Abstract
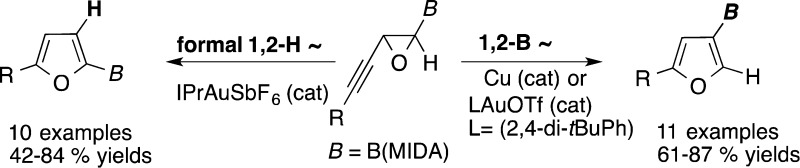
A regioselective transition metal-catalyzed cycloisomerization reaction of boron-containing alkynyl epoxides toward C2- and C3-borylated furans has been developed. It was found that the copper catalyst as well as the gold catalyst with more basic triflate counterion favor boryl migration toward C3-borylated furans, whereas employment of the cationic gold hexafluoroantimonate affords C2-borylated furan via a formal 1,2-hydrogen shift.
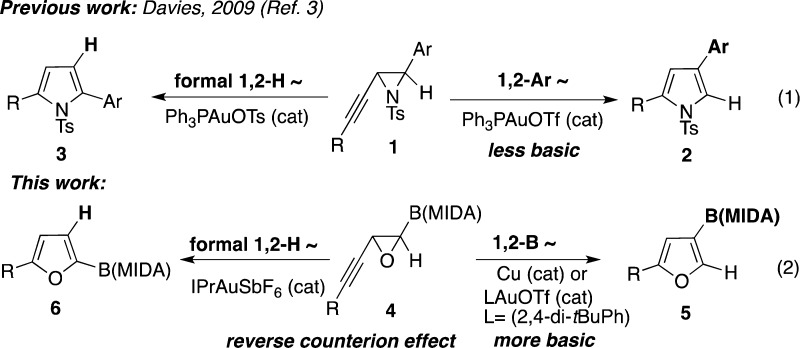
Migratory cycloisomerization is a powerful approach effectively used in synthesis of densely functionalized heterocycles.1 Our group and others have developed a set of transition metal-catalyzed heterocyclization reactions proceeding with migration of various functional groups.2 Recently, Davies’ group efficiently employed alkynyl aziridine 1 in a gold-catalyzed cycloisomerization toward either 2,4- or 2,5-substituted pyrroles 2 and 3 (Scheme 1, eq 1).3 The regioselectivity of this reaction is governed by the basicity of counterion, where the less basic triflate favors migration of an aryl group to afford 2, while a more basic tosylate leads to pyrrole 3, the product of a formal 1,2-hydrogen shift. Herein we report a regiodivergent copper- and gold-catalyzed cycloisomerization reactions of boron-containing alkynyl epoxide 4 toward C3- and C2-borylated furans 5 and 6 (Scheme 1, eq 2). To the best of our knowledge, this represents the first example of synthesis of heterocycles involving a 1,2-boryl group migration.4 Moreover, this gold-catalyzed cycloisomerization exhibits a reverse counterion effect, where the gold catalyst with more basic triflate counterion favors boryl migration to produce C3-borylated furan 5, whereas employment of a cationic gold hexafluoroantimonate triggers a formal 1,2-hydrogen migration producing C2-borylated furan 6.
Scheme 1. Regiodivergent Synthesis of Furans and Pyrroles via Cycloisomerization of Alkynyl Epoxides and Aziridines.
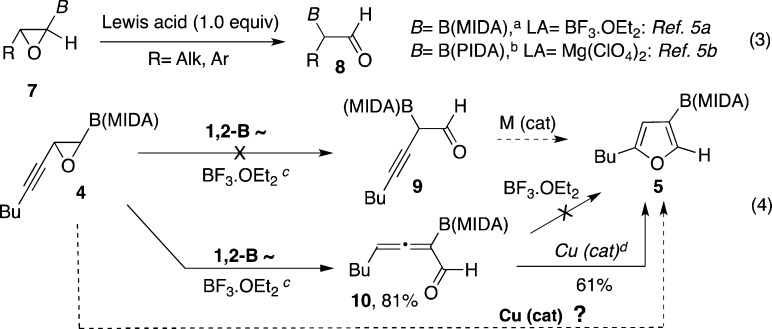
Inspired by the recently reported Lewis acid-mediated 1,2-boryl migration in oxirane 7 into α-borylated aldehyde 8 (Scheme 2, eq 3),5 we attempted analogous transformation on boron-containing alkynyl epoxide 4. We expected formation of a skipped propargyl aldehyde 9 (analogue of 8), which was thought to be a key precursor for the synthesis of very useful, yet otherwise challenging to access borylated furans such as 5,6,7 via a subsequent cycloisomerization reaction (Scheme 2, eq 4).1,8 However, the reaction of 4 in the presence of BF3·OEt25a did not provide 9; allenyl aldehyde 10 was efficiently formed instead (Scheme 2, eq 4).9 All attempts to cyclize allene 10 into furan 5 under forcing reaction conditions failed. Since allenyl aldehydes and ketones are capable substrates for the Cu-catalyzed cycloisomerization reaction toward furans,1,10 we tested transformation of allenyl boronate 10 into furan 5 in the presence of copper catalysts (Scheme 2, eq 4). Indeed, employment of [CuOTf]2·PhH catalyst led to the formation of borylated furan 5 in a reasonable yield. Naturally, next, we explored the possibility of the direct transformation of alkynyl epoxide 4 into furan 5 in the presence of Cu catalyst (Table 1). To our delight, boron-containing alkynyl epoxide 4a under the same copper-catalyzed conditions produced furan 5 in a good yield; although, a detectable amount of unexpected C2-borylated furan 6 was also formed (entry 1).11 Likewise, phenyl-substituted alkynyl epoxide 4b produced furan 5b in good regioselectivity albeit in a low yield (entry 2). Cyclization of alkynyl epoxide 4c possessing an electron-rich aryl group proceeded smoothly to produce furan 5c in an excellent regioselectivity (entry 3). However, attempts to cycloisomerize 4d possessing an electron-deficient aryl group failed (entry 4). Employing trisubstituted alkynyl epoxide 4e in this transformation resulted in complete decomposition of starting material (entry 5).
Scheme 2. Metal-Catalyzed 1,2-Boryl Migration in the Synthesis of 3-Borylated Furans.
MIDA = N-methyliminodiacetic acid; PIDA = pinene-derived iminodiacetic acid; BF3OEt2 (1.0 equiv), DCM, −30 °C.; [CuOTf]2·PhH (5 mol %), DCE, 30 °C.
Table 1. Copper-Catalyzed Migratory Cycloisomerization Reaction toward Borylated Furans.
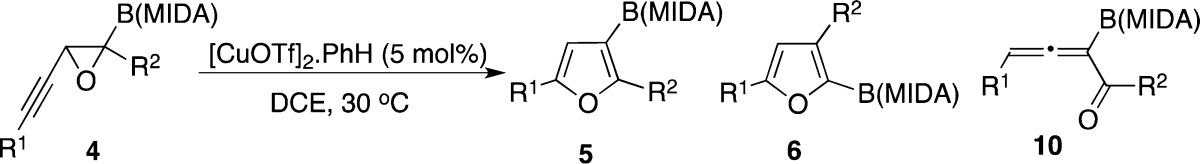
| entry | substrate | R1, R2 | yield, %a (5:6:10)b |
|---|---|---|---|
| 1 | 4a | nBu, H | 80 (97:3:0) |
| 2 | 4b | Ph, H | 36 (89:11:0)c |
| 3 | 4c | 2-OMe-Ph, H | 51 (99:1:0) |
| 4 | 4d | 2-CF3-Ph, H | 96 (0:0:100)d |
| 5 | 4e | nBu, nBu | 0e |
Isolated yields.
NMR ratios.
T = 55 °C.
Heating of the reaction did not lead to furan.
Decomposition of 4.
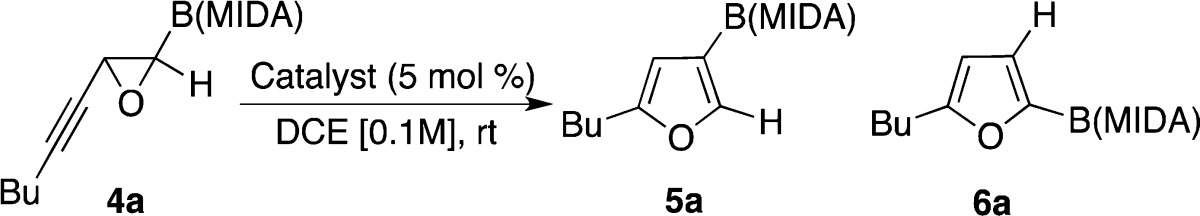
Seeking for more general and efficient conditions for regioselective synthesis of borylated furans, we next turned our attention to π-philic gold catalysts (Table 2).3,11,12 We found that in the presence of Ph3PAuCl, the reaction was more regioselective for 5a with triflate counterion (entry 2) rather than with hexafluoroantimonate (entry 1). Gratifyingly, employment of a gold catalyst possessing electron-rich phosphite ligand with triflate led to furan 5a with an excellent regioselectivity and yield (entry 3). Conversely, switching counterion to hexafluoroantimonate favored formation of 6a (entry 4). Analogous results were obtained in the presence of IPrAuCl with triflate counterion (entry 5). Remarkably, employment of the same gold catalyst with hexafluoroantimonate led to furan 6a, exclusively (entry 6).13 Control gold-free experiments indicated that the reactions in the presence of silver triflate (entry 7) and silver hexafluoroantimonate (entry 8) were both less efficient and regioselective.
Table 2. Optimization of the Catalyst.
| entry | catalyst | NMR yield, % | 5a:6a |
|---|---|---|---|
| 1 | Ph3PAuCl/AgSbF6 | >99 | 50:50 |
| 2 | Ph3PAuCl/AgOTf | 85 | 82:18 |
| 3 | (ArO)3PAuCla/AgOTfb,c | 81d | 96:4e |
| 4 | (ArO)3PAuCla/AgSbF6b | 76 | 22:78 |
| 5 | IPrAuCl/AgOTf | 79 | 33:67 |
| 6 | IPrAuCl/AgSbF6 | 81 | 0:100 |
| 7 | AgOTfb | 39 | 87:13 |
| 8 | AgSbF6 | 39 | 86:14 |
Ar = 2,4-di-tBuPh.
Solution [0.04M].
T = −30 °C.
76% NMR yield at rt.
Ratio of 91:9 at rt.
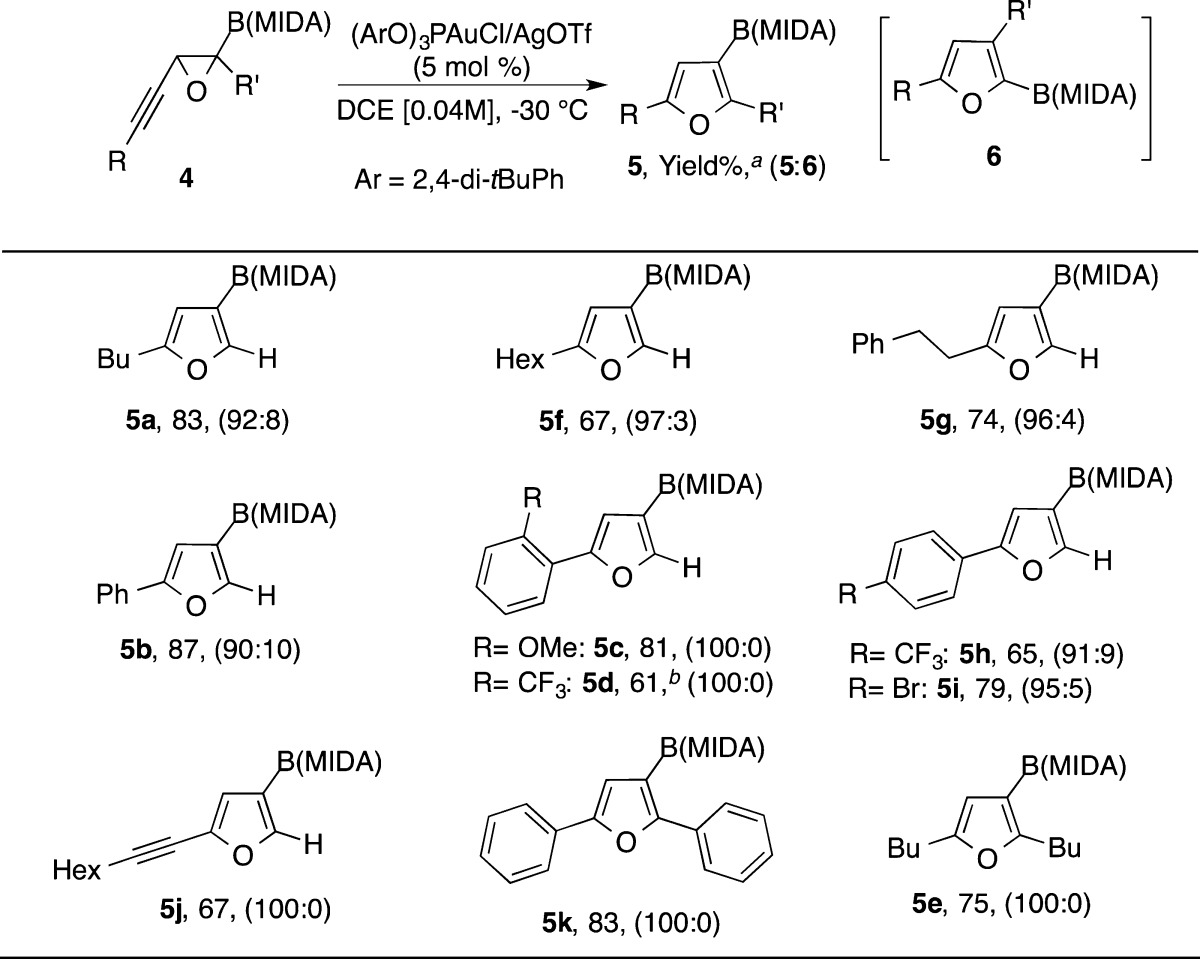
After finding conditions for regiodivergent synthesis of boryl furans 5 and 6, we first examined the scope of the migratory cycloisomerization reaction toward C3-borylated furan 5 (Table 3). Thus, C2-alkyl-substituted furans 5a, 5f, and 5g were produced in good yields and excellent regioselectivity. Phenyl-substituted alkynyl epoxide 4b reacted smoothly to afford 5b in 87% yield and good regioselectivity. Likewise, ortho-substituted aryl-containing furans 5c and 5d were exclusively isolated in good yields. Analogously, cyclization of alkynyl epoxides 4h and 4i provided furans 5h and 5i possessing para-substituted aryls. Diynyl epoxide 4j afforded alkynylated furan 5j exclusively in 67% yield. Remarkably, employment of trisubstituted oxirane 4k in this transformation led to the exclusive formation of furan 5k in 83% yield, thus, indicating overwhelming preference of boryl group versus aryl group1,2a−2c,3 migration. Analogously, dialkyl-containing furan 5e was selectively obtained under these conditions.
Table 3. Gold-Catalyzed Synthesis of C3-Borylated Furans.
Isolated yield.
Reaction was performed at rt.
Next, we investigated the gold-catalyzed cycloisomerization reaction of alkynyl epoxide 4 leading to C2-borylated furan 6 (Table 4). Thus, alkyl-containing furans 6a, 6f, and 6g were exclusively obtained in reasonable to good yields. Analogously, alkynyl epoxide 4b possessing phenyl substituent worked well under these reaction conditions. Likewise, electronically different ortho- and para-substituted aryl-containing furans 6c, 6d, 6h, and 6i were obtained in modest to good yields and excellent regioselectivity. In addition, diynyl epoxide 4j was smoothly converted into alkynylated furan 6j in good yield. Finally, cyclization of 4l afforded monosubstituted boryl furan 6l in 60% yield.14
Table 4. Gold-Catalyzed Synthesis of C2-Borylated Furans.
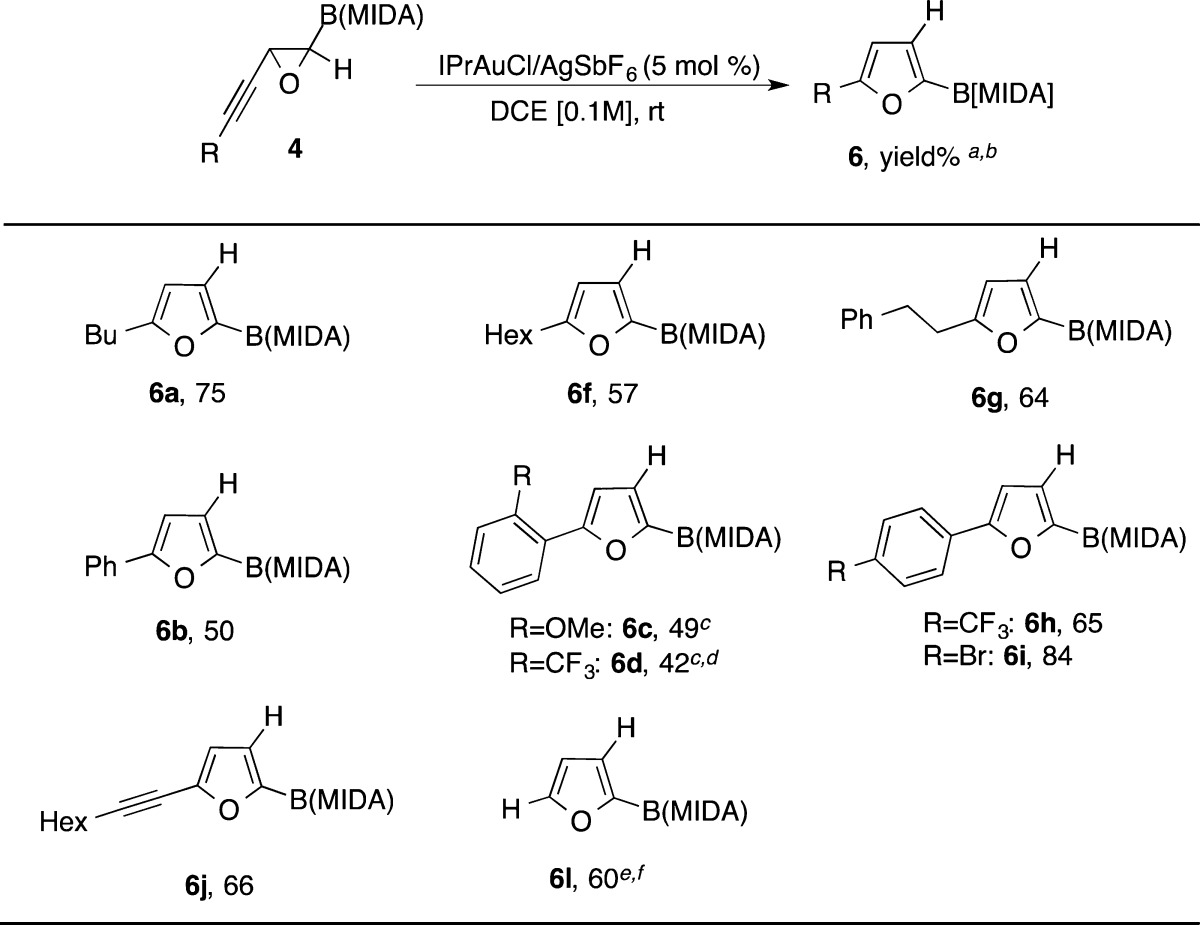
Isolated yields.
Exclusive formation of C2-borylated furans was observed in all examples.
Substantial decomposition of substrate was observed.
At 60 °C.
THF as the solvent.
NMR yield.
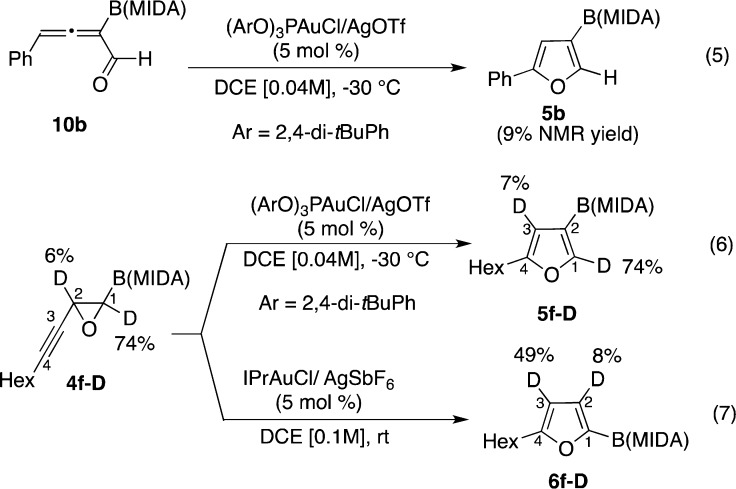
We also performed some initial mechanistic experiments. First, we verified whether the Au-catalyzed reaction proceeds via intermediacy of allene 10 (Scheme 3, eq 5). However, a very low yield of furan 5b was observed when 10b was subjected to the reaction conditions. This indicates that in contrast to the Cu-catalyzed version (vide supra), generation of allene intermediate 10 in the gold-catalyzed transformation is less likely. Next, cycloisomerization of the deuterium-labeled alkynyl epoxide 4f-D was examined. It was found that under the gold-catalyzed migratory conditions, furan 5f-D was formed in which deuterium label stayed intact at the C1 position (Scheme 3, eq 6). However, subjecting 4f-D to the nonmigratory cycloisomerization conditions produced furan 6f-D with deuterium label at the C3 position substantially scrambled (Scheme 3, eq 7).15
Scheme 3. Mechanistc Studies of the Gold-Catalyzed Cycloisomerization Reactions.
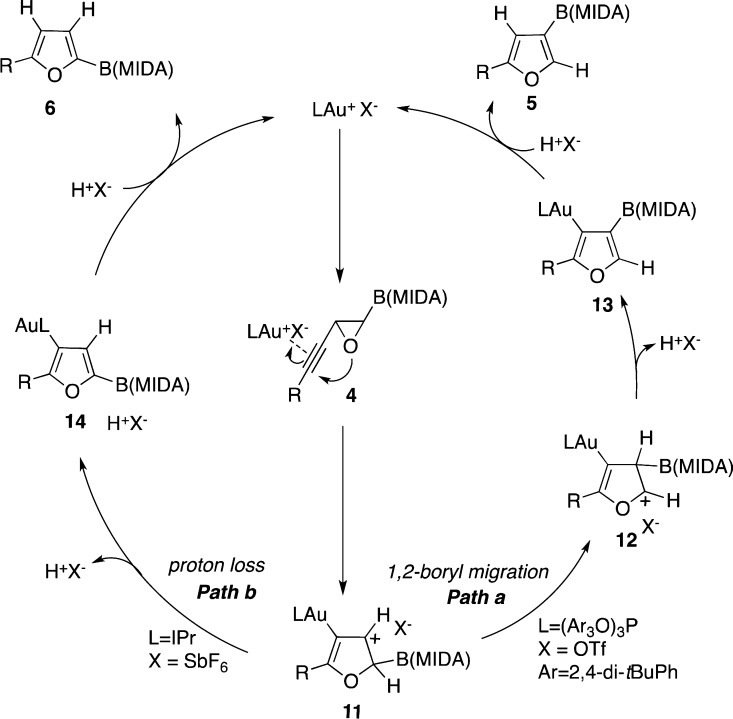
Obviously, more detailed studies toward understanding the role of both ligand and counterion on the regioselectivity of this cycloisomerization reaction are required. At this point, based on the above observations, we propose the following working rationale for this regiodivergent transformation (Scheme 4). First, activation of the π system of 4 by the gold catalyst triggers a nucleophilic attack of the oxygen at the distal position of alkyne moiety producing the heterocyclic cation 11.12 In the case of triflate counterion (Path a), a 1,2-boryl migration to the cationic center of 11 takes place to generate cation 12, which, upon proton loss affords the furyl gold specie 13. Protiodemetalation of the latter produces the C3-borylated furan 5. Alternatively, in the presence of hexafluoroantimonate counterion (Path b), a proton loss15 in intermediate 11 takes place to afford the furyl gold species 14, which upon protiodeauration produces C2-borylated furan 6. It should be mentioned that the observed counterion effect on the regioselectivity of boryl group versus hydrogen migration is in a sharp contrast with the migration trend for carbon and silyl group versus hydrogen migration previously reported by our2d,2k and other16 groups. Further studies to elucidate the origins of the observed migratory trend are underway in our group.
Scheme 4. Mechanistic Proposal for the Regidivergent Gold-Catalyzed Cycloisomerizations.
In summary, complementary regioselective copper- and gold-catalyzed cycloisomerization reactions of boron-containing alkynyl epoxides toward C2- and C3-borylated furans have been developed. It was found that the copper-catalyzed transformation triggers the initial 1,2-boryl group migration forming an allenyl derivative, which upon cycloisomerization produces C3-borylated furan. However, the Cu-catalyzed cycloisomerization appeared to be not general. Accordingly, we developed two efficient and general Au-catalyzed protocols, where depending on the choice of ligand and counterion, regioisomeric boryl furans can be selectively obtained. Thus, phosphite gold complex with triflate counterion strongly favors a 1,2-boryl migration during the cycloisomerization process to produce the C3-borylated furans. In contrast, employment of NHC gold hexafluoroantimonate affords C2-borylated furans, exclusively. MIDA boronate moiety proven to be a highly valuable group for a variety of useful transformations.17 Thus, it is believed that the obtained regioisomeric MIDA boronate-containing furans would become useful building blocks for organic synthesis.
Acknowledgments
We thank National Institutes of Health (GM-64444) and National Science Foundation (CHE-1362541) for financial support of this work.
Supporting Information Available
Experimental details, compound characterization, and spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- For recent reviews, see for example:; a Gulevich A. V.; Dudnik A. S.; Chernyak N.; Gevorgyan V. Chem. Rev. 2013, 113, 3084. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dudnik A.; Chernyak N.; Gevorgyan V. Aldrichimica Acta 2010, 43, 37. [PMC free article] [PubMed] [Google Scholar]; c Weibel J.-M.; Blanc A.; Pale P. Chem. Rev. 2008, 108, 3149. [DOI] [PubMed] [Google Scholar]; d Garayalde D.; Nevado C. Beilstein J. Org. Chem. 2011, 7, 767. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Shapiro N. D.; Toste F. D. Synlett 2010, 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples of carbon migrations in synthesis of heterocycles, see:; a Dudnik A. S.; Gevorgyan V. Angew. Chem., Int. Ed. 2007, 46, 5195. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Crone B.; Kirsch S. F. Chem.—Eur. J. 2008, 14, 3514. [DOI] [PubMed] [Google Scholar]; c Gorin D. J.; Davis N. R.; Toste F. D. J. Am. Chem. Soc. 2005, 127, 11260. [DOI] [PubMed] [Google Scholar]; For silicon migrations, see:; d Dudnik A. S.; Xia Y. Z.; Li Y. H.; Gevorgyan V. J. Am. Chem. Soc. 2010, 132, 7645. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Nakamura I.; Sato T.; Terada M.; Yamamoto Y. Org. Lett. 2007, 46, 2284. [DOI] [PubMed] [Google Scholar]; For tin and germanium migrations, see:; f Seregin I. V.; Gevorgyan V. J. Am. Chem. Soc. 2006, 128, 12050. [DOI] [PMC free article] [PubMed] [Google Scholar]; For examples of sulfur and selenium migrations, see:; g Dudnik A. S.; Sromek A. W.; Rubina M.; Kim J. T.; Kel’in A. V.; Gevorgyan V. J. Am. Chem. Soc. 2008, 130, 1440. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Teo W. T.; Rao W. D.; Koh M. J.; Chan P. W. H. J. Org. Chem. 2013, 78, 7508. [DOI] [PubMed] [Google Scholar]; For selected examples of acyloxy and phosphatyloxy group migrations, see:; i Sromek A. W.; Kel’in A. V.; Gevorgyan V. Angew. Chem., Int. Ed. 2004, 43, 2280. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Zhang L. J. Am. Chem. Soc. 2005, 127, 16804. [DOI] [PubMed] [Google Scholar]; k Rao W. D.; Susanti D.; Chan P. W. H. J. Am. Chem. Soc. 2011, 133, 15248. [DOI] [PubMed] [Google Scholar]; For halogen migration, see:; l Xia Y.; Dudnik A. S.; Gevorgyan V.; Li Y. J. Am. Chem. Soc. 2008, 130, 6940. [DOI] [PMC free article] [PubMed] [Google Scholar]; For alkyne migration, see:; m Wang T.; Shi S.; Hansmann M. M.; Rettenmeier E.; Rudolph M.; Hashmi A. S. K. Angew. Chem., Int. Ed. 2014, 53, 3715. [DOI] [PubMed] [Google Scholar]; For nitro group migration, see:; n Stokes B. J.; Liu S.; Driver T. G. J. Am. Chem. Soc. 2012, 134, 6928.22489740 [Google Scholar]
- a Davies P. W.; Martin N. Org. Lett. 2009, 11, 2293. [DOI] [PubMed] [Google Scholar]; b Davies P. W.; Martin N.; Spencer N. Beilstein. J. Org. Chem. 2011, 7, 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a 1,3-boryl migration from oxygen to carbon for the synthesis of borylated benzofurans, see:Hirner J. J.; Faizi D. J.; Blum S. A. J. Am. Chem. Soc. 2014, 136, 4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a He Z.; Yudin A. K. J. Am. Chem. Soc. 2011, 133, 13770. [DOI] [PubMed] [Google Scholar]; b Li J.; Burke M. D. J. Am. Chem. Soc. 2011, 133, 13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, 2nd ed.; Hall D. G., Ed.; Wiley-VCH: Weinheim, 2011. [Google Scholar]
- For examples on synthesis of borylated furans via metal-catalyzed C-H borylation, see:; a Ishiyama T.; Takagi J.; Yonekawa Y.; Hartwig J. F.; Miyaura N. Adv. Synth. Catal. 2003, 345, 1103. [Google Scholar]; b Obligacion J. V.; Semproni S. P.; Chirik P. J. J. Am. Chem. Soc. 2014, 136, 4133. [DOI] [PubMed] [Google Scholar]
- For cycloisomerization of skipped propargylic aldehydes and ketones, see:; a Umland K.-D.; Palisse A.; Haug T. T.; Kirsch S. F. Angew. Chem., Int. Ed. 2011, 50, 9965. [DOI] [PubMed] [Google Scholar]; b Xiao Y.; Zhang J. Angew. Chem., Int. Ed. 2008, 47, 1903. [DOI] [PubMed] [Google Scholar]
- Although, 9 has never been detected in crude reaction mixtures, its intermediacy in the formation of allene 10 cannot be completely ruled out.
- a Kel’in A. V.; Gevorgyan V. J. Org. Chem. 2002, 67, 95. [DOI] [PubMed] [Google Scholar]; b Miao M. Z.; Cao J.; Zhang J. J.; Huang X.; Wu L. L. J. Org. Chem. 2013, 78, 2687. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for details.
- For leading references on the gold-catalyzed cycloisomerizations, see:; a Blanc A.; Alix A.; Weibel J. M.; Pale P. Eur. J. Org. Chem. 2010, 9, 1644. [Google Scholar]; b Yoshida M.; Al-Amin M.; Shishido K. Synthesis 2009, 14, 2454. [Google Scholar]; c Sethofer S. G.; Mayer T.; Toste F. D. J. Am. Chem. Soc. 2010, 132, 8276. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lu B.; Li Y. X.; Wang Y. L.; Aue D. H.; Luo Y. D.; Zhang L. J. Am. Chem. Soc. 2013, 135, 8512. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Hashmi A. S. K.; Frost T. M.; Bats J. W. J. Am. Chem. Soc. 2000, 122, 11553. [Google Scholar]; f Simonneau A.; Harrak Y.; Jeanne-Julien L.; Lemiere G.; Mouries-Mansuy V.; Goddard J. P.; Malacria M.; Fensterbank L. Chemcatchem. 2013, 5, 1096. [Google Scholar]; g Obradors C.; Leboeuf D.; Aydin J.; Echavarren A. M. Org. Lett. 2013, 15, 1576. [DOI] [PubMed] [Google Scholar]; h Teller H.; Flugge S.; Goddard R.; Fürstner A. Angew. Chem., Int. Ed. 2010, 49, 1949. [DOI] [PubMed] [Google Scholar]; i Prechter A.; Henrion G.; Bel P. F. D.; Gagosz F. Angew. Chem., Int. Ed. 2014, 53, 4959. [DOI] [PubMed] [Google Scholar]; j Liu L. P.; Malhotra D.; Paton R. S.; Houk K. N.; Hammond G. B. Angew. Chem., Int. Ed. 2010, 49, 9132. [DOI] [PubMed] [Google Scholar]; k Garayalde D.; Gomez-Bengoa E.; Huang X. G.; Goeke A.; Nevado C. J. Am. Chem. Soc. 2010, 132, 4720. [DOI] [PubMed] [Google Scholar]; l Pawar S. K.; Wang C. D.; Bhunia S.; Jadhav A. M.; Liu R. S. Angew. Chem., Int. Ed. 2013, 52, 7559. [DOI] [PubMed] [Google Scholar]
- Hashmi A. S. K.; Sinha P. Adv. Synth. Catal. 2004, 346, 432. [Google Scholar]
- Trisubstituted 4e and 4k afforded products 5e and 5k in 81% and 67%, respectively. No competitive alkyl or aryl migration was observed.
- For substantial D-label scrambling observed in processes involving protropic isomerization, see, for example:; a Xia Y. Z.; Dudnik A. S.; Li Y. H.; Gevorgyan V. Org. Lett. 2010, 12, 5538. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gorin D. J.; Dube P.; Toste F. D. J. Am. .Chem. Soc. 2006, 128, 14480. [DOI] [PubMed] [Google Scholar]; Also, see ref (3b).
- a Basak A.; Chakrabarty K.; Ghosh A.; Das G. K. J. Org. Chem. 2013, 78, 9715. [DOI] [PubMed] [Google Scholar]; b Li W. B.; Li Y. Y.; Zhou G. H.; Wu X. S.; Zhang J. L. Chem.—Eur. J. 2012, 18, 15113. [DOI] [PubMed] [Google Scholar]; c Fang R.; Yang L. Z. Organometallics 2012, 31, 3043. [Google Scholar]; d Huang G. P.; Cheng B.; Xu L.; Li Y. H.; Xia Y. Z. Chem.—Eur. J. 2012, 18, 5401. [DOI] [PubMed] [Google Scholar]
- Gillis E. P.; Burke M. D. Aldrichimica Acta 2009, 42, 17. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.