Abstract
Until now, physiological mechanisms and downstream targets responsible for the cadmium (Cd) tolerance mediated by endogenous hydrogen sulfide (H2S) have been elusive. To address this gap, a combination of pharmacological, histochemical, biochemical and molecular approaches was applied. The perturbation of reduced (homo)glutathione homeostasis and increased H2S production as well as the activation of two H2S-synthetic enzymes activities, including L-cysteine desulfhydrase (LCD) and D-cysteine desulfhydrase (DCD), in alfalfa seedling roots were early responses to the exposure of Cd. The application of H2S donor sodium hydrosulfide (NaHS), not only mimicked intracellular H2S production triggered by Cd, but also alleviated Cd toxicity in a H2S-dependent fashion. By contrast, the inhibition of H2S production caused by the application of its synthetic inhibitor blocked NaHS-induced Cd tolerance, and destroyed reduced (homo)glutathione and reactive oxygen species (ROS) homeostases. Above mentioned inhibitory responses were further rescued by exogenously applied glutathione (GSH). Meanwhile, NaHS responses were sensitive to a (homo)glutathione synthetic inhibitor, but reversed by the cotreatment with GSH. The possible involvement of cyclic AMP (cAMP) signaling in NaHS responses was also suggested. In summary, LCD/DCD-mediated H2S might be an important signaling molecule in the enhancement of Cd toxicity in alfalfa seedlings mainly by governing reduced (homo)glutathione and ROS homeostases.
Introduction
Cadmium (Cd) contamination is a non-reversible accumulation process, with the estimated half-life and high plant-soil mobility, thus resulting in a serious threat to human health through food chains. Normally, Cd exposure leads to the inhibition of plant growth, decrease of crop yield, and even plant cell death [1], [2]. Indirectly stimulated generation of reactive oxygen species (ROS) that modify the antioxidant defence and bring out oxidative stress is ascribed to one of the Cd toxicities in plants, and therefore lipid peroxidation is considered as a hallmark of Cd exposure [3].
In plants, there are a lot of antioxidant defence mechanisms, which could keep the normally formed ROS at a low level and prevent them from exceeding toxic thresholds [3], [4]. The glutathione (GSH) and ascorbate were subsequently recognized as the heart of the redox hub [5]. In plants, GSH is synthesized by two ATP-dependent steps: γ-glutamylcysteine (γ-EC) is synthesized from L-glutamate and L-cysteine by γ-glutamyl cysteine synthetase (γ-ECS, also called as γ-GCS); and the second step, glycine is conjunct to γ-EC by glutathione synthetase (GS) [6], [7]. In soybean and alfalfa plants, GSH homolog homoglutathione (hGSH) synthesized by homoglutathione synthetase (hGS) from β-alanine and γ-EC, is more abundant than GSH [8]. The rate of glutathione reductase (GR) reaction was the same with either oxidized glutathione (GSSG) or oxidized homoglutathione (hGSSGh) as the substrate [7]. Upon Cd exposure, it was confirmed that the rapid accumulation of peroxides and depletion of GSH and hGSH causes redox imbalance in Medicago sativa [9]. Subsequent experiments with comparing ten pea genotypes showing that, activities of ascorbate peroxidase (APX) decreased, but concentrations of GSH increased in the less Cd-sensitive genotypes [10].
Another sulphur-containing compound, hydrogen sulfide (H2S), previously known as a toxic gas, has been progressively recognized as a gaseous signaling molecule with multiple functions in animals [11], [12]. For example, H2S has been revealed as a cytoprotectant and a regulator in various biological processes, such as oxidative stress suppression, smooth muscle relaxation, proliferation inhibition and apoptosis triggering [13]–[16]. Meanwhile, although previous reports observed that many plants can emit H2S [17]–[19], there have been few studies on the physiological role of H2S in planta during the last century.
In mammals, the majority of endogenous H2S was produced by two enzymes, cystathionine β-synthase (CBS, EC 4.2.1.22) and cystathionine γ-lyase (CSE, EC 4.4.1.1), from L-cysteine [20]. Cysteine-degrading enzymes such as cysteine desulfhydrases are hypothesized to be involved in H2S release in plants [21]. Previously, two specific desulfhydrases, L-cysteine desulfhydrase (LCD, EC 4.4.1.1; also called L-CDes or L-DES) and D-cysteine desulfhydrase (DCD, EC 4.4.1.15; also called D-CDes or D-DES), have been isolated and partially analyzed from Arabidopsis thaliana [22]–[24]. The LCD, which is considered as the most important enzyme with H2S production in plants, shares a 100% sequence homolog with CSE in mammals [25]. By using sodium hydrosulfide (NaHS) as a H2S donor, ample evidence further suggested that H2S can protect plants against various stress-induced damage, such as salinity stress [26], drought [27]–[29], heavy metal exposure [30], [31], and heat shock [32]. Additionally, H2S can act as an inducer in several developmental processes, including adventitious root formation [33] and flower senescence [34]. However, exogenously applied H2S donor without checking the kinetics of H2S synthesis including corresponding metabolic enzyme activities or transcripts, may not fully replicate the function of endogenous H2S in plants.
Cyclic AMP (adenosine 3′, 5′-cyclic monophosphate, cAMP) is a well-known second messenger playing important roles in many physiological processes. The cAMP is synthesized by adenylyl cyclase and broken down by cNMP phosphodiesterase. Dedioxyadenosine (DDA) and 1,3-diazinane-2,4,5,6-tetrone (alloxan) are well characterized as the inhibitors of adenylyl cyclase. Likewise, cNMP phosphodiesterase is sensitive to the inhibitor 1-methyl-3-(2-methylpropyl)-7H-purine-2,6-dione (IBMX) [35], [36]. In animals, there is ample evidences to show H2S-activited cAMP level or H2S-regulated cAMP homeostasis [37], [38]. It was found that H2S acted via cAMP-mediated PI3K/Akt/p70S6K signal pathways to inhibit hippocampal neuronal apoptosis and protect neurons from OGD/R-induced injury [39]. However, the functions of cAMP signaling in H2S-alleviated Cd stress in plants are still poorly understood.
Thus, the aim of this study was to investigate the signaling role of endogenous H2S in the tolerance of Medicago sativa seedlings to Cd stress. For this purpose, we preliminarily investigated the synthesis of endogenous H2S under Cd stress, which has not been fully performed. Furthermore, the effects of H2S on GSH and hGSH metabolism, as well as ROS homeostasis were checked. Our results further indicated that Cd stress triggered endogenous H2S production catalyzed by LCD/DCD pathways, and the elevated H2S acts as a signal improving the homeostasis of GSH pool and keeping ROS under control, both of which finally contributed to Cd tolerance. Finally, the possible involvement of cAMP signaling in NaHS responses was also suggested.
Materials and Methods
Plant material, growth condition
Commercially available alfalfa (Medicago sativa L. Victoria) seeds were surface-sterilized with 5% NaClO for 10 min, and rinsed extensively in distilled water before being germinated for 1 d at 25°C in the darkness. Uniform seedlings were then selected and transferred to the plastic chambers and cultured with nutrient medium (quarter-strength Hoagland's solution) in the illuminating incubator (14 h light with a light intensity of 200 µmol·m−2·s−1, 25±1°C, and 10 h dark, 23±1°C). Five-day-old seedlings were then incubated in quarter-strength Hoagland's solution with or without varying concentrations of NaHS (Sigma-Aldrich; St Louis, MO, USA) or the other indicated chemicals (2 mM DL-propargylglycine (PAG), 1 mM GSH, 1 mM L-buthionine-sulfoximine (BSO), 50 µM 8-Br-cAMP (8Br), 200 µM alloxan (All), 1 mM DDA, and 500 µM IBMX) alone, or the combination of treatments for 6 h followed by the indicated time points of incubation in 200 µM CdCl2. Seedlings without chemicals were used as the control (Con). The pH for both nutrient medium and treatment solutions was adjusted to 6.0.
After various treatments, above-ground parts and root tissues of seedlings were sampled immediately or flash-frozen in liquid nitrogen, and stored at −80°C for further analysis. Among these, above-ground parts and root tissues of 240 seedlings were respectively used for the determination of Cd contents. Seedling root tissues were also used for fresh weight determination (10 seedlings), thiobarbituric acid reactive substances (TBARS) content determination (120 seedlings), and other indicated tests (30 seedlings).
Determination of H2S content, LCD and DCD activity
Hydrogen sulfide content was determined according to the method previously reported [19], [34]. 100 mg of alfalfa seedling roots from 30 seedlings were ground under liquid nitrogen and extracted by 1 ml phosphate buffered saline (50 mM, pH 6.8) containing 0.1 M EDTA and 0.2 M ascorbic acid. After centrifugation at 13000 g for 15 min at 4°C, 400 µl of the supernatant was injected to 200 µl 1% zinc acetate and 200 µl 1 N HCl. After 30 min reaction, 100 µl 5 mM dimethyl-p-phenylenediamine dissolved in 7 mM HCl was added to the trap followed by the injection of 100 µl 50 mM ferric ammonium sulfate in 200 mM HCl. After 15 min incubation at room temperature, the amount of H2S was determined at 667 nm. Solutions with different concentrations of Na2S were used in a calibration curve.
100 mg of alfalfa seedling roots from 30 seedlings were used for activity determination. The activities of LCD and DCD were determined as described by the methods previously reported [23], [40]. L-cysteine desulfhydrase (LCD) activity was measured by the release of H2S from L-cysteine in the presence of dithiothreitol (DTT). The formation of methylene blue was determined at 670 nm. To removal of the background, content of H2S in the extracted protein solution was measured by same way with 50% trichloroacetic acid (TCA) instead of L-cysteine. The final LCD activity was calculated from the difference between the measured LCD activity and the background. D-cysteine desulfhydrase (DCD) activity was measured by the same method with following modifications: D-cysteine instead of L-cysteine, the pH of Tris-HCl was 8.0 rather than 9.0. Solutions with different concentrations of Na2S were prepared, treated in the same way as the assay samples and were used for the quantification of enzymatically formed H2S.
Determination of thiobarbituric acid reactive substances (TBARS), (h)GSH and (h)GSSG(h) contents
Lipid peroxidation was estimated by measuring the amount of TBARS as previously described [41]. About 400 mg of root tissues from 120 seedlings was ground in 0.25% 2-thiobarbituric acid (TBA) in 10% TCA using a mortar and pestle. After heating at 95°C for 30 min, the mixture was quickly cooled in an ice bath and centrifuged at 10,000×g for 10 min. The absorbance of the supernatant was read at 532 nm and corrected for unspecific turbidity by subtracting the absorbance at 600 nm. The concentration of lipid peroxides together with oxidatively modified proteins of plants were thus quantified in terms of TBARS amount using an extinction coefficient of 155 mM−1 cm−1 and expressed as nmol g−1 fresh weight (FW).
(h)GSH (GSH + hGSH) and (h)GSSG(h) (GSSG + hGSSGh) contents were measured by the 5,5′dithio-bis-(2-nitrobenzoic acid) (DTNB)-glutathione reductase (GR) recycling assay [41], [42]. Frozen root tissues from 30 seedlings were homogenized in cold 5% 5-sulfosalicylic acid. The homogenate was centrifuged at 12,000×g for 20 min at 4°C and the supernatant was collected. Total glutathione ((h)GSH plus (h)GSSG(h)) was determined in the homogenates spectrophotometrically at 412 nm, using GR, DTNB, and NADPH. (h)GSSG(h) contents were determined by the same method in the presence of 2-vinylpyridine and (h)GSH contents were calculated from the difference between total glutathione and (h)GSSG(h).
Thiol analysis by reversed-phase HPLC
Low-molecular-weight thiols and their corresponding disulfides contents in root tissues from 30 seedlings were measured according to the methods previously reported [43]–[45], through derivatization with monobromobimane (mBBr) after reduction with DTT with or without previously blocked with N-ethylmaleimide (NEM), and separation by reversed-phase HPLC (Agilent Technologies, 1200 series Quaternary, Foster city, USA).
Histochemical analyses
Histochemical detection of lipid peroxidation and loss of plasma membrane integrity was performed with Schiff's reagent and with Evans blue described by previous reports [41], [45].
Real-time quantitative RT-PCR analysis
Total RNA from root tissues of 30 seedlings was extracted using Trizol reagent (Invitrogen) according to the manufacturer's instructions. DNA-free total RNA (2 µg) from different treatments was used for first-strand cDNA synthesis in a 20-µL reaction volume containing 2.5 units of avian myeloblastosis virus reverse transcriptase XL (TaKaRa) and oligo dT primer.
Real-time quantitative RT-PCR reactions were performed with Mastercycler realplex2 real-time PCR system (Eppendorf, Hamburg, Germany) using the SYBR Premix Ex Taq (TaKaRa) according to the user manual. The cDNA was amplified using primers (Table S1). The expression levels of the genes are presented as values relative to the corresponding control samples under the indicated conditions, with normalization of data to the geometic average of two internal control genes MSC27 and Actin2 [46].
Visualization of endogenous ROS by LSCM
Endogenous ROS was imaged using the fluorescent probe H2DCFDA, and then scanned described by [45], [47].
Statistical analysis
Values are means ± SD of three different experiments with three replicated measurements. Differences among treatments were analysed by one-way ANOVA, taking P<0.05 as significant according to Duncan's multiple range test.
Results
(h)GSH depletion and increased endogenous H2S synthesis triggered by Cd stress
Considering alfalfa plants contain a thiol tripeptide homolog, hGSH, instead of or in addition to GSH [8], [9], we detected the concentrations of GSH and hGSH. As shown in Table 1, the content of hGSH in alfalfa seedling roots under the control conditions, was about 8-fold higher than that of GSH. Similarly, hGSSGh is the main component of (h)GSSG(h) (total of hGSSGh and GSSG), because the GSSG content was almost negligible.
Table 1. Concentrations of low molecular weight thiols and their disulfides, and hGSH/hGSSGh ratio in root tissues.
| Treatment | cysteine (nmol g−1 FW) | cysteine disulfide (nmol g−1 FW) | γ-EC (nmol g−1 FW) | γ-EC disulfide (nmol g−1 FW) | GSH (nmol g−1 FW) | GSSG (nmol g−1 FW) | hGSH (nmol g−1 FW) | hGSSGh (nmol g−1 FW) | hGSH/hGSSGh |
| Con→Con | 30±1 d | 3.8±0.8 c | 10±1 e | 1.5±0.1 | 27±2 bc | 0.2±1.9 | 252±16 b | 28±2 c | 8.86 |
| Con→Cd | 33±1 cd | 5.7±0.8 b | 14±2 d | 1.7±0.1 | 21±2 c | 0.2±1.4 | 112±13 f | 33±1 bc | 3.41 |
| NaHS→Cd | 40±2 c | 4.0±0.6 c | 18±2 bc | 1.4±0.5 | 26±4 c | 0.1±1.4 | 163±14 de | 33±4 bc | 4.89 |
| NaHS→Con | 34±2 cd | 3.4±0.7 c | 8±0 e | 1.2±0.5 | 36±1 b | 0.2±1.1 | 309±14 a | 30±2 bc | 10.23 |
| NaHS + PAG→Cd | 54±7 b | 4.3±0.4 bc | 21±3 b | 1.2±0.5 | 29±5 bc | 0.3±0.7 | 144±8 e | 41±6 a | 3.55 |
| NaHS + PAG + GSH→Cd | 65±8 a | 4.7±1.6 bc | 27±1 a | 1.4±0.5 | 46±11 a | 0.7±0.6 | 179±7 d | 36±5 ab | 4.91 |
| PAG→Cd | 52±6 b | 7.4±0.7 a | 21±1 b | 1.6±0.2 | 29±1 bc | 0.9±0.9 | 82±12 g | 33±3 bc | 2.41 |
| PAG→Con | 56±4 b | 4.0±0.3 c | 17±3 cd | 1.2±0.5 | 29±2 bc | 0.7±0.7 | 206±28 c | 36±2 ab | 5.67 |
Seedlings were pretreated with or without 100 µM NaHS, 2 mM PAG, 1 mM GSH, individual or combination for 6 h, and then exposed to 200 µM CdCl2 for another 12 h. Values are means ± SD of three independent experiments with three replicates for each. Different letters within columns indicate significant differences (P<0.05) according to Duncan's multiple range test.
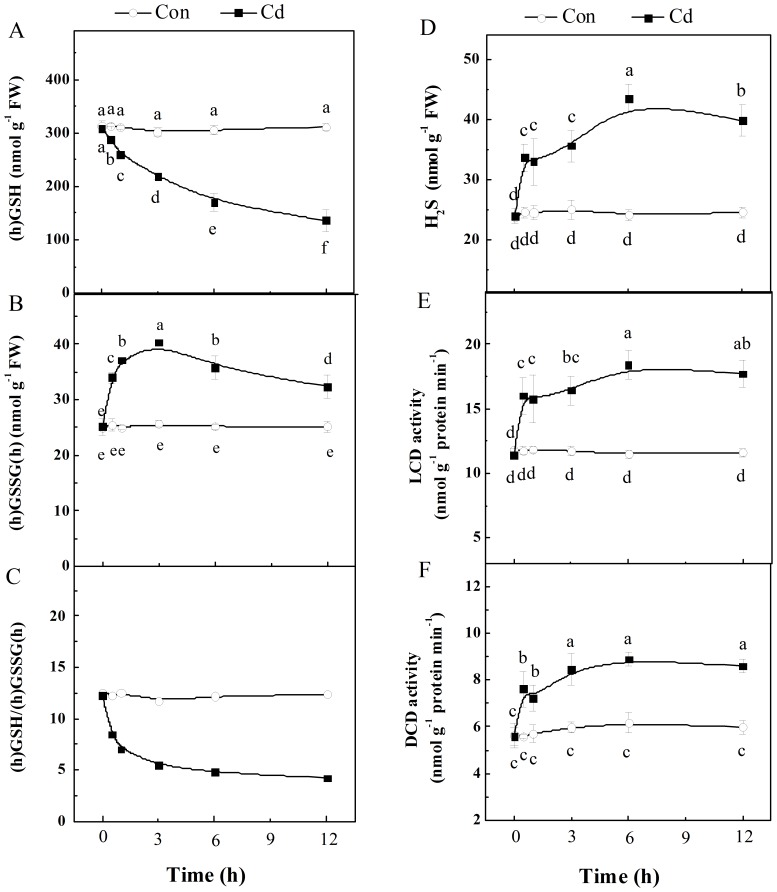
To further elucidate the correlation among GSH pool, H2S and Cd tolerance, the time course of (homo)glutathione ((h)GSH; total of hGSH and GSH, and (h)GSSG(h)) contents, and H2S synthesis were investigated in alfalfa seedling roots upon Cd stress. As expected, a decrease of (h)GSH content (especially hGSH) and an increase of (h)GSSH(h) (especially hGSSGh) level were progressively triggered by Cd stress within 12 h, thus leading to a decreased (h)GSH/(h)GSSH(h) ratio (12 h; Figure 1A-C), an important parameter for the intracellular redox status in planta upon Cd stress [3], [45]. The ratio of hGSH/hGSSGh exhibited the similar tendency (Table 1). These results were consistent with the observed Cd toxicity, confirmed by the histochemical staining detecting the aggravated loss of plasma membrane integrity and lipid peroxidation with Evans blue and Schiff's reagent, increased TBARS content and growth stunt of seedling roots (Figure S1).
Figure 1. Time course changes of GSH pool and H2S synthesis upon Cd stress.
Upon 200 µM CdCl2 treatment for 12 h, contents of (h)GSH (A), (h)GSSG(h) (B) and H2S (D), the ratio of (h)GSH/(h)GSSG(h) (C), and the activities of LCD (E) and DCD (F) in root tissues were analyzed. Values are means ± SD of three independent experiments with three replicates for each. Bars denoted by the same letter did not differ significantly at P<0.05 according to Duncan's multiple range test.
Because H2S synthesis could be induced by oxidative stress and depletion of GSH both in animals and plants [48]–[50], we simultaneously investigated the production of H2S in seedling roots after the exposure to Cd. Similar to the recent report [51], the production of H2S was continuously increased after the exposure to Cd alone for 12 h (Figure 1D). The changes in activities of two H2S synthetic enzymes LCD and DCD displayed similar tendencies (Figure 1E and F). Apparently, the reduced (homo)glutathione depletion and increased endogenous H2S synthesis preceded Cd toxicity in alfalfa seedlings.
NaHS not only mimics intracellular H2S content, but also alleviates Cd toxicity
Previous results revealed that the exogenously applied NaHS, a H2S donor, alleviates Cd toxicity in bermudagrass seedlings [51]. Therefore, a preliminary work was carried out to compare the oxidative damage and growth performance of alfalfa seedlings upon Cd exposure with or without the indicated concentrations of NaHS pretreatment. Firstly, the results of histochemical staining and TBARS contents revealed that NaHS at 100 (in particular) and 500 µM was able to significantly decreased Cd-induced lipid peroxidation (Figure S1A and B). These beneficial roles were also supported by the changes of fresh weight of ten alfalfa seedling roots, showing that NaHS at 100 and 500 µM had the greatest effects on the alleviation of the inhibition of root growth caused by Cd stress (Figure S1C). The beneficial roles of 100 µM NaHS alone were also observed. Subsequent work confirmed that H2S rather than other sulphur-containing derivatives and sodium exhibited the cytoprotective role in the improvement of Cd toxicity by using a series of sulphur- and sodium-containing chemicals including Na2S, Na2SO4, Na2SO3, NaHSO4, NaHSO3, and NaAc, in comparison with the positive roles of NaHS (Figure S2).
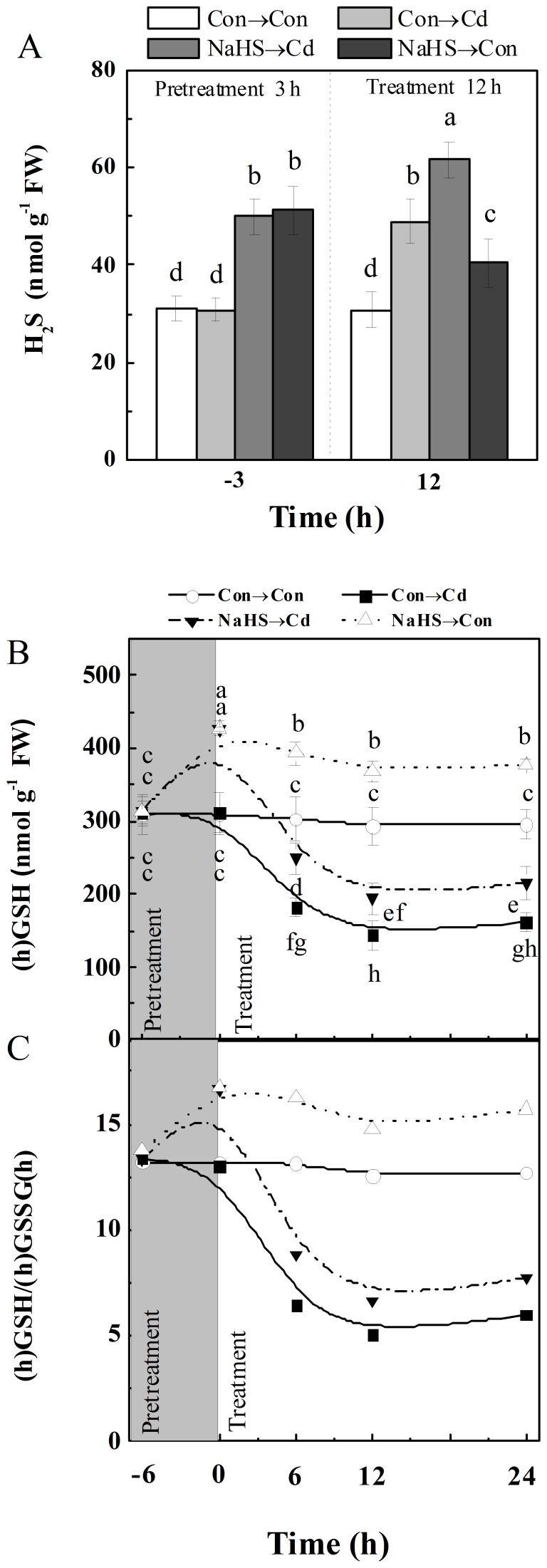
Accordingly, we observed that the treatment with 100 µM NaHS for 3 h resulted in the enhancement of endogenous H2S level in alfalfa seedling roots, which also mimicked a physiological response elicited by Cd alone for 12 h (Figure 2A). The addition of Cd to the NaHS-pretreated plants further strengthened the increased H2S content. Therefore, 100 µM NaHS was used to mimic the physiological role of intracellular H2S in the subsequent experiments.
Figure 2. NaHS increased endogenous H2S and (h)GSH contents, and the ratio of (h)GSH/(h)GSSG(h) upon Cd stress.
Endogenous H2S concentration in root tissues (A) was detected at 3 h after the beginning of 100 µM NaHS pretreatment (−3 h), and 200 µM CdCl2 or chemical-free control treatments for 12 h (12 h). Meanwhile, contents of (h)GSH (B) and the ratio of (h)GSH/(h)GSSG(h) (C) in root tissues were detected at the indicated time points of treatments. Values are means ± SD of three independent experiments with three replicates for each. Bars denoted by the same letter did not differ significantly at P<0.05 according to Duncan's multiple range test.
Changes of low molecular weight thiols and their disulfides as well as representative transcripts in response to NaHS
To determine the influence of H2S at physiologically concentrations on (h)GSH depletion, GSH pool and corresponding metabolism associated genes were investigated. As shown in Figure 2B, the time-course analysis revealed that (h)GSH contents in seedling roots were significantly enhanced by the pretreatment with NaHS for 6 h, and remained high through 24 h of further incubation in the control solution. Meanwhile, NaHS pretreatment was able to slow down the decreased (h)GSH levels caused by Cd exposure. Changes of the (h)GSH/(h)GSSG(h) ratio also exhibited the similar tendencies (Figure 2C). Comparatively, Cd-induced cysteine and γ-EC (in particular), and cysteine disulfide contents were differentially strengthened or blocked by NaHS pretreatment, respectively (Table 1).
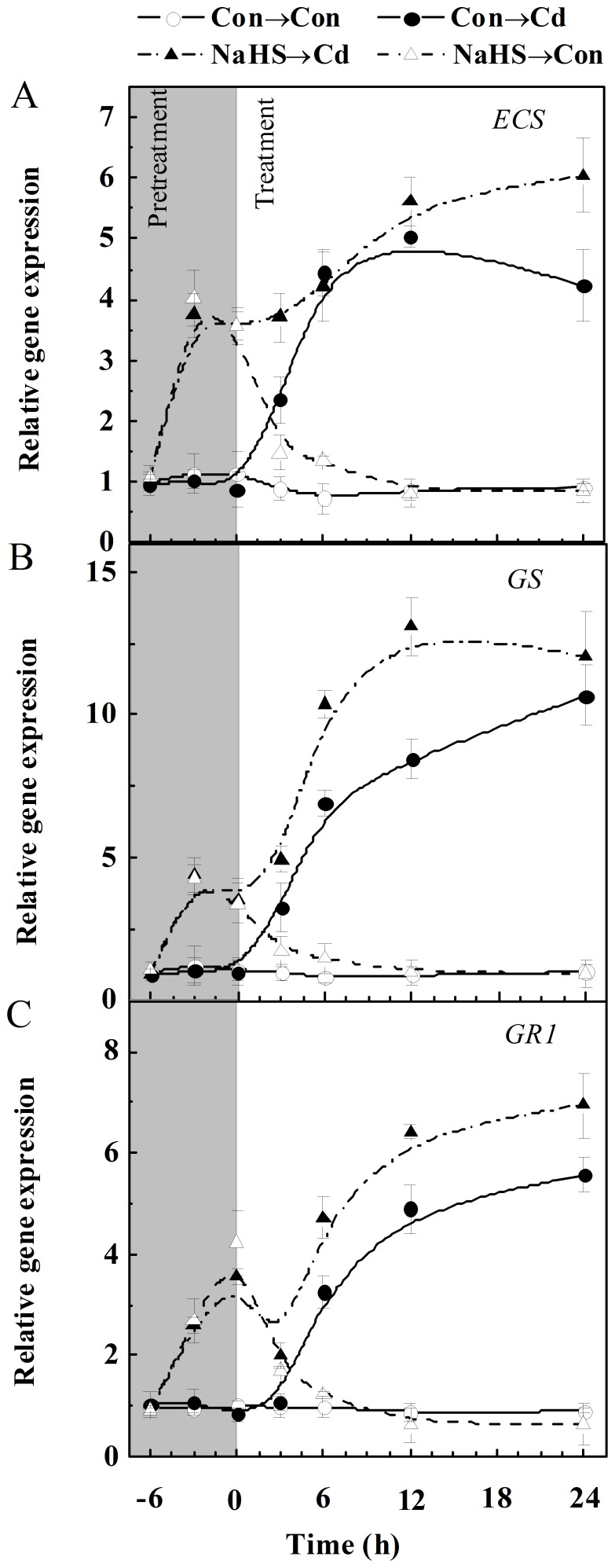
These results arises the question that, whether this increases in metabolites are, at least in part, duo to changes in the expression of genes involved in (h)GSH metabolism. Therefore, the expression of ECS, GS, and GR1 genes, were analyzed by real-time RT-PCR. Results of Figure 3A and B revealed that the transcripts of ECS, GS and GR1 (especially) in seedling roots approximately displayed a time-dependent increase during Cd stress for 24 h, while the transcriptional profiles of these genes in the control samples were relatively constant during the same period. The pretreatment with NaHS for 6 h in culture solution increased above transcripts, which were differentially strengthened by thereafter Cd stress.
Figure 3. Time course of transcripts responsible for (h)GSH metabolism regulated by NaHS and Cd.
Seedlings were pretreated with or without 100 µM NaHS for 6 h and then exposed to 200 µM CdCl2 for another 24 h. The expression levels of ECS (A), GS (B) and GR1 (C) in root tissues analyzed by real-time RT-PCR are presented as values relative to the control at the beginning of pretreatment, normalized against expression of two internal reference genes in each sample. Values are means ± SD of three independent experiments with three replicates for each.
NaHS-induced Cd tolerance, (h)GSH and ROS homeostases were sensitive to PAG, but rescued by GSH
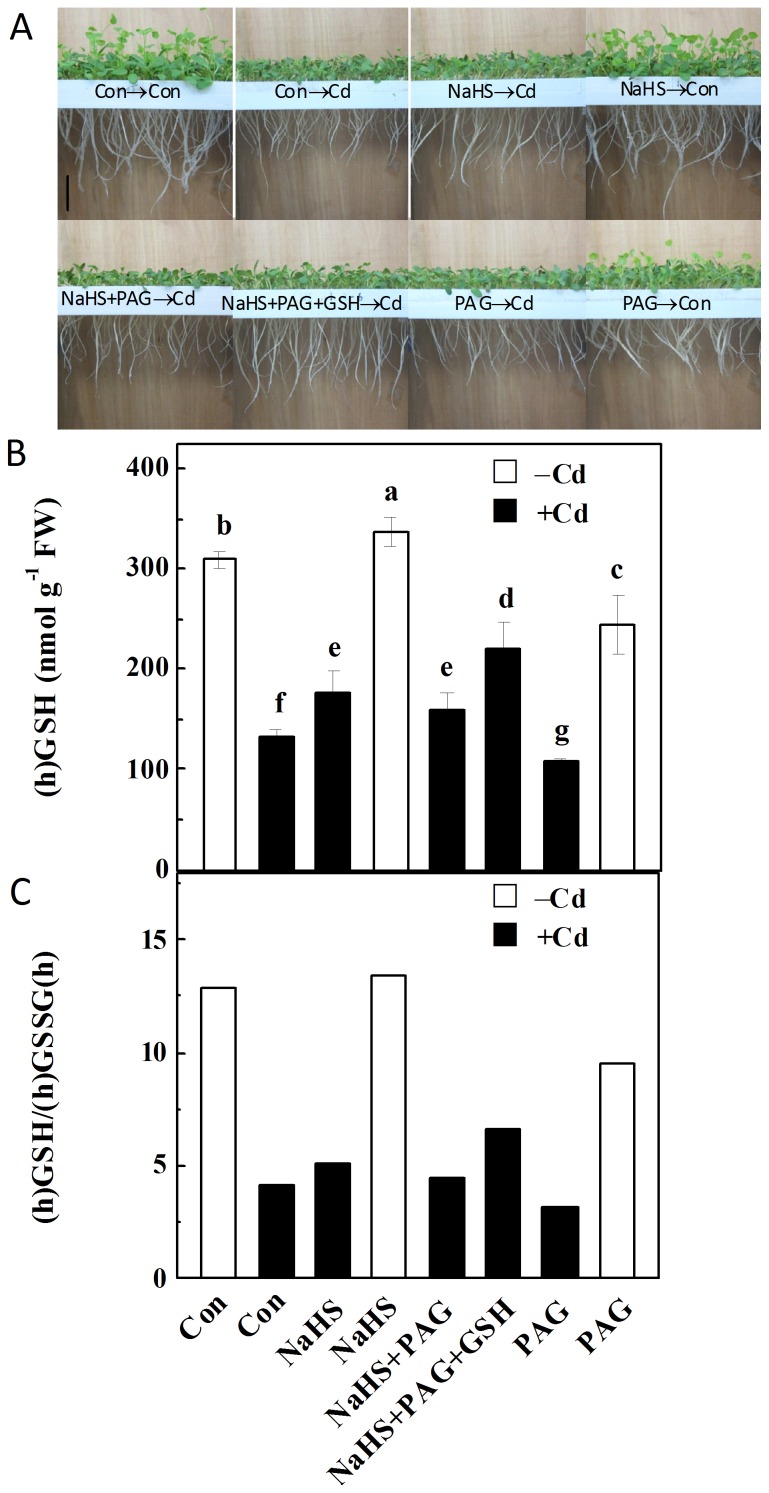
To further verify the involvement of endogenous H2S in Cd tolerance, DL-propargylglycine (PAG), an effective H2S synthetic inhibitor [27], and GSH, applied individually and in combination, were used in the subsequent experiment. After 72 h exposure to Cd, the alfalfa seedlings displays severe growth inhibition both in roots and above ground parts, compared to control samples, both of which were improved by NaHS pretreatment (Figure 4A). By contrast, the improvement of seedling growth inhibition as well as the reestablishment of (h)GSH homeostasis triggered by NaHS were sensitive to PAG, but blocked by exogenously applied GSH (Figure 4, Figure S3A). An aggravated Cd toxicity in seedling growth inhibition was also observed when PAG was pretreated.
Figure 4. NaHS, PAG and GSH pretreatments differentially regulated seedling growth, (h)GSH content, and (h)GSH/(h)GSSG(h) ratio.
Corresponding phenotypes were photographed after 200 µM CdCl2 treatment for 72 h, with or without 100 µM NaHS, 2 mM PAG, 1 mM GSH, individual or combination pretreatments for 6 h (A). Scale bar, 2 cm. Contents of (h)GSH (B), and the ratio of (h)GSH/(h)GSSG(h) (C) in root tissues were also analyzed after 200 µM CdCl2 treatment for 12 h, with or without 100 µM NaHS, 2 mM PAG, 1 mM GSH, individual or combination pretreatment for 6 h. Values are means ± SD of three independent experiments with three replicates for each. Bars denoted by the same letter did not differ significantly at P<0.05 according to Duncan's multiple range test.
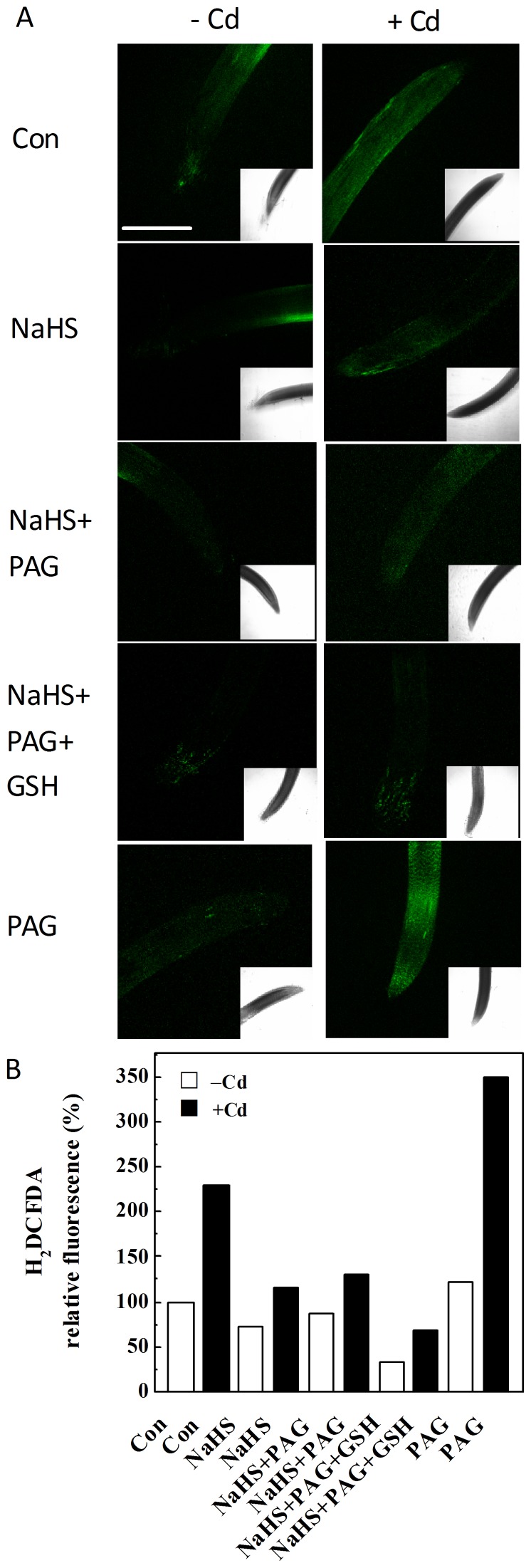
In an attempt to assess the potential role of endogenous H2S in ROS homeostasis in Cd-stressed seedlings, ROS production was visualized by staining with H2DCFDA. As expected, ROS in root tips with Cd alone were produced considerably, suggesting a perturbation in ROS homeostasis (Figure 5). However, the pretreatment with NaHS reduced the ROS abundance. Further results revealed that PAG pretreatment increased the H2DCFDA fluorescence in Cd-stressed seedling roots, which was further blocked by the addition of GSH. The changes of TBARS content, an indictor of lipid peroxidation, exhibited the similar tendencies (Figure S3B).
Figure 5. NaHS and GSH pretreatments alleviated Cd-induced ROS production, but blocked by PAG.
LSCM results (A). Seedlings were pretreated with or without 100 µM NaHS, 2 mM PAG, 1 mM GSH, individual or combination for 6 h, and then exposed to 200 µM CdCl2 for another 6 h. After various treatments, the roots were respectively stained with H2DCFDA, then washed thoroughly to removal extra dye and immediately photographed by LSCM. Scale bar, 0.5 mm. The relative DCF fluorescence intensity in the corresponding roots (B).
Cd treatment caused the accumulation of Cd contents both in shoot and root (particularly) tissues (Figure S4). Similar to the previous reports [31], NaHS decreased Cd accumulation, which was significantly reversed by PAG, but was further blocked by the cotreatment with GSH.
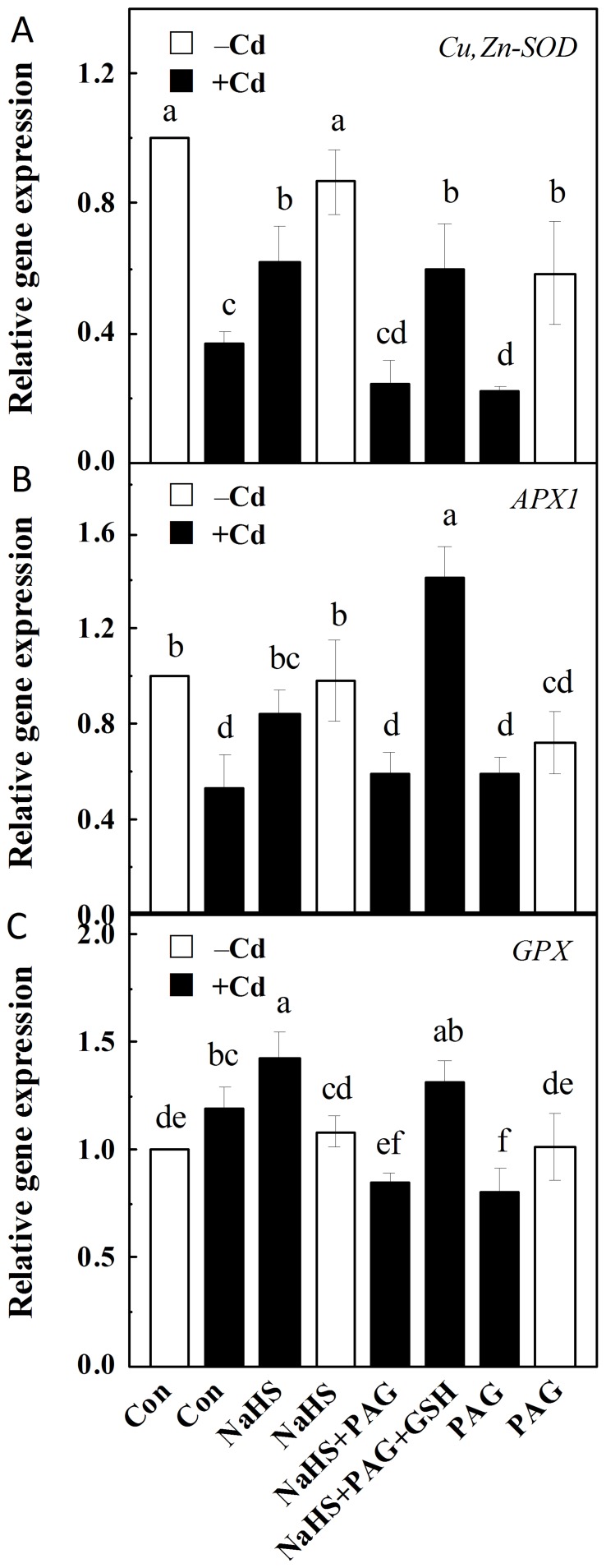
Transcripts of representative antioxidant defense genes were sensitive to PAG, but rescued by GSH
Since ROS homeostasis was reestablished by NaHS in stressed conditions, the real-time RT-PCR test of corresponding genes involved in their metabolism, i.e. Cu, Zn-SOD, APX1, and GPX [3], [5], were analysed. The results of Figure 6 revealed that in comparison with Cd alone samples, NaHS pretreatment followed by Cd exposure resulted in the enhancement in the transcript levels of Cu, Zn-SOD, APX1, and GPX in alfalfa seedling roots. The addition of PAG, however, significantly blocked the increases in the transcripts levels of these representative antioxidant enzymes induced by NaHS, all of which were reversed when GSH was added together with PAG.
Figure 6. Transcripts of Cu, Zn-SOD, APX1, and GPX regulated by NaHS, PAG, GSH and Cd.
Seedlings were pretreated with or without 100 µM NaHS, 2 mM PAG, 1 mM GSH, individual or combination for 6 h, and then exposed to 200 µM CdCl2 for another 12 h. The expression levels of Cu,Zn-SOD (A), APX1 (B), and GPX (C) transcripts in root tissues analyzed by real-time RT-PCR are presented as values relative to the control, normalized against expression of two internal reference genes in each sample. Values are means ± SD of three independent experiments with three replicates for each. Bars denoted by the same letter did not differ significantly at P<0.05 according to Duncan's multiple range test.
NaHS responses were sensitive to a (h)GSH synthetic inhibitor, but reversed by the added GSH
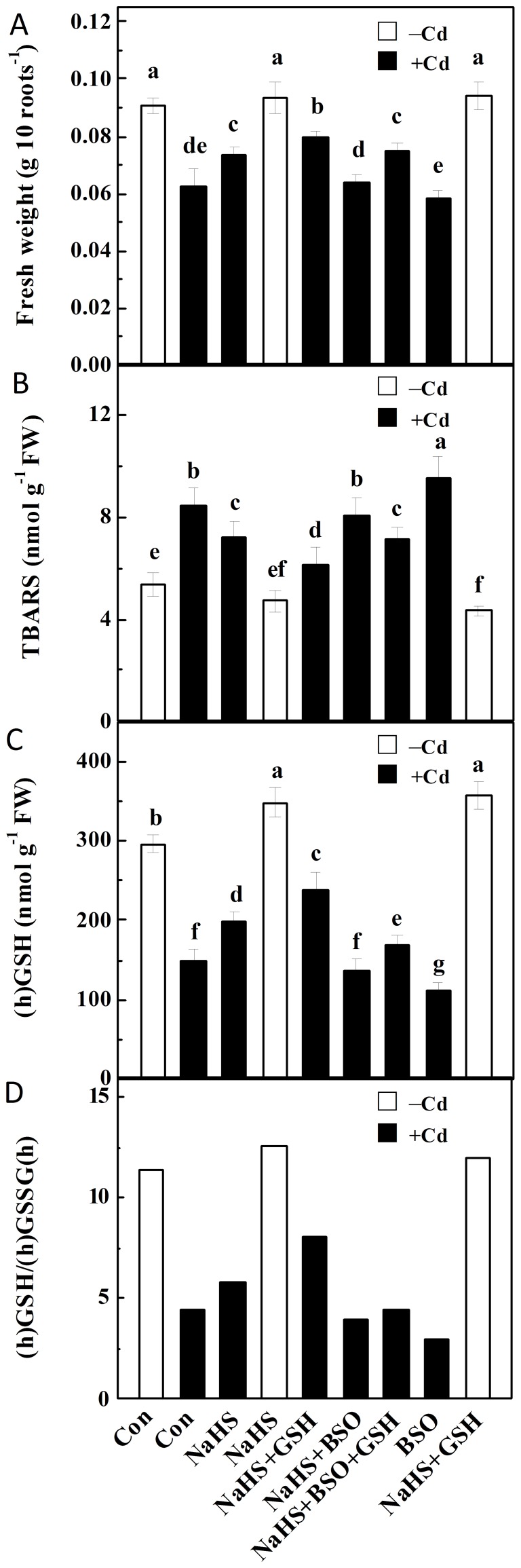
The involvement of (h)GSH homeostasis in NaHS-induced cytoprotective against Cd stress were further investigated using a (h)GSH synthetic inhibitor and GSH applied exogenously. Pretreatment with NaHS, and L-buthionine-sulfoximine (BSO) at 1 mM, a concentration expected to be effective [52], exhibited an aggravated Cd toxicity, which was confirmed by the severe growth stunt and TBARS overproduction, in comparison with Cd plus NaHS (Figure 7A and B). Similarly, NaHS-mediated reestablishment of (h)GSH homeostasis in Cd stressed alfalfa seedling roots was also perturbed by BSO (Figure 7C and D), which was confirmed by the significant decreased (h)GSH content and the ratio of (h)GSH/(h)GSSG(h), respect to Cd alone. By contrast, above BSO responses were sensitive to the addition of GSH when applied together. Above results clearly indicated a requirement for (h)GSH homeostasis in NaHS-mediated alleviation of Cd toxicity.
Figure 7. NaHS, GSH and BSO pretreatments differentially regulated seedling growth, TBARS accumulation, (h)GSH content, and (h)GSH/(h)GSSG(h).
Fresh weight of 10 roots (A), TBARS accumulation (B), (h)GSH contents (C), and (h)GSH/(h)GSSG(h) ratio (D) in root tissues were determined after seedlings were pretreated with or without 100 µM NaHS, 1 mM GSH, 1 mM BSO, individual or combination for 6 h, and then exposed to 200 µM CdCl2 for 72 h (A), 24 h (B) and 12 h (C and D). Values are means ± SD of three independent experiments with three replicates for each. Bars denoted by the same letter did not differ significantly at P<0.05 according to Duncan's multiple range test.
cAMP signaling might be involved in NaHS responses
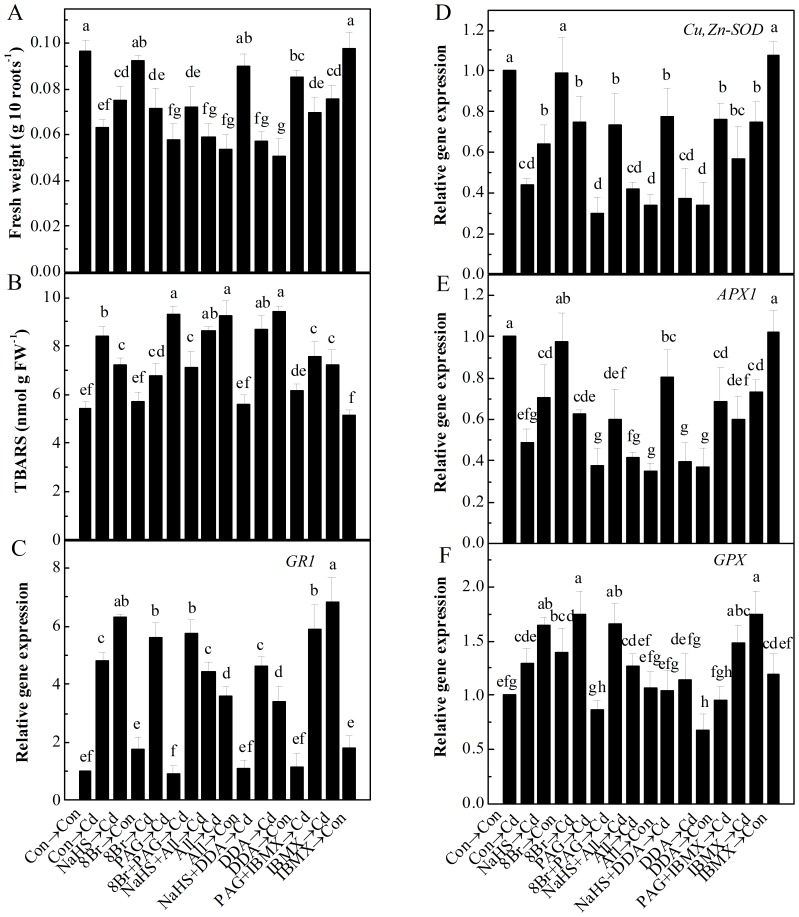
To testify the hypothesis that H2S response is associated with cAMP signaling pathway, a pharmacological approach was used to manipulate endogenous cAMP. Results presented in Figure 8A and B indicated that the pretreatment with 8-Br-cAMP, a membrane-permeable analogue of cAMP, alleviated Cd-induced decrease of fresh weight and increase of TBARS content in alfalfa seedling roots. Both of two adenylyl cyclase inhibitors, alloxan and DDA, blocked NaHS-alleviated Cd stress. Moreover, similar to the beneficial actions of 8-Br-cAMP (when was cotreated with PAG followed by Cd stress), a cNMP phosphodiesterase inhibitor IBMX also reversed the PAG responses in the aggravation of fresh weight loss and lipid peroxidation caused by Cd stress. Results from the real-time RT-PCR showed that 8-Br-cAMP and IBMX pretreatments followed by Cd stress, mimicked the effect of NaHS on GR1 up-regulation, regardless of whether PAG was added or not (Figure 8C). Two inhibitors alloxan and DDA partially blocked NaHS plus Cd-induced GR1 transcripts. A similar tendency was found in the changes in GPX transcripts (Figure 8F). Results presented in Figure 8D and E further revealed the negative effects of adenylyl cyclase inhibitors on the transcripts of Cu, Zn-SOD and APX1 in NaHS-pretreated seedling roots upon Cd, in comparison with the positive responses of 8-Br-cAMP and IBMX in the presence or absence of PAG.
Figure 8. cAMP pathway might be involved in H2S-alleviated Cd toxicity.
Fresh weight of 10 roots (A), TBARS accumulation (B), GR1 (C), Cu,Zn-SOD (D), APX1 (E), and GPX (F) gene expression in alfalfa seedling roots upon Cd stress. Seedlings were pretreated with or without 100 µM NaHS, 50 µM 8-Br-cAMP (8Br), 2 mM PAG, 200 µM alloxan (All), 500 µM IBMX, 1 mM DDA alone, or the combination of treatments for 6 h, and then exposed to 200 µM CdCl2 for 72 h (A), 24 h (B) and 12 h (C–F). The expression levels of corresponding genes analyzed by real-time RT-PCR are presented as values relative to the control, normalized against expression of two internal reference genes in each sample. Values are means ± SD of three independent experiments with three replicates for each. Bars denoted by the same letter did not differ significantly at P<0.05 according to Duncan's multiple range test.
Discussion
Although H2S is a hazardous gaseous molecule with a strong odor of rotten eggs, it has been described as an important regulator with a variety of biological roles in animals and recently in plants [11]–[16], [25]–[34], [53]–[56]. Moreover, recent works on Populus euphratica cells [57] and bermudagrass seedlings [51], demonstrated that exogenously applied NaHS, a H2S donor, resulted in an enhanced Cd tolerance in these species. However, possible physiological mechanisms and downstream targets responsible for the observed Cd tolerance triggered by intracellular H2S remain elusive. In this report, we discovered endogenous H2S production in response to Cd stress, and further provided evidence demonstrating a requirement of (h)GSH and ROS homeostases, at least partially, in the intracellular H2S-medaited plant adaptation against Cd toxicity. Therefore, our results presented in this work are vital for both fundamental and applied plant biology.
Endogenous H2S production in response to Cd stress: the possible involvement of LCD/DCD
In animals, it was previously reported that diverse stress-inducing stimuli could result in the production of H2S, including oxidative stress [49], depletion of cysteine (or its derivatives) [58] and glutathione [50]. Recent work in Arabidopsis [25] and bermudagrass seedlings [51] reported drought- and Cd-induced H2S production. Because the signal compound H2S is very reactive [53], the rapid regulation of the activity of H2S biosynthetic enzymes seems essential to fulfill H2S-depenent functions. In this work, we further showed that Cd-triggered endogenous H2S production might be related to LCD/DCD pathways (Figure 1D-F), since the similar increasing changes in the levels of intracellular H2S as well as LCD/DCD activities were observed in the seedling roots of alfalfa challenged with Cd for 12 h. Meanwhile, similar to previous reports in wheat [30], bermudagrass [51], Spinacia oleracea seedlings [59], and strawberry plants [60], NaHS-induced H2S production in alfalfa plants was also observed (Figure 2A).
In plants, both LCD and DCD are hypothesized to be involved in intracellular H2S synthesis [21] [27]. Several LCD/DCD candidates have been cloned and partially analyzed from the model plant Arabidopsis to Brassica napus [24] [61]. Our above findings are consistent with those reported by Bloem et al. [40], in which they found that Brassica napus was able to react to Pyrenopeziza brassicae infection with a greater potential to release H2S, which was reflected by an increasing LCD activity with fungal infection. More recently, auxin-induced DES-mediated H2S generation was also found to be involved in lateral root formation in tomato seedlings [62]. In view of the fact that all H2S synthetic enzymes are not fully elucidated, our results suggested that LCD/DCD pathways might be, at least partially, related to Cd-induced H2S production in alfalfa seedlings. In a future study, the role of other enzymatic and non-enzymatic pathways-mediated induction of H2S synthesis in alfalfa seedlings upon Cd stress need be further elucidated.
The mechanism underlying the role of intracellular H2S in the alleviation of Cd toxicity: reestablishment of reduced (homo)glutathione and ROS homeostases
Ample evidence revealed a clear relationship between metal stress and redox homeostasis and antioxidant capacity [3] [9] [63]–[66]. Also, GSH could function as a heavy metal-ligand and an antioxidant [5] [67]. In plants, H2S serves as a signal as well as a novel antioxidant in hormonal and defense responses against abiotic stress [53] [60]. Genetic evidence further revealed that the GSH deficiency mutant pad2-1 shows the more oxidized redox state in contrast to wild type [68]. Arabidopsis mutants deficient in phytochelatins (PCs) and GSH biosynthesis respectively, cad1 and cad2, are consequently more sensitive to Cd [6] [69] [70], that showed the crucial role of PCs, especially their precursor GSH in responding to Cd challenge. In the assays described here, as expected, when alfalfa seedling plants were upon Cd exposure, (h)GSH homeostasis is altered, which is reflected by the fact that the concentrations of reduced GSH and hGSH dropped (Table 1, Figure 1A–C), possible as a consequence of initiated PCs biosynthesis [71] [72]. Similarly, a low ratio of (h)GSH/(h)GSSH(h), an important redox index related to Cd tolerance in alfalfa plants [45], was also observed. These changes thereafter cause redox imbalance and in turn Cd toxicity (Figures S1A, S3 and S4; Figures 4A and 5).
Our further experiments provide strong evidence to support the existence of a causal relationship between the endogenous H2S signal and the alleviation of Cd toxicity in alfalfa seedlings partly by reestablishment of (h)GSH and ROS homeostases, which might be associated with the cAMP pathway. This conclusion is based on several pieces of evidence: (i) increased H2S metabolism as well as the perturbation of (h)GSH homeostasis in alfalfa seedling roots are two early responses to the exposure of Cd (Figure 1, Table 1). These changes were consistent with the phenotypes of Cd toxicity (Figure 4A, Figure S1C). (ii) Application of a H2S-releasing compound NaHS (also called as H2S donor), not only mimics intracellular H2S content triggered by Cd, but also alleviates Cd toxicity (Figures 2 and 4). Consistently, we also detected reestablishment of (h)GSH homeostasis, which was reflected by a higher (h)GSH content and ratio of (h)GSH/(h)GSSG(h) upon Cd stress. The observed Cd tolerance might be due to the available (h)GSH by the up-regulation of (h)GSH synthesis related genes, ECS and GS (Figure 3A and B), as well as GR1 (Figure 3C), because besides the synthesis of PCs, availability of GSH and concerted activity of GR seem to play a important role for plants to combat oxidative stress and Cd toxicity [7] [72] [73]. While, the inhibition of H2S production caused by its synthetic inhibitor PAG blocked NaHS-induced Cd tolerance and reestablishment of (h)GSH and ROS homeostases, the latter of which was confirmed by the histochemical staining detecting the alleviation of plasma membrane integrity and lipid peroxidation, decreased ROS content and up-regulation of Cu,Zn-SOD, APX1 and GPX transcripts, as well as declined TBARS level (Table 1, Figures 2–6, and Figures S1, S3 and S4). (iii) Above mentioned PAG responses were further rescued by exogenously applied GSH (Table 1, Figures 4–6). (iv) NaHS responses were sensitive to a (h)GSH synthetic inhibitor, but reversed by the added GSH (Figure 7), both of which suggesting a requirement of (h)GSH homeostasis for NaHS cytoprotective roles; and (v) Previous reports in animals showed H2S-activited cAMP level or H2S-regulated cAMP homeostasis [37] [38]. Here, we found that two adenylyl cyclase inhibitors, alloxan and DDA, blocked the beneficial responses conferred by NaHS in alfalfa seedlings subjected to Cd stress (Figure 8). On the contrary, an analogue of cAMP 8-Br-cAMP and a cNMP phosphodiesterase inhibitor IBMX mimicked the effects of NaHS on the alleviation of Cd toxicity as well as the regulation of (h)GSH homeostasis and ROS metabolism (GR1, Cu,Zn-SOD, APX1, and GPX, etc). Above pharmacological evidence indicated the involvement of cAMP signaling in NaHS responses. Additionally, NaHS-triggered cytoprotective roles were confirmed to act as a H2S-dependent fashion (Figure S2). Above results clearly established a casual link between intracellular H2S in the alleviation of Cd toxicity and reestablishment of (h)GSH and ROS homeostases.
Conclusions
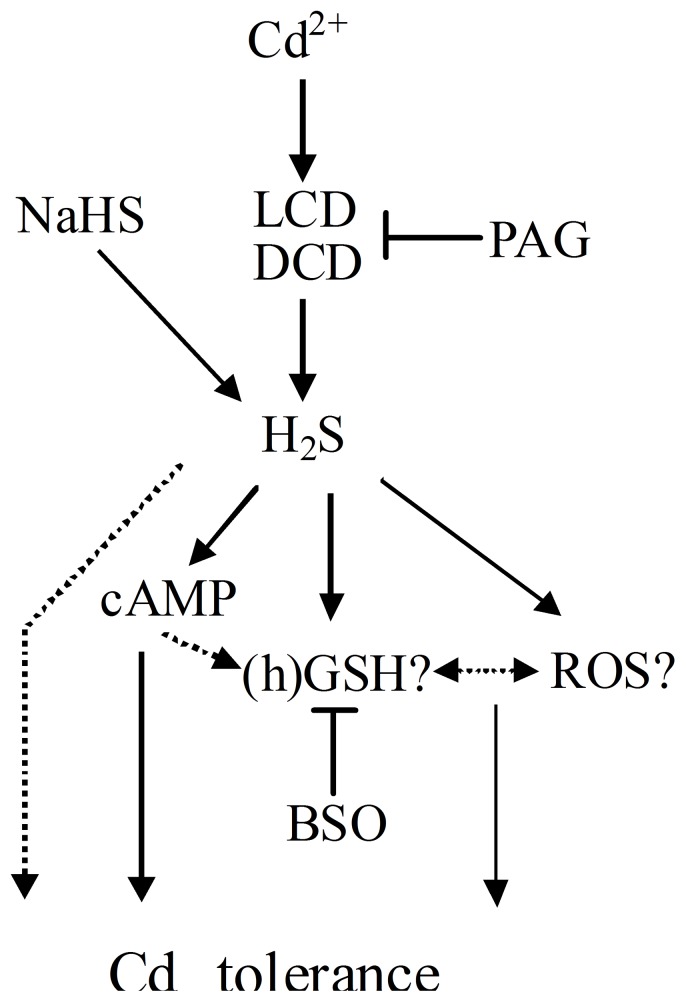
In summary, our pharmacological, histochemical, biochemical and molecular evidence suggested that the intracellular H2S was able to ameliorate Cd toxicity in alfalfa seedlings at least partly by reestablishment of (h)GSH and ROS homeostases. Figure 9 illustrates a simplified scheme of mechanisms involved in Cd tolerance by LCD/DCD-produced H2S-modulated (h)GSH and ROS homeostases, since 1) LCD/DCD-produced H2S acts as a signal triggered by Cd to regulated (h)GSH metabolisms; 2) both (h)GSH and ROS homeostases could be reestablished by H2S and further linked to Cd tolerance; 3) cAMP signaling pathway might be related to NaHS-triggered Cd tolerance, partially through the regulation of GSH homeostasis and ROS metabolism. Taking into account that H2S participates in stressful responses and developmental process, our study therefore may extend our understanding of the complex system integrating environmental and developmental signals.
Figure 9. Simplified scheme of mechanisms involved in Cd tolerance by LCD/DCD-produced H2S-modulated (h)GSH and ROS homeostases.
Abbreviations: NaHS, sodium hydrosulfide; PAG, DL-propargylglycine; LCD, L-cysteine desulfhydrase; DCD, D-cysteine desulfhydrase; H2S, hydrogen sulfide; ROS, reactive oxygen species; (h)GSH, reduced (homo)glutathione; BSO, L-buthionine-sulfoximine; cAMP, cyclic AMP. The dashed line denotes possible signaling cascade. T bars, inhibition.
Supporting Information
NaHS pretreatment alleviates Cd toxicity.
(DOC)
H2S or HS−, but not other compounds derived from NaHS contribute to NaHS responses.
(DOC)
Effects of NaHS, PAG and GSH pretreatments on the fresh weight (A) and TBARS concentrations (B) in alfalfa seedling roots upon Cd stress.
(DOC)
Effects of NaHS, PAG and GSH pretreatments on Cd concentrations in alfalfa seedlings upon Cd stress.
(DOC)
The sequences of primers for real-time RT-PCR.
(DOC)
Acknowledgments
Conceived and designed the experiments: WS. Performed the experiments: WC KZ QJ. Analyzed the data: WC Y. Xie WS. Contributed reagents/materials/analysis tools: HC JC Y. Xia JZ. Wrote the paper: WC HC WS.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Fundamental Research Funds for the Central Universities (KYZ201316), the National Natural Science Foundation of China (grants no. 30971711, J1210056, J1310015), and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gao J, Sun L, Yang X, Liu JX (2013) Transcriptomic analysis of cadmium stress response in the heavy metal hyperaccumulator Sedum alfredii hance. PLoS ONE 8(6): e64643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ye Y, Li Z, Xing D (2013) Nitric oxide promotes MPK6-mediated caspase-3-like activation in cadmium-induced Arabidopsis thaliana programmed cell death. Plant Cell Environ 36: 1–15. [DOI] [PubMed] [Google Scholar]
- 3. Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14: 43–50. [DOI] [PubMed] [Google Scholar]
- 4. Tkalec M, Štefanić PP, Cvjetko P, Šikić S, Pavlica M, et al. (2012) The effects of cadmium-zinc interactions on biochemical responses in tobacco seedlings and adult plants. PLoS ONE 9(1): e87582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cobbett CS, May MJ, Howden R, Rolls B (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J 16: 73–78. [DOI] [PubMed] [Google Scholar]
- 7. Cruz de Carvalho MH, Brunet J, Bazin J, Kranner I, d' Arcy-Lameta A, et al. (2010) Homoglutathione synthetase and glutathione synthetase in drought-stressed cowpea leaves: expression patterns and accumulation of low-molecular-weight thiols. J Plant Physiol 167: 480–487. [DOI] [PubMed] [Google Scholar]
- 8. Matamoros MA, Moran JF, Iturbe-Ormaetxe I, Rubio MC, Becana M (1999) Glutathione and homoglutathione synthesis in legume root nodules. Plant Physiol 121: 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ortega-Villasante C, Rellán-Álvarez R, Del Campo FF, Carpena-Ruiz RO, Hernández LE (2005) Cellular damage induced by cadmium and mercury in Medicago sativa . J Exp Bot 56: 2239–2251. [DOI] [PubMed] [Google Scholar]
- 10. Metwally A, Safronova VI, Belimov AA, Dietz KJ (2005) Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J Exp Bot 56: 167–178. [DOI] [PubMed] [Google Scholar]
- 11. Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li L, Rose P, Moore PK (2011) Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51: 169–187. [DOI] [PubMed] [Google Scholar]
- 13. Hosoki R, Matsuki N, Kimura H (1997) The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531. [DOI] [PubMed] [Google Scholar]
- 14. Du J, Hui Y, Cheung Y, Bin G, Jiang H, et al. (2004) The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels 19: 75–80. [DOI] [PubMed] [Google Scholar]
- 15. Yang G, Sun X, Wang R (2004) Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J 18: 1782–1784. [DOI] [PubMed] [Google Scholar]
- 16. Kimura Y, Goto Y, Kimura H (2010) Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 12: 1–13. [DOI] [PubMed] [Google Scholar]
- 17. Wilson LG, Bressan RA, Filner P (1978) Light-dependent emission of hydrogen sulfide from plants. Plant Physiol 61: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hällgren JE, Fredriksson SÅ (1982) Emission of hydrogen sulfide from sulfur dioxide-fumigated pine trees. Plant Physiol 70: 456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sekiya J, Schmidt A, Wilson LG, Filner P (1982) Emission of hydrogen sulfide by leaf tissue in response to L-cysteine. Plant Physiol 70: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang R (2002) Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798. [DOI] [PubMed] [Google Scholar]
- 21. Papenbrock J, Riemenschneider A, Kamp A, Schulz-Vogt HN, Schmidt A (2007) Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants – from the field to the test tube and back. Plant Biol 9: 582–588. [DOI] [PubMed] [Google Scholar]
- 22. Léon S, Touraine B, Briat JF, Lobréaux S (2002) The AtNFS2 gene from Arabidopsis thaliana encodes a NifS-like plastidial cysteine desulphurase. Biochem J 366: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riemenschneider A, Wegele R, Schmidt A, Papenbrock J (2005) Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana . FEBS J 272: 1291–1304. [DOI] [PubMed] [Google Scholar]
- 24. Álvarez C, Calo L, Romero LC, García I, Gotor C (2010) An O-acetylserine(thiol)lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol 152: 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jin Z, Shen J, Qiao Z, Yang G, Wang R, et al. (2011) Hydrogen sulfide improves drought resistance in Arabidopsis thaliana . Biochem Biophys Res Commun 414: 481–486. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Li L, Cui W, Xu S, Shen W, et al. (2012) Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 351: 107–119. [Google Scholar]
- 27. García-Mata C, Lamattina L (2010) Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol 188: 977–984. [DOI] [PubMed] [Google Scholar]
- 28. Lisjak M, Srivastava N, Teklic T, Civale L, Lewandowski K, et al. (2010) A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol Biochem 48: 931–935. [DOI] [PubMed] [Google Scholar]
- 29. Zhang H, Jiao H, Jiang CX, Wang SH, Wei ZJ, et al. (2010) Hydrogen sulfide protects soybean seedlings against drought-induced oxidative stress. Acta Physiol Plant 32: 849–857. [Google Scholar]
- 30. Zhang H, Hu LY, Hu KD, He YD, Wang SH, et al. (2008) Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50: 1518–1529. [DOI] [PubMed] [Google Scholar]
- 31. Li L, Wang Y, Shen W (2012) Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. Biometals 25: 617–631. [DOI] [PubMed] [Google Scholar]
- 32. Li ZG, Gong M, Xie H, Yang L, Li J (2012) Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci 185–186: 185–189. [DOI] [PubMed] [Google Scholar]
- 33. Lin YT, Li MY, Cui WT, Lu W, Shen WB (2012) Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J Plant Growth Regul 31: 519–528. [Google Scholar]
- 34. Zhang H, Hu SL, Zhang ZJ, Hu LY, Jiang CX, et al. (2011) Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol Tec 60: 251–257. [Google Scholar]
- 35. Ma W, Qi Z, Smigel A, Walker RK, Verma R, et al. (2009) Ca2+, cAMP, and transduction of non-self perception during plant immune responses. Proc Natl Acad Sci U S A 106: 20995–21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jin XC, Wu WH (1998) Involvement of cyclic AMP in ABA- and Ca2+-mediated signal transduction of stomatal regulation in Vicia faba . Plant Cell Physiol 40: 1127–1133. [Google Scholar]
- 37. Kimura H (2000) Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun 267: 129–133. [DOI] [PubMed] [Google Scholar]
- 38. Lu M, Liu YH, Ho CY, Tiong CX, Bian JS (2012) Hydrogen sulfide regulates cAMP homeostasis and renin degranulation in As4.1 and rat renin-rich kidney cells. Am J Physiol Cell Physiol 302: C59–C66. [DOI] [PubMed] [Google Scholar]
- 39. Shao JL, Wan XH, Chen Y, Bi C, Chen HM, et al. (2011) H2S protects hippocampal neurons from anoxia-reoxygenation through cAMP-mediated PI3K/Akt/p70S6K cell-survival signaling pathways. J Mol Neurosci 43: 453–460. [DOI] [PubMed] [Google Scholar]
- 40. Bloem E, Riemenschneider A, Volker J, Papenbrock J, Schmidt A, et al. (2004) Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J Exp Bot 55: 2305–2312. [DOI] [PubMed] [Google Scholar]
- 41. Cui W, Gao C, Fang P, Lin G, Shen W (2008) Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J Hazard Mater 260: 715–724. [DOI] [PubMed] [Google Scholar]
- 42. Smith IK (1985) Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol 79: 1044–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herschbach C, Pilch B, Tausz M, Rennenberg H, Grill D (2002) Metabolism of reduced and inorganic sulphur in pea cotyledons and distribution into developing seedlings. New Phytol 153: 73–80. [Google Scholar]
- 44. Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, et al. (2007) Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J 52: 973–986. [DOI] [PubMed] [Google Scholar]
- 45. Cui W, Li L, Gao Z, Wu H, Xie Y, et al. (2012) Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress duo to cadmium stress in Medicago sativa . J Exp Bot 63: 5521–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kováčik J, Babula P, Klejdus B, Hedbavny J, Jarošová M (2014) Unexpected behavior of some nitric oxide modulators under cadmium excess in plant tissue. PLos ONE 9(3): e91685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rennenberg H, Filner P (1982) Stimulation of H2S emission from pumpkin leaves by inhibition of glutathione synthesis. Plant Physiol 69: 766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kwak WJ, Kwon GS, Jin I, Kuriyama H, Sohn HY (2003) Involvement of oxidative stress in the regulation of H2S production during ultradian metabolic oscillation of Saccharomyces cerevisiae . FEMS Microbiol Lett 219: 99–104. [DOI] [PubMed] [Google Scholar]
- 50. Sohn HY, Kum EJ, Kwon GS, Jin I, Adams CA, et al. (2005) GLR1 plays an essential role in the homeodynamics of glutathione and the regulation of H2S production during respiratory oscillation of Saccharomyces cerevisiae . Biosci Biotechnol Biochem 69: 2450–2454. [DOI] [PubMed] [Google Scholar]
- 51. Shi H, Ye T, Chan Z (2014) Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol Biochem 74: 99–107. [DOI] [PubMed] [Google Scholar]
- 52. Rüegsegger A, Schmutz D, Brunold C (1990) Regulation of glutathione synthesis by cadmium in Pisum sativum L. Plant Physiol. 93: 1579–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lisjak M, Teklic T, Wilson ID, Whiteman M, Hancock JT (2013) Hydrogen sulfide: environmental factor or signalling molecule? Plant Cell Environ 36: 1607–1616. [DOI] [PubMed] [Google Scholar]
- 54. García-Mata C, Lamattina L (2013) Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci 201–202: 66–73. [DOI] [PubMed] [Google Scholar]
- 55. Kimura Y, Kimura H (2004) Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18: 1165–1167. [DOI] [PubMed] [Google Scholar]
- 56. Li ZG, Gong M, Liu P (2012) Hydrogen sulfide is a mediator in H2O2-induced seed germination in Jatropha Curcas . Acta Physiol Plant 34: 2207–2213. [Google Scholar]
- 57. Sun J, Wang R, Zhang X, Yu Y, Zhao R, et al. (2013) Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol Biochem 65: 67–74. [DOI] [PubMed] [Google Scholar]
- 58. Sohn HY, Kuriyama H (2001) The role of amino acids in the regulation of hydrogen sulfide production during ultradian respiratory oscillation of Saccharomyces cerevisiae . Arch Microbiol 176: 69–78. [DOI] [PubMed] [Google Scholar]
- 59. Chen J, Wu FH, Wang WH, Zheng CJ, Lin GH, et al. (2011) Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J Exp Bot 62: 4481–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Christou A, Manganaris GA, Papadopoulos I, Fotopoulos V (2013) Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot 64: 1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xie Y, Lai D, Mao Y, Zhang W, Shen W, et al. (2013) Molecular cloning, characterization, and expression analysis of a novel gene encoding L-cysteine desulfhydrase from Brassica napus . Mol Biotechnol 54: 737–746. [DOI] [PubMed] [Google Scholar]
- 62. Fang T, Cao Z, Li J, Shen W, Huang L (2014) Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol Biochem 76: 44–51. [DOI] [PubMed] [Google Scholar]
- 63. Dawood M, Cao F, Jahangir MM, Zhang G, Wu F (2012) Alleviation of aluminum toxicity by hydrogen sulfide is related to elevated ATPase, and suppressed aluminum uptake and oxidative stress in barley. J Hazard Mater 209–210: 121–128. [DOI] [PubMed] [Google Scholar]
- 64. Jin CW, Mao QQ, Luo BF, Lin XY, Du ST (2013) Mutation of mpk6 enhances cadmium tolerance in Arabidopsis plants by alleviating oxidative stress. Plant Soil 371: 387–396. [Google Scholar]
- 65. Lagorce A, Fourçans A, Dutertre M, Bouyssiere B, Zivanovic Y, et al. (2012) Genome-wide transcriptional response of the archaeon Thermococcus gammatolerans to cadmium. PLoS ONE 7(7): e41935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thapa G, Sadhukhan A, Panda SK, Sahoo L (2012) Molecular mechanistic model of plant heavy metal tolerance. Biometals 25: 489–505. [DOI] [PubMed] [Google Scholar]
- 67. Dixit P, Mukherjee PK, Ramachandran V, Eapen S (2013) Glutathione transferase from Trichoderma virens enhances cadmium tolerance without enhancing its accumulation in transgenic Nicotiana tabacum . PLoS ONE 6(1): e16360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dubreuil-Maurizi C, Vitecek J, Marty L, Branciard L, Frettinger P, et al. (2011) Glutathione deficiency of the Arabidopsis mutant pad2-1 affects oxidative stress-related events, defense gene expression, and the hypersensitive response. Plant Physiol 157: 2000–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Howden R, Andersen CR, Goldsbrough PB, Cobbett CS (1995) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana . Plant Physiol 107: 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Howden R, Goldsbrough PB, Andersen CR, Cobbett CS (1995) Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol 107: 1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grill E, Löffler S, Winnacker EL, Zenk MH (1989) Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci USA 86: 6838–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, et al. (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem. 44: 25–37. [DOI] [PubMed] [Google Scholar]
- 73. Verbruggen N, Juraniec M, Baliardini C, Meyer CL (2013) Tolerance to cadmium in plants: the special case of hyperaccumulators. Biometals 26: 633–638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NaHS pretreatment alleviates Cd toxicity.
(DOC)
H2S or HS−, but not other compounds derived from NaHS contribute to NaHS responses.
(DOC)
Effects of NaHS, PAG and GSH pretreatments on the fresh weight (A) and TBARS concentrations (B) in alfalfa seedling roots upon Cd stress.
(DOC)
Effects of NaHS, PAG and GSH pretreatments on Cd concentrations in alfalfa seedlings upon Cd stress.
(DOC)
The sequences of primers for real-time RT-PCR.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.