SUMMARY
Proopiomelanocortin (POMC) neurons within the hypothalamic arcuate nucleus are vital anorexigenic neurons. Although both the leptin receptor and insulin receptor are coupled to activation of phosphatidylinositide3-kinase (PI3K) in POMC neurons, they are thought to have disparate actions on POMC excitability. Using whole-cell recording and selective pharmacological tools, we have found that similar to leptin, purified insulin depolarized POMC, and adjacent kisspeptin neurons via activation of TRPC5 channels, which are highly expressed in these neurons. In contrast, insulin hyperpolarized and inhibited NPY/AgRP neurons via activation of KATP channels. Moreover, Zn2+, which is found in insulin formulations at nanomolar concentrations, inhibited POMC neurons via activation of KATP channels. Finally as predicted, insulin given intracerebroventrically robustly inhibited food intake and activated c-fos expression in arcuate POMC neurons. Our results show that purified insulin excites POMC neurons in the arcuate nucleus, which we propose is a major mechanism by which insulin regulates energy homeostasis.
INTRODUCTION
The hypothalamic arcuate nucleus is one of the primary CNS regions involved in the regulation of food intake (see (Elmquist et al., 1999; Gao and Horvath, 2007; Hill et al., 2008a) for review). There are two critical neuropeptide cell groups within the arcuate nucleus controlling feeding behavior: the neuropeptide Y/agouti-related peptide (NPY/AgRP) neurons, which are orexigenic, and the proopiomelanocortin (POMC) neurons which are anorexigenic (Elmquist et al., 1999; Gao and Horvath, 2007; Hill et al., 2008a). Thus, optogenetic or pharmacogenetic stimulation of NPY/AgRP neurons robustly stimulates food intake (Aponte et al., 2011; Krashes et al., 2011); whereas optogenetic stimulation of POMC neurons inhibits feeding and weight gain (Aponte et al., 2011).
POMC and NPY/AgRP neurons within the arcuate nucleus are juxtaposed and send dendritic projections into the median eminence, which is a circumventricular organ and outside of the blood-brain-barrier (Horvath, 2005). Therefore, these neurons are situated for detecting circulating levels of leptin, insulin and glucose (Cowley et al., 2001; Faouzi et al., 2007; Ibrahim et al., 2003). Cowley et al. (2001) showed that leptin depolarizes POMC neurons via activation of a non-selective cation current (Cowley et al., 2001), which was consistent with previous anatomical findings on the activational effects (i.e., c-fos, pStat expression) on POMC neurons (Elias et al., 1999). In contrast, leptin has been shown to hyperpolarize and thereby inhibit NPY/AgRP neurons via activation of KATP channels (Van den Top et al., 2004). The identification of the non-selective cation channel activated by leptin in POMC neurons was elusive for almost 10 years until through rigorous biophysical and molecular analysis we were able to identify that canonical transient receptor potential (TRPC) channels were responsible for the depolarizing effects of leptin (Qiu et al., 2010).
Insulin receptors, like leptin receptors, are expressed in POMC neurons and intracerebroventricular (ICV) administration of insulin increases the expression of POMC mRNA (Benoit et al., 2002). Insulin receptors are also expressed in other unidentified, presumed arcuate NPY/AgRP neurons (Benoit et al., 2002), since ICV insulin treatment prevents fasting-induced increases in arcuate NPY expression (Schwartz et al., 1991). Insulin binding to its receptor generates phosphorylation of insulin receptor substrate (IRS) proteins, which leads to activation of phosphatidylinositide3-kinase (PI3K) that has multiple downstream actions in POMC and other neurons (Morton et al., 2006; Xu et al., 2005). The leptin receptor (LRb) is coupled to the Janus kinase (Jak2)-IRS- PI3K signaling pathway, and this pathway activates TRPC channels in mouse POMC neurons (Qiu et al., 2010). PI3K is essential for leptin-induced POMC neuronal activation (Hill et al., 2008b; Qiu et al., 2010). Although both the leptin receptor and insulin receptor are coupled to activation of PI3 kinase in POMC neurons, previous reports suggest that insulin, as opposed to leptin, inhibits POMC neuronal excitability through activation of an inwardly rectifying K+ (Kir6.2/KATP) channel (Hill et al., 2008b; Plum et al., 2006b; Williams et al., 2010).
Therefore, a long standing enigma is that although insulin and leptin have similar actions in controlling food intake and energy balance (Benoit et al., 2002; Brown et al., 2006) and appear to share a common signaling pathway in POMC neurons (Morton et al., 2006; Xu et al., 2005), they have opposing actions on POMC cell excitability (Hill et al., 2008b; Plum et al., 2006b; Williams et al., 2010). To address these issues, we undertook a series of cellular electrophysiological and pharmacological studies to elucidate the actions of insulin in guinea pig and mouse POMC neurons. Our results demonstrate that insulin like leptin excites POMC and kisspeptin (Kiss1) neurons through activating TRPC channels, which may be a common downstream target of not only metabolic hormones but neurotransmitters (e.g., serotonin) involved in the control of energy homeostasis.
RESULTS
Purified insulin excites arcuate POMC neurons in guinea pig and mouse
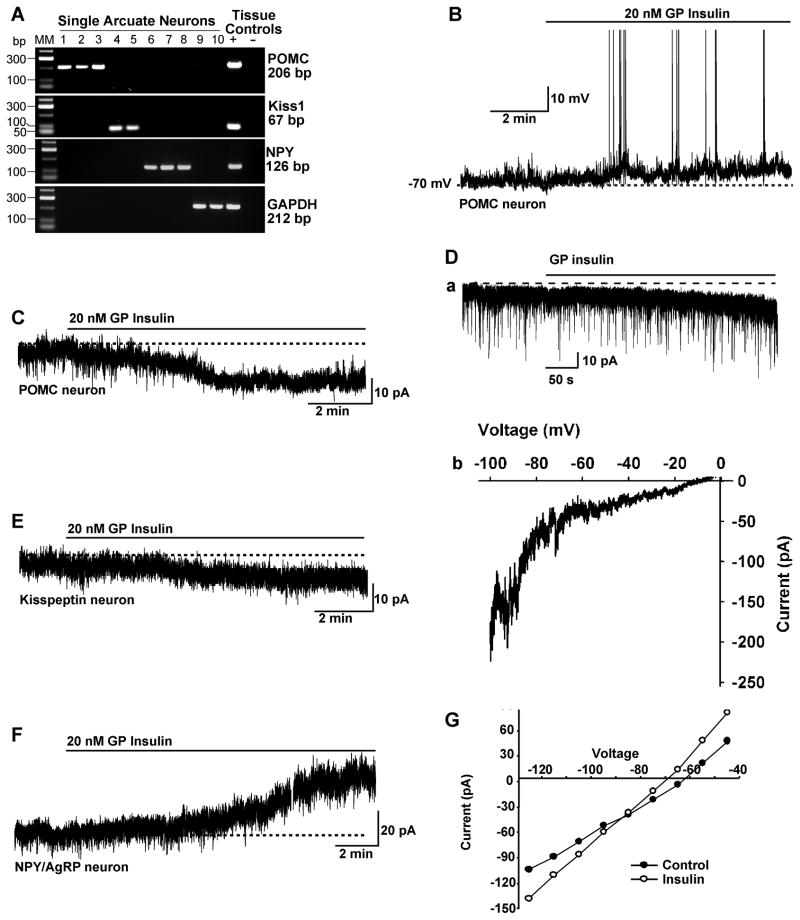
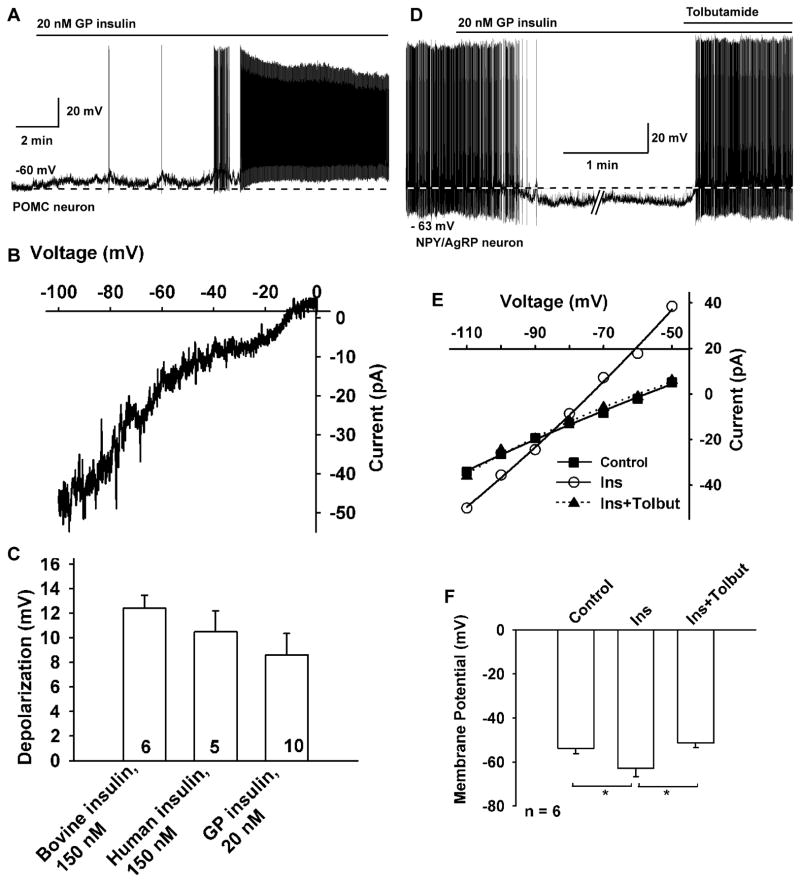
POMC and kisspeptin neurons are highly expressed in the middle to caudal region of the arcuate nucleus of the guinea pig hypothalamus (Bosch et al., 2012; Thornton et al., 1994). Therefore, we targeted neurons in this area of male and female guinea pigs using visualized patch recording. Of the neurons examined (targeted using infrared DIC optics), we identified post hoc 30% (19/64) as POMC, 20% (9/45) as kisspeptin and 32% (12/37) as NPY/AgRP neurons using scRT-PCR (Figure 1A). The electrotonic properties of these guinea pig POMC, kisspeptin and NPY/AgRP neurons are summarized in Table 1 and are similar to what we have previously published for POMC and kisspeptin neurons (Qiu et al., 2003; Qiu et al., 2011). Guinea pig insulin depolarized and increased action potential firing in 74% (14/19) of POMC neurons and 89% (8/9) of kisspeptin neurons (Figure 1B, C and E). In contrast, guinea pig insulin hyperpolarized and inhibited firing in 92% (11/12) of NPY/AgRP neurons (Figure 1F). The remaining POMC, kisspeptin and NPY/AgRP neurons did not respond to insulin. In the presence of TTX and under whole-cell voltage clamp, GP insulin (20 nM) induced an inward current in all identified POMC neurons (9.4 ± 0.8 pA, n=19) and kisspeptin neurons (8.8 ± 3.8 pA, n=6). The I–V relationship reversed near −10 mV, indicative of activation of a non-selective cationic conductance (Figure 1D). As opposed to its actions in POMC and kisspeptin neurons, GP insulin induced an outward current (mean = 9.8 ± 4.3 pA, n=6) in NPY/AgRP neurons that reversed at EK+ (−90 mV) (Figure 1G). Since previously it has been reported that insulin activates KATP channels in mouse POMC neurons (Hill et al., 2010; Plum et al., 2006a), we targeted EGFP-labeled POMC neurons in mice to determine if the insulin response was unique to guinea pig neurons. Interestingly, pure guinea pig insulin also depolarized mouse POMC neurons (9.7 ± 1.5 mV, n = 10) (Figure 2A), and the double-rectifying I/V plot revealed a reversal potential of −10 mV (Figure 2B). Guinea pig insulin had a similar depolarizing effect on mouse kisspeptin neurons (8.3 ± 1.3 mV, n = 4) (Figure S1). Then we asked if other pure insulin reagents, like bovine and recombinant human insulin, also depolarize POMC neurons (Figure 2A–C). Indeed, both human and bovine insulin, albeit at higher concentrations (150 nM), also depolarized mouse POMC neurons (recombinant human insulin, 10.5 ± 1.7 mV, n=5; bovine insulin, 12.4 ± 1.1 mV, n=6; guinea pig insulin, 9.7 ± 1.5 mV, n=10). Therefore, unadulterated insulin depolarizes POMC neurons via activation of a cationic conductance. In contrast, guinea pig insulin induced membrane hyperpolarization of NPY/AgRP neurons in mice, which was abrogated by the SUR1 (KATP channel) antagonist tolbutamide (Figure 2D–F).
Figure 1. Insulin excites guinea pig POMC and kisspeptin neurons and inhibits NPY /AgRP neurons.
(A) Identification of arcuate neurons following whole-cell recording with scRT-PCR. Representative gels illustrating the mRNA expression of POMC, Kiss1 and NPY in ten arcuate neurons that were harvested after whole-cell recording. The expected sizes for the PCR products are as follows: for POMC, 206 bp; for Kiss1, 67 bp; for NPY, 126 bp; and for GAPDH, 212 bp. Cells that were negative for POMC, Kiss1 and NPY were tested for GAPDH to confirm the presence of mRNA. Hypothalamic tissue RNA was also included as positive control (+, with RT) and negative control (−, without RT). MM, molecular markers. (B) GP insulin (20 nM) depolarized POMC neurons and induced firing. (C) In voltage clamp, insulin induced an inward current. (D) The insulin-activated inward current and its I–V relationship in POMC neurons. a, Rapid application of 20 nM insulin induced a robust inward current with an internal solution containing 130 mM Cs+ gluconate and Ca2+ and K+ channel blockers in the extracellular CSF (see the Experimental Procedures), Vhold = −40 mV. b, Voltage ramps from 0 to 100 mV were applied (over 2 s) before and during the treatment with insulin. The I–V relationship for the insulin-induced current was obtained by digital subtraction of the control I–V from the I–V in the presence of insulin (20 nM). The reversal potential of the nonselective cation current was 10 mV. (E) Similar to POMC neurons, insulin induced an inward current in kisspeptin neurons that reversed at −10 mV (I/V not shown). (F and G) GP insulin induced an outward current in NPY/AgRP neurons (F), and the I–V plot of the cell shows that the reversal potential is close to EK+ (−90 mV; G).
Table 1.
Electrotonic properties of guinea pig POMC, kisspeptin and NPY/AgRP neurons
| Cell Type | RMP (mV) | Rin (Mω) | Cm (pF) |
|---|---|---|---|
| POMC (n=18) | −55.9 ± 1.9 | 1110 ± 208 | 24.1 ± 2.4 |
| Kisspeptin (n=20) | −60.5 ± 1.2 | 870 ± 65 | 24.2 ± 1.3 |
| NPY/AgRP (n=12) | −57.8 ± 2.1 | 1154 ± 147 | 18.3 ±1.2 |
Note: RMP, Resting membrane potential; Rin, Input resistance; Cm, Cell membrane capacitance
Figure 2. Insulin depolarizes and excites mouse POMC neurons and hyperpolarizes and inhibits NPY/AgRP neurons.
(A) GP insulin (20 nM) depolarized and induced firing in a mouse POMC neuron. (B) The I–V relationship for the insulin-induced current was obtained by digital subtraction of the control I–V from the I–V in the presence of insulin (20 nM) with a Cs+-based internal solution and K+ channel blockers in the extracellular CSF (see the Experimental Procedures). The reversal potential of the nonselective cation current was −10 mV (n=3). The I–V relationship showed a typical doubly rectifying shape. (C) Summary of the effects of bovine insulin (150 nM, Sigma-Aldrich I1882), and human recombinant insulin (150 nM, Sigma-Aldrich I9278) to depolarize mouse POMC neurons in comparison to purified guinea pig insulin (20 nM, Nationl Institutes of Health). (D) GP insulin (20 nM) inhibited the firing and hyperpolarized (−10.7 mV) a NPY/AgRP neuron, and the effects of insulin were antagonized by the KATP channel blocker tolbutamide (200 μM). (E) I/V plot revealed that the insulin-induced outward current had a reversal potential close to EK+. The voltage protocol consisted of 1s steps every 10 mV from −50 to −110 mV (Data not shown). Vhold = −60 mV. (F) Change in the membrane potential of arcuate NPY/AgRP neurons with application of 20 nM guinea pig insulin and after the addition of 200 μM tolbutamide (n=6). Data points represent the mean ± SEM. *p < 0.05.
Pharmacological analysis of insulin signaling in POMC and kisspeptin neurons
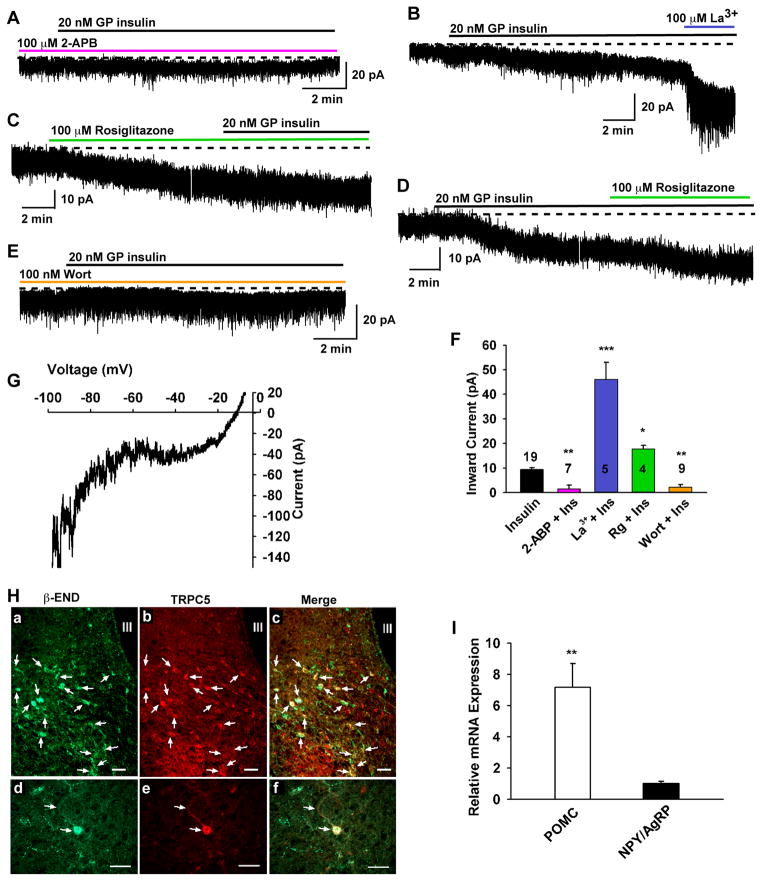
Since leptin depolarizes POMC and kisspeptin neurons via activation of TRPC channels (Qiu et al., 2010; Qiu et al., 2011), we hypothesized that insulin would also activate this cation-selective channel. We first examined whether the insulin-activated currents in POMC neurons (Figure 3) were sensitive to 2-APB (2-aminoethyl diphenylborinate), which is a potent blocker of TRPC3, 4, 5 and 6 channels (Clapham et al., 2005). Extracellular 2-APB (100 μM) robustly blocked the insulin-induced currents by 83.4% (1.5 ± 0.7 pA, n = 7 versus control 9.4 ± 0.8 pA, n = 19, p < 0.01; Figure 3). Furthermore, blocking KATP channels with tolbutamide had no effect on the ability of insulin to depolarize POMC neurons (Figure S2). Since in heterologous expression systems micromolar concentrations of the lanthanide La3+ potentiate TRPC4 and TRPC5 (Strübing et al., 2001) but inhibit TRPC1, 3, 6 and 7 channels (Clapham et al., 2005), we tested the effects of La3+ on the insulin-activated current. Indeed, 100 μM La3+ greatly potentiated the insulin-induced current by 5 fold in every neuron tested (45.8 ± 7.2 pA, n = 5, p< 0.001 versus control) (Figure 3B, F). The potentiated inward insulin-activated TRPC current reversed near −10 mV, indicative of activation of a non-selective cation channel. Furthermore, we found that rosiglitazone, an enhancer of TRPC5 channel activity in adipocytes (Sukumar et al., 2012), potentiated the TRPC channel current by two-fold (17.5 ± 1.6 pA n =4, p<0.05), and the I/V revealed a double-rectifying plot that reversed at −10 mV (Figure 3G), which is consistent with the enhancement of a TRPC-mediated cationic conductance.
Figure 3. Insulin response requires TRPC channel activation and PI3 kinase.
(A–E) Representative traces of the insulin-induced currents in the presence of the TRPC channel blocker 2-APB (100 μM) (A) or TRPC5 channel enhancers La3+ (100 μM) (B) and rosiglitazone (C and D) or PI3K inhibitor wortmannin (100 nM) (E). Vhold = −60 mV. Similar to La3+, rosiglitazone (100 μM) potentiated insulin-activated inward currents. (F) Bar graphs summarizing the effects of Wort, 2-APB, and TRPC 4,5 channel enhancers La3+ and rosiglitazone (100 μM) on the insulin-induced inward currents. La 3+ (100 μM) augmented the current by 5 fold and rosiglitazone (Rg) by about 2 fold. ***p < 0.001; **p < 0.01, *p < 0.05 significantly different from the control group (black bar). Cell numbers are indicated. (G) The I–V relationship for the rosiglitazone (100 μM) -induced current was obtained by digital subtraction. Note the similar double rectifying I/V plots and reversal potentials for insulin and rosiglitazone. (H) Immunoreactive TRPC5 channel protein is expressed in POMC neurons. Representative photomicrographs illustrating low- (a, b, c) and high- power (d, e, f) images of immunoreactive β-endorphin (a and d; POMC product labeled with Cy2, green), Immunoreactive TRPC5 (b and e; labeled with Cy3, red), and the combined images of the same cells (c and f; yellow). TRPC5 was expressed in the majority of β-endorphin neurons. Note, arrows indicate the cells with co-localization of β-endorphin and TRPC5. IR-TRPC5 is also present in unidentified arcuate neurons (red cells without arrow). Scale bars = 45 μm. (I) Based on qPCR, TRPC5 transcipts are expressed many fold higher in POMC versus NPY/AgRP neurons.
Since PI3K is essential for mediating the effects of leptin (Hill et al., 2008b; Xu et al., 2005) and is also critical for the membrane insertion of TRPC channels (Bezzerides et al., 2004), we next examined the role of PI3K in the insulin-induced inward current. The selective PI3K inhibitor wortmannin (100 nM) robustly blocked the insulin-induced inward current by 94.2% (0.5 ± 0.5 pA, n = 9, p<0.01 versus control) (Figure 3E, F).
TRPC1, 4 and 5 are the predominant subfamily members expressed in POMC and kisspeptin neurons, and previously we found that TPRC5 channel protein is expressed in kisspeptin neurons (Qiu et al., 2010; Qiu et al., 2011). Therefore, we used immunocytochemical staining to identify TRPC5 channel protein in POMC neurons. We found that the TRPC5 channel subtype 5 is co-localized with β-endorphin in the majority of POMC neurons in the arcuate nucleus (Figure 3H). In addition, our qPCR analysis comparing expression of TRPC5 mRNA in POMC versus NPY/AgRP neurons revealed that TRPC5 mRNA was highly expressed in POMC neurons, but was expressed at many-fold lower levels in NPY/AgRP neurons (Figure 3I; p<0.01; n=4 animals). Taken together, it appears that the insulin-induced excitation of POMC and kisspeptin neurons, in contrast to NPY neurons, occurs via a receptor-mediated activation of PI3K and opening of TRPC5 channels.
Insulin formulations and Zn2+ hyperpolarize POMC neurons
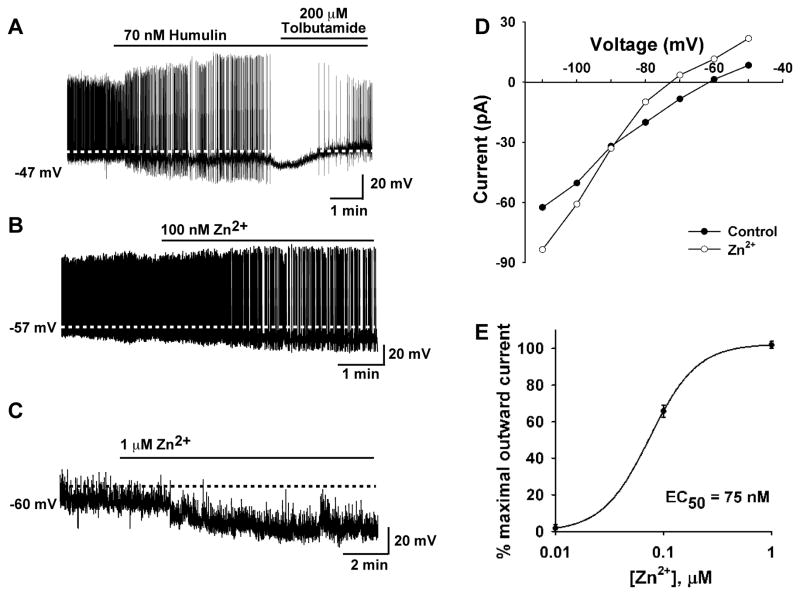
Previous reports suggest that insulin inhibits POMC neuronal activity (Hill et al., 2008b; Könner et al., 2007; Plum et al., 2006b; Williams et al., 2010), but in our hands pure guinea pig and bovine insulin and recombinant human insulin depolarized and excited guinea pig and mouse POMC neurons. Since leptin and insulin can activate a common PI3K signaling pathway in POMC neurons (Hill et al., 2008b; Xu et al., 2005), we asked the question why one hormone would increase and the other hormone would inhibit POMC neuronal excitability if they share a common signaling pathway? Therefore, we decided to measure the effects of the insulin formulation Humulin, which has been commonly used by other groups, on POMC neuronal excitability. We targeted POMC-EGFP neurons in mice and applied Humulin R (50–400 nM). Humulin at all concentrations hyperpolarized (ranging from 3.5 mV to 10.5 mV, n=5) mouse POMC neurons and inhibited firing (Figure 4 and Figure S3A, B). Likewise, Novolin (200 nM) hyperpolarized and inhibited the spontaneous firing of POMC neurons (Figure S3C). We know that insulin formulations such as Humulin (Lilly) and Novolin (Novo Nordisk) contain Zn2+ as an additive to help prolong the effects of insulin (Owens, 2011). Humulin R and Novolin R formulations contain human insulin (rDNA origin) and Zn2+ at about a 2 to 1 molar ratio, respectively. Furthermore, Zn2+ activates KATP channels in heterologous (COS-7) cells (Prost et al., 2004). Therefore, we postulated that the insulin formulation-induced depolarization may be overwhelmed by Zn2+-induced hyperpolarization in POMC cells. Indeed, nanomolar concentration of Zn2+ hyperpolarized and inhibited firing of POMC neurons (Figure 4B, C) and in voltage clamp induced an outward current (6.3 ± 1.3 pA, n =3) that reversed at EK+ (Figure 4D), consistent with Zn2+ activating KATP channels. In fact, the EC50 for Zn2+ was 75 nM (Figure 4E), which is in the range of the Zn2+ concentrations in 150 nM insulin formulations (e.g., Humulin, Novolin). Finally as proof of principle, we co-perfused Zn2+ (75 nM) and purified guinea pig insulin (150 nM) and found that zinc abrogated the ability of insulin to depolarize POMC neurons (Figure S3D, E). In voltage clamp and in the presence of TTX, one could clearly detect the outward (K+) current induced by Zn2+ in POMC neurons (Figure S3F).
Figure 4. Human insulin formulation and Zn2+ inhibit POMC neurons.
(A) Human insulin preparation (Humulin R) hyperpolarized and inhibited the firing of mouse POMC neurons, which was reversed by tolbutamide (200 μM). (B) The effect was mimicked by Zn2+ (0.1–10 μM). (C) In the presence of TTX to block action potential firing, Zn2+ hyperpolarized POMC neurons. (D) A representative I–V plot illustrating that the Zn2+-activated currents, measured in voltage clamp and in the presence of TTX, reversed at −90 mV (EK+), indicative of the activation of a K+ current. (E) A logistics fit of the concentration-response curve for the Zn2+-induced outward current yielded an EC50 = 75 nM, which is similar to the Zn2+ concentration in 150 nM human insulin preparations. The maximum outward current was obtained at 1 μM Zn2+ with higher concentrations showing no further increase in outward current.
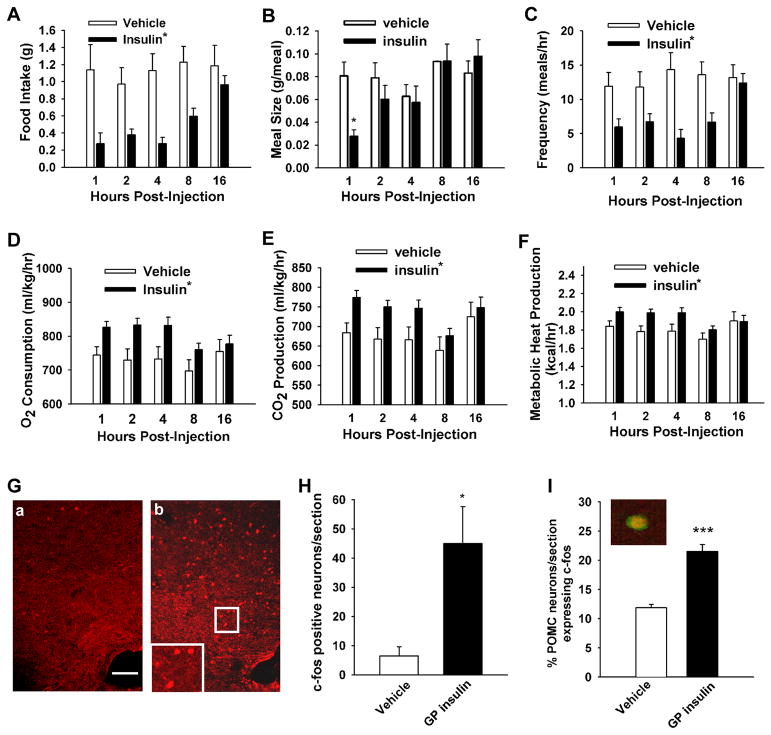
Intracerebroventricular (ICV) insulin decreases food intake and increases metabolism in guinea pigs
We next evaluated the ability of centrally administered insulin to modulate energy intake, meal pattern and energy expenditure in male and late follicular phase female guinea pigs fed a standard, grain-based diet. In preliminary experiments, we found no difference between males and females in the response to purified guinea pig insulin. Therefore, the data sets from both sexes were combined (n=3 males and 2 females in each group). Insulin (4 mU) delivered directly into the third cerebroventricle decreased hourly food intake across most of the time points examined with a trend toward a return to control levels by the end of the sampling period (F = 37.24, df = 1, p < 0.0001; repeated measures, multi-factorial ANOVA; Figure 5A). This insulin-induced decrease in hourly consumption was associated with a significant main effect of insulin on meal size (F = 5.34, df = 1, p < 0.03), and a significant interaction between insulin and time (F = 3.27, df = 4, p < 0.02). This resulted in a significant reduction in the meal size observed at 1 hr following insulin administration (Figure 5B). Similarly, ICV insulin decreased meal frequency, which lasted for at least 8 hr and appeared to return to control levels by 16 hr following insulin exposure (F = 29.05, df = 1, p < 0.0001; Figure 5C). On the other hand, insulin did not affect meal duration (F = 0.74, df = 1, p < 0.39; data not shown).
Figure 5. Guinea pig insulin given intracerebroventrically robustly inhibited food intake and increased energy expenditure and expression of c-fos in the arcuate nucleus.
(A–C) Insulin decreased food intake (A) concomitant with decreases in meal size (B) and frequency (C). (D–F) Insulin increased O2 consumption (D) as well as CO2 (E) and metabolic heat production (F). Bars represent means ± SEM (of the incremental hourly intake, meal size, and meal frequency or of the O2 consumption, CO2 production and metabolic heat production observed, respectively) in animals fed a standard, grain-based diet and treated with either insulin (4 mU; ICV), or its 0.9% saline vehicle (2 μl; ICV). The asterisk shows values from insulin-treated animals that are significantly different (repeated measures, multifactorial ANOVA/ least significant difference; p< 0.05) than those from vehicle-treated controls (n = 5 in each group). (G and H) Insulin stimulated c-fos expression in the arcuate nucleus. c-fos immunofluorescent micrographs of the arcuate nucleus from a saline (G–a)- and insulin (G–b)- treated guinea pig. The scale bar represents 100 μm. (H) The bar graph represents mean (± SEM) of c-fos positive neurons per section. (I) The bar graph depicts the mean percentage (± SEM) of POMC neurons that express c-fos following ICV insulin versus vehicle in anesthetized mice. Inset displays coexpression (yellow) of a POMC neuron and c-fos. Insulin increased c-fos expression by 2-fold (p<0.001, Student’s t-test).
These centrally mediated, insulin-induced changes in energy intake and meal pattern also were correlated with alterations in energy expenditure. Thus, insulin increased O2 consumption (F = 18.92, df = 1, p < 0.0001; Figure 5D) and CO2 production (main effects of insulin: F = 14.31, df = 1, p < 0.0003 and time: F = 2.67, df = 4, p < 0.04). Significant increases were seen for at least 4 hr following insulin exposure (Figure 5E). Moreover, insulin significantly increased metabolic heat production over the same time period (F = 11.51, df = 1, p < 0.0009; Figure 5F).
To investigate the potential link between the rapid excitatory effects of insulin on POMC neuronal excitability and the above changes in energy homeostasis, we measured the expression of the immediate early gene c-fos in control and insulin treated guinea pigs. Indeed, ICV administration of insulin significantly (p<0.02) increased the expression of c-fos positive neurons in the arcuate nucleus (45.0 ± 12.6 c-fos positive neurons/section, n=4 guinea pigs) compared with the vehicle -treated animals (6.5 ± 3.1 c-fos positive neurons/section, n=3 guinea pigs; Figure 5G, H). Since the arcuate nucleus includes POMC, kisspeptin and potentially other cellular groups that are excited by insulin, we investigated the effects of ICV insulin in anesthetized POMC-EGFP mice. Indeed, ICV insulin significantly (p<0.001) increased the percent of POMC neurons expressing c-fos (21.5 ± 1.2 %; n=4) versus vehicle (11.9 ± 0.6 %; n=4) (Figure 5I and Figure S4). Therefore, the insulin-induced increase in POMC cell excitability translates into heightened transcriptional activity and presumably downstream postsynaptic actions of POMC peptides to control energy homeostasis.
Since in guinea pigs insulin (4 mU) was injected into the third ventricle at the level of the hypothalamus, we believe that insulin acted within the hypothalamus to alter feeding behavior as revealed by neuronal c-fos expression in the arcuate nucleus of the hypothalamus. Hence, our ICV insulin regimen had no effect on serum glucose levels (130.0 ± 16.4 mg/dl, n = 4, in saline- versus 108.0 ± 10.8 mg/dl, n = 5, in insulin-treated guinea pigs, p>0.05). We also observed no effect of insulin on serum cholesterol levels (40.3 ± 7.5 mg/dl in saline, n=4 and 30.1 ± 4.1 mg/dl in insulin-treated, n=5). However, serum triglyceride was significantly reduced by the insulin treatment (83.8 ± 8.3 mg/dl, n = 4 with saline versus 56.9 ± 7.9 mg/dl, n =5 with insulin; p<0.05), which is consistent with observations in the rat that insulin infused into the hypothalamus suppresses lipolysis (Scherer et al., 2011).
DISCUSSION
Insulin is a critical regulator of energy homeostasis. In particular, it is known to inhibit feeding in a number of animal models via a hypothalamic site of action that includes POMC neurons. Still the cellular mechanisms by which insulin affects hypothalamic POMC neuronal excitability have been controversial. Here, we have used two animal models to elucidate the effects of insulin: the guinea pig, which is physiologically more akin to primates than other rodent species (Bosch et al., 2013), and mice expressing POMC-EGFP or NPY-GFP neurons. Our results indicate that pure guinea pig insulin, similar to leptin, depolarizes POMC and adjacent kisspeptin neurons via activation of TRPC5 channels, which is enhanced by the insulin sensitizer rosiglitazone. In contrast, guinea pig insulin hyperpolarizes and inhibits NPY/AgRP neurons via activation of KATP channels. With these pronounced cellular actions, it is not surprising that guinea pig insulin given intracerebroventrically robustly inhibited food intake and meal pattern. Finally we identified Zn2+, which is found in insulin formulations at nanomolar concentrations, as the mediator of the inhibitory effects of formulated insulin on POMC neurons via activation of K+ channels. Therefore, pure insulin, like leptin, excites POMC and kisspeptin neurons. Our cellular electrophysiological, behavioral and pharmacological studies may help resolve the longstanding controversy between the in vitro versus in vivo effects of insulin on POMC neuronal excitability and gene expression.
In targeting neurons in the guinea pig arcuate nucleus, we were able to identify over 80% of the recorded neurons post hoc as POMC, kisspeptin or NPY/AgRP neurons using scRT-PCR. Importantly, pure guinea pig insulin depolarized and increased action potential firing in the majority of POMC and kisspeptin neurons. In the presence of TTX to synaptically isolate the cells, insulin induced an equivalent inward current in both POMC and kisspeptin neurons. This response, in terms of both magnitude and reversal, was reminiscent of the leptin-mediated effects that we described in mouse POMC and kisspeptin neurons (Qiu et al., 2010; Qiu et al., 2011). In contrast, pure insulin hyperpolarized NPY/AgRP neurons via activation of KATP channels (current findings). Whereas POMC and NPY/AgRP neurons are critical for the control of energy homeostasis, kisspeptin neurons control reproductive functions (Bosch et al., 2012; Hill et al., 2010; Qiu et al., 2011), and insulin excitation of these neurons is consistent with its role in controlling fertility (Hill et al., 2010).
Given that leptin depolarizes POMC and kisspeptin neurons via activation of TRPC channels (Qiu et al., 2010; Qiu et al., 2011), we hypothesized that insulin would also activate these cation-selective channels. 2-APB is a potent blocker of TRPC 4, 5 channels (Clapham et al., 2005), and abrogated greater than 80% of the insulin-induced current in POMC and kisspeptin neurons. However, the critical pharmacological experiment was the use of the lanthanide, La3+, to augment the insulin current. La3+ potentiates TRPC4 and TRPC5 channels (Strübing et al., 2001) but inhibits TRPC1, 3, 6 and 7 channels in heterologous expression systems (Clapham et al., 2005). Previously, we documented that La3+ potentiated the leptin-induced current in mouse POMC neurons (Qiu et al., 2010). Here, we observed that La3+ potentiated the insulin-induced current by at least five-fold in guinea pig POMC and kisspeptin neurons. Furthermore, we found that the insulin sensitizer rosiglitazone, which has been found to potentiate TRPC5 channel activity in adipocytes (Sukumar et al., 2012), also enhanced the TRPC channel current. Again, the current-voltage plot revealed that these actions resulted from an enhancement of a double-rectifying cationic conductance, which is the hallmark of TRPC5 channels (Clapham, 2003). Moreover, immunocytochemical staining demonstrated that TRPC5 channel protein is expressed in guinea pig POMC neurons, which is similar to what we observed in kisspeptin neurons (Qiu et al., 2011). In addition, TRPC5 mRNA was highly expressed in POMC neurons but not in NPY/AgRP neurons. Therefore, we have compelling evidence that insulin, like leptin, activates TRPC channels in guinea pig POMC and kisspeptin neurons to depolarize these cells and increase their excitability.
Initially we thought that there was a species difference (guinea pig versus mouse), given that previously it was reported in mouse POMC neurons that insulin activates KATP channels (Claret et al., 2007; Hill et al., 2008b; Könner et al., 2007; Plum et al., 2006b; Williams et al., 2010). Therefore, we documented that pure guinea pig insulin, as well as pure bovine and human recombinant insulin, depolarized mouse POMC neurons. In addition, insulin hyperpolarized and inhibited the firing of NPY/AgRP neurons in the mouse, which was blocked by the SUR1 (KATP channel) antagonist tolbutamide. As predicted, tolbutamide did not affect the ability of insulin to depolarize POMC neurons. The fact that pure insulin (guinea pig, bovine) as well as recombinant human insulin depolarized and excited POMC neurons in both guinea pig and mouse, led us to investigate the constituents of insulin formulations on the electrophysiological response. Initially, we tested a commonly used insulin preparation, Humulin, and found that it hyperpolarized and inhibited firing of POMC neurons (Figure 4A). Humulin and Novolin formulations contain zinc that prolongs the effects of insulin (Owens, 2011). Interestingly, Zn2+ inhibits neurotransmitter release and binds to and promotes the closure of glutamate ionotropic GluN2A receptors (IC50 ~20 nM) (Fayyazuddin et al., 2000). Also, Zn2+ activates KATP channels in heterologous (COS-7) cells (Prost et al., 2004). Indeed, we found that Zn2+, within the range of concentrations found in Humulin and Novolin, potently hyperpolarized and inhibited firing of mouse POMC neurons through activating a K+ current, consistent with Zn2+ activating KATP channels. As further proof of principle we found that Zn2+ potently inhibited the ability of purified insulin and leptin to depolarize and excite POMC neurons (Figure S3). Probably the cellular effects of Zn 2+ are mitigated by the buffering capacity of the CSF and glial cells (Takeda, 2000) so the direct inhibitory effects of this divalent cation on POMC neuronal activity are not realized with ICV injections of insulin formulations. Therefore, we believe that the in vitro inhibitory effects of insulin formulations are due to Zn2+ and not insulin itself.
The increase in POMC cell excitability induced by insulin translated into heightened transcriptional activity and presumably downstream postsynaptic actions of POMC peptides to regulate food intake and energy homeostasis. Insulin delivered directly into the third ventricle decreased hourly food intake over 8 hr, which is comparable with previous findings in mice (Benoit et al., 2002; Brown et al., 2006) and rats (Clegg et al., 2011). Moreover, the catabolic effects of insulin are blocked by melanocortin receptor 3,4 antagonists (Benoit et al., 2002). In the guinea pig the insulin-induced decrease in hourly consumption was associated with a significant decrease in meal frequency over the same time period, which would further argue for a hypothalamic site of action (Eckel, 2004). In addition, these centrally mediated, insulin-induced changes in food intake and meal pattern also were correlated with alterations in energy expenditure. There was an increase in O2 consumption and CO2 production. Insulin also significantly increased metabolic heat production as one would predict from the increased metabolism. As further evidence of insulin’s “excitatory” actions in the hypothalamic arcuate nucleus we measured increased expression of the immediate early gene c-fos in the insulin-treated guinea pigs and specifically in POMC neurons in transgenic (POMC-EGFP) mice. Therefore, the insulin-induced increase in POMC cell excitability boosted transcriptional activity to control energy homeostasis.
The pleiotropic effects of leptin and insulin in POMC neurons are most critical for both the short term (excitability) and long term (transcriptional) modulation of POMC neuronal activity and the control of food intake and energy homeostasis. Our electrophysiological findings are congruent with the effects of optogenetic and pharmacogenetic stimulation of NPY/AgRP neurons to rapidly increase food intake (Aponte et al., 2011; Krashes et al., 2011) and prolonged stimulation of POMC neurons to attenuate food intake and weight gain (Aponte et al., 2011). Moreover, POMC and NPY/AgRP neurons in the arcuate nucleus have been identified as major CNS targets of insulin and leptin actions (Belgardt and Brüning, 2010; Morton et al., 2006; Schwartz et al., 2000). We found no evidence for insulin and leptin acting at different sub-populations of POMC neurons, as has been previously reported (Williams et al., 2010). In fact, insulin and leptin depolarize the same population of POMC neurons in guinea pigs and mice (Figure S5).
Over the past decade, there has been a major thrust to identify the critical signaling molecules in POMC and NPY/AgRP neurons and one common convergent point in both IR and LRb signaling is PI3 kinase, and specifically p110β (Al-Qassab et al., 2009; Hill et al., 2008b; Xu et al., 2005). Leptin-mediated excitation (inward current) of POMC neurons is abrogated by inhibition of PI3K activity (Al-Qassab et al., 2009; Hill et al., 2008b; Qiu et al., 2010), and we presently show that blockade of PI3 kinase prevents the insulin-mediated depolarization. Activation of PI3K generates phosphatidylinositol-3,4,5-triphosphate (PIP3), which appears to contribute to the translocation and activation of PLCγ at the plasma membrane (Bae et al., 1998; Falasca et al., 1998; Rameh et al., 1998). Through its phospholipase catalytic domains, PLCγ hydrolyzes PIP2, which affects multiple channels including TRPC channels (Rodríguez-Menchaca et al., 2012; Zhang et al., 2013). In addition, in hippocampal neuronal cultures, nerve growth factor and brain-derived neurotrophic factor activate PI3K and increase the rapid membrane insertion of TRPC5 channels from intracellular vesicular pools (Bezzerides et al., 2004), all of which also may be involved in actions of leptin and insulin in POMC neurons (Figure 6). The similar mechanisms of insulin and leptin signaling in hypothalamic neurons that are important for regulating metabolic functions argue for common CNS-mediated actions of these two hormones in maintaining energy homeostasis. In this respect, it was recently shown that leptin acting through a hypothalamic-dependent mechanism is capable of maintaining euglycemia in the absence of insulin (Fujikawa et al., 2013).
Figure 6. A cellular model of insulin and leptin signaling via TRPC channel activation in the POMC neurons.
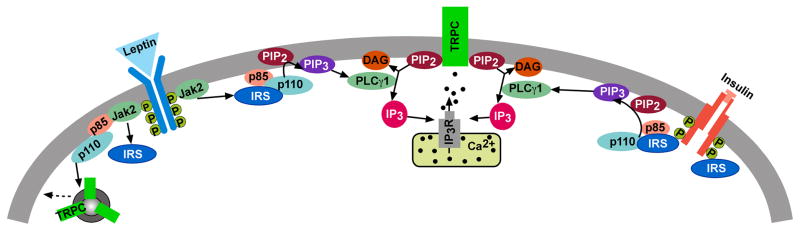
Based on the current findings and other published data, we propose convergence of leptin and insulin signaling via IRS-PI3K to activate TRPC1 and TRPC5 channels in POMC neurons, which generates an inward cationic current to depolarize POMC neurons and increase their excitability. PI3 kinase (p85/p110) will also accelerate the insertion of TPRC channels into the membrane (Bezzerides et al., 2004).
Based on the current and previous findings, we conclude that insulin binds to its receptor to directly phosphorylate IRS proteins to activate PI3K (Figure 6). PI3K subsequently activates PLCγ1 to augment TRPC channel activity and POMC neuronal excitability. Since it is evident that insulin and leptin are essential for regulation of food intake and energy homeostasis, this signaling pathway, and in particular TRPC5 channels, represent potential targets for the critical uncoupling events leading to insulin and leptin resistance in obesity and type II diabetes.
EXPERIMENTAL PROCEDURES
Animals and treatments
All animal procedures described in this study are in accordance with institutional guidelines based on National Institutes of Health standards and approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University or Western University of Health Sciences.
For the in vivo studies at Western University male and female Topeka guinea pigs (390–620 g) either were bred in-house or purchased from Elm Hill Breeding Labs (Chelmsford, MA, USA), and housed under controlled temperature (22 C) and light (12 hrs on/ 12 hrs off). Food and water were provided ad libitum. The animals were weaned at 3 weeks of age, at which time they were subdivided and fed a standard, grain-based diet (TestDiet, Richmond, IN, USA), from which 11% of the calories were derived from fat. In this study gonadally intact animals were used throughout experimentation. Female animals were checked daily, through two consecutive estrous cycles, to identify the temporal window of genital swelling and vaginal opening. This was done to ensure that we performed the behavioral testing during the late follicular phase. For this reason we surgically implanted ICV guide cannulae (for details see supplemental Methods) during the mid-luteal phase to minimize the impact of the procedure on the estrous cycle. Either 4 mU (Clegg et al., 2011) of pure guinea pig insulin or 2 μl of filtered 0.9% saline vehicle was delivered into the third ventricle.
At OHSU, female Topeka guinea pigs (400–600 g), bred in our institutional breeding facility, and female multicolor guinea pigs (400–500 g; Elm Hill Breeding Labs, Chelmsford, MA) were used in these experiments. The guinea pigs were maintained under constant temperature (23° C) and light (on between 0630 and 2030 h) and had free access to food and water. Female guinea pigs were used intact during the late follicular phase of the reproductive cycle. Female and male POMC-EGFP and NPY-GFP transgenic mice (Qiu et al., 2010; Roepke et al., 2011) were selectively bred in-house, and maintained under controlled temperature (22°C) and photoperiod (12/12 hr light/dark cycle) conditions with food and water ad libitum.
Electrophysiological solutions/drugs
A standard artificial cerebrospinal fluid (aCSF) was used with the exception of when La3+ was added to the bath, in which case a HEPES-buffered CSF solution was used (Qiu et al., 2011). All drugs were purchased from Calbiochem (La Jolla, CA) unless otherwise specified. Purified guinea pig insulin was purchased from Dr. Al Parlow (Harbor-UCLA Medical Center, Torrance, CA) through the National Hormone and Peptide Program. Bovine pancreatic insulin ( I-1882) and human recombinant insulin (I-9278) were purchased from Sigma (St. Louis); Humulin R (human insulin) was purchased from Lilly USA (Indianapolis, IN). The TRPC5 channel activator rosiglitazone (Sukumar et al., 2012) (Enzo Life Sciences, Inc., Farmingdale, NY) (100 mM) was dissolved in dimethylsulfoxide (DMSO). The relatively selective TRPC channel blocker 2-aminoethyldiphenylborinate (2-APB, 100 mM), the phosphatidylinositol 3 kinase (PI3K) inhibitor wortmannin (Alomone Laboratories, Jerusalem, Israel) (100 μM) and the KATP channel blocker tolbutamide (100 mM, Sigma-Aldrich) were dissolved in DMSO. Aliquots of the stock solutions were stored as appropriate until needed
Whole-cell patch recording from guinea pig POMC, kisspeptin and NPY/AgRP neurons
Coronal slices (250 μm) were prepared from gonadally intact male and female guinea pigs as previously described (Qiu et al., 2011). Whole-cell patch recordings were made in arcuate nucleus neurons using an Olympus BX51 W1 fixed stage scope out-fitted with IR-DIC video imaging (Qiu et al., 2011). Patch pipettes (A–M Systems, Seattle; 1.5 mm O.D. borosilicate glass) were pulled on a Brown/Flaming puller (Sutter Instrument Co., Model P-97) and filled with the following solution (in mM): 128 potassium gluconate, 10 NaCl, 1 MgCl2, 11 EGTA, 10 HEPES, 2 ATP, 0.25 GTP; adjusted to pH 7.3 with KOH; 295 mOsm. For generating full range current-voltage (I/V) plots, K+-gluconate in the normal internal solution was replaced with Cs+-gluconate consisting of the following (in mM): 100 Cs+ gluconate, 20 TEA-Cl, 10 NaCl, 1 MgCl2, 10 HEPES, 11 EGTA, 1 ATP, 0.25 GTP, the pH was adjusted to 7.3 with CsOH at 300 mOsm. The extracellular solution contained Na+, K+, Ih (HCN), Ca2+, and GABAA channel blockers (in mM: tetrodotoxin, 0.001; 4-aminopyridine, 5; CsCl, 2; CoCl2, 1; nifedipine, 0.01; picrotoxin, 0.1 (Qiu et al., 2010). Pipette resistances ranged from 3–5 MΩ. In whole cell configuration, access resistance was less than 20 MΩ; the access resistance was 80% compensated. The input resistance (Rin) was calculated by measuring the slope of the current-voltage relationship curve between −70 and −50 mV. Standard whole-cell patch recording procedures and pharmacological testing were performed as previously described (Qiu et al., 2010). Electrophysiological signals were digitized with Digidata 1322A (Axon Instruments), and the data were analyzed using p-Clamp software (version 9.2, Molecular Devices, Foster City, CA). The liquid junction potential was corrected for all data analysis.
Single cell RT-PCR identification of recorded cells
After electrophysiological recording, the content of the cell was aspirated into the tip of the patch-pipette under visual control. In all cases, the contents of the pipette were expelled into a 500 μl test tube containing 5 μl of reverse transcriptase solution including 5× reverse transcription buffer and 15 U of RNasin and 10 mM of dithiothreitol to protect the RNA. Each harvested cell was reverse transcribed as described previously (Qiu et al., 2011). Primers for the scPCR (POMC, Kiss1, NPY) were as described previously (Qiu et al., 2011). The PCR was performed using 3–4 μl of cDNA template from each RT reaction in a 20 μl PCR mix. Fifty cycles of amplification were performed using a BIO-RAD C1000 Thermal Cycler (Bio-Rad, Hercules, CA) according to established protocols (Bosch et al., 2013).
Quantitative real-time PCR (qPCR) of TRPC channels in POMC and NPY/AgRP neurons
Adult female POMC-EGFP and NPY-GFP mice (C57BL/6) were used for these experiments. Brain removal, slicing, dispersion of arcuate neurons, harvesting, RT-PCR and qPCR processing were done as described previously using designed TRPC5 and β-actin primers (Bosch et al., 2013). cDNA samples from POMC and NPY/AgRP neurons were run in duplicate and the mean ΔCT of the NPY/AgRP pool values was used as a calibrator when comparing the mRNA quantities between POMC and NPY/AgRP neuronal pools. Data are expressed as mean ± SEM, and differences were analyzed using Student’s t-test.
Visualized patch recording from mouse POMC-EGFP and NPY-GFP neurons
Both POMC-EGFP and NPY-GFP male and female transgenic mice were used for these experiments (Qiu et al., 2010; Roepke et al., 2011).
Under basal conditions, mouse POMC cells in this study showed a mean RMP of −56.6 ± 2.2mV (n=61) and average input resistance of 1165 ± 72 MΩ (n=61). Mouse NPY/AgRP neurons showed a mean RMP of −49.1 ± 2.4 mV (n=14) and average input resistance of 1121± 152 MΩ (n=14). To characterize the insulin-mediated depolarizing or hyperpolarizing response in POMC and NPY/AgRP neurons, we perfused either bovine (150 nM), human (150 nM) or guinea pig insulin (20 nM). For the ZnCl2 experiments, POMC neurons were perfused with 0.5 μM TTX for 5 min after obtaining a stable whole-cell configuration. ZnCl2 was consecutively applied at 10 nM, 100 nM and 1 μM while the outward current was measured. The data were normalized to percent maximal outward current and the EC50 of 75 nM was determined by a logistics equation fit to the data (Prism GraphPad software, San Diego, CA).
Electrophysiology data analysis
Comparisons between different drug treatments were performed using a one-way ANOVA analysis with the Newman-Keuls’s post hoc test. Differences were considered statistically significant if p < 0.05. All data are presented as mean ± SEM.
Behavioral Monitoring and Tissue Processing
We evaluated insulin-induced changes in energy intake, meal pattern and energy expenditure using a four-cage Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH, USA) as previously described and validated (Farhang et al., 2010). Briefly, we monitored incremental food intake, meal frequency, meal duration and meal size, along with metabolic parameters such as O2 consumption, CO2 and heat production, in animals fed a grain-based diet. Animals were allotted 2–5 days of acclimation in their experimental cages before the 5-day testing period, during which they were weighed and injected ICV with either insulin or its filtered 0.9% saline vehicle every morning at 8:00 a.m., and then monitored around the clock for changes in the aforementioned indices of energy balance.
After the testing phase the guinea pigs again were injected ICV either with insulin or vehicle and euthanized 2 hr later under ketamine (100 mg/kg; s.c.) anesthesia via rapid decapitation. Core blood was collected for measurements of glucose, cholesterol and triglyceride in guinea pigs (See supplemental methods for serum measurement details). In addition, POMC-EGFP male mice received a third cerebroventricle injection of either insulin or vehicle under isoflurane anesthesia (see supplemental methods for more detail) and euthanized two hr later under ketamine (10 mg i.p.) anesthesia via rapid decapitation (See supplemental section for tissue collection and fixation).
Tissue sections from guinea pigs and POMC-EGFP mice were used for immuncytochemical analysis of c-fos, an immediate early gene commonly used to locate neuronal activation, in the arcuate nucleus. Immunocytochemistry for c-fos was performed using previously described methods (Roepke et al., 2011) with minor modifications. Briefly, sections were incubated at 4°C for 48 hr with rabbit antiserum for c-fos (sc-52; Santa Cruz, CA) diluted 1:500 in a tris buffer solution containg 0.7 % λ-carrageenan, 0.4 % Triton X-100 and 3 % bovine serum albumin (Sigma). Sections were then washed in 0.1 M phosphate buffer (pH=7.4) for 30 min followed by a 90 min incubation at room temperature with a Cy3-conjugated Donkey-anti rabbit IgG antibody (1:300; Jackson Immunoresearch, West Grove, PA). Next sections were washed in phosphate buffer for 2 hr and coverslipped with a glycerol-glycine buffer solution (Roepke et al., 2011) and stored at −20°C until analysis. Data are expressed as mean ± SEM, and differences were analyzed using the Student’s t-test (see supplemental section for additional detail on data analysis).
Sections from additional guinea pigs were used for co-localization of β-endorphin and TRPC5. For colocalization of POMC and TRPC5, double-label immunocytochemistry was performed as described previously (Qiu et al., 2011) using our rabbit β-endorphin polyclonal antibody (1:5,000) (Thornton et al., 1994) and a mouse monoclonal TRPC5 antibody (1:500, UC Davis/NIH NeuroMab Facility, Davis, CA). Immunoreactive β-endophin was visualized using biotinylated IgG followed by streptavidin-conjugated to Cy2 and TRPC5 with IgG-conjugated to Cy3. Immunostained cells were photographed using a Nikon E800 microscope equipped with a digital Nikon DS camera. Brightness and contrast of digitized images were adjusted using Adobe Photoshop (Mountain View, CA) software to match images observed in the microscope.
Supplementary Material
Highlights.
Purified insulin excites POMC and kisspeptin neurons but inhibits NPY/AgRP neurons
Insulin excitation is through activation of PI3K and TRPC5 channels
Zn2+ in insulin formulations inhibits POMC neurons via activation of KATP channels
Insulin inhibits food intake and activates c-fos expression in arcuate POMC neurons
Acknowledgments
We thank Mr. M. Rick Rollins, Ms. Marina V. Rulevskaya and Ms. Uyen-Vy Navarro for excellent technical support. We also thank Dr. Francis Pau (ONPRC) for his help with establishing guinea pig assays for serum sample analyzes, and Dr. Yuan Fang for his initial technical contributions on the TRPC5 immunostaining in POMC neurons. The POMC-EGFP mice were originally obtained from Dr. Malcolm Low, currently at the University of Michigan. The NPY-GFP mice were originally obtained from Dr. Brad Lowell, Harvard University. We thank Drs. Show-Ling Shyng and Wei Fan (OHSU) for their comments on an earlier draft of the manuscript. This research was funded by US National Institute of Health (NIH) grants: NS38809 (to MJK), NS43330 (to OKR), DK68098 (to MJK and OKR), DA024314 (to EJW) and P30-NS061800 for the microscopy core. CCN was supported by NIH training grants T32 DK007680 and T32 HD007133.
Footnotes
Author Contribution
JQ, CZ and AWS performed and analyzed the electrophysiology experiments. MAB did the single cell RT-PCR analysis of the harvested cells. CCN and OKR performed the histological, immunocytochemical and microscopy analysis. AB, SK and EJW did the behavioral in vivo analysis. MJK, OKR, JQ, CZ, CCN and EJW designed the experiments, analyzed the data and prepared the manuscript.
Competing Financial Interests
The authors declare no competing financial interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJH, Batterham RL, Ashford MLJ, Vanhaesebroeck B, Withers DJ. Dominant role of the p110β isoform of PI3K over p110α in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YS, Cantley LG, Chen CS, Kim SR, Kwon KS, Rhee SG. Activation of phospholipase C-γ by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Brüning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann NY Acad Sci. 2010;1212:97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Bosch MA, Tonsfeldt KJ, Ronnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17β-Estradiol. J Mol Cell Endocrinol. 2013;367:85–97. doi: 10.1016/j.mce.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MA, Xue C, Ronnekleiv OK. Kisspeptin expression in guinea pig hypothalamus: Effects of 17β-estradiol. J Comp Neurol. 2012;520:2143–2162. doi: 10.1002/cne.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–689. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G. International union of pharmacology. XLIX nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LGD, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet C, Vaulont S, Ashford MLJ, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D’Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103:10–16. doi: 10.1016/j.physbeh.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82:35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbæk C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faouzi M, Leshan R, Björnhom M, Hennessey T, Jones J, Münzberg H. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinolgy. 2007;148:5414–5423. doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- Farhang B, Pietruszewski L, Lutfy K, Wagner EJ. The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology. 2010;59:190–200. doi: 10.1016/j.neuropharm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyazuddin A, Villarroel A, Le Goff A, Lerma J, Neyton J. Four residues of the extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors. Neuron. 2000;25:683–694. doi: 10.1016/s0896-6273(00)81070-3. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Berglund ED, Patel VR, Ramadori G, Vianna CR, Vong L, Thorel F, Chera S, Herrera PL, Lowell BB, Elmquist JK, Baldi P, Coppari R. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab. 2013;18:431–444. doi: 10.1016/j.cmet.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Bruning JC, Elmquist JK. Direct insulin and leptin action on pro-poiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008a;294:E827–E832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signalng in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008b;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci. 2005;8:561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose-responsive and express K-ATP channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Owens DR. Insulin preparations with prolonged effect. Diabetes Technol Ther. 2011;13:S5–S14. doi: 10.1089/dia.2011.0068. [DOI] [PubMed] [Google Scholar]
- Plum L, Belgardt BF, Brüning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006a;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Münzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft F, Brüning JC. Enhanced PIP3 signaling in POMC neurons causes Katp channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006b;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost AL, Bloc A, Hussy N, Derand R, Vivaudou M. Zinc is both an intracellular and extracellular regulator of KATP channel function. J Physiol. 2004;559:157–167. doi: 10.1113/jphysiol.2004.065094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology. 2011;152:1503–1514. doi: 10.1210/en.2010-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh LE, Rhee SG, Spokes K, Kazlauskas A, Cantley LC, Cantley LG. Phosphoinositide 3-kinase regulates phopholipase cγ-mediated calcium signaling. J Biol Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Menchaca AA, Adney SK, Zhou L, Logothetis DE. Dual regulation of voltage-sensitive ion channels by PIP2. Front Pharmacol. 2012;3:170. doi: 10.3389/fphar.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Smith AW, Rønnekleiv OK, Kelly MJ. Fasting and 17β-estradiol differentially modulate the M-current in neuropeptide Y neurons. J Neurosci. 2011;17:11825–11835. doi: 10.1523/JNEUROSCI.1395-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer T, O’Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S, Milsom T, Puchowicz M, Scheja L, Zechner R, Fisher SJ, Previs SF, Buettner C. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Marks JL, Sipols AJ, Baskin DG, Woods SC, Kahn SE, Porte D., Jr Central insulin administration reduces neuropeptide Y mRNA expression in the arcuate nucleus of food-deprived lean (Fa/Fa) but not obese (fa/fa) Zucker rats. Endocrinology. 1991;128:2645–2647. doi: 10.1210/endo-128-5-2645. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Sukumar P, Sedo A, Li J, Wilson LA, O’Regan D, Lippiat JD, Porter KE, Kearney MT, Ainscough JFX, Beech DJ. Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ Res. 2012;111:191–200. doi: 10.1161/CIRCRESAHA.112.270751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A. Movement of zinc and its functional significance in the brain. Brain Res. Brain Res. Rev. 2000;34:137–148. doi: 10.1016/s0165-0173(00)00044-8. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Loose MD, Kelly MJ, Rønnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- Van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. Kisspeptin activation of TRPC4 channels in female GnRH neurons requires PIP2 depletion and cSrc kinase activation. Endocrinology. 2013;154:2772–2783. doi: 10.1210/en.2013-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.