Abstract
The mechanisms causing cognitive problems in chronic pain patients are not well understood. We used the Stroop color word task (SCWT) to investigate distraction-induced analgesia, cognitive performance, and cerebral activation patterns in 29 fibromyalgia (FM) patients (mean age 49.8 years, range 25–64 years) and 31 healthy controls (HC) (mean age 46.3 years, range 20–63 years). In the first study, SCWT was used to investigate distraction-induced analgesia in FM patients. Two versions of the task were applied, one with only congruent color-word images and one with incongruent images. Pressure pain thresholds were assessed using a pressure algometer before, during, and following SCWT. In the second study, reaction times (RTs) were assessed and functional magnetic resonance imaging (fMRI) was used to investigate cerebral activation patterns in FM patients and HC during the SCWT. An event-related task mixing incongruent and congruent images was used. In study one, we found reduced pressure pain sensitivity during SCWT in both groups alike and no statistically significant differences were seen between the incongruent and congruent conditions. The study two revealed longer RTs during the incongruent compared to the congruent condition in both groups. FM patients had longer RTs than HC in both conditions. Furthermore, we found a significant interaction between group and congruency; that is, the group differences in RTs were more pronounced during the incongruent condition. This was reflected in a reduced activation of the caudate nucleus, lingual gyrus, temporal areas, and the hippocampus in FM patients compared to HC. In conclusion, we found normal pain inhibition during SWTC in FM patients. The cognitive difficulties seen in FM patients, reflected in longer RTs, were related to reduced activation of the caudate nucleus and hippocampus during incongruent SCWT, which most likely affected the mechanisms of cognitive learning in FM patients.
Introduction
Fibromyalgia (FM) is a chronic pain syndrome, however, many FM patients also suffer from disturbed sleep, fatigue, mood disorders and cognitive impairment. The cognitive dysfunctions reported by FM patients [1]–[3] are referred to as “fibrofog” and can be more disabling than the pain itself [2], [4]. In particular, FM patients are easily distracted with difficulties focusing and redirecting attention [5], and have been reported to perform more poorly on tests assessing attention/executive function such as the Stroop Color Word Test (SCWT). The poor performance of FM patients was not related to disturbances in mood or sleep [6]–[8], but has been reported to correlate with high pain intensity [6] and high pain sensitivity [8].
In accordance with this, pain intensity has been shown to influence cognitive task performance in chronic pain patients using a Stroop paradigm [9]. Recently, Glass et al. (2011), reported aberrant brain activation in FM patients during a test targeting response inhibition. The authors hypothesized that overlapping networks are responsible for executive functioning tasks and pain processing, and that resources taken up by pain processing in FM patients would explain the inability to activate these networks during cognitive tasks [10].
Attentional resources are limited and therefore different stimuli compete with each other for attentional space. Based on this, a bi-directional interference between the perception of pain and the performance of cognitive tasks would be expected, i.e., not only would pain interfere with cognition but focusing on a cognitive task would also decrease pain perception. This is in line with previous findings showing that focusing on the pain can enhance pain perception and that pain perception can be decreased by distraction or cognitive tasks [11]–[13]. Modified versions of the Stroop interference test [14] have been used to assess cognitive modulations of pain perception and pain-related brain activity in healthy subjects. Incongruent Stroop was found to reduce heat pain sensitivity and increase activation of the cingulo-frontal cortex including the orbitofrontal and perigenual anterior cingulate cortex (ACC) as well as the periaqueductal gray (PAG) and the posterior thalamus in healthy controls [15]. Bantick et al. (2002) found that pain intensity scores for heat stimuli were significantly reduced when subjects took part in the cognitively more demanding task (incongruent stimuli) compared to the less demanding neutral task and this was accompanied by reduced activation in pain relevant brain areas such as insula, mid cingulate and thalamus. They also found that the peringual cingulate cortex and orbitofrontal cortex were more activated when painful stimuli and cognitive stimuli were presented together than what would be expected by a simple additive effect of the two [16].
However, using a more tonic pain stimulus it has been reported that the cognitive modulation of pain-related brain responses was not uniform but depended on behavioral strategy and that none of the brain areas showing attention-related responses, including dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex, were modulated by pain [13].
The authors concluded that pain stimuli in healthy subjects did not interfere with brain activities evoked by cognitively demanding tasks. Further, mild or moderate pain was not found to affect performance on a cognitive task, nor did the pain alter brain activity related to this task [17]. Therefore, the interference between pain and cognitive processing is complicated and state dependent.
To our knowledge, no previous studies have investigated the effects of chronic pain on attention-related cerebral responses during the SCWT, or the effects of Stroop on pain sensitivity in chronic pain patients. The aims of this study were two-fold. The first aim was to investigate the effect of cognitive load on distraction induced analgesia in FM patients and HC using the SCWT. In the SCWT the subjects are required to respond to the font color of color words, either congruent (“yellow” written in yellow) or incongruent (“yellow” written in green) [16]. Considering earlier findings of high cognitive load being related to increased distraction induced analgesia [16], we hypothesized that if FM patients have a normal ability to activate ACC during cognitive tasks, the higher cognitive load during the incongruent condition would cause a larger reduction of pain sensitivity compared to the congruent condition and no group differences in distraction-induced analgesia would be found between FM patients and healthy controls. If however, FM patients have a general inability to activate ACC, in line with their reduced ability to activate this structure during painful stimuli [18], then we would expect to find reduced distraction-induced analgesia in FM patients compared to healthy controls and no difference between the incongruent and congruent conditions.
Secondly, we wanted to use SCWT to investigate the effects of chronic ongoing pain on cerebral correlates of cognition in FM patients. As mentioned earlier, structures in the brain that are involved in cognition and pain perception are highly overlapping so mapping the neural correlates of this interference test would provide valuable knowledge as to which cerebral structures are impaired during cognition in FM. We chose the SCWT since it is a purely cognitive task known to activate the dorsal ACC (dACC) [19]. In healthy individuals, reciprocity has been found between activation of the dACC and dlPFC; individuals with high SCWT inference (longer reaction times (RT) during incongruent trials) exhibited higher task related dACC and lower dlPFC activation and vice versa [19]. Providing that FM patients do not have a generalized dysfunction of ACC, then an increased SCWT inference, reflected as higher task related dACC and lower dlPFC activation compared to HC would be expected. However, if FM patients have a more generalized dysfunction of ACC, either due to primary pathology or as a consequence of processing chronic pain [18], [20], [21], a reduced activation of dACC and no reciprocity between dACC and dlPFC would be expected during SCWT.
Methods
Subjects
Subjects were recruited by newspaper advertisement to participate in a multi-center experimental study (ClinicalTrials.gov identification number: NCT01226784) where FM patients were randomized to physical exercise or relaxation therapy. The current study was performed in the Stockholm cohort only and relies on baseline data before start of treatment, therefore no outcome of the clinical trial is reported in this manuscript.
FM patients: 31 women with FM were initially included in the study. However 2 patients had to be excluded (one due to not meeting MRI safety criteria and one due to inability to participate). The final cohort consisted of 29 FM patients average age 49.8 years (range 25–64 years). In study one, one participant was excluded due to inability to participate, leaving 28 participants. Four participants were unwilling or unable to participate in the fMRI part of the study; one participant reported falling asleep during the scan and one participant was not scanned due to technical failure of the equipment, leaving 23 participants.
Inclusion criteria for women with FM were to be of working age, 20–65 years, and meeting the ACR-1990 classification criteria for FM [22]. Exclusion criteria were high blood pressure (>160/90 mmHg), osteoarthritis in hip or knee, other severe somatic or psychiatric disorders, other primary causes of pain than FM, high consumption of alcohol (Audit >6), participation in a rehabilitation program within the past year, regular resistance exercise training or relaxation exercise training twice a week or more, inability to understand or speak Swedish, and not being able to refrain from analgesics, NSAID or hypnotics for 48 hours prior to examinations. One patient was on anticonvulsants and eleven were taking antidepressants (4 tricyclic antidepressants, 4 selective serotonin re-uptake inhibitors and 3 serotonin-noradrenalin re-uptake inhibitors). The daily intake of other drugs was as follows: NSAIDs 2 patients, acetaminophen 4 patients and tramadol 1 patient (no other opioid containing drugs were used on daily basis). All patients declared that they had refrained from hypnotics, NSAIDs, acetaminophen and tramadol/other analgesics at least 48 hours prior to study participation (48 hours before study one and 72 hours before study two (fMRI)). All patients had a physical exam by a specialist in rehabilitation medicine and filled in questionnaires regarding the impact of fibromyalgia (fibromyalgia impact questionnaire (FIQ) [23], hospital anxiety and depression scale (HADS) [24] and health related quality of life (Short Form - 36 (SF-36)) [25].
Healthy controls: 32 healthy female controls were recruited, one had to be excluded due to MR scan showing signs of neuroinflammatory changes, resulting in the final cohort consisting of 31 women, average age 46.3 years (range 20–63 years). All healthy subjects were interviewed regarding their health and completed relevant questionnaires. In study two one participant was unwilling to participate in the fMRI part of the study, one participant was not able to use the response box and one participant’s data was missing due to technical failure, this leaving 28 participants in study 2.
The subject characteristics are presented in table 1.
Table 1. Descriptive data for 29 FM patients and 31 HC, average values and range are presented.
| FM | HC | Group differences | |
| Age (years) | 49.8 (25–64) | 46.3 (20–63) | NS |
| FM dur (years) | 8.9 (0.5–19) | NA | NA |
| Pain VAS (mm) | 45.3 (5–92) | 0.6 (0–10) | P<0.0001 |
| FIQ | 63.1 (42.5–85.0) | 6.8 (0–22.8) | P<0.0001 |
| HADS-D | 7.3 (3.0–16.3) | 2.1 (0–7.0) | P<0.0001 |
| HADS-A | 8.8 (0–18) | 3.2 (0–13.0) | P<0.0001 |
| SF-36 PCS | 30.2 (11.8–46.7) | 55.2 (48.5–62.3) | P<0.0001 |
| SF-36 MCS | 36.5 (18.4–58.9) | 50.6 (31.6–58.6) | P<0.0001 |
FM dur = duration of FM, Pain VAS = pain intensity rated on 100 mm visual analogue scale, FIQ = fibromyalgia impact questionnaire, HADS-D = Hospital anxiety and depression scale, depression ratings, HADS-A = Hospital anxiety and depression scale, anxiety ratings, SF-36 PCS = short form 36 physical compact score, SF-36 MCS = short form 36 mental compact score (original 0–100 scoring algorithms based on the summated ratings method). NA = not applicable.
The regional ethics committee in Stockholm approved the study and written informed consent was obtained from all participants.
Material
Pressure algometry
Pressure pain thresholds (PPTs) were assessed using a pressure algometer (Somedic Sales AB). The pressure algometer had a gun shaped handle with a 1 cm2, circular, flat rubber tip on the end. It had a display that informed the experimenter how forcefully they were pressing and at which rate. The chosen rate was approximately 50 kPa/s. A response button was attached to the algometer and the subjects were instructed to push the button at the instant the pressure become painful. When this button was pressed, the current pressure froze on the screen. The algometer was calibrated for accuracy before each participant.
Stroop Color Word Test (SCWT)
Images were presented on a 17″ LCD screen with a resolution of 1024×768 pixels. Participants were given a response button box (Current designs) with four response buttons in the colors red, green, yellow and blue. The colors red, green and yellow together with the Swedish translation of these words made up the stimuli.
Functional magnetic resonance imaging
FMRI data was collected using a 3.0 tesla scanner (Discovery MR750, GE) and a 32-channel head-coil (MR instruments Inc). Foam wedges and headphones were used to minimize head motion and reduce perceived scanner noise.
Procedure
Subjects were familiarized with the SCWT and assessed regarding pressure pain modulation during SCWT. They returned on the following day for the SCWT fMRI scan. The patients had to refrain from analgesics, NSAIDS and hypnotics 48 hours before the assessment of pain modulation during SCWT and 72 hours before the fMRI scan. Prior to fMRI a 3-plane localizer was performed followed by ASSET-calibration for parallel imaging.
Pressure pain modulation during SCWT
Two paradigms were used in the SCWT test, one paradigm using only classic Stroop images: color words written in incongruent colors (for instance the word “green” in red color), and one control task with color words written in congruent colors (the word “red” in red color). Both paradigms lasted 10 minutes, each stimulus was presented on the screen for 2 seconds and the interstimulus interval (ISI) was three seconds. During the ISI a fixation cross appeared after a second and stayed on the screen for one second. The experimenter orally instructed the participants to respond based on the color of the text and not the word that they were reading. Participants were explicitly instructed to only use the red, green and yellow buttons as blue stimuli was not used. A test run was performed using congruent images and the experimenter ended the run when confident that the participant had familiarized themselves with the speed of the experiment and the location of the buttons. Participants pressed the response buttons with the index, middle and ring finger on the right hand.
To acquaint the participants with the algometer it was demonstrated at the upper arm before testing at the thigh. PPTs were assessed eight times during the congruent and incongruent condition respectively. Twice before the beginning of the paradigm, then at 60, 180, 300, 420 and 540 seconds into the task, and finally once 10 minutes after the task had ended. Blood pressure and heart rate was measured using an automated blood pressure monitor once before the beginning of the task, then 90, 210, 330 and 450 seconds into the task, and finally 10 minutes following task conclusion. The last measurement taken 10 minutes following the conclusion of the first task was also used as the initial measurement prior to the second task.
Half of the FM patients as well as healthy controls started with the incongruent condition followed by the congruent condition, and vice versa. In order to avoid sensitization, PPTs were initially assessed at the right thigh following the left thigh in half of the participants and vice versa in a counterbalanced order.
SCWT during fMRI
Imaging parameters were as following: single shot EPI with a flip angle of 90°, TE 30 and TR 2500. Field of view (FOV) 28.8, 48 axial slices with a slice thickness of 3 mm, no slice spacing, which were acquired interleaved. There were two runs lasting 10 minutes. There were 84 images in each run with an even distribution of congruent and incongruent trials; the order of presentation of congruent and incongruent images was randomized. Interstimulus asynchrony was jittered between six and ten seconds, averaging at eight seconds. The stimulus was presented on a screen (24×32 cm) behind the participants, which was made visible via a mirror placed on the coil. A fixation-cross preceded each stimulus 500 ms followed by a blank screen for 1500 ms. The stimulus was on for 3000 ms. The word “Ready” appeared on the screen prior to the first fMRI run, the experimenter asked the participant to read the word on the screen out loud to ensure that they were positioned in such a way that they could see the words on the screen, as well as to confirm their ability to read the words used as stimuli. The paradigm was run using Neurobehavioural Systems Presentation software and is illustrated in figure 1. T1 weighted structural images were acquired in coronal orientation for anatomical reference purposes and T2 weighted anatomical images for screening for structural anomalies.
Figure 1. Example of one congruent and incongruent trial showing the fMRI set-up.
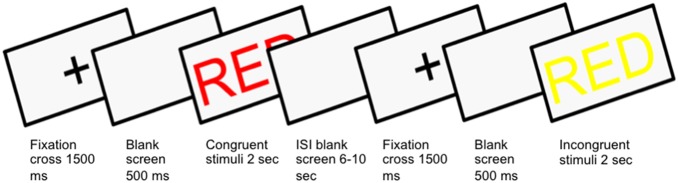
Stimuli were presented in Swedish, but the English translation is used here for illustrative purposes.
Statistics
Analysis of pressure pain modulation during SCWT
In order to assess the effect of SCWT on pressure pain sensitivity the relative change in PPTs at m. quadriceps during SCWT was analyzed. In analogy with our previous studies of pain inhibitory mechanisms the PPTs were normalized (i.e., each PPT value was divided by the individual’s first PPT measure) [26]. The effect of SCWT on PPT was analyzed using a repeated measures ANOVA with the within-subject factors CONGRUENCY (2 levels, congruent and incongruent SCWT) and TIME (7 levels, before, 5 times during and 10 minutes following SCWT) and the between-subject factor GROUP (FM patients and controls). The effect of SCWT on blood pressure and heart rate was analyzed by repeated measures ANOVA with the within-subject factors CONGRUENCY (2 levels, congruent and incongruent SCWT) and TIME (6 levels, before, 4 times during and 10 minutes following SCWT) and the between-subject factor GROUP (FM patients and controls). The difference in reaction time was assessed by repeated measures ANOVA with the within-subject factors CONGRUENCY (2 levels, congruent and incongruent SCWT) and the between-subject factor GROUP (FM patients and controls).
Greenhouse-Geisser corrections were used in case of significant test of sphericity.
Group differences in absolute PPTs at baseline and reaction times were assessed by Students’ independent t-test. Post hoc analysis of intraindividual differences in PPTs over time and reaction times were assessed by Students’ paired t-test. Group differences in the number of errors during the SCWT, were analyzed by Independent Samples Mann-Whitney U-test.
Analyses of fMRI-data
Data was analyzed using SPM8 (Wellcome Trust Centre for Neuroimaging, University College London, UK). EPI images were realigned and then normalized to the canonical EPI-template in standard Montreal Neurological Insititute (MNI) space. Finally the images were smoothed using an 8 mm full-width-at-half-maximum (FWHM)-kernel. For each individual subject, first-level analysis was performed using a fixed effects analysis compounding both scans into the same general linear model (GLM). Stimulus onset times of congruent and incongruent stimuli were entered as regressors of interest, and movement parameters were entered as covariates of no interest. All regressors were convolved with the canonical hemodynamic response function (peak positive BOLD response after approximately 5–6 seconds after stimulus onset, return to pre-simuli baseline after 10–12 seconds) as implemented in SPM8 before they were entered into the general first level linear model [27]. Brain activity at a group level was assessed using a random, second level analysis performed in SPM8 using the parameter files from the individual contrasts files that pertained to the regressors of interest at the first level. To evaluate within-group increases/decreases in brain activity, one-sample T-tests were used (activation maps thresholded at p<0.05, family-wise error corrected), whereas between-group differences in brain activity was carried out using 2-sample T-tests (activation maps thresholded at p<0.001 uncorrected). Clusters of activity smaller than 20 contiguous voxels were not reported. All T- and 2 sample T-tests at the group level were performed with the general linear model framework as implemented in SPM8.
Results
Subject characteristics
The subject characteristics are presented in table 1. As expected FM patients had higher ratings of depression and anxiety as well as reduced health related quality of life compared to HC.
Pressure pain modulation during SCWT
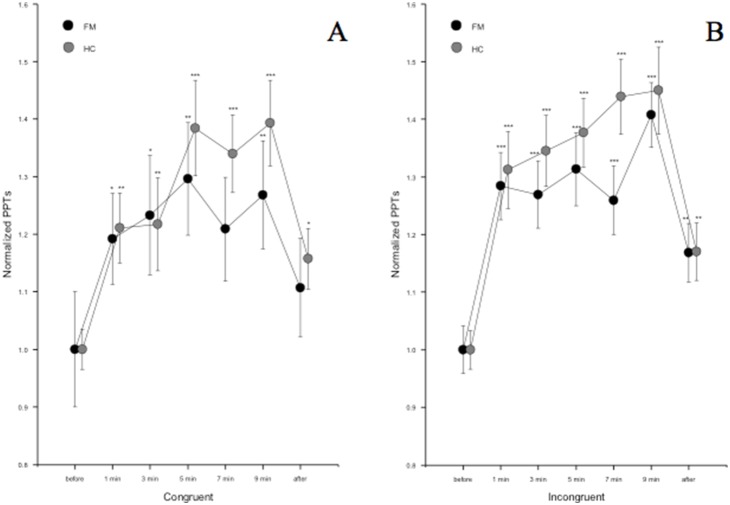
The average absolute PPT at m. quadriceps was 161 kPa in FM patients and 292 kPa in healthy controls (p<0.0001) at baseline and the absolute PPT was lower in FM patients compared to controls at all times during incongruent as well as congruent SCWT (p<0.0001). The effect of SCWT on normalized PPTs is shown in figure 2. There was a statistically significant effect of TIME (d = 4.69, F = 35.28, p<0.0001), but no statistically significant effect of CONGRUENCY or GROUP and no statistically significant interactions were seen. The normalized PPTs were higher at all times during incongruent SCWT compared to baseline in both groups (p<0.0001) and also at 10 minutes following the SCWT in both groups (p = 0.001).
Figure 2. Average normalized PPTs ± SEM during a) congruent and b) incongruent SCWT in FM patients and HC.
PPTs increased in both groups alike during congruent as well as incongruent SCWT. There were no statistically significant differences between congruent and incongruent SCWT regarding the modulation of PPTs. * = p<0.05, ** = p<0.01, *** = p<0.001. The normalization was performed as follows: each PPT value was divided with the individual’s first PPT at baseline and the curves were adjusted (by adding a coefficient) so that the baseline value always corresponded to 1.
PPTs were significantly higher in FM patients during congruent SCWT at all times compared to baseline (p<0.05) with the exception at 7 min, but no statistically significant difference was seen 10 min following SCWT compared to baseline. In HC PPTs were higher at all times during congruent SCWT compared to baseline (p<0.01) and remained elevated 10 min following Stroop (p = 0.016).
Blood pressure (BP) and heart rate (HR) during SCWT
There were no statistically significant group differences in BP or HR at baseline. Regarding systolic BP, there was a statistically significant effect of TIME (d = 3.20, F = 5.40, p = 0.001), CONGRUENCY (d = 1, F = 9.57, p = 0.003) but not GROUP and no statistically significant interactions were seen. During incongruent SCWT systolic BP increased at 90 s compared to baseline (from 117 to 120 mmHg, p = 0.002) and then returned to baseline. No statistically significant differences from baseline were seen during congruent SCWT.
Regarding diastolic BP there was a statistically significant effect of TIME (d = 2.43, F = 7.03, p<0.0001), but not CONGRUENCY or GROUP and no statistically significant interactions were seen. During incongruent SCWT diastolic blood pressure increased at 90 s (84 mmHg, p<0.0001), 210 s (83 mm Hg, p = 0.008) and 330 s (83 mmHg, p = 0.013) and then returned to baseline values (81 mm Hg), whereas it increased at 90 s (from 81 to 83 mmHg, p = 0.003) during congruent SCWT and then returned to baseline.
Regarding HR there was a statistically significant effect of TIME (d = 2.34, F = 3.92, p = 0.017) but not CONGRUENCY or GROUP and no statistically significant interactions were seen. During incongruent SCWT there was a statistically significant HR increase from baseline (69 bmp) at 210 s (71 bmp, p = 0.027) and at 330 s (71 pbm, p = 0.022), no other significant increases from baseline were seen during incongruent or congruent SCWT.
Stroop prestanda
Average RTs for HC were 870.57 ms (standard deviation (SD) = 165.42 ms) for incongruent stimuli, and 758.20 ms (SD = 121.47 ms) for congruent stimuli. The average RTs for FM patients were 1017.81 ms, (SD = 207.10 ms) for incongruent stimuli and 836.53 ms (SD = 149.25 ms) for congruent stimuli (Fig. 3). There was a statistically significant effect of CONGRUENCY (d = 1, F = 106.87, p<0.0001) and GROUP (d = 1, F = 7.61, p = 0.008) and a significant CONGRUENCY×GROUP interaction (d = 1, F = 5.49, p = 0.023).
Figure 3. Average reaction times (s) ± SEM during congruent and incongruent SCWT.
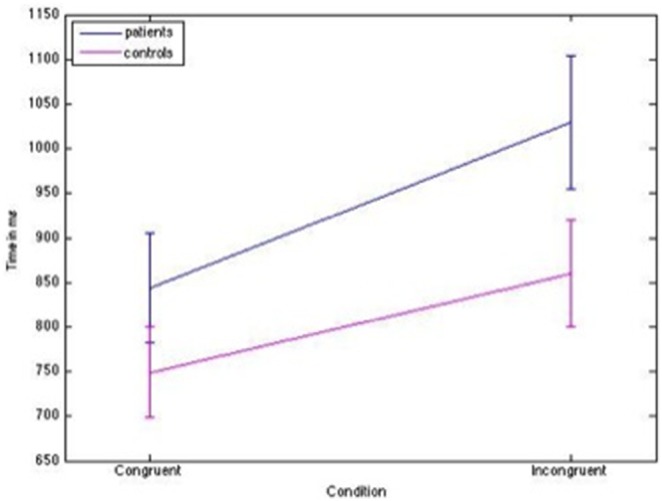
FM patients had longer reaction times than controls in both conditions (congruent p = 0.027, incongr p = 0.005), but the difference was more pronounced during incongruent SCWT (p = 0.023).
Compared to HC, FM patients had longer RTs during incongruent (p = 0.005) as well as congruent (p = 0.027) SCWT, but the group difference was more pronounced during the incongruent than the congruent task. This is illustrated in figure 3.
There were no significant group differences regarding the number of errors made during congruent (HC hit ratio 99.99%, FM hit ratio 99.99%, p = 0.850) or incongruent (HC hit ratio 99.81%, FM hit ratio 99,76% p = 0.426) SCWT.
fMRI results
Main effect of SCWT
The main effect of the contrast incongruent – congruent when both groups were analyzed together revealed an expected activation network including the parietal lobe, dorsal ACC (dACC), posterior cingulate cortex (PCC), precuneus, insula and dlPFC, (Figure 4, Table 2).
Figure 4. Main areas of interest representing cerebral activation when contrasting incongruent >congruent stimuli for FM patients (n = 23) and HC (n = 28) together.
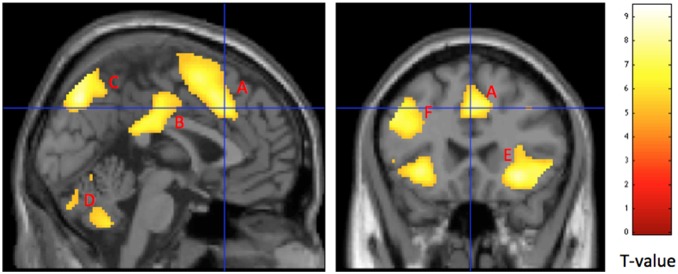
Results are reported at p<0.05, FWE corrected for multiple comparisons. A: dorsal anterior cingulate cortex (dACC), B: posterior cingulate cortex (PCC), C: precuneus, D: cerebellum, E: Insula (bilateral) and F: dorsolateral prefrontal cortex (dlPFC).
Table 2. Representation of cerebral activation when contrasting incongruent >congruent stimuli for FM patients and HC together.
| Anatomical Region | ClusterSize | X | Y | Z | PeakZ-value | P-value |
| Left Inferior Parietal Lobe | 14730 | −36 | −52 | 46 | 7.29 | p<0.0001 |
| Cerebellum | 3867 | 30 | −52 | −24 | 7.20 | p<0.0001 |
| dACC | 2252 | 6 | 18 | 42 | 7.03 | p<0.0001 |
| Right Insula | 1358 | 30 | 22 | −8 | 6.92 | p<0.0001 |
| Left Fusiform Gyrus/Cerebellum | 2573 | −44 | −52 | −18 | 6.77 | p<0.0001 |
| Right Mid Frontal Lobe | 847 | 38 | −2 | 64 | 6.14 | p<0.0001 |
| Right Mid/PosteriorCingulate Cortex | 716 | −4 | −20 | 32 | 6.00 | p<0.0001 |
| Right Superior Frontal Lobe | 133 | 24 | 46 | 38 | 5.53 | p = 0.001 |
| Right Mid Frontal Lobe | 210 | 40 | 38 | 34 | 5.07 | p = 0.001 |
| Left Thalamus | 69 | −8 | −18 | 4 | 5.05 | p = 0.004 |
| Right Calcarine Sulcus | 68 | 12 | −70 | 8 | 4.94 | p = 0.03 |
Results are reported at p<0.05, FWE corrected for multiple comparisons at cluster level.
Between group effects
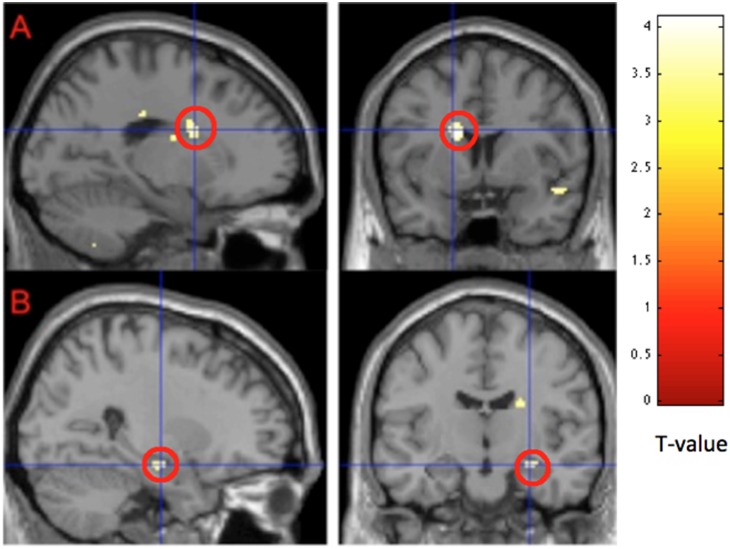
FM patients showed no regions with more activity than HC for incongruent >congruent images. When contrasting HC>FM we found greater activation in the caudate nucleus (bilaterally), temporal areas encompassing the hippocampus and the lingual gyrus (Figure 5, Table 3). The results remained also when FM patients on antidepressants and anticonvulsants were excluded from the analysis, but at a lower level of significance.
Figure 5. Representation of cerebral activation when contrasting HC>FM for incongruent >congruent stimuli.
HC had significantly higher activation than FM patients in A) the caudate nucleus (p<0.0001, peak voxel) and B) the hippocampus (p<0.001, peak voxel). Results are reported a threshold setting of p<0.001, p values are at peak voxel, not corrected for multiple comparisons.
Table 3. Representation of cerebral activation when contrasting HC>FM for incongruent >congruent stimuli.
| Anatomical Region | ClusterSize | X | Y | Z | PeakZ-value | P-value |
| Right Caudate Nucleus | 76 | 22 | −14 | 24 | 3.78 | p<0.0001 |
| Right Mid Temporal Lobe | 154 | 42 | −2 | −16 | 3.73 | p<0.0001 |
| Right Hippocampus | 26 | 26 | −14 | −16 | 3.52 | p<0.0001 |
| Right Superior Temporal Pole | 32 | 44 | −40 | 24 | 3.50 | p<0.0001 |
| Right Lingual Gyrus | 37 | 6 | −56 | 0 | 3.50 | p<0.0001 |
| Left Hippocampus | 28 | −38 | −24 | −8 | 3.50 | p<0.0001 |
| Left Caudate Nucleus | 49 | −14 | 6 | 22 | 3.49 | p<0.0001 |
Results are reported at threshold setting of p<0.001, p values are at peak voxel, not corrected for multiple comparisons. Smallest reported cluster-size is 20 voxels.
Discussion
We found a normal inhibition of pressure pain sensitivity in FM patients compared to healthy controls when engaging in the cognitive task. This was independent of the cognitive demand/load thus suggesting that distraction of attention away from painful stimuli, rather than engaging in the cognitive task per see was mediating the decreased pain sensitivity. To our knowledge, this is the first evidence that FM patients have a normal ability to regulate pain sensitivity during distraction. As expected, FM patients had slower RTs during the cognitive task, but did not make more mistakes. Several brain regions showed more profound activation in both groups alike during the cognitively more demanding (incongruent) task compared to the congruent task, mainly cerebellum, inferior parietal lobe, dACC, PCC and right insula. FM patients did not show increased task related activation of ACC. Instead reduced task related cerebral activations were seen when FM patients were compared to healthy controls in brain regions implicated in cognitive processing and memory such as the caudate nucleus, and hippocampus.
Pain modulation during SCWT
We found the same relative reduction in pressure pain sensitivity in both groups when engaging in the SCWT task. Furthermore, the cognitive load did not influence pain sensitivity. To our knowledge, this is the first evidence of normal activation of top-down pain inhibitory mechanisms in FM patients and our results indicate that distraction from pain stimuli can reduce, although not normalize, pain sensitivity in FM patients.
The effect of distraction on heat pain sensitivity has been studied previously in healthy individuals, and it was found that the PAG is activated during distraction away from the painful stimuli. The authors also reported a correlation between the degree of PAG activation and reduced pain sensitivity [28]. These findings were later supported by a study showing that distraction from heat pain stimuli by SCWT reduced pain sensitivity and decreased cerebral pain related activations, particularly in the so called “medial pain system” [15]. However, compared to pain stimulation without distraction, distraction increased the pain related activation in OBFC, ACC, thalamus and PAG, and these activations were related to reduced pain sensitivity, indicating the role of these structures in top-down pain modulation [15]. In fact, PAG and rostral ACC (rACC) has been shown to be implicated in opioid as well as placebo analgesia [11]. We have previously demonstrated that FM patients have an augmented processing of evoked pressure pain stimuli but impaired ability to activate cerebral sites related to descending pain inhibition, i.e., rACC and brainstem/PAG [18]. Furthermore, FM patients had reduced connectivity between rACC and the brain’s pain inhibitory network, including the brainstem/PAG, during evoked pressure pain [21]. Our findings are in accordance with behavioral studies showing an inability of FM patients to activate endogenous pain inhibitory mechanisms, such as conditioned pain modulation (CPM) [29], [30] and exercise induced analgesia (EIA) [26], [31], [32]. However, since CPM and EIA involves using a painful conditioning stimulus it has been proposed that deficient CPM and EIA in FM patients could be due to an ongoing activation of these pain inhibitory networks by the chronic pain, thus precluding further activation [18], [26] and/or by activation of pain facilitatory mechanisms by the painful conditioning stimuli [26]. That FM patients are able to modulate pain sensitivity during a cognitive task would support the latter. Moreover our data would indicate that the higher order cognitive areas, such as the OBFC are functioning normally in FM patients during SCWT thus enabling the patients to distract from pain stimulation and explaining the normal pain modulation seen during SCWT, however this requires further study.
The current findings are in accordance with a pilot study conducted in our group showing normal ability of FM patients to inhibit pressure pain sensitivity during a stressful variant of the incongruent SCWT accompanied by profound BP and HR increases (unpublished data). The paradigm of the present study was chosen to ensure that the increase in BP and HR would be very modest, in fact, it was only seen during the first part of the SCWT, offsetting baroreceptor analgesia as an likely explanation [33].
Cerebral activation during SCWT
FM patients had longer RTs during SCWT compared to controls and the group difference was even more pronounced during the incongruent trials, however they did not make more mistakes. This is in accordance with previous studies [6]–[8], [34], [35] and indicates impaired regulation of attention/executive function in FM. However, it was not reflected in an increase of task related dACC activation as hypothesized based on the previous findings in healthy controls [19]. This evokes two new hypotheses regarding the dACC in FM; either that FM patients have a general dysfunction of the ACC, as they are not showing activation patterns as healthy poor performers would be expected to, or that other systems are failing. Further exploratory analysis of our findings provided support for the latter showing reduced activation in the caudate nucleus and hippocampus during SCWT. Malfunction of the caudate nucleus in FM has been suggested before based on findings of decreased cerebral blood flow [36], although this is not a uniform finding [37], [38]. Furthermore, in hippocampus, aberrations of metabolites as well as glutamate concentrations have been reported in FM [39], [40]. Although caution should be made when drawing parallels between study one and study two in the current article, the behavioural results indicate normal functioning of higher order cortical areas, which was supported by the fMRI data.
The ability to learn new action schemas is required in order to perform well on the SCWT. Caudate nucleus and hippocampus are areas that are connected to each other and involved in learning as well as pain modulation, making them highly clinically relevant in FM syndrome. Normal functioning of the caudate nucleus depends on dopamine (DA), and DA modulates reciprocally with serotonin [41]. Reduced concentrations of DA, noradrenaline (NA) and serotonin metabolites have been reported in the cerebrospinal fluid of FM patients [42], and furthermore, treatment with a DA increasing drug has shown promising results in FM [43]. Studies that have investigated pain and cognition in DA related disorders, such as attention deficit hyperactivity disorder (ADHD) and Parkinson’s disease, have found similarities with FM. Patients with Parkinson’s disease had more errors and longer RTs when doing the Stroop task [44], [45], and presented other cognitive problems such as difficulties shifting between action schemas, which has been suggested to be related to low DA levels in the caudate nucleus [46]. ADHD patients have been reported to experience not only difficulties with attention, but also problems with motor skills and they often report pain [46]. Although studies of pain in ADHD patients are scarce, one study reported widespread pain in 80% of the ADHD participants [47] and ADHD subjects had lower tolerance to evoked pain than HCs [48]. Following treatment with methylphenidate (Ritalin), a substance increasing extracellular DA and NA, pain tolerance was elevated, though not completely normalized, in ADHD patients [48]. A history of childhood ADHD has been reported to be more frequent in FM patients [49] and a larger proportion of FM patients scored high on an ADHD diagnostic scale compared to other chronic pain patients [50]. Therefore, it is possible that ADHD and FM share some common DA related pathology. Further support for this comes from PET studies showing reduced dopamine (D2) receptor binding potential in the caudate nucleus in FM [51] as well as in ADHD [52].
Limitations
Contrary to our hypothesis and to previous findings [51], the distraction-induced analgesia was not influenced by cognitive load in our HC or FM patients. However, we cannot exclude the possibility that the difference in difficulty between the incongruent and congruent task in this study was insufficient. Therefore it is possible that a more difficult task would have further improved the distraction-induced analgesia in both groups.
We did not assess the cerebral correlates of pain modulation during SCWT with fMRI, which would have provided additional important information. Also, contrary to previous studies, we did not assess the effects of evoked painful stimuli on cognitive function. Combining these two approaches during fMRI in future studies could improve our understanding of the cerebral interactions during pain processing and cognition. Finally, we did not want to exclude patients on antidepressants/anticonvulsants since this would risk bias our FM cohort towards less affected individuals.
Conclusions
To our knowledge, this is the first evidence that FM patients have a normal ability to regulate pain sensitivity when focusing on a cognitive task. We found evidence of slower cognitive processing in FM patients related to task difficulty. Contrary to our hypothesis, no evidence of ACC dysfunction during SCWT was seen. Instead FM patients had reduced activation of caudate nucleus and hippocampus during SCWT in line with problems learning new actions schemas.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. There are ethical restrictions on the data set underlying the findings. A de-identified data set is available upon request from Eva Kosek (eva.kosek@ki.se).
Funding Statement
This study was supported by grants from the Swedish Rheumatism Association, the Stockholm County Council, the Swedish Foundation for Strategic Research, the Swedish Research Council K2013-52X-22199-01-3, and Karolinska Institute Foundation. No financial interests reported. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Reyes Del Paso GA, Pulgar A, Duschek S, Garrido S (2012) Cognitive impairment in fibromyalgia syndrome: the impact of cardiovascular regulation, pain, emotional disorders and medication. Eur J Pain 16: 421–429. [DOI] [PubMed] [Google Scholar]
- 2. Bertolucci PH, de Oliveira FF (2013) Cognitive impairment in fibromyalgia. Curr Pain Headache Rep 17: 344. [DOI] [PubMed] [Google Scholar]
- 3. Oliveira PJ, Costa ME (2013) Relationships among affect, emotional distress, and physical health status in fibromyalgia. Journal of Automimmune Diseases and Rheumatology 1: 11–18. [Google Scholar]
- 4. Cherry BJ, Weiss J, Barakat BK, Rutledge DN, Jones CJ (2009) Physical performance as a predictor of attention and processing speed in fibromyalgia. Arch Phys Med Rehabil 90: 2066–2073. [DOI] [PubMed] [Google Scholar]
- 5. Glass JM (2009) Review of cognitive dysfunction in fibromyalgia: a convergence on working memory and attentional control impairments. Rheum Dis Clin North Am 35: 299–311. [DOI] [PubMed] [Google Scholar]
- 6. Baudic S, Attal N, Mhalla A, Ciampi de Andrade D, Perrot S, et al. (2013) Unilateral repetitive transcranial magnetic stimulation of the motor cortex does not affect cognition in patients with fibromyalgia. J Psychiatr Res 47: 72–77. [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez JL, Mercado F, Barjola P, Carretero I, Lopez-Lopez A, et al. (2010) Generalized hypervigilance in fibromyalgia patients: an experimental analysis with the emotional Stroop paradigm. J Psychosom Res 69: 279–287. [DOI] [PubMed] [Google Scholar]
- 8. Veldhuijzen DS, Sondaal SF, Oosterman JM (2012) Intact cognitive inhibition in patients with fibromyalgia but evidence of declined processing speed. J Pain 13: 507–515. [DOI] [PubMed] [Google Scholar]
- 9. Grisart MG, Plaghki LH (1999) Impaired selective attention in chronic pain patients. European Journal of Pain 3: 325–333. [DOI] [PubMed] [Google Scholar]
- 10. Glass JM, Williams DA, Fernandez-Sanchez ML, Kairys A, Barjola P, et al. (2011) Executive function in chronic pain patients and healthy controls: different cortical activation during response inhibition in fibromyalgia. J Pain 12: 1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petrovic P, Ingvar M (2002) Imaging cognitive modulation of pain processing. Pain 95: 1–5. [DOI] [PubMed] [Google Scholar]
- 12. Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M (2000) Pain-related cerebral activation is altered by a distracting cognitive task. Pain 85: 19–30. [DOI] [PubMed] [Google Scholar]
- 13. Seminowicz DA, Mikulis DJ, Davis KD (2004) Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain 112: 48–58. [DOI] [PubMed] [Google Scholar]
- 14. Stroop JR (1935) Studies of interference in serial verbal reactions. Journal of experimental psychology 18: 643–662. [Google Scholar]
- 15. Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, et al. (2004) Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain-an fMRI analysis. Pain 109: 399–408. [DOI] [PubMed] [Google Scholar]
- 16. Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, et al. (2002) Imaging how attention modulates pain in humans using functional MRI. Brain 125: 310–319. [DOI] [PubMed] [Google Scholar]
- 17. Seminowicz DA, Davis KD (2007) Interactions of pain intensity and cognitive load: the brain stays on task. Cereb Cortex 17: 1412–1422. [DOI] [PubMed] [Google Scholar]
- 18. Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, et al. (2009) Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain 144: 95–100. [DOI] [PubMed] [Google Scholar]
- 19. Floden D, Vallesi A, Stuss DT (2010) Task context and frontal lobe activation in the stroop task. Journal of Cognitive Neuroscience 23: 867–879. [DOI] [PubMed] [Google Scholar]
- 20. Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, et al. (2013) Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum 65: 3293–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, et al. (2012) Patients with fibromyalgia display less functional connectivity in the brain's pain inhibitory network. Mol Pain 8: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33: 160–172. [DOI] [PubMed] [Google Scholar]
- 23. Bennett R (2005) The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol 23: S154–162. [PubMed] [Google Scholar]
- 24. Bjelland I, Dahl AA, Haug TT, Neckelmann D (2002) The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 25. Contopoulos-Ioannidis DG, Karvouni A, Kouri I, Ioannidis JP (2009) Reporting and interpretation of SF-36 outcomes in randomised trials: systematic review. BMJ 338: a3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lannersten L, Kosek E (2010) Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain 151: 77–86. [DOI] [PubMed] [Google Scholar]
- 27. Worsley KJ, Friston KJ (1995) Analysis of fMRI time-series revisited-again. Neuroimage 2: 173–181. [DOI] [PubMed] [Google Scholar]
- 28. Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, et al. (2002) Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci 22: 2748–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lautenbacher S, Rollman GB (1997) Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain 13: 189–196. [DOI] [PubMed] [Google Scholar]
- 30. Kosek E, Hansson P (1997) Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain 70: 41–51. [DOI] [PubMed] [Google Scholar]
- 31. Kosek E, Ekholm J, Hansson P (1996) Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. Pain 64: 415–423. [DOI] [PubMed] [Google Scholar]
- 32. Staud R, Robinson ME, Price DD (2005) Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain 118: 176–184. [DOI] [PubMed] [Google Scholar]
- 33. Fechir M, Breimhorst M, Kritzmann S, Geber C, Schlereth T, et al. (2012) Naloxone inhibits not only stress-induced analgesia but also sympathetic activation and baroreceptor-reflex sensitivity. Eur J Pain 16: 82–92. [DOI] [PubMed] [Google Scholar]
- 34. Leavitt F, Katz RS (2008) Speed of mental operations in fibromyalgia: a selective naming speed deficit. J Clin Rheumatol 14: 214–218. [DOI] [PubMed] [Google Scholar]
- 35. Cherry BJ, Zettel-Watson L, Shimizu R, Roberson I, Rutledge DN, et al. (2014) Cognitive performance in women aged 50 years and older with and without fibromyalgia. J Gerontol B Psychol Sci Soc Sci 69: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mountz JM, Bradley LA, Modell JG, Alexander RW, Triana-Alexander M, et al. (1995) Fibromyalgia in women. Abnormalities of regional cerebral blood flow in the thalamus and the caudate nucleus are associated with low pain threshold levels. Arthritis Rheum 38: 926–938. [DOI] [PubMed] [Google Scholar]
- 37. Kwiatek R, Barnden L, Tedman R, Jarrett R, Chew J, et al. (2000) Regional cerebral blood flow in fibromyalgia: single-photon-emission computed tomography evidence of reduction in the pontine tegmentum and thalami. Arthritis Rheum 43: 2823–2833. [DOI] [PubMed] [Google Scholar]
- 38. Gur A, Karakoc M, Erdogan S, Nas K, Cevik R, et al. (2002) Regional cerebral blood flow and cytokines in young females with fibromyalgia. Clin Exp Rheumatol 20: 753–760. [PubMed] [Google Scholar]
- 39. Fayed N, Garcia-Campayo J, Magallon R, Andres-Bergareche H, Luciano JV, et al. (2010) Localized 1H-NMR spectroscopy in patients with fibromyalgia: a controlled study of changes in cerebral glutamate/glutamine, inositol, choline, and N-acetylaspartate. Arthritis Res Ther 12: R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Emad Y, Ragab Y, Zeinhom F, El-Khouly G, Abou-Zeid A, et al. (2008) Hippocampus dysfunction may explain symptoms of fibromyalgia syndrome. A study with single-voxel magnetic resonance spectroscopy. J Rheumatol 35: 1371–1377. [PubMed] [Google Scholar]
- 41. Esposito E, Dimatteo V, Digiovanni G (2008) Serotonin–dopamine interaction: an overview. 172: 3–6. [DOI] [PubMed] [Google Scholar]
- 42. Legangneux E, Mora JJ, Spreux-Varoquaux O, Thorin I, Herrou M, et al. (2001) Cerebrospinal fluid biogenic amine metabolites, plasma-rich platelet serotonin and [3H]imipramine reuptake in the primary fibromyalgia syndrome. Rheumatology (Oxford) 40: 290–296. [DOI] [PubMed] [Google Scholar]
- 43. Holman AJ, Myers RR (2005) A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum 52: 2495–2505. [DOI] [PubMed] [Google Scholar]
- 44. Hsieh Y, Chen K, Wang C, Lai C (2008) Cognitive and motor components of response speed in the stroop test in Parkinson's diease patients. Kaohsiung J Med Sci 24: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Djamshidian A, O'Sullivan SS, Lees A, Averbeck BB (2011) Stroop test performance in impulsive and non impulsive patients with Parkinson's disease. Parkinsonism Relat Disord 17: 212–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grahn JA, Parkinson JA, Owen AM (2008) The cognitive functions of the caudate nucleus. Prog Neurobiol 86: 141–155. [DOI] [PubMed] [Google Scholar]
- 47. Stray LL, Kristensen O, Lomeland M, Skorstad M, Stray T, et al. (2013) Motor regulation problems and pain in adults diagnosed with ADHD. Behav Brain Funct 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Treister R, Eisenberg E, Demeter N, Pud D (2013) Alterations in Pain Response are Partially Reversed by Methylphenidate (Ritalin) in Adults with Attention Deficit Hyperactivity Disorder (ADHD). Pain Pract. doi:10.1111/papr.12129 [DOI] [PubMed] [Google Scholar]
- 49. Reyero F, Ponce G, Rodriguez-Jimenez R, Fernandez-Dapica P, Taboada D, et al. (2011) High frequency of childhood ADHD history in women with fibromyalgia. Eur Psychiatry 26: 482–483. [DOI] [PubMed] [Google Scholar]
- 50. Krause K, Krause J, Magyarosy I, Ernst E, Pongratz D (1998) Fibromyalgia syndrome and attention deficit disorder: Is there a comorbidity and are there consequences for the therapy of fibromyalgia syndrome? J Muscoskeletal Pain 6: 111–116. [Google Scholar]
- 51. Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, et al. (2007) Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci 25: 3576–3582. [DOI] [PubMed] [Google Scholar]
- 52. Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, et al. (2009) Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA 302: 1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. There are ethical restrictions on the data set underlying the findings. A de-identified data set is available upon request from Eva Kosek (eva.kosek@ki.se).