Abstract
The indolamine melatonin (MEL) is described as an antioxidant and a free radical scavenger. However occasionally, the indoleamine has been reported to increase free radicals with insufficient mechanistic explanation. In an attempt to find a reason for those controversial results, a potential mechanism that explains MEL prooxidant activity is investigated. The current controversy about redox detection methods has prompted us to search a possible interaction between MEL and dichlorodihydrofluorescein (DCFH2), perhaps the most widely fluorescence probe employed for free radicals detection in cellular models. Here, it is demonstrated that melatonin potentiates the photooxidation of DCFH2 in a cell-free system, increasing the production of its fluorescent metabolite. Indeed, MEL works as an antioxidant scavenging hydroxyl radicals in this system. Thus, this reaction between MEL and DCFH2 produces N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), a biogenic amine with antioxidant properties too. This reaction is O2 and light dependent and it is prevented by antioxidants such as N-acetylcysteine or ascorbic acid. Furthermore, when DCFH2 has been employed to evaluate antioxidant or prooxidant activities of MEL in cellular models it is confirmed that it works as an antioxidant but these results can be modulated by light misleading to a prooxidant conclusion. In conclusion, here is demonstrated that DCFH2, light and melatonin interact and results obtained using these fluorescence probes in studies with melatonin have to be carefully interpreted.
Introduction
Oxidative stress has an important impact in human health. Its implication in several disorders including atherosclerosis, diabetes, neurodegeneration or cancer has been widely investigated. The principal components of oxidative stress are a variety of chemical species such as nitric oxide (NO), superoxide anions (O2 •−), hydroxyl radicals (•OH) and hydrogen peroxide (H2O2) among others. Some of these molecules are generated exogenously or produced endogenously from several sources including oxidative phosphorylation in mitochondria. Given its important role in physiology and pathology, there is an increasing interest in developing accurate methods to measure free radical production in cells.
One of the principal drawbacks of oxidative stress research has been the accuracy when measuring ROS production in in vivo systems. There are currently several methods developed for measuring free radicals inside cells including chemiluminescence of luminol or lucigenin [1], cytochrome c reduction [2] or ferrous oxidation of xylenol orange [3] as well as some other commercially available fluorescence probes. However, among all of them, 2′,7′-dichlorofluorescein (DCF) staining is by far the most widely employed for the analysis of ROS and cellular oxidative stress [4], [5]. To measure ROS in cells [6], DCFH2-DA is used because it can be easily taken up and it is more resistant to oxidation than DCFH2. Upon internalization it is rapidly de-acetylated and after that it reacts with ROS to produce a fluorescence product [7]. Given its simplicity and sensitivity, DCFH2-DA [4] has been employed to study the production of H2O2 [8] in several reports by using microplate reader [9] or flow cytometry methods [10].
N-acetyl-5-methoxy-tryptamine or melatonin is an indolamine produced endogenously and secreted into circulation mainly by pineal gland though it is also synthesized in many other locations. In all species studied thus far, its synthesis from tryptophan occurs during darkness [11], [12]. Considering its nocturnal synthesis, melatonin has been linked to sleep promotion [13], a chemical signal of light∶dark cycle [14], and a regulator of reproductive physiology in seasonal breeding mammals among others [15]. Besides regulating circadian and circannual rhythms, melatonin is a major endogenous antioxidant and a free radical scavenger [16]. Melatonin functions as a direct-scavenging molecule and it also stimulates indirectly gene expression and activities of antioxidant enzymes [17]. As a direct scavenger, melatonin reacts with different free radicals including •OH, O2 •−, NO• and alkyl-peroxyl radicals [18]–[20] and indirectly, it stimulates glutathione production and the activities of both, glutathione peroxidase and superoxide dismutase [21], [22]. There is an inverse relationship between melatonin levels and tumour growth, in terms of initiation but also, of progression and metastasis [23]. Although numerous mechanisms have been identified to explain melatonin inhibition of cancer [24], its role as an intracellular redox regulator has been well documented as one of the mechanism by which it could modulate cancer growth [25]. Melatonin has been mostly reported to inhibit cell growth by reducing free radicals production or activity [26] but also, it has been suggested that melatonin by itself promotes cell toxicity and death of some tumour cells through a prooxidant pathway [27]–[30].
Antioxidant and prooxidant activities of melatonin have been previously evaluated by using DCFH2 or DCFH2-DA staining by other researchers. Furthermore, there are several cases of interactions between DCFH2 or DCFH2-DA with other molecules. So, a set of experiments to assess any potential interaction between melatonin with DCFH2 or DCFH2-DA are performed to clarify discrepancies observed about antioxidant or prooxidant properties of the pineal neuroindoleamine when this probe are used.
Material and Methods
Chemicals and solutions
2′-7′-dichlorodihydrofluorescein diacetate (DCFH2-DA) was purchased from Invitrogen (Life Technologies, Alcobendas, Madrid, Spain). All other chemicals were purchased from Sigma-Aldrich (Tres Cantos, Madrid, Spain). Melatonin (Merck, Darmstadt, Germany) stock (1 M) was prepared in DMSO and then diluted until desired concentration directly in phosphate buffer saline (PBS). Other reagents including catalase (CAT), superoxide dismutase (SOD), ascorbic acid (AA), N-acetyl cysteine (NAC) or H2O2 were freshly prepared in PBS and used immediately for all assays.
Light-dark experiments
Light-dark experiments were performed in a hermetic box protected from external light and equipped with a light bulb located at 15 cm from samples. Light used was a 6W linear fluorescent (F6T5/D, GE lighting # 10028) with the following features: Initial Lumen (NOM) 230, Median Lumen (NOM) 185, Colour temperature 6500 K, Nominal initial lumen per Watt (NOM) 38. Other specific parameters such as spectral, power distribution or electric characteristic can be checked at the company web site (www.gelighting.com). Light power reaching samples was 25000 lux. All the experiments were performed at RT. All solutions were placed in open tubes and at the same time for each experiment. Dark experiments were carried out in the same conditions than light experiments but in this case light of box was turned off.
DCFH2 preparation
For cell-free experiments DCFH2-DA was deacetilated to DCFH2 prior to each experiment following the method described before [31]. Briefly, 0.5 ml DCFH2-DA (1.0 mM in methanol) was mixed with 2 ml of NaOH (0.01 M) for 30 minutes at RT. Then, mixture was neutralized by adding 10 ml of NaH2PO4 (25 mM, pH 7.4). Final solution 1 mM DCFH2 was employed within 15 minutes after dilution.
Fluorescence and absorbance spectroscopy
Absorption spectra of samples containing DCFH2-DA or DCFH2 in PBS at pH 7.4 with or without MEL, H2O2, AA, SOD or CAT were measured by using a Cary 50 Bio UV-Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) at room temperature. Changes in absorption were quantified at 501 nm (λmax of DCF).
Fluorescence were measured in quartz cuvettes using a Cary Eclipse fluorimeter (Agilent Technologies, Santa Clara, CA USA) at RT (λexc = 480 nm, λem = 500–700 nm). Voltage was set between 400 and 800 V. Since voltage was changed to get enough acquisition, all groups from the same set of experiments were measured at the same time, using the same voltage intensity. For studies under a N2 atmosphere, an atmosbag glove bag (Sigma-Aldrich) was used.
HPLC measurements
HPLC analysis was performed on 1260 Infinity HPLC system (Agilent Technologies, USA) equipped with a binary pump with solvent selection valves, online degasser and a programmable auto-sampler. A tracer Extrasil ODS1 column (250 mm×0.46 mm, 5 µm) (Teknokroma, Barcelona, Spain), operating at 35°C was used. An ODS guard column was placed previously to protect the analytical column. Mobile phase solution was always filtered through a 0.45 µm membrane filter. Identification of the compounds was determined by their retention time (RT) and UV spectrum. All measurements were performed using Chemstation software.
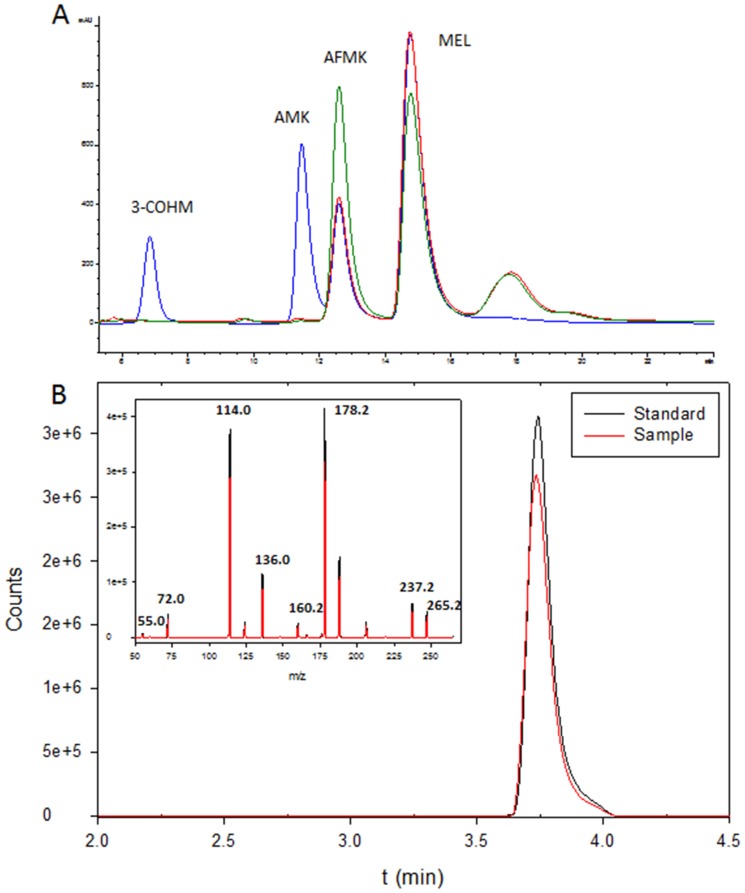
HPLC analysis of MEL, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), N1-acetyl-5-methoxykynuramine (AMK) or cyclic 3-hydroxymelatonin (3-COHM) was performed as previously described [32]. Briefly, sodium acetate (20 mM, pH 5.1) in 35% methanol was used as mobile phase. A flow of 0.9 ml/min and different wavelengths (190 at 800 nm) were employed to obtain the spectrum of absorbance for each compound. The elution order was 3-COHM, AMK, MEL and AFMK and absorbance was set at 230/279 nm (absolute/relative maximum) for MEL, 233/380 nm for AMK, 233/337 nm for AFMK and 231/306 for 3COHM. Quantification was performed at 231 nm. Standards of AFMK, AMK and 3COHM were synthesized by using the method reported by Tan et al [33]. Thus, H2O2 was diluted to 50 mM with PBS (50 mM, pH 7.0) and deferoxamine was dissolved in this solution at a final concentration of 1 mM to chelate any possible trace of free iron. MEL was then added to this solution to make a final concentration of 1 mM. The mixture was incubated for 2 h at RT. The majority components of this solution were then mixed with an equal volume of dichloromethane and shaken horizontally for 10 min. The water phase was discarded and the organic phase was dried under vacuum. The residue was dissolved in a small volume of methanol and fractionated by analytical thin layer chromatography with silica gel on polyester, fluorescent indicator, layer of 250 mm and 20 3 20 cm (TLC) using ethyl acetate as the solvent. The major spot (about 90% in all metabolites), which migrated with an RF of 0.2 (detected with UV lamp at 254 nm) was scraped from the TLC plate and extracted with methanol. The TLC purification was repeated two additional times. The purified product was identified to be AFMK by simple 1H-NMR. For AMK synthesis, the above purified AFMK was dissolved in PBS buffer (50 mM, pH 7.0) at a final concentration of 7 mM and incubated with catalase (2500 U/ml) at room temperature for 24 h. The solution was mixed with two portions of dichloromethane (per volume) and shaken horizontally for 10 min. The water phase was discarded and the organic phase was dried under vacuum. The residue was then dissolved in a small volume of methanol and the enzyme metabolite was fractionated by analytical TLC using ethyl acetate as the solvent. The single metabolite produced by catalase was isolated from TLC plate as described above and identified to be the AMK by 1H-NMR.
DCFH2 and DCF were separated by HPLC in an isocratic mode following the method previously reported [34]. A mixture of NaH2PO4 (20 mM, pH 6.8) and methanol (43∶57) was used as mobile phase. Flow was set at 1 ml/min, at RT and 20 µl of sample were injected. Wavelengths between 190 and 800 nm were used.
HPLC-MS was used to confirm presence of AFMK in samples. Agilent 1290 Infinity (HPLC) and Agilent 6460 triple quad (MS) equipped with a Zorbax Eclipse Plus C18 column (Agilent, 2.1×50 mm, 1.8 µm particle) were used. Mobile phase consisting in two components (A 0.1% formic acid; B ACN with 0.1% formic acid) in gradient mode (5% B to 90% B, 1 to 6 min) with a flow of 250 µl/min at 30°C and 2 µl of injection volume were the optimal parameters chosen. Flow of 5 L/min and temperature of 300°C of nebulization gas was chosen. ESI positive at 3500 V, product ion mode (m/z ion 265 (M+H)+) and 10 eV as Collision Energy to fragment precursor ion was used.
Cell culture experiments
Hippocampal neuronal (HT22) and prostate cancer (PC3) cell lines were cultured in DMEM and DMEM/F12 respectively, supplemented with 10% FBS and 1% antibiotic-antimycotic cocktail. Cells were grown at 37°C in a humidified 5% CO2 environment, seeded at a density of 25,000 cell/mL of complete media in 6 or 96 well plates and allowed to attach overnight before experiments. Cells were incubated 24 hours with or without 1 mM MEL. Thereafter, medium was replaced and KRH buffer (50 mM HEPES, 137 mM NaCl, 4.7 mM KCl, 1.85 mM CaCl2, 1.3 mM MgSO4, 0.1% BSA, pH: 7.4) with 10 µM of 2,7-dichlorofluorescein diacetate (DCFH-DA) was added for 30 min at 37°C in darkness. Fluorescence was measured after 30 min in a microplate reader (λex 485 nm, λem 530 nm - μQuant, Biotek) or from flow cytometer (Beckman-Coulter EPICS-XL Cytometer) as previously described [9], [35].
Results
Evaluation of DCFH2-DA photooxidation in the presence of melatonin
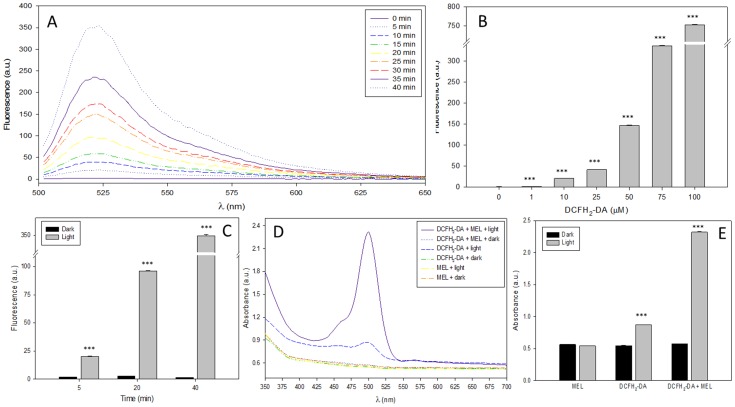
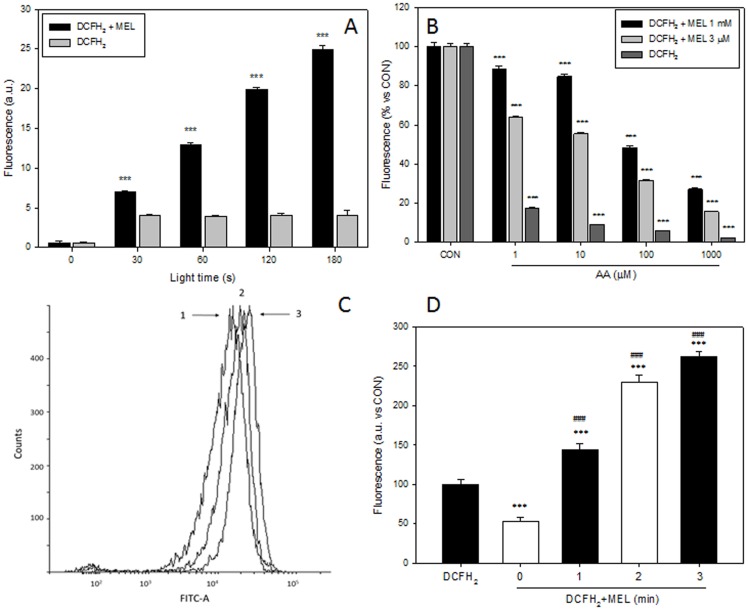
DCFH2-DA is one of the most widely employed fluorescence probe to measure redox state inside cells. It is a cell permeable precursor of DCFH2 that can readily cross membrane. After internalization, it is cleaved by intracellular esterases giving DCFH-DA obtaining DCFH2. Therefore, to evaluate a possible interference in the fluorescence of DCF caused by MEL and light reaction, both molecules (DCFH2 and DCFH2-DA) were employed. Thus, DCFH2-DA photooxidation was evaluated by measuring fluorescence emission of its oxidant product in the presence or absence of MEL in both, under light or in darkness. When DCFH2-DA was mixed with MEL and exposed to light at different times, a significant increase in fluorescence emission was observed (Fig. 1A). This increase of fluorescence was clearly dependent on time, DCFH2-DA concentration (Fig. 1B) and light (Fig. 1C). Similarly, when DCFH2-DA alone or plus MEL were exposed to light/dark and absorbance was measured, MEL increased significantly the absorbance of DCFH2-DA (Fig. 1D, E).
Figure 1. Effect of melatonin on light-induced DCFH2-DA oxidation.
A) Fluorescence spectrum (λexc = 485 nm, λem = 500–700 nm) of DCFH2-DA (100 µM) plus MEL (1 mM) under light (0–40 min). B) Fluorescence of several concentrations of DCFH2-DA plus MEL (1 mM) and light 60 minutes. ***p<0.001 vs no treat. C) Fluorescence of DCFH2-DA plus MEL (1 mM) during 5, 20 or 40 minutes under light or dark conditions. ***p<0.001 vs Darkness. D) Absorbance Spectrum (350–700 nm) of DCFH2-DA (100 µM) or MEL (1 mM) alone or mixed under 30 minutes of dark or light conditions. E) Absorbance measurement at 505 nm of MEL (1 mM), DCFH2-DA (100 µM), alone or mixed under 30 minutes of light or dark conditions. ***p<0.001 vs Darkness.
Evaluation of DCFH2 photooxidation in the presence of melatonin
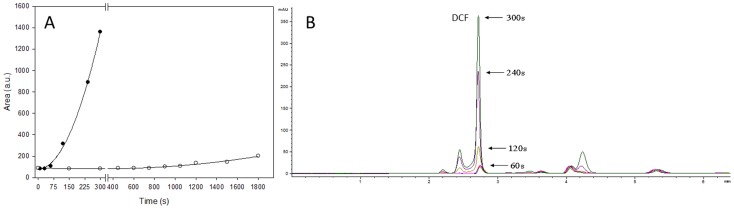
Once it was observed the enhancement of DCFH2-DA photooxidation by MEL, the interaction of DCFH2 and MEL was also studied. DCFH2-DA was deacetylated to DCFH2 which was then mixed with MEL under light. As reported above, a significant increase of time-dependent fluorescence when 100 µM DCFH2 was exposed to light was observed. By using 10 µM DCFH2 plus 1 mM MEL under light, fluorescence was rapidly increased after few seconds (Fig. 2A). Chromatogram presented in figure 2B showed a production of DCF compound after 60, 120, 240 and 300 seconds plus light and MEL. As shown, after only 60 seconds of exposition to MEL and light, DCF peak is 10 times higher than control.
Figure 2. Time-dependence in melatonin effect on DCFH2 photooxidation.
A) Time course of DCF production by DCFH2 (10 µM) alone (-○-) or plus MEL 1 mM (-•-) under light. B) Chromatogram of DCF after DCFH2 (10 µM) plus MEL (1 mM) were exposed to light for 60, 120, 240 or 300 seconds.
Evaluation of DCFH2 and DCFH2-DA photooxidation in the presence of melatonin under UV light or in a N2 atmosphere
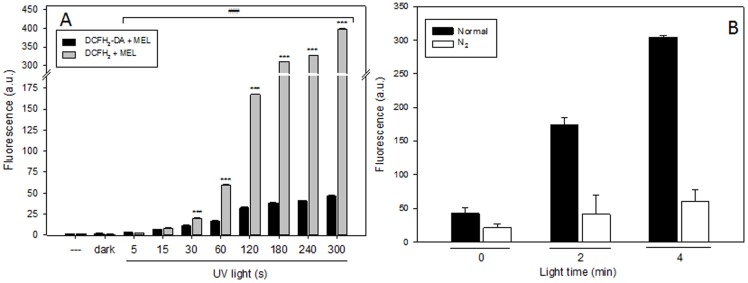
In addition to visible light, UV light was employed to evaluate the photooxidation of DCFH2 and DCFH2-DA. After DCFH2 exposure to UV light, there was an increase in fluorescence, and again, that increase was dependent on time. Similarly to what happens under visible light, when DCFH2 was incubated with MEL under UV light, fluorescence emission was significantly higher (Fig. 3A). The increment of fluorescence under UV light is much higher than under visible light since even lower compound concentration gives a much faster time of reaction. The spectrum of fluorescence after light exposure at different times is shown in supplementary material. The increment of fluorescence is 10 times higher when DCFH2 was combined with MEL under UV light than when DCFH2-DA was employed (Figure S1A). Likewise, MEL was able to increase by 100 fold the fluorescence of DCFH2 when they were exposed to UV light for several minutes (Figure S1B).
Figure 3. Role of UV light and O2 in DCFH2-DA and DCFH2 photooxidation.
A) Fluorescence of DCFH2 (10 µM) or DCFH2-DA (100 µM) plus MEL (1 mM) under UV light. B) Fluorescence of DCFH2 (10 µM) plus MEL (1 mM) under N2 or normal atmosphere.
To check if atmospheric O2 has an important role in photooxidation of DCFH2 by MEL, an experiment under N2 was performed. When O2 was eliminated from solution fluorescence did not increase. After 2 minutes under light, fluorescence intensity under N2 atmosphere is clearly lower than under normal atmosphere (Fig. 3B). For these experiment it is possible to conclude that O2 plays an instrumental role in the photooxidation process.
Participation of H2O2 generation by melatonin in DCFH2 or DCFH2-DA photooxidation
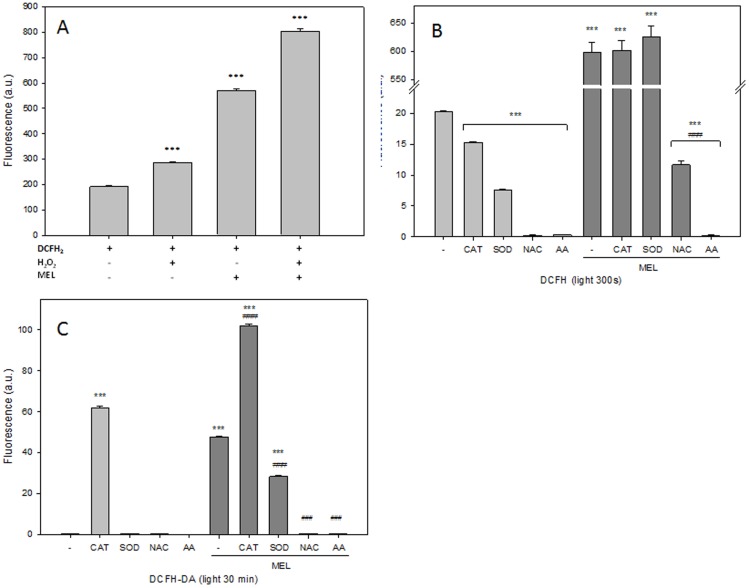
In order to understand the mechanism of DCFH2 photooxidation by MEL, H2O2 was included in the DCFH2 plus MEL mixture solution. After 300 seconds under visible light, the increment of fluorescence was measured. As previously described by others [7], an increment of DCF was observed after either H2O2 or MEL addition (Fig. 4A). In previous reports [7], [36], [37], the activity of antioxidant enzymes in preventing DCF formation was studied to demonstrate its dependence on ROS production. Consequently, catalase (CAT), superoxide dismutase (SOD), N-acetyl-cysteine (NAC) or ascorbic acid (AA) were employed to inhibit DCF formation after DCFH2 or DCFH2-DA plus MEL under light. CAT or SOD did not inhibit DCF formation after DCFH2 plus MEL exposure under light but they clearly reduced its formation after DCFH2 exposure alone (Fig. 4B). On the contrary, antioxidants such as AA or NAC inhibited DCF fluorescence when both DCFH2 (Fig. 4B) or DCFH2-DA (Fig. 4C) were incubated alone or plus MEL under light [38], [39].
Figure 4. Impact of antioxidants on melatonin enhancement of DCFH2 and DCFH2-DA photooxidation.
A) Fluorescence of DCFH2 (10 µM), MEL (1 mM), H2O2 (10 µM) alone or in combination under light for 300 second. B) Evaluation of fluorescence of DCFH2 (10 µM) with CAT (200 U), SOD (200 U), NAC (10 mM) and AA (10 mM) with or without supplementation of MEL (1 mM) under light for 300 seconds. C) Evaluation of fluorescence of DCFH2-DA (100 µM) with CAT (200 U), SOD (200 U), NAC (10 mM) and AA (10 mM) with or without supplementation of MEL (1 mM) under 30 min seconds of light exposure.
Production of kynureamines after DCFH2 and melatonin reaction
Previous studies focused on photooxidation of MEL by protoporphyrin IX [40] or by 2-hydroxyquinoxaline [41] showed the presence of several kynureamines as metabolites. For this reason, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), N1-acetyl-5-methoxykynuramine (AMK) or cyclic 3-hydroxymelatonin (3-COHM) were studied after DCFH2 exposure to light in the presence of MEL. When DCFH2 plus MEL was exposed for 30 seconds under light, we found a significant reduction of MEL concomitant with the presence of some new products. By comparing retention time as well as uv-spectrum with AFMK, AMK or 3-COHM standards, it was confirmed that AFMK was found after DCFH2 plus MEL were exposed to light (Fig. 5A). To ensure that AFMK is the compound generated in this reaction, a molecules produced and AFMK standard were compared by HPLC-MS obtained a positive confirmation of AFMK generation (Fig. 5B). The formation of AFMK requires the presence of two oxygen atom. Thus, when these experiments were performed in pure DMSO, DCF fluorescence was not found (data not shown).
Figure 5. Presence of melatonin metabolites in DCFH2 photooxidation enhanced by melatonin.
A) Chromatogram of standards of 3-COHM, AMK, AFMK and MEL (blue line), chromatogram of MEL (1 mM) with DCFH (10 µM) under light 5 min (red) or 10 min (green). B) Chromatogram and mass-spectrum obtained by HPLC-MS of AFMK standard (black) and AFMK present in sample (red) after MEL (1 mM) incubation with DCFH (10 µM) after 5 min of exposure to light.
Dose response study of DCFH2 photooxidation by melatonin
A dose response study was made by using 0.1 µM of DCFH2 and 3 µM MEL, the concentration of the indole found inside prostate LNCaP cells when they are incubated with 1 mM MEL for 6 hours [42]. Under these conditions, an increase of fluorescence was observed even after only 30 seconds (Fig. 6A). In addition, by using AA as antioxidant, there was a clear reduction in DCF formation also in a dose dependent manner (Fig. 6B). Furthermore a higher dose response study was done. So, in all MEL concentrations studied −1 nM to 1 mM- an increase in fluorescence was observed (Figure S2).
Figure 6. Dose dependence of DCFH2 in melatonin enhancement of photooxidation in vitro and in cellular models.
A) Fluorescence of DCFH2 (0.1 µM) alone or plus MEL (3 µM) under light. B) Fluorescence of DCFH2 (0.1 µM) alone plus MEL (3 µM) or MEL (1 mM) in combination with AA (1–1000 µM) under light exposure for 60 seconds. C) Fluorescence, detected by flow cytometer, of HT22 cells incubated with 10 µM DCFH2 alone (1) or with 1 mM MEL plus 10 µM DCFH2 in darkness (2) or after 2 minutes of light exposure (3). D) Fluorescence, detected by microplate fluorimeter, of PC3 cells incubated with DCFH2 (10 µM) alone or with 1 mM MEL plus DCFH2 (10 µM) in darkness or after 1, 2 and 3 minutes of light exposure.
DCFH2 photooxidation by MEL in culture cells
Prostate cancer (PC3) and hippocampal neuronal (HT22) cells were incubated with or without 1 mM MEL for 24 hours. Then, 10 µM of DCFH2-DA was added for 30 min prior to cytometer or fluorometric measurement. Those experimental conditions were chosen because there were normally employed by investigations describing pro-oxidant activity of the indoleamine [27], [43]–[45]. Changes in fluorescence among experimental groups were detected in both cell lines. Thus, when cells are incubated with MEL, a decrease in fluorescence is observed only when all experiment is performed in complete darkness (Fig. 6 C,D). When HT22 cells were exposed to light only for 1 minute, an increase of fluorescence and therefore DCF formation was observed. Same results were found in PC3 cells but light effect was lower. Thus, after 2 min under light an increase in fluorescence was also observed.
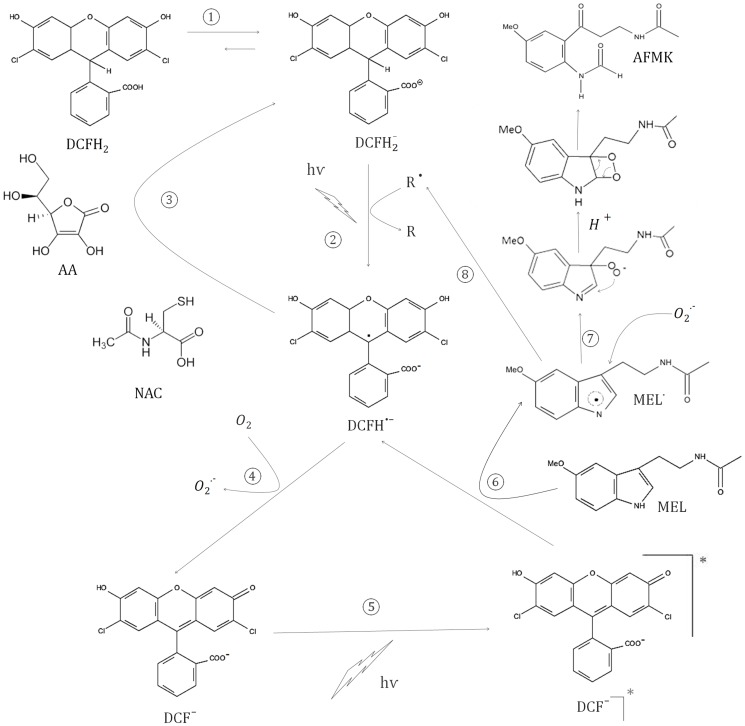
According to our results, a hypothetical pathway describing the potential reactions between DCFH2 and MEL are shown in Figure 7.
Figure 7. Diagram of proposal hypothesis about the mechanism of DCFH2, MEL and light reactions.
Discussion
This study tried to understand an apparent dual role of MEL as pro-oxidant or anti-oxidant molecule. Mostly, the indolamine has been considered to scavenges free radicals or stimulates cell antioxidant defense [11], [17] while some reports described a pro-oxidant activity that in some context might induce cell death [27], [28]. The number of references that describe MEL as a pro-oxidant factor are considerably fewer that those describing antioxidant properties of the indole and also, few mechanistic explanations are proposed to explain its activity in promoting free radicals.
There is a clear controversy about the challenges and limitations of assay methods for measuring ROS [46]. In fact, some investigators considered essential to keep this limitations in mind for proper interpretation of data obtained [47]. Several reports have showed that DCFH2 is even oxidized in processes that do not actually involve ROS. Also, photo-irradiation incidental to spectrofluorometric or fluorescence microscopy observation has also been reported, therefore causing serious problems for the correct interpretation of DCFH2 as an indicator of ROS production [39]. For this reason and in order to evaluate the convenience of using DCFH2 in the evaluation of ROS production by melatonin, here it was performed an in vitro study about possible interactions between both, DFCH2-DA or DCFH2 and MEL, since those are probably the most widely employed probes for ROS analysis inside living cells.
Photo-oxidation of MEL has been previously reported in several occasions [40], [41], [48]. But while there was no increase in fluorescence when MEL was exposed to light alone in a free cell system, a clear increment was found when DCFH2-DA alone was exposed to light for a long time as previously described by others [38], [39], [49], [50].
The mechanism of DCFH2 oxidation is not clear yet [47], [51], [52]. In a previous report, Wrona et al. [53] have shown that a radical product DCFH•− occur as an intermediate. DCFH•− is necessary since its elimination by reaction with AA or NAC results in no DCF formation. Accordingly, when DCFH2 and MEL were incubated together in the absence of light, DCF was not detected, thus indicating that light is necessary for fluorescence enhancement.
On the other hand, high concentrations of DCFH2-DA (100 µM) and MEL (1 mM) are necessary to increase fluorescence in a cell-free system. Interestingly those experimental conditions are normally employed by investigations describing pro-oxidant activity of the indoleamine [27], [43]–[45]. This might explain the increment observed in DCF after MEL incubation under some situations without any net increase in ROS production. Our results prove this fact since antioxidants such as CAT or SOD are unable to inhibit DCF formation after MEL incubation. Furthermore, our results by using two different cell lines showed that under light, DCF assay might induce wrong in conclusions. Thus, MEL is inhibiting DCF formation when the experiment was performed in complete darkness but after a short exposition to light DCF fluorescence increase.
An accumulation of DCFH2 in V79 hamster cells after incubation with 10 µM of DCFH2 has been documented [46], [54]. Considering that we have used high concentrations of both, DCFH2 (10 µM) and MEL (1 mM) and the uptake of high concentrations of MEL might be compromised, being intracellular concentrations of the indole much lower than those applied in the culture media [55]. Here we studied the ability of MEL to increase DCF formation when employed at micromolar range concentration to assure that these observations were feasible to occur in the intracellular environment. In vitro experiments when MEL increases DCF fluorescence, high concentration of MEL (1 mM) in culture medium was used. For this reason, photooxidation of DCFH2 by MEL is possible as shown here.
Results obtained suggest that the mechanism by which DCF is produced from DCFH2 and DCFH2-DA is mechanistically different. As expected, these results confirmed that DCFH2 and DCFH2-DA are not the most adequate probes to test the ability of MEL to depurate free radicals in biological systems since fluorescence is a consequence of a side reaction that do not involve ROS participation. Also, considering mechanistic differences between DCFH2 and DCFH2-DA, it seems that DCFH2-DA could be a better choice since it is necessary a longer light exposure and a higher concentration to obtain less than 10 times of fluorescence when employed.
In conclusion, by using DCFH2 staining to measure redox control by MEL, it could be concluded than MEL might be a pro-oxidant molecule, while the real situation is very different since it is still working as an antioxidant compound and scavenging free radicals as shown in the diagram (Fig. 7). Most of the reactions shown in the depicted diagram (1–5) have already been demonstrated in previous reports. Thus, step 1 is due to physiological pH and step 2 was also previously described [53], [56]. By the effect of radical species or light, DCFH2 − is rapidly converted into DCFH•− (2). AA and NAC acting as direct scavengers react with DCFH•− (3) and inhibit DCF− formation. DCF− is generated from DCFH•− when it reacts with oxygen to form superoxide (4). Under light, DCF− absorbs energy and changes to the excited state DCF−]* (5) and MEL would be able to react with it to give DCFH•− and MEL• (6). This last reaction has been described when other molecules [57], such as GSH, are employed and it might be the reason why MEL is able to augment DCF fluorescence without increasing ROS production. Furthermore MEL• can react with H2O, O2 or O2 •− to render AFMK (7). Other possibility is the role of this MEL• as catalyst of the reaction 2 obtained MEL as product (R• to R) (8). Thus, the increment in DCF production by MEL might not be a result of a pro-oxidant activity, but rather it seems that MEL is still working as an antioxidant in this context (6).
Altogether results presented here led us to propose that unless performed under dim red light all time of the performance of the assay, DCFH2 should not be employed for ROS measuring when working with melatonin since depending on time, DCFH2 or MEL concentration, it is possible to detect an increment in DCF− fluorescence without any increment of ROS more on the contrary, while melatonin is still working as an antioxidant and a radical scavenger. Results published in the literature concerning pro-oxidant activity of melatonin in certain cell types should be re-evaluated, as this pro-oxidant action does not seem to be the underlying mechanism by which the indole induces cell death.
Supporting Information
Fluorescence spectrum (λexc = 480 nm, λem = 500–700 nm) of DCFH2-DA (100 µM) plus MEL (1 mM) under UV light (A), DCFH2 (10 µM) alone (C) or plus MEL (1 mM) under UV light at short times (B) or long times (D).
(TIF)
Fluorescence of DCFH2 (10 µM) plus several concentrations of MEL under light exposure (120 s).
(TIF)
Acknowledgments
The authors would like to thank Dr. Sergio Cueto for performing HPLC-MS analysis. We are grateful to Javier Iglesias and Javier Fernandez for their helpful technical assistance with the light chamber.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work has been supported by an ISCIII (FISS-09-PS09/02204) grant. ARG acknowledges support from the “Severo Ochoa” fellowship program (FICYT). DH and JCM acknowledge sponsorship from Instituto Universitario Oncologico del Principado de Asturias (IUOPA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gyllenhammar H (1987) Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J Immunol Methods 97: 209–213. [DOI] [PubMed] [Google Scholar]
- 2. Dahlgren C, Karlsson A (1999) Respiratory burst in human neutrophils. J Immunol Methods 232: 3–14. [DOI] [PubMed] [Google Scholar]
- 3. Nourooz-Zadeh J (1999) Effect of dialysis on oxidative stress in uraemia. Redox Rep 4: 17–22. [DOI] [PubMed] [Google Scholar]
- 4. Brandt R, Keston AS (1965) Synthesis of Diacetyldichlorofluorescin: A Stable Reagent for Fluorometric Analysis. Anal Biochem 11: 6–9. [DOI] [PubMed] [Google Scholar]
- 5. LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5: 227–231. [DOI] [PubMed] [Google Scholar]
- 6. Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, et al. (1983) Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol 130: 1910–1917. [PubMed] [Google Scholar]
- 7. Hempel SL, Buettner GR, O'Malley YQ, Wessels DA, Flaherty DM (1999) Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med 27: 146–159. [DOI] [PubMed] [Google Scholar]
- 8. Keston AS, Brandt R (1965) The Fluorometric Analysis of Ultramicro Quantities of Hydrogen Peroxide. Anal Biochem 11: 1–5. [DOI] [PubMed] [Google Scholar]
- 9. Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27: 612–616. [DOI] [PubMed] [Google Scholar]
- 10. Hafer K, Iwamoto KS, Schiestl RH (2008) Refinement of the dichlorofluorescein assay for flow cytometric measurement of reactive oxygen species in irradiated and bystander cell populations. Radiat Res 169: 460–468. [DOI] [PubMed] [Google Scholar]
- 11. Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, et al. (2010) The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol Rev Camb Philos Soc 85: 607–623. [DOI] [PubMed] [Google Scholar]
- 12. Stehle JH, Saade A, Rawashdeh O, Ackermann K, Jilg A, et al. (2011) A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J Pineal Res 51: 17–43. [DOI] [PubMed] [Google Scholar]
- 13. Lemoine P, Zisapel N (2012) Prolonged-release formulation of melatonin (Circadin) for the treatment of insomnia. Expert Opin Pharmacother 13: 895–905. [DOI] [PubMed] [Google Scholar]
- 14. Reiter RJ (1991) Melatonin: the chemical expression of darkness. Mol Cell Endocrinol 79: C153–158. [DOI] [PubMed] [Google Scholar]
- 15. Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, et al. (2009) Melatonin and reproduction revisited. Biol Reprod 81: 445–456. [DOI] [PubMed] [Google Scholar]
- 16. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 51: 1–16. [DOI] [PubMed] [Google Scholar]
- 17. Mayo JC, Sainz RM, Antoli I, Herrera F, Martin V, et al. (2002) Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci 59: 1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, et al. (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem 2: 181–197. [DOI] [PubMed] [Google Scholar]
- 19. Hardeland R, Reiter RJ, Poeggeler B, Tan DX (1993) The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neurosci Biobehav Rev 17: 347–357. [DOI] [PubMed] [Google Scholar]
- 20. Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, et al. (2003) The chemistry of melatonin's interaction with reactive species. J Pineal Res 34: 1–10. [DOI] [PubMed] [Google Scholar]
- 21. Quiros I, Sainz RM, Hevia D, Garcia-Suarez O, Astudillo A, et al. (2009) Upregulation of manganese superoxide dismutase (SOD2) is a common pathway for neuroendocrine differentiation in prostate cancer cells. Int J Cancer 125: 1497–1504. [DOI] [PubMed] [Google Scholar]
- 22. Fischer TW, Kleszczynski K, Hardkop LH, Kruse N, Zillikens D (2012) Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res [DOI] [PubMed] [Google Scholar]
- 23. Blask DE, Sauer LA, Dauchy RT (2002) Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem 2: 113–132. [DOI] [PubMed] [Google Scholar]
- 24. Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, et al. (2011) Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res 51: 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sainz RM, Mayo JC, Tan DX, Lopez-Burillo S, Natarajan M, et al. (2003) Antioxidant activity of melatonin in Chinese hamster ovarian cells: changes in cellular proliferation and differentiation. Biochem Biophys Res Commun 302: 625–634. [DOI] [PubMed] [Google Scholar]
- 26. Mediavilla MD, Sanchez-Barcelo EJ, Tan DX, Manchester L, Reiter RJ (2010) Basic mechanisms involved in the anti-cancer effects of melatonin. Curr Med Chem 17: 4462–4481. [DOI] [PubMed] [Google Scholar]
- 27. Osseni RA, Rat P, Bogdan A, Warnet JM, Touitou Y (2000) Evidence of prooxidant and antioxidant action of melatonin on human liver cell line HepG2. Life Sci 68: 387–399. [DOI] [PubMed] [Google Scholar]
- 28. Wolfler A, Caluba HC, Abuja PM, Dohr G, Schauenstein K, et al. (2001) Prooxidant activity of melatonin promotes fas-induced cell death in human leukemic Jurkat cells. FEBS Lett 502: 127–131. [DOI] [PubMed] [Google Scholar]
- 29. Zhang HM, Zhang Y, Zhang BX (2011) The role of mitochondrial complex III in melatonin-induced ROS production in cultured mesangial cells. J Pineal Res 50: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bejarano I, Espino J, Barriga C, Reiter RJ, Pariente JA, et al. (2011) Pro-oxidant effect of melatonin in tumour leucocytes: relation with its cytotoxic and pro-apoptotic effects. Basic Clin Pharmacol Toxicol 108: 14–20. [DOI] [PubMed] [Google Scholar]
- 31. Cathcart R, Schwiers E, Ames BN (1983) Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem 134: 111–116. [DOI] [PubMed] [Google Scholar]
- 32. Hevia D, Botas C, Sainz RM, Quiros I, Blanco D, et al. (2010) Development and validation of new methods for the determination of melatonin and its oxidative metabolites by high performance liquid chromatography and capillary electrophoresis, using multivariate optimization. J Chromatogr A 1217: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 33. Tan DX, Manchester LC, Reiter RJ, Plummer BF, Limson J, et al. (2000) Melatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformation. Free Radic Biol Med 29: 1177–1185. [DOI] [PubMed] [Google Scholar]
- 34. Possel H, Noack H, Augustin W, Keilhoff G, Wolf G (1997) 2,7-Dihydrodichlorofluorescein diacetate as a fluorescent marker for peroxynitrite formation. FEBS Lett 416: 175–178. [DOI] [PubMed] [Google Scholar]
- 35. Eruslanov E, Kusmartsev S (2010) Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol 594: 57–72. [DOI] [PubMed] [Google Scholar]
- 36. Liochev SI, Fridovich I (2001) Copper,zinc superoxide dismutase as a univalent NO(-) oxidoreductase and as a dichlorofluorescin peroxidase. J Biol Chem 276: 35253–35257. [DOI] [PubMed] [Google Scholar]
- 37. Kim YM, Lim JM, Kim BC, Han S (2006) Cu,Zn-superoxide dismutase is an intracellular catalyst for the H(2)O(2)-dependent oxidation of dichlorodihydrofluorescein. Mol Cells 21: 161–165. [PubMed] [Google Scholar]
- 38. Bilski P, Belanger AG, Chignell CF (2002) Photosensitized oxidation of 2′,7′-dichlorofluorescin: singlet oxygen does not contribute to the formation of fluorescent oxidation product 2′,7′-dichlorofluorescein. Free Radic Biol Med 33: 938–946. [DOI] [PubMed] [Google Scholar]
- 39. Afzal M, Matsugo S, Sasai M, Xu B, Aoyama K, et al. (2003) Method to overcome photoreaction, a serious drawback to the use of dichlorofluorescin in evaluation of reactive oxygen species. Biochem Biophys Res Commun 304: 619–624. [DOI] [PubMed] [Google Scholar]
- 40. Hardeland R, Balzer I, Poeggeler B, Fuhrberg B, Uria H, et al. (1995) On the primary functions of melatonin in evolution: mediation of photoperiodic signals in a unicell, photooxidation, and scavenging of free radicals. J Pineal Res 18: 104–111. [DOI] [PubMed] [Google Scholar]
- 41. Behrends A, Riediger S, Grube S, Poeggeler B, Haldar C, et al. (2007) Photocatalytic mechanisms of indoleamine destruction by the quinalphos metabolite 2-hydroxyquinoxaline: a study on melatonin and its precursors serotonin and N-acetylserotonin. J Environ Sci Health B 42: 599–606. [DOI] [PubMed] [Google Scholar]
- 42. Hevia D, Mayo JC, Quiros I, Gomez-Cordoves C, Sainz RM (2010) Monitoring intracellular melatonin levels in human prostate normal and cancer cells by HPLC. Anal Bioanal Chem 397: 1235–1244. [DOI] [PubMed] [Google Scholar]
- 43. Sanchez-Sanchez AM, Martin V, Garcia-Santos G, Rodriguez-Blanco J, Casado-Zapico S, et al. (2011) Intracellular redox state as determinant for melatonin antiproliferative vs cytotoxic effects in cancer cells. Free Radic Res 45: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 44. Albertini MC, Radogna F, Accorsi A, Uguccioni F, Paternoster L, et al. (2006) Intracellular pro-oxidant activity of melatonin deprives U937 cells of reduced glutathione without affecting glutathione peroxidase activity. Ann N Y Acad Sci 1091: 10–16. [DOI] [PubMed] [Google Scholar]
- 45. Buyukavci M, Ozdemir O, Buck S, Stout M, Ravindranath Y, et al. (2006) Melatonin cytotoxicity in human leukemia cells: relation with its pro-oxidant effect. Fundam Clin Pharmacol 20: 73–79. [DOI] [PubMed] [Google Scholar]
- 46. Wardman P (2007) Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med 43: 995–1022. [DOI] [PubMed] [Google Scholar]
- 47. Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, et al. (2012) Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poeggeler B, Hardeland R (1994) Detection and quantification of melatonin in a dinoflagellate, Gonyaulax polyedra: solutions to the problem of methoxyindole destruction in non-vertebrate material. J Pineal Res 17: 1–10. [DOI] [PubMed] [Google Scholar]
- 49. Chignell CF, Sik RH (2003) A photochemical study of cells loaded with 2′,7′-dichlorofluorescin: implications for the detection of reactive oxygen species generated during UVA irradiation. Free Radic Biol Med 34: 1029–1034. [DOI] [PubMed] [Google Scholar]
- 50. Marchesi E, Rota C, Fann YC, Chignell CF, Mason RP (1999) Photoreduction of the fluorescent dye 2′-7′-dichlorofluorescein: a spin trapping and direct electron spin resonance study with implications for oxidative stress measurements. Free Radic Biol Med 26: 148–161. [DOI] [PubMed] [Google Scholar]
- 51. Myhre O, Andersen JM, Aarnes H, Fonnum F (2003) Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65: 1575–1582. [DOI] [PubMed] [Google Scholar]
- 52. Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142: 231–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wrona M, Wardman P (2006) Properties of the radical intermediate obtained on oxidation of 2′,7′-dichlorodihydrofluorescein, a probe for oxidative stress. Free Radic Biol Med 41: 657–667. [DOI] [PubMed] [Google Scholar]
- 54. Wrona M, Patel K, Wardman P (2005) Reactivity of 2′,7′-dichlorodihydrofluorescein and dihydrorhodamine 123 and their oxidized forms toward carbonate, nitrogen dioxide, and hydroxyl radicals. Free Radic Biol Med 38: 262–270. [DOI] [PubMed] [Google Scholar]
- 55. Hevia D, Sainz RM, Blanco D, Quiros I, Tan DX, et al. (2008) Melatonin uptake in prostate cancer cells: intracellular transport versus simple passive diffusion. J Pineal Res 45: 247–257. [DOI] [PubMed] [Google Scholar]
- 56. Rota C, Chignell CF, Mason RP (1999) Evidence for free radical formation during the oxidation of 2′-7′-dichlorofluorescin to the fluorescent dye 2′-7′-dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free Radic Biol Med 27: 873–881. [DOI] [PubMed] [Google Scholar]
- 57. Wrona M, Patel KB, Wardman P (2008) The roles of thiol-derived radicals in the use of 2′,7′-dichlorodihydrofluorescein as a probe for oxidative stress. Free Radic Biol Med 44: 56–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescence spectrum (λexc = 480 nm, λem = 500–700 nm) of DCFH2-DA (100 µM) plus MEL (1 mM) under UV light (A), DCFH2 (10 µM) alone (C) or plus MEL (1 mM) under UV light at short times (B) or long times (D).
(TIF)
Fluorescence of DCFH2 (10 µM) plus several concentrations of MEL under light exposure (120 s).
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.