Abstract
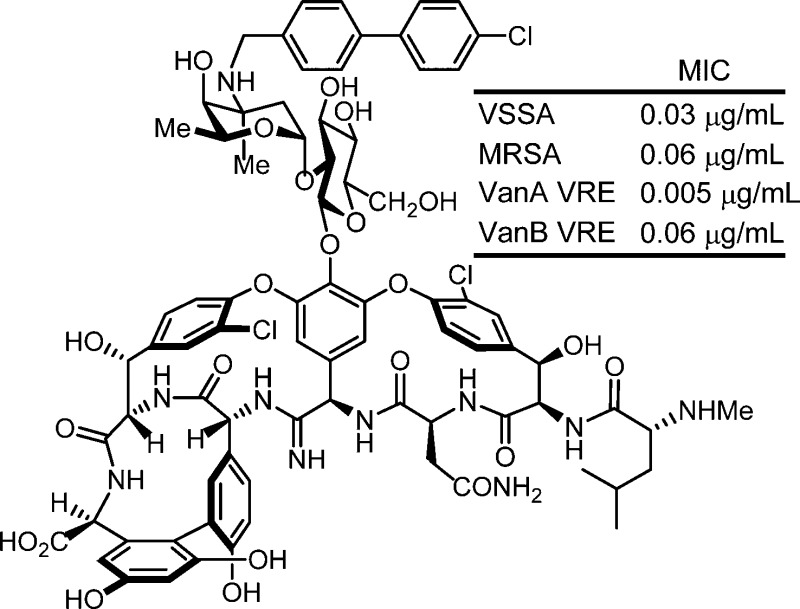
The total synthesis of two key analogues of vancomycin containing single-atom exchanges in the binding pocket (residue 4 amidine and thioamide) are disclosed as well as their peripherally modified (4-chlorobiphenyl)methyl (CBP) derivatives. Their assessment indicates that combined pocket amidine and CBP peripherally modified analogues exhibit a remarkable spectrum of antimicrobial activity (VSSA, MRSA, VanA and VanB VRE) and impressive potencies (MIC = 0.06–0.005 μg/mL) against both vancomycin-sensitive and -resistant bacteria and likely benefit from two independent and synergistic mechanisms of action. Like vancomycin, such analogues are likely to display especially durable antibiotic activity not prone to rapidly acquired clinical resistance.
Vancomycin (1) is the key member of the glycopeptide antibiotics that are the most important class of drugs used against resistant bacterial infections.1 Although it was disclosed in 19562 and introduced into the clinic in 1958, the structure of vancomycin was only established 25–30 years later (Figure 1).3 After more than 50 years of clinical use and with the even more widespread use of glycopeptide antibiotics for livestock (avoparcin), vancomycin-resistant pathogens have slowly emerged worldwide. This was first restricted to vancomycin-resistant enterococci (VRE)4 but more recently includes vancomycin-resistant Staphylococcus aureus (VRSA).5 This has intensified interest in the development of alternative treatments for resistant pathogens that display the remarkable durability of vancomycin, including new derivatives of the glycopeptide antibiotics.1,6,7
Figure 1.
Structure of vancomycin (1), its (4-chlorobiphenyl)methyl derivative 4, and targeted synthetic analogues.
Recently, we described studies of the binding pocket redesign of vancomycin in efforts to directly address the molecular basis of bacterial resistance.8 Vancomycin inhibits bacterial cell wall synthesis by binding the precursor peptidoglycan peptide terminus d-Ala-d-Ala, inhibiting transpeptidase-catalyzed cell wall cross-linking and maturation.9 In the clinically resistant phenotypes (VanA and VanB), the synthesis of the precursor lipid intermediates I and II continues, but resistant bacteria sense the antibiotic challenge10 and conduct a late-stage remodeling of their peptidoglycan termini from d-Ala-d-Ala to d-Ala-d-Lac11 to avoid the antibiotic action. The binding affinity of vancomycin for the altered ligand11 is reduced 1000-fold,12 resulting in a corresponding 1000-fold loss in antimicrobial activity.
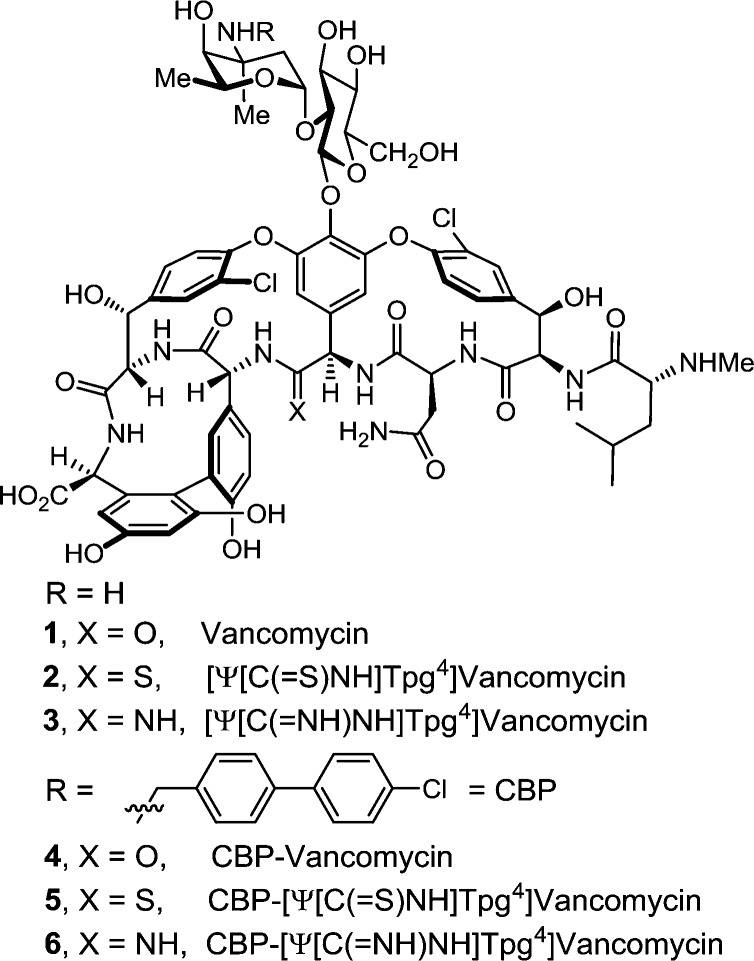
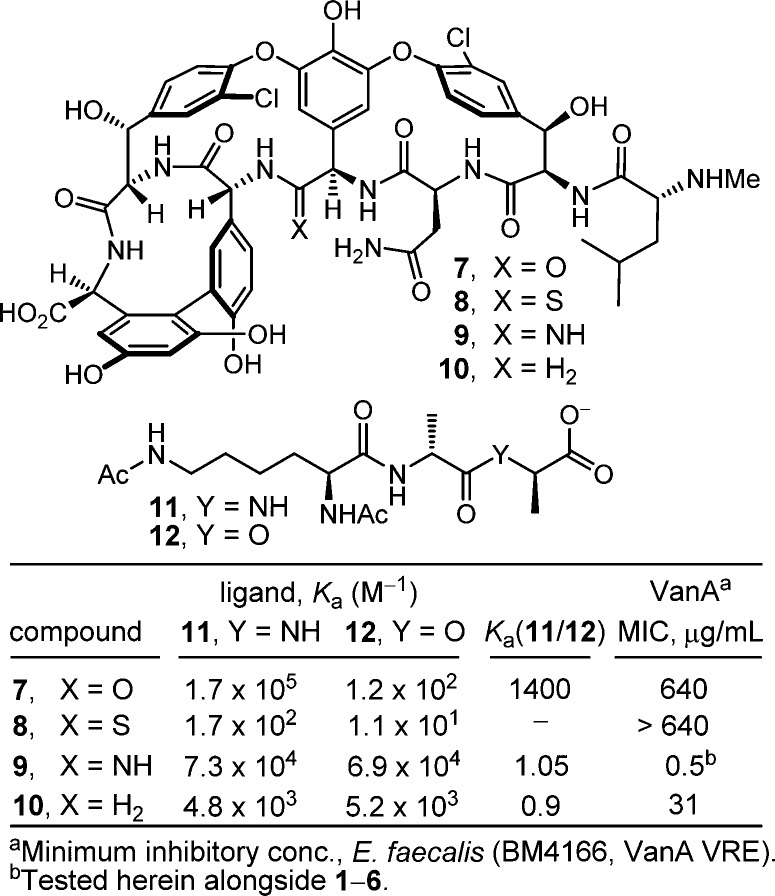
These studies also revealed that the redesign of the vancomycin binding pocket for use against vancomycin-resistant bacteria must target compounds that not only establish binding to d-Ala-d-Lac but also maintain d-Ala-d-Ala binding. Subsequent to a validation in which [Ψ[CH2NH]Tpg4]vancomycin aglycon (10)13 displayed such dual binding properties that reinstated activity against VanA VRE, the total synthesis of [Ψ[C(=NH)NH]Tpg4]vancomycin aglycon (9)14 was reported in efforts that improved the dual binding affinities and antimicrobial activity (Figure 2). Amidine 9 displayed balanced binding affinity for both target ligands within 2-fold that of vancomycin aglycon for d-Ala-d-Ala and exhibited effective antimicrobial activity against VanA VRE, being equipotent to the activity that vancomycin displays against sensitive bacterial strains. Not only did this represent replacement of a single atom in the antibiotic aglycon (O → NH) to counter a complementary exchange in the cell wall precursors of resistant bacteria (NH → O), but the modified antibiotic maintained the ability to bind d-Ala-d-Ala in the unaltered peptidoglycan. Detailed herein is the extension of these studies to the total synthesis of [Ψ[C(=NH)NH]Tpg4]vancomycin (3) with the introduction of the l-vancosaminyl-1,2-d-glucosyl disaccharide, representing a binding pocket analogue of vancomycin itself containing this single-atom change. Although the attached carbohydrate in vancomycin does not alter the in vitro antimicrobial activity or influence target d-Ala-d-Ala or d-Ala-d-Lac binding, it impacts in the vivo activity, increasing the water solubility, influencing the pharmacokinetic and distribution properties, and contributing a potential second mechanism of action.
Figure 2.
Synthetic analogues of vancomycin aglycon (7) that contain key modifications to the binding pocket.
Because of their structural complexity, essentially all analogues of the glycopeptide antibiotics consist of semisynthetic derivatives of the natural products.1,6 The most significant of the modifications introduce peripheral hydrophobic groups, and the most widely studied one entails 4-chlorobiphenyl substitution of a peripheral carbohydrate.6 This has been explored in a variety of glycopeptide antibiotics and at range of positions, most notably in oritavancin,15 the N-(4-chlorobiphenyl)methyl derivative of chloroeremomycin, and in vancomycin itself (4, CBP-vancomycin).16 Studies of their mechanism of action have shown that the chlorobiphenyl side chain promotes antibiotic dimerization and membrane anchoring and establishes antimicrobial activity against vancomycin-resistant organisms despite a lack of improved binding with d-Ala-d-Ala or d-Ala-d-Lac.17 It is possible such semisynthetic changes to vancomycin also avoid bacterial sensing of the antibiotic challenge, which may account for their VanB VRE activity (like teicoplanin).10 Alternatively, they may entail a second mechanism of action. The direct inhibition of transglycosylases mediated by the modified carbohydrate has been implicated as a second mechanism by which the lipophilic glycopeptides with impaired d-Ala-d-Ala binding properties exhibit antimicrobial activity.18 Others, including telavancin, have been shown to function both through the traditional mechanism of inhibition of cell wall synthesis by binding d-Ala-d-Ala and also through the disruption of bacterial membrane integrity, a mechanism typically not observed for glycopeptides.19 Regardless of the origin of the effects, such derivatives often increase the antibiotic potency as much as 100-fold. While bacterial sensitivity to the antibiotics is increased, VanA vancomycin-resistant bacterial strains (MIC = ca. 10 μg/mL) remain 1000-fold less sensitive than susceptible strains (MIC = ca. 0.01 μg/mL).
Given the distinct origins of their impact on the antimicrobial activity of vancomycin, we expected that incorporation of such peripheral hydrophobic modifications into the structure of a binding-pocket-modified vancomycin would further increase their antimicrobial activity against not only sensitive but also vancomycin-resistant bacteria to truly remarkable potencies. Aside from the merits of such analogues as new therapeutics, their increased potencies would have the additional impact of reducing the amount of material needed for preclinical exploration. Although this conceivably could be demonstrated by chlorobiphenyl substitution of the synthetic aglycon 9, the most definitive assessment of the dual impact would be a direct comparison of CBP-vancomycin 4 with 6, wherein a change in a single atom in the binding pocket was introduced, despite the synthetic challenges this poses. Herein we report not only the total synthesis of [Ψ[C(=NH)NH]Tpg4]vancomycin (3) from synthetic [Ψ[C(=S)NH]Tpg4]vancomycin aglycon (8)14 but also the synthesis of its (4-chlorobiphenyl)methyl derivative 6. This was accomplished by using two sequential enzymatic glycosylations to first provide [Ψ[C(=S)NH]Tpg4]vancomycin (2) followed by a Ag(I)-promoted20 conversion of the thioamide to an amidine. An additional single-step introduction of the (4-chlorobiphenyl)methyl group into 2 to provide 5 followed by Ag(I)-promoted conversion to the amidine afforded 6. In addition to the opportunity to assess amidine 3 and the impact of combining the vancomycin pocket redesign with a key peripheral modification, the approach was designed to shed light on the role of the chlorobiphenyl modification through an examination of thioamide 2 and its (4-chlorobiphenyl)methyl derivative, which fail to effectively bind d-Ala-d-Ala or d-Ala-d-Lac.
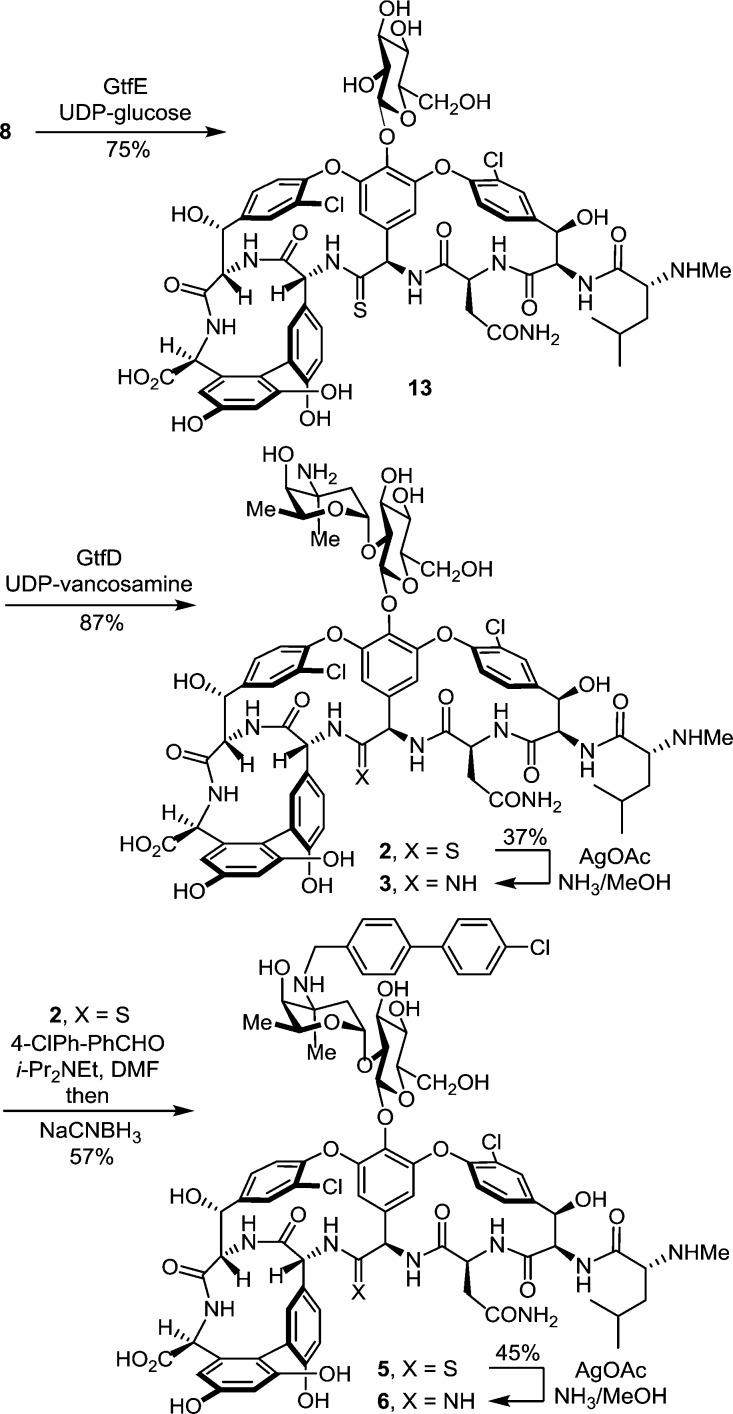
The two sequential glycosylations of synthetic 8(14) were conducted with the purified recombinant glycosyltransferases21 GtfE and GtfD and the synthetic glycosyl donors (UDP-glucose22 for GtfE and UDP-vancosamine23 for GtfD) under recently described conditions23 to provide pseudoaglycon 13 (75%, HPLC conversion 86–92%) and 2 (87%, HPLC conversion >95%) (Scheme 1).24 Direct conversion of thioamide 2 to the corresponding amidine (10 equiv of AgOAc, sat. NH3–MeOH, 25 °C, 7 h, 37% unoptimized)20 provided 3.24 Significantly, the latter reaction was implemented without competitive deglycosylation and the entire sequence (conversion of 8 to 3) was conducted without protecting groups.
Scheme 1.
Subsequent introduction of the chlorobiphenyl group into 2 by selective reductive amination [1.5 equiv of 4-(4-chlorophenyl)benzaldehyde, 5 equiv of i-Pr2NEt, DMF, 70 °C, 2 h; NaCNBH3, 70 °C, 5 h] provided 5 (57%), without the observation of competitive reactions of either the thioamide (reduction) or the N-terminal free amine (reductive amination), using conditions modified from those disclosed for CBP-vancomycin itself.25 Direct AgOAc-promoted (10 equiv, sat. NH3–MeOH, 25 °C, 7 h) conversion of the thioamide to the amidine provided 6 (45%, unoptimized), the chlorobiphenyl derivative of 3, without the need for intervening protecting groups throughout the four-step sequence. By design, the final reaction introducing the amidine was conducted effectively on fully functionalized substrates (2 and 5) lacking protecting groups and incorporating the vancomycin disaccharide.
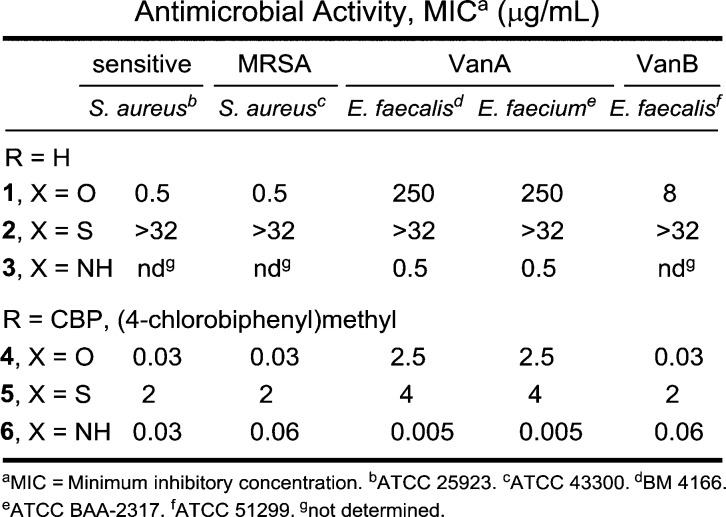
The antimicrobial activity of the compounds was evaluated against a panel of Gram-positive bacteria that included vancomycin-sensitive S. aureus (VSSA), methicillin-resistant S. aureus (MRSA), and both VanA (Enterococcus faecalis and Enterococcus faecium) and VanB (E. faecalis) VRE, of which VanA is the most stringent of the resistant organisms (Figure 3). In line with reports of its impact, introduction of the (4-chlorobiphenyl)methyl group into vancomycin (4 vs 1) results in 100-fold improvements in the activity against VanA and VanB VRE and 20-fold improvements against VSSA and MRSA in the strains examined. Like the aglycon thioamide 8, vancomycin thioamide 2 was ineffective as an antimicrobial agent (>32 μg/mL). Notably, this behavior is derived from a single-atom exchange in the binding pocket (O → S) and is analogous to that of other analogues bearing altered binding pockets incapable of binding d-Ala-d-Ala.18,25 However, introduction of the (4-chlorobiphenyl)methyl group into thioamide 2 to give thioamide 5 reinstates impressive and equally potent activity (MIC = 2–4 μg/mL) against all vancomycin-sensitive and -resistant strains despite its inability to bind the primary cell wall target. It is unlikely such effective activity can be achieved simply by the effects of antibiotic membrane anchoring or dimerization. Rather, it likely reflects potent antimicrobial activity derived from a second mechanism of action impacting cell wall synthesis unrelated to d-Ala-d-Ala/d-Ala-d-Lac binding, such as transglycosylase inhibition.18 Importantly, the vancomycin amidine 3, like the aglycon amidine 9, was found to exhibit potent activity against VanA vancomycin-resistant bacteria (MIC = 0.5 μg/mL), reinstating activity equal to the potency observed with vancomycin against sensitive bacteria. Most significantly, introduction of the chlorobiphenyl group into vancomycin amidine 3 to give 6 resulted in a compound with a remarkable spectrum of activity and impressive potencies. Not only does 6 match the activity that CBP-vancomycin 4 displays against vancomycin-sensitive bacteria (VSSA and MRSA), but it also exhibits this extraordinary potency against VanA and VanB vancomycin-resistant bacteria. In fact, the activity of 6 against the most stringent of the resistant bacteria, VanA VRE, was nearly 10-fold better than the potency it displays against the sensitive bacteria. Because of the insights derived from examination of thioamides 2 and 5, this behavior of 6 likely represents a spectrum of activity and potency derived from bacterial cell wall synthesis inhibition through two independent mechanisms, suggesting that resistance is less likely to emerge.
Figure 3.
In vitro antibacterial activity.
Herein we have detailed the completion of the total syntheses of [Ψ[C(=S)NH]Tpg4]vancomycin (2) and [Ψ[C(=NH)NH]Tpg4]vancomycin (3), two fully adorned analogues of vancomycin that contain a single-atom exchange in the binding pocket, as well as the syntheses of the corresponding chlorobiphenyl derivatives 5 and 6. By design, the sequential enzyme-catalyzed glycosylation reactions of the thioamide aglycon, the final amidine introduction, and the intermediate reductive amination used for the chlorobiphenyl introduction were conducted without the need of protecting groups, establishing the foundation and providing the methodology for potential semisynthetic or biosynthetic preparations of such glycopeptide analogues. In line with expectations based on the behavior of the corresponding aglycons and in stark contrast to one another, the vancomycin amidine reestablishes potent antimicrobial activity against VanA VRE, whereas vancomycin thioamide is inactive even against vancomycin-sensitive bacteria. The introduction of a peripheral chlorobiphenyl modification converts vancomycin thioamide into an effective antimicrobial agent that is active against vancomycin-sensitive and -resistant bacteria (MIC = 2–4 μg/mL) even though it is not capable of effective d-Ala-d-Ala/d-Ala-d-Lac binding and converts the vancomycin amidine into a compound with a remarkable spectrum of activity and truly impressive potencies (MIC = 0.06–0.005 μg/mL) that are likely derived from cell wall biosynthesis inhibition through two independent mechanisms. In addition to indicating that such peripheral and pocket synthetic modifications are synergistic, such analogues are likely to display durable antibiotic activity8 and may be less prone to rapidly acquired clinical resistance.
Acknowledgments
We gratefully acknowledge the financial support of the National Institutes of Health (CA041101 to D.L.B.). We especially thank Professor C. T. Walsh for the gift of the plasmid constructs pET22b-gtfD-his6 and pET22b-gtfE-his6 for GtfD and GtfE expression, Dr. K. C. Collins for expressing and purifying the enzymes in facilities provided by K. D. Janda (TSRI), Dr. J. C. Collins for initial exploration of the enzymatic glycosylations, and Dr. Y. Feng for a supply of UDP-vancosamine. We thank Dr. M. Weiss for advanced intermediates en route to additional 8 in the early stages of the work and D. Ogasawara and Dr. T. Michaels for quantities of the amino acid subunits and early-stage intermediates in the synthesis of additional 8.
Supporting Information Available
Full experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Kahne D.; Leimkuhler C.; Lu W.; Walsh C. T. Chem. Rev. 2005, 105, 425. [DOI] [PubMed] [Google Scholar]
- McCormick M. H.; Stark W. M.; Pittenger G. E.; Pittenger R. C.; McGuire J. M. Antibiot. Annu. 1955–1956, 606. [PubMed] [Google Scholar]
- Harris C. M.; Kopecka H.; Harris T. M. J. Am. Chem. Soc. 1983, 105, 6915. [Google Scholar]
- a Leclercq R.; Derlot E.; Duval J.; Courvalin P. N. Engl. J. Med. 1988, 319, 157. [DOI] [PubMed] [Google Scholar]; b Courvalin P. Clin. Infect. Dis. 2006, 42, S25. [DOI] [PubMed] [Google Scholar]
- a Weigel L. M.; Clewell D. B.; Gill S. R.; Clark N. C.; McDougal L. K.; Flannagan S. E.; Kolonay J. F.; Shetty J.; Killgore G. E.; Tenover F. C. Science 2003, 302, 1569. [DOI] [PubMed] [Google Scholar]; b Walsh T. R.; Howe R. A. Annu. Rev. Microbiol. 2002, 56, 657. [DOI] [PubMed] [Google Scholar]
- a Malabarba A.; Nicas T. I.; Thompson R. C. Med. Res. Rev. 1997, 17, 69. [DOI] [PubMed] [Google Scholar]; b Najarajan R. J. Antibiot. 1993, 46, 1181.8407579 [Google Scholar]; c Van Bambeke F. V.; Laethem Y. V.; Courvalin P.; Tulkens P. M. Drugs 2004, 64, 913. [DOI] [PubMed] [Google Scholar]
- a Süssmuth R. D. ChemBioChem 2002, 3, 295. [DOI] [PubMed] [Google Scholar]; b Walker S.; Chen L.; Helm J.; Hu Y.; Rew Y.; Shin D.; Boger D. L. Chem. Rev. 2005, 105, 449. [DOI] [PubMed] [Google Scholar]; c von Nussbaum F.; Brands M.; Hinzen B.; Weigand S.; Häbich D. Angew. Chem., Int. Ed. 2006, 45, 5072. [DOI] [PubMed] [Google Scholar]
- a James R. C.; Pierce J. G.; Okano A.; Xie J.; Boger D. L. ACS Chem. Biol. 2012, 7, 797. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Boger D. L. Med. Res. Rev. 2001, 21, 356. [DOI] [PubMed] [Google Scholar]; c Boger D. L.; Miyazaki S.; Kim S. H.; Wu J. H.; Castle S. L.; Loiseleur O.; Jin Q. J. Am. Chem. Soc. 1999, 121, 10004. [Google Scholar]; d Boger D. L.; Kim S. H.; Mori Y.; Weng J.-H.; Rogel O.; Castle S. L.; McAtee J. J. J. Am. Chem. Soc. 2001, 123, 1862. [DOI] [PubMed] [Google Scholar]; e Crowley B. M.; Mori Y.; McComas C. C.; Tang D.; Boger D. L. J. Am. Chem. Soc. 2004, 126, 4310. [DOI] [PubMed] [Google Scholar]
- a Perkins H. R. Pharmacol. Ther. 1982, 16, 181. [DOI] [PubMed] [Google Scholar]; b Williams D. H.; Williamson M. P.; Butcher D. W.; Hammond S. J. J. Am. Chem. Soc. 1983, 105, 1332. [Google Scholar]
- a Hong H.-J.; Hutchings M. I.; Buttner M. J. Adv. Exp. Med. Biol. 2008, 631, 200. [DOI] [PubMed] [Google Scholar]; b Koteva K.; Hong H.-J.; Wang X. D.; Nazi I.; Hughes D.; Naldrett M. J.; Buttner M. J.; Wright G. D. Nat. Chem. Biol. 2010, 6, 327. [DOI] [PubMed] [Google Scholar]; c Ikeda S.; Hanaki H.; Yanagisawa C.; Ikeda-Dantsuji Y.; Matsui H.; Iwatsuki M.; Shiomi K.; Nakae T.; Sunakawa K.; Omura S. J. Antibiot. 2010, 63, 533. [DOI] [PubMed] [Google Scholar]; d Kwun M. J.; Novotna G.; Hesketh A. R.; Hill L.; Hong H. Antimicrob. Agents Chemother. 2013, 57, 4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg T. D. H.; Wright G. D.; Dutka-Malen S.; Arthur M.; Courvalin P.; Walsh C. T. Biochemistry 1991, 30, 10408. [DOI] [PubMed] [Google Scholar]
- McComas C. C.; Crowley B. M.; Boger D. L. J. Am. Chem. Soc. 2003, 125, 9314. [DOI] [PubMed] [Google Scholar]
- Crowley B. M.; Boger D. L. J. Am. Chem. Soc. 2006, 128, 2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Xie J.; Pierce J. G.; James R. C.; Okano A.; Boger D. L. J. Am. Chem. Soc. 2011, 133, 13946. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Xie J.; Okano A.; Pierce J. G.; James R. C.; Stamm S.; Crane C. M.; Boger D. L. J. Am. Chem. Soc. 2012, 134, 1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nicas T. I.; Mullen D. L.; Flokowitsch J. E.; Preston D. A.; Snyder N. J.; Zweifel M. J.; Wilkie S. C.; Rodriguez M. J.; Thompson R. C.; Cooper R. D. Antimicrob. Agents Chemother. 1996, 40, 2194. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Nagarajan R.; Schabel A. A.; Occolowitz J. L.; Counter F. T.; Ott J. L.; Felty-Duckworth A. M. J. Antibiot. 1989, 42, 63. [DOI] [PubMed] [Google Scholar]
- Cooper R. D. G.; Snyder N. J.; Zweifel M. J.; Staszak M. A.; Wilkie S. C.; Nicas T. I.; Mullen D. L.; Butler T. F.; Rodriguez M. J.; Huff B. E.; Thompson R. C. J. Antibiot. 1996, 49, 575. [DOI] [PubMed] [Google Scholar]
- a Allen N. E.; Hobbs J. N. Jr.; Nicas T. I. Antimicrob. Agents Chemother. 1996, 40, 2356. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sharman G. J.; Try A. C.; Dancer R. J.; Cho Y. R.; Staroske T.; Bardsley B.; Maguire A. J.; Cooper M. A.; O’Brien D. P.; Williams D. H. J. Am. Chem. Soc. 1997, 119, 12041. [Google Scholar]; c Allen N. E.; Nicas T. I. FEMS Microbiol. Rev. 2003, 26, 511. [DOI] [PubMed] [Google Scholar]
- a Ge M.; Chen Z.; Onishi H. R.; Kohler J.; Silver L. L.; Kerns R.; Fukuzawa S.; Thompson C.; Kahne D. Science 1999, 284, 507. [DOI] [PubMed] [Google Scholar]; b Chen L.; Walker D.; Sun B.; Hu Y.; Walker S.; Kahne D. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Higgins D. L.; Chang R.; Debabov D. V.; Leung J.; Wu T.; Krause K. M.; Sandvik E.; Hubbard J. M.; Kaniga K.; Schmidt D. E. Jr.; Gao Q.; Cass R. T.; Karr D. E.; Benton B. M.; Humphrey P. P. Antimicrob. Agents Chemother. 2005, 49, 1127. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Corey G. R.; Stryjewski M. E.; Weyenberg W.; Yasothan U.; Kirkpatrick P. Nat. Rev. Drug Discovery 2009, 8, 929. [DOI] [PubMed] [Google Scholar]
- Okano A.; James R. C.; Pierce J. G.; Xie J.; Boger D. L. J. Am. Chem. Soc. 2012, 134, 8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Losey H. C.; Peczuh M. W.; Chen Z.; Eggert U. S.; Dong S. D.; Pelczer I.; Kahne D.; Walsh C. T. Biochemistry 2001, 40, 4745. [DOI] [PubMed] [Google Scholar]; b Oberthur M.; Leimkuhler C.; Kruger R. G.; Lu W.; Walsh C. T.; Kahne D. J. Am. Chem. Soc. 2005, 127, 10747. [DOI] [PubMed] [Google Scholar]; c Losey H. C.; Jiang J.; Biggins J. B.; Oberthur M.; Ye X.; Dong S. D.; Kahne D.; Thorson J. S.; Walsh C. T. Chem. Biol. 2002, 9, 1305. [DOI] [PubMed] [Google Scholar]; d Thayer D. A.; Wong C. H. Chem.—Asian J. 2006, 1, 445. [DOI] [PubMed] [Google Scholar]
- Commercially available (Sigma-Aldrich).
- Nakayama A.; Okano A.; Feng Y.; Collins J. C.; Collins K. C.; Walsh C. T.; Boger D. L. Org. Lett. 2014, 16, 3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The sequence was conducted only twice on a preparative scale and consequently is not yet optimized.
- a Chen Z.; Eggert U. S.; Dong S. D.; Shaw S. J.; Sun B.; LaTour J. V.; Kahne D. Tetrahedron 2002, 58, 6585. [Google Scholar]; b Kerns R.; Dong S. D.; Fukuzawa S.; Carbeck J.; Kohler J.; Silver L.; Kahne D. J. Am. Chem. Soc. 2000, 122, 12608. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.