
Prior to TMA staining specificity of primary ATP7b antibody was ensured using an isotype control (PP501P, Acris, Hiddenhausen, Germany). Proceeding staining deparaffinization was carried out by immersing slides in 100% xylol (3×3 min), followed by 90%, 80%, 70% and 50% ethanol (2×3 min each). Finally, slides were washed in distilled water (2×3 min). Antigen retrieval was performed in an autoclave at 1 bar, 125°C for 20 min using antigen retrieval buffer (DAKO, Hamburg, Germany) at pH 6.1. Incubation with primary and secondary antibodies as well as detection with Vectastain ELITE ABC Kit (Vector Laboratories, Burlingame, USA) was carried out as described [28].
Antigen expression was pre-tested in a set of HNSCC tissues to establish suitable antigen evaluation categories based on antigen expression variability. Grading scores with uniform distribution of antigen expression levels among individual grading categories were chosen for the final TMA evaluation. Slides were scanned at a 20× magnification by the Nanozoomer Digital Pathology (NDP) System (Hamamatsu Photonics, Hamamatsu, Japan) by the BIOQUANT TIGA Center of the University Heidelberg. Scanned TMAs were afterwards evaluated with the help of the NDP Viewer software (Hamamatsu Photonics, Hamamatsu, Japan). Each tumor biopsy was scored semiquantitatively on the basis of a well-established immunoreactivity scoring system (IRS) [29]. Each investigator (DT, JW) ranked a value for the expression intensity from 1 (no staining) to 4 (very strong expression) and a value describing the extent of tumor staining (1, no expression; 2, 0–24%; 3, 25–49%; 4, 50–74%, 5, 75–100%). These values were multiplied. The final score of each tumor was then calculated as the mean of these two independent evaluations. Consequently, lowest score was 1, highest score was 20.
Data base query
The Cancer Genome Atlas (TCGA; URL: https://tcga-data.nci.nih.gov/tcga/) validation data were retrieved as level 3 normalized RNAseq gene expression files. Age, sex, clinical staging, survival times, vital status, drug and radiation treatment were annotated clinical data provided by TCGA. Only patients of clinical stage II-IVa were selected for survival analyses. Patient characteristics are shown in Table 3.
Table 3. Clinical characteristics of TCGA dataset including n = 317 HNSCC patients used for correlation of RNAseq mRNA expression with survival.
| Mean ± SD | Range | |
| Characteristics | ||
| Age (years) | 61±12 | 19–90 |
| OS (weeks) | 113±124 | 1–917 |
| PFS (weeks) | 98±93 | 6–561 |
| Frequency | Percent | |
| Gender | ||
| M | 230 | 72.6 |
| F | 87 | 27.4 |
| Localisation | ||
| Oropharynx | 42 | 13.2 |
| Hypopharynx | 3 | 0.9 |
| Larynx | 77 | 24.3 |
| Oral Cavity | 195 | 61.5 |
| Therapy | ||
| Radiation | 39 | 12.3 |
| Chemotherapy | 0 | 0.0 |
| Radio/Chemotherapy | 72 | 22.7 |
| Surgery | 317 | 100.0 |
| Clinical stage | ||
| II | 70 | 22.1 |
| III | 81.00 | 25.5 |
| IV | 166.00 | 52.4 |
Statistical analysis
Differences in drug transporter expression levels between tumor and control samples were analyzed using two-sided t-tests. RNAseq data were analyzed using Mann-Whitney U test. Probability values of p<0.05 were considered significant. Survival associations were calculated using a log rank test and displayed as Kaplan-Meier plots. Calculations were performed using Prism 5 statistics software (GraphPad Software, La Jolla, USA). Heatmaps were drawn using the SUMO statistic software (http://angiogenesis.dkfz.de/oncoexpress/software/sumo/).
Results
mRNA expression of drug transporters in HNSCC
mRNA expression levels of a subset of drug transporters (ABCB1, ABCC1, ABCC2, ABCG2, SLC31A1) were determined by qRT-PCR in a study sample of therapy naïve stage IVa HNSCC tumors (n = 40) and normal control samples (n = 14) (Fig. 1A). In general, ABCB1 and ABCC2 were very low expressed in HNSCC, whereas ABCC1 was highly expressed. ABCG2 and SLC31A1 exhibited intermediate mRNA expression levels. Comparing tumor and normal tissues, three of the five analyzed genes showed a significant lower expression in tumors than in healthy control tissues (ABCB1, p<0.0001; ABCC2, p<0.005; ABCG2, p<0.0001), whereas ABCC1 expression was significantly up-regulated in the carcinomas (p<0.0001). These differences resulted in a 9.4- , 2.1- and 4.9-fold lower median expression of ABCB1, ABCC2 and ABCG2 in tumor samples compared to control samples, respectively. ABCC1 exhibited a 1.8-fold higher median expression in tumor samples. SLC31A1 expression did not differ between HNSCC and control samples.
Figure 1. mRNA expression of drug transporters in HNSCC.
(A) Determination of mRNA expression levels of drug transporters by qRT-PCR in a study sample of therapy naïve stage IVa HNSCC tumors (n = 40, grey boxes) and normal control samples (n = 14, white boxes) (relative mRNA expression normalized to the lowest value). (B) External validation by HNSCC RNAseq mRNA expression data derived from the ‘The Cancer Genome Atlas’ (TCGA) consortium. Comparison of normalized counts of paired tumor (n = 37, grey boxes) and adjacent noncancerous normal tissue (n = 37, white boxes). Whisker indicates 5–95 percentile; Mann-Whitney U test; **, p<0.01; ***, p<0.001.
To validate our findings in an independent dataset, we extracted RNAseq mRNA expression data from the HNSCC database derived from the TCGA consortium (Fig. 1B). ABCB1 (p<0.0001) and ABCG2 (not significantly) also showed lower expression in tumors, whereas ABCC1 was also significantly (p<0.0001) higher expressed in tumors. In contrast to our study sample, SLC31A1 expression was significantly down-regulated in the TCGA dataset of HNSCC (p = 0.0036) and ABCC2 exhibited significantly higher expression in tumors (p = 0.0002), whereas it was significantly down-regulated in our samples.
Impact of mRNA expression of drug transporter genes on patient survival
To analyze association of drug transporter gene expression with survival of HNSCC patients, patients were assigned into two groups differing by either higher or lower gene expression than the median of all samples. Based on this grouping the genes showed a divergent correlation with survival in the univariate log rank survival analysis.
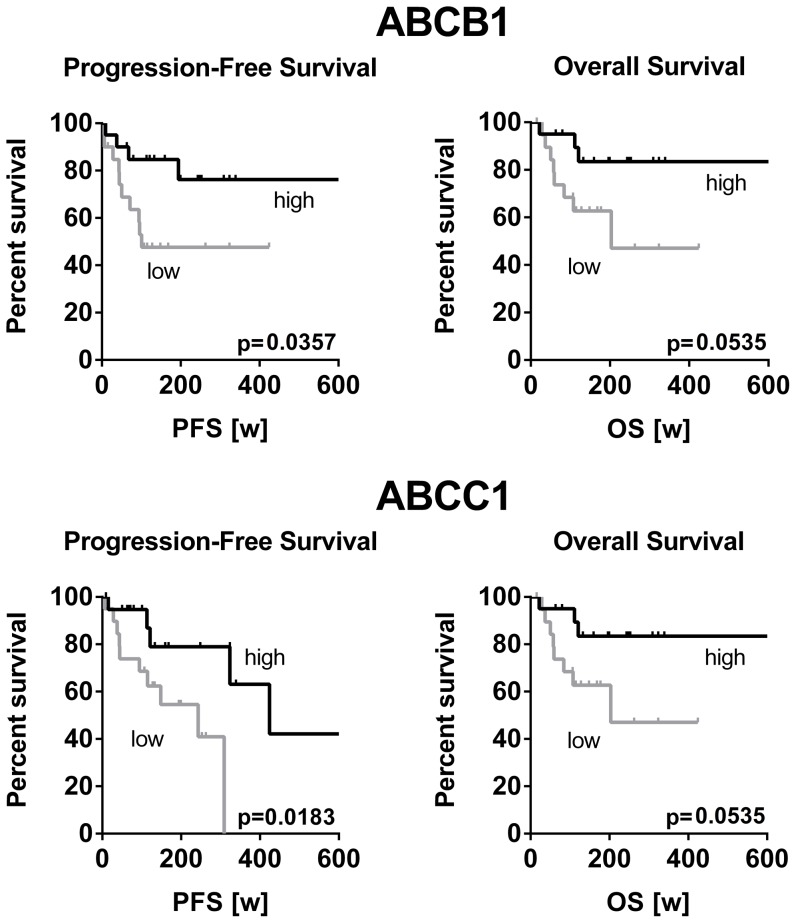
High ABCB1 as well as high ABCC1 mRNA gene expression was significantly associated with longer progression free survival (p = 0.0357 and p = 0.0183, respectively) and overall survival (p = 0.0535 and p = 0.038, respectively) (Fig. 2). In contrast, high expression levels of ABCG2, ABCC2 and SLC31A1 tended to be associated with a shorter survival time of HNSCC patients (Fig. 3). However, these associations did not reach statistical significance.
Figure 2. Correlation of low mRNA expression and shortened survival of HNSCC patients.
Classification of HNSCC patients according to the gene expression level in the high (n = 20) or low (n = 20) expression group. Survival analysis by Kaplan-Meier curves and log rank test revealed a significant correlation of lower expression and shortened progression-free and overall survival.
Figure 3. Correlation of high mRNA expression and shortened survival of HNSCC patients.
Classification of HNSCC patients according to the expression level of the respective gene in high (n = 20) or low (n = 20) expression group. Survival analysis by Kaplan-Meier curves and log rank test revealed that patients with high gene expression tended to accompany a shorter progression-free and overall survival.
Because there was a substantial difference in expression levels, potential mutual exclusive up- or down-regulation of genes was analyzed through hierarchical clustering the median normalized log2 transformed mRNA expression values. The respective heatmap uncovered three distinct groups (Fig. 4): First group (n = 14) constantly exhibiting low expression of all drug transporters evaluated (#1, red framed), second group (n = 19) with both high ABCB1 and high ABCC1 expression (#2, yellow framed) and a third group showing low ABCB1/ABCC1 expression but high levels of either ABCG2, SLC31A1, or ABCC2 (#3, green framed). Best survival was observed for the first group with constantly low expression of all drug transporters evaluated. In contrast, lowest survival was observed for the third group of patients with tumors exhibiting high expressions of ABCG2, SLC31A1, and ABCC2. The second group (high ABCB1 and high ABCC1) exhibited intermediate survival times.
Figure 4. Hierarchical clustering of mRNA expression and survival of HNSCC patients.
(A) Heatmap of the median normalized log2 transformed mRNA expressions hierarchically clustered with Euclidean distance matrix and complete linkage. Three distinct groups were uncovered: First group (n = 14) low expression of all drug transporters evaluated (#1, red framed), second group (n = 19) high ABCB1 and high ABCC1 expression (#2, yellow framed) and a third group (n = 7) showing low ABCB1/ABCC1 expression but high levels of either ABCG2, SLC31A1, or ABCC2 (#3, green framed). (B) Survival analysis by Kaplan-Meier curves and log rank test revealed that patients of group #3 (n = 7) tended to survive shorter than those of group #2 (n = 19). Best survival was seen for patients of group #1 (n = 14) who show a lower expression of drug transporters.
Validation of survival in the independent TCGA dataset
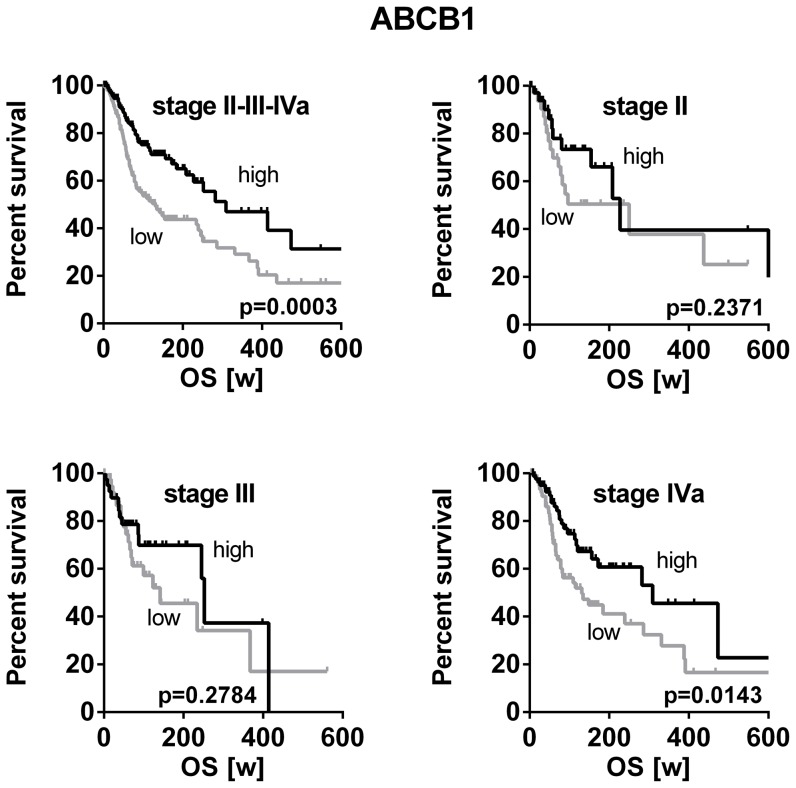
To challenge our findings, the publicly available TCGA HNSCC dataset containing high patient numbers of all clinical stages was again used for validation in anindependent dataset. Patients with HNSCC of stage II (n = 70), stage III (n = 81) or stage IVa (n = 166) were again grouped according to our previous approach into lower or higher than the median of all samples, respectively. Consistent with our initial finding, high ABCB1 expression significantly correlated with a better overall survival (p = 0.0003) (Fig. 5). Stratification according to clinical stage indicates significant correlation with survival within the group of stage IVa patients (p = 0.0143). In stage II and III the same trend was obvious, but it did not reach statistical significance (Fig. 5).
Figure 5. Correlation of RNAseq mRNA expression and overall survival of HNSCC patients in the TCGA dataset.
Classification of stage II-III-IVa TCGA HNSCC patients according to the ABCB1 expression level in high or low. Survival analysis by Kaplan-Meier curves and log rank test of all stages together (upper left; high, n = 159; low, n = 158) or either stage II (upper right; high, n = 34; low, n = 36), stage III (lower left; high, n = 40; low, n = 41) and stage IVa (lower right; high, n = 83; low, n = 83) revealed consistently shorter survival times for patients with high ABCB1 expression, which reached significance in the whole group and the subgroup of stage IVa tumors.
ATP7b protein expression evaluated by tissue microarray
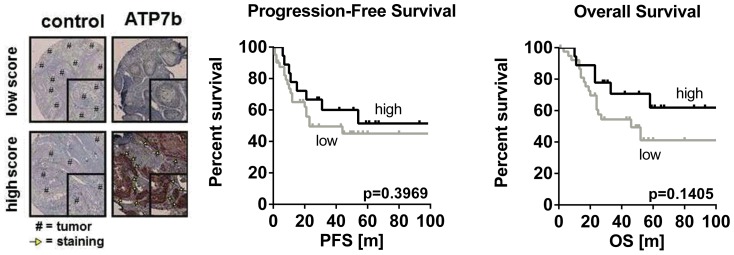
There was a high variation of ATP7b protein expression among the 61 HNSCC samples evaluated with immunoreactive scores between 1 to 19 (median 14). Grouping of these patients according to their immunoreactive score (cutoff 15) and subsequent survival analysis showed that ATP7b expression levels did not correlate with progression-free survival (p = 0.3969) or overall survival (p = 0.1405), respectively (Fig. 6). However, concordant to ABCB1 and ABCC1 patients with high ATP7B protein expression tended to survive longer.
Figure 6. Immunohistochemical staining of ATP7b protein expression in HNSCC.
Immunohistochemical staining of ATP7b protein expression in HNSCC patients (n = 61) revealed a high range of immunoreactive scores (left). Grouping of patients according to their immunoreactive scores (cutoff = 15) into low (n = 41) or high (n = 18) showed a trend towards a longer progression-free and overall survival in patients with a higher ATP7b expression (right).
Discussion
Identification of tissue biomarkers in biopsy specimens of HNSCC may not only select patients that may benefit from more aggressive treatment modalities but may also indicate prognosis. To date, robust clinical, molecular, or radiographic markers in HNSCC are still rare [30]. Therefore, we intended to investigate the expression of drug transporters in these tumors, because their clinical relevance is unclear so far. Besides their role as mediators of cytostatic drug resistance, ABC-transporters have also been proposed as markers of malignancy in HNSCC. In parotid mucoepidermoid carcinoma advanced grades exhibited higher Pgp expression levels than tumors of lower grades [31]. Moreover, Pgp expression has been reported to generally increase during the course of the disease [32]–[33]. Consequently, our samples of higher stage HNSCC were expected to also show higher expression levels of drug transporters than non-tumor controls. However, the opposite was demonstrated. ABCB1, ABCC2, and ABCG2 were significantly lower expressed in tumors than in normal epithelium of tonsils. The same trend was observed for SLC31A1 but without reaching statistical significance. ABCC1 was the only drug transporter evaluated that was overexpressed in tumors (Fig. 1A). To validate these unexpected findings and to rule out that our observations were biased by unrelated factors, the TCGA dataset was analyzed for drug transporter expression in HNSCC and adjacent non-tumor control tissue. Because here 37 pairs of tumors and their normal counterparts of the very same patient were compared, the results are considered independent of confounders such as gender, age, or smoking status. Except ABCC2, the TCGA dataset generally confirmed our results by demonstrating that drug transporters such as ABCB1 (Pgp) are highly significantly (P<0.0001) down-regulated in HNSCC (Fig. 1B). In contrast to the intuitive dogma, such low expression seems to be associated with a malignant phenotype and advanced tumor disease. This assumption was supported by the subsequent survival analysis. HNSCC patients with low ABCB1 expression had significantly shorter progression-free survival times and tended to die earlier (overall survival, p = 0.0535) than patients with ABCB1 expression higher than the median (Fig. 2). This finding was again confirmed using the TCGA dataset by demonstrating that low expression of ABCB1 correlates with poor overall survival in HNSCC stage II - IVa while high expression of ABCB1 rather indicates favorable survival. Stratification for tumor stages confirmed this trend and again showed that in advanced tumors (stage IVa), poor survival in association with low ABCB1 expression (Fig. 5). Mechanistically, it is hard to understand why high expression of ABCB1 and ABCC1 was related to improved survival while low expressions indicated poor survival. Recently, experimental studies suggested that overexpression of ABC-transporters leads to enhanced efflux of glutathione and diminished cellular glutathione content. When intracellular glutathione levels are low, platinum drug species are rarely complexed and thus remain pharmacologically active [34]. In consequence, increased expression of glutathione export transporters can indeed promote efficacy of platinum drugs in HNSCC and lead to a clinical benefit. This observation has been confirmed clinically in HNSCC [20]. Second, cancer is frequently accompanied by inflammatory processes in the microenvironment of the tumor, especially with advancing disease [35]–[36]. Secreted inflammation-associated cytokines (e.g. tumor necrosis factors, interleukines, etc) are in turn very well known to subsequently downregulate drug transporters [37]. In consequence, the observed downregulation of drug transporters in HNSCC tissue might simply be an indicator of enhanced inflammation which is typically observed in advanced stages of cancer and accompanied by poor survival [38].
In contrast to ABCB1 and ABCC1, high expression of ABCG2, ABCC2, and SLC31A1 tended to indicate poor progression-free survival and overall survival, but none of these associations reached statistical significance (Fig. 3). Due to these contradicting results, a cluster analysis was subsequently performed in order to detect dependencies or expression patterns that concertedly determine survival. Three distinct groups were identified. Best survival was observed when all drug transporters showed a reduced expression, whereas both progression-free and overall survival was shortened with coordinated high expression of SLC31A1, ABCG2, and ABCC2 (Fig. 4). The latter two genes are known cancer-stem cell markers in HNSCC [39]–[40]. In consequence, it is comprehensible that patients with high expression of ABCG2 and ABCC2 (and thus potentially high cancer-stem cell burden) exhibit poor survival. Together, the results of the cluster analysis finally suggest that the course of the disease or survival cannot be estimated by a single drug transporter gene, but rather by the whole ‘transportome’ or at least the combination of certain drug transporters (e.g. cancer-stem cell marking drug transporters).
ATP7b expression was evaluated at the protein level using tissue microarray methodology (Fig. 6A). A high variation of the immunoreactive score was observed among the 61 HNSCC samples evaluated. However, survival analysis revealed that ATP7b expression does not correlate with survival times (Fig. 6B) contradicting earlier findings by others [23].
In conclusion, this study contradicts the intuitive dogma whereupon high expression of ABC-transporters such as Pgp is unfavorable for survival of HNSCC patients. In contrast, overexpression of distinct drug transporters might even indicate improved survival. Cluster analysis evaluating a subset of drug transporters including cancer-stem cell markers such as ABCG2 is suggested for further studies on the significance of drug transporters for HNSCC disease.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper or given as URL.
Funding Statement
This project was funded by grant WE 4135/3-1 und HE 2357/2-1 from the German Research Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Colevas AD (2006) Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 24: 2644–2652. [DOI] [PubMed] [Google Scholar]
- 2. Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2: 48–58. [DOI] [PubMed] [Google Scholar]
- 3. Kamazawa S, Kigawa J, Minagawa Y, Itamochi H, Shimada M, et al. (2000) Cellular efflux pump and interaction between cisplatin and paclitaxel in ovarian cancer cells. Oncology 59: 329–335. [DOI] [PubMed] [Google Scholar]
- 4. Lagas JS, Vlaming ML, van Tellingen O, Wagenaar E, Jansen RS, et al. (2006) Multidrug resistance protein 2 is an important determinant of paclitaxel pharmacokinetics. Clin Cancer Res 12: 6125–6132. [DOI] [PubMed] [Google Scholar]
- 5. Duan Z, Brakora KA, Seiden MV (2004) Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther 3: 833–838. [PubMed] [Google Scholar]
- 6. Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W, et al. (2005) The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther 4: 855–863. [DOI] [PubMed] [Google Scholar]
- 7. Guminski AD, Balleine RL, Chiew YE, Webster LR, Tapner M, et al. (2006) MRP2 (ABCC2) and cisplatin sensitivity in hepatocytes and human ovarian carcinoma. Gynecol Oncol 100: 239–246. [DOI] [PubMed] [Google Scholar]
- 8. Weaver DA, Crawford EL, Warner KA, Elkhairi F, Khuder SA, et al. (2005) ABCC5, ERCC2, XPA and XRCC1 transcript abundance levels correlate with cisplatin chemoresistance in non-small cell lung cancer cell lines. Mol Cancer 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeh JJ, Hsu NY, Hsu WH, Tsai CH, Lin CC, et al. (2005) Comparison of chemotherapy response with P-glycoprotein, multidrug resistance-related protein-1, and lung resistance-related protein expression in untreated small cell lung cancer. Lung 183: 177–183. [DOI] [PubMed] [Google Scholar]
- 10. Yoh K, Ishii G, Yokose T, Minegishi Y, Tsuta K, et al. (2004) Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res 10: 1691–1697. [DOI] [PubMed] [Google Scholar]
- 11. Surowiak P, Materna V, Matkowski R, Szczuraszek K, Kornafel J, et al. (2005) Relationship between the expression of cyclooxygenase 2 and MDR1/P-glycoprotein in invasive breast cancers and their prognostic significance. Breast Cancer Res 7: R862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burger H, Foekens JA, Look MP, Meijer-van Gelder ME, Klijn JG, et al. (2003) RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin Cancer Res 9: 827–836. [PubMed] [Google Scholar]
- 13. Soini Y, Virkajärvi N, Raunio H, Pääkkö P (1996) Expression of P-glycoprotein in hepatocellular carcinoma: a potential marker of prognosis. J Clin Pathol 49: 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duensing S, Dallmann I, Grosse J, Buer J, Lopez Hänninen E, et al. (1994) Immunocytochemical detection of P-glycoprotein: initial expression correlates with survival in renal cell carcinoma patients. Oncology 51: 309–313. [DOI] [PubMed] [Google Scholar]
- 15. Uematsu T, Hasegawa T, Hiraoka BY, Komatsu F, Matsuura T, et al. (2001) Multidrug resistance gene 1 expression in salivary gland adenocarcinomas and oral squamous-cell carcinomas. Int J Cancer 92: 187–194. [DOI] [PubMed] [Google Scholar]
- 16. Chen CL, Sheen TS, Lou IU, Huang AC (2001) Expression of multidrug resistance 1 and glutathione-S-transferase-Pi protein in nasopharyngeal carcinoma. Hum Pathol 32: 1240–1244. [DOI] [PubMed] [Google Scholar]
- 17. Lo Muzio L, Staibano S, Pannone G, Mignogna MD, Serpico R, et al. (2000) The human multidrug resistance gene (MDR-1): immunocytochemical detection of its expression in oral SCC. Anticancer Res 20: 2891–2897. [PubMed] [Google Scholar]
- 18. Larbcharoensub N, Leopairat J, Sirachainan E, Narkwong L, Bhongmakapat T, et al. (2008) Association between multidrug resistance-associated protein 1 and poor prognosis in patients with nasopharyngeal carcinoma treated with radiotherapy and concurrent chemotherapy. Hum Pathol 39: 837–845. [DOI] [PubMed] [Google Scholar]
- 19. Tsuzuki H, Fujieda S, Sunaga H, Sugimoto C, Tanaka N, et al. (1998) Expression of multidrug resistance-associated protein (MRP) in head and neck squamous cell carcinoma. Cancer Lett 126: 89–95. [DOI] [PubMed] [Google Scholar]
- 20. van den Broek GB, Wildeman M, Rasch CR, Armstrong N, Schuuring E, et al. (2009) Molecular markers predict outcome in squamous cell carcinoma of the head and neck after concomitant cisplatin-based chemoradiation. Int J Cancer 124: 2643–2650. [DOI] [PubMed] [Google Scholar]
- 21. Song IS, Savaraj N, Siddik ZH, Liu P, Wie Y, et al. (2004) Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther 3: 1543–1549. [PubMed] [Google Scholar]
- 22. Nakayama K, Kanzaki A, Ogawa K, Miyazaki K, Neamati N, et al. (2002) P-type adenosine triphophatase (ATP7B) as a cisplatin-based chemoresistance marker in ovarian carcinoma: comparative analysis with expression of MDR1, MRP, LRP and BCRP. Int J Cancer 101: 488–495. [DOI] [PubMed] [Google Scholar]
- 23. Miyashita H, Nitta Y, Mori S, Kanzaki A, Nakayama K, et al. (2003) Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) as a chemoresistance marker in human oral squamous cell carcinoma treated with cisplatin. Oral Oncol 39: 157–162. [DOI] [PubMed] [Google Scholar]
- 24. Theile D, Ketabi-Kiyanvash N, Herold-Mende C, Dyckhoff G, Efferth T, et al. (2011) Evaluation of drug transporters' significance for multidrug resistance in head and neck squamous cell carcinoma. Head Neck 33: 959–968. [DOI] [PubMed] [Google Scholar]
- 25. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3 RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albermann N, Schmitz-Winnenthal FH, Z'graggen K, Volk C, Hoffmann MM, et al. (2005) Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem Pharmacol 70: 949–958. [DOI] [PubMed] [Google Scholar]
- 27. Freier K, Bosch FX, Flechtenmacher C, Devens F, Benner A, et al. (2003) Distinct sitespecific oncoprotein overexpression in head and neck squamous cell carcinoma: a tissue microarray analysis. Anticancer Res 23: 3971–3977. [PubMed] [Google Scholar]
- 28. Karcher S, Steiner HH, Ahmadi R, Zoubaa S, Vasvari G, et al. (2006) Different angiogenic phenotypes in primary and secondary glioblastomas. Int J Cancer 118: 2182–2189. [DOI] [PubMed] [Google Scholar]
- 29. Remmele W, Stegner HE (1987) Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8: 138–140. [PubMed] [Google Scholar]
- 30. Thomas GR, Nadiminti H, Regalado J (2005) Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. Int J Exp Pathol 86: 347–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Furusaka T1, Sasaki CT, Matsuda A, Susaki Y, Matsuda H, et al. (2013) Multidrug resistance in mucoepidermoid carcinoma of the parotid gland–immunohistochemical investigations of P-glycoprotein expression. Acta Otolaryngol 133: 552–557. [DOI] [PubMed] [Google Scholar]
- 32. Ralhan R1, Narayan M, Salotra P, Shukla NK, Chauhan SS (1997) Evaluation of P-glycoprotein expression in human oral oncogenesis: correlation with clinicopathological features. Int J Cancer 72: 728–734. [DOI] [PubMed] [Google Scholar]
- 33. Jain V1, Das SN, Luthra K, Shukla NK, Ralhan R (1997) Differential expression of multidrug resistance gene product, P-glycoprotein, in normal, dysplastic and malignant oral mucosa in India. Int J Cancer 74: 128–133. [DOI] [PubMed] [Google Scholar]
- 34. Theile D, Grebhardt S, Haefeli WE, Weiss J (2009) Involvement of drug transporters in the synergistic action of FOLFOX combination chemotherapy. Biochem Pharmacol 78: 1366–1373. [DOI] [PubMed] [Google Scholar]
- 35. Royuela M, Ricote M, Parsons MS, Garcia-Tunon I, Paniagua R, et al. (2004) Immunohistochemical analysis of the IL-6 family of cytokines and their receptors in normal, hyperplastic, and malignant human prostate. J Pathol 202: 41–49. [DOI] [PubMed] [Google Scholar]
- 36. Brozek W, Bises G, Girsch T, Cross HS, Kaiser HE, et al. (2005) Differentiation dependent expression and mitogenic action of interleukin-6 in human colon carcinoma cells: relevance for tumour progression. Eur J Cancer 41: 2347–2354. [DOI] [PubMed] [Google Scholar]
- 37. Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, et al. (2008) Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos 36: 205–216. [DOI] [PubMed] [Google Scholar]
- 38.Rassouli A, Saliba J, Castano R, Hier M, Zeitouni AG (2013) Systemic inflammatory markers as independent prognosticators of Head and Neck Squamous cell carcinoma. Head Neck 2013. [DOI] [PubMed]
- 39. Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH (2007) Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res 67: 3716–3724. [DOI] [PubMed] [Google Scholar]
- 40. Lee SH1, Nam HJ, Kang HJ, Kwon HW, Lim YC (2013) Epigallocatechin-3-gallate attenuates head and neck cancer stem cell traits through suppression of Notch pathway. Eur J Cancer 49: 3210–3218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper or given as URL.