Abstract
Background
Hydatid liver cysts are rare in North America. The objective of this study was to determine the optimal surgical management for hydatid liver cysts treated outside endemic areas.
Methods
We reviewed the cases of consecutive patients who underwent management of hydatid liver cysts. Radical liver resections were compared with other types of procedures. Clinical presentation, investigations, perioperative outcomes and long-term follow-up were evaluated. We evaluated disease recurrence using the Kaplan–Meier method.
Results
Forty patients underwent surgery for hydatid liver cysts. Most patients had single (68%) right-sided (46%) cysts with a median size of 10 cm. Most (83%) underwent liver resection with or without drainage/marsupialization. Radical liver resection was carried out in 60% (19 major, 5 minor). Additional procedures were required in 50% (biliary fistulization 30%, diaphragmatic fistulization 20% or paracaval location/ fusion 8%). Postoperative complications occurred in 48%. The median follow-up was 39 months. The 3-year recurrence-free survival was significantly different between patients who had radical resection and those who had other procedures (100% v. 71%, p = 0.002).
Conclusion
The surgical management of hydatid liver cysts in North America remains rare and challenging and is frequently associated with fistulizing complications. Excellent long-term outcomes are best achieved using principles of radical liver resection that are familiar to North American surgeons.
Abstract
Contexte
L’hydatidose (kyste hydatique du foie) est une affection rare en Amérique du Nord. Cette étude visait à déterminer quelle était la meilleure façon de prendre en charge cette maladie à l’extérieur de zones où elle est endémique.
Méthodes
On a revu les cas de patients consécutifs traités pour des kystes hydatiques du foie. L’ablation radicale du foie a été comparée à d’autres types d’intervention. Le tableau clinique, les examens exploratoires, les résultats périopératoires et le suivi de longue durée ont été évalués. On a aussi évalué la récurrence de la maladie en utilisant la méthode Kaplan–Meier.
Résultats
Quarante patients avaient été opérés pour des kystes hydatiques du foie. La plupart présentaient des kystes simples (68 %) dans le foie droit (46 %), qui mesuraient en moyenne 10 cm de diamètre. La plupart (83 %) avaient subi une résection hépatique avec ou sans drainage ou marsupialisation. Une résection radicale a été pratiquée chez 60 % des patients (19 cas majeurs, 5 cas mineurs). D’autres interventions se sont avérées nécessaires dans 50 % des cas (fistulisation dans les voies biliaires 30 %, fistulisation dans le diaphragme 20 %, localisation paracave ou fusion 8 %). Des complications postopératoires sont survenues dans 48 % des cas. La durée moyenne du suivi a été de 39 mois. On a observé une différence significative entre le taux de survie sans récidive sur 3 ans entre les patients ayant subi une résection radicale et ceux ayant subi une autre intervention (100 % c. 71 %, p = 0.002).
Conclusion
En Amérique du Nord, le traitement chirurgical de l’hydatidose reste rare, difficile et souvent compliqué par une fistulisation. La résection hépatique radicale, que les chirurgiens nord-américains maîtrisent bien, est l’intervention permettant d’obtenir les meilleurs résultats à long terme.
Hydatid disease is an important parasitic ailment that affects primarily the liver in 75% of cases.1 Most cases in humans are caused by Echinococcus granulosus or Echinococcus multilocularis, which account for cystic and alveolar echinococcosis, respectively.1 The worldwide annual incidence of cystic echinococcosis is 1–200 per 100 000.1 Dogs and other canids are the definitive hosts for the adult tapeworm, whereas ungulates (typically sheep) act as intermediate hosts by ingesting shed eggs.1,2 Humans living in proximity to definitive and intermediate hosts can become accidental intermediate hosts by the same mechanism. Hydatid disease is endemic in many sheep- and cattle-raising parts of the world, namely Mediterranean countries, the Middle East, Eastern Europe and South America.
The management of hydatid cysts of the liver is controversial. Therapeutic options can be broadly divided into 3 categories: medical treatment, conservative surgery and radical surgery. Medical therapy, using the antihelminthic agents albendazole or mebendazole, has been recommended for asymptomatic and uncomplicated cysts smaller than 5 cm.2 Conservative surgical options include external drainage and unroofing procedures in addition to various types of residual cavity management strategies. Radical surgical options include formal liver resection and complete pericystectomy.
The surgical management of hydatid liver cysts is most common in endemic regions of the world. Not surprisingly, surgeons in these areas have accumulated extensive experience and reported large series of patients, documenting recurrence rates of 7.7%–30% and morbidity of 21%–80% with conservative surgical therapies.3–6 In contrast, liver surgeons working in Western centres — particularly North American hospitals — can be expected to treat only a handful of such patients over the course of their careers.7–11 In this context, it becomes particularly important to define surgical treatment strategies that are specific to the expertise of Western liver surgeons, which is lacking in the literature. We sought to review a single North American tertiary care centre’s experience with patients who underwent liver surgery for hydatid cysts of the liver.
Methods
This work was a retrospective review of our institutional experience with hydatid disease of the liver. All consecutive patients who underwent inpatient management or day procedures for hydatid liver cysts at our provincial referral hepatobiliary academic unit were included (1988–2012). The Centre Hospitalier de l’Université de Montréal Research Center Ethics Committee approved our study protocol.
In order to identify study patients, we searched discharge abstracts for diagnoses of hydatid disease based on the International Classification of Diseases (ICD)-10 (codes 122.8, 122.9) and ICD-9 (codes B67.8, B67.9). All other patients treated with albendazole were identified through the pharmacy database, as this drug is not approved for routine use in Canada and is thus dispensed on an individual patient basis after approval by Health Canada. Individual surgeon records were also mined for any missing cases. Cases of hydatid liver cysts were defined on the basis of characteristic imaging findings, ecchinococcal serology, microscopic identification of scolices within cyst fluid and pathological examination of resected specimens. We retrieved the medical records of potential study patients and checked them manually for inclusion. Relevant data pertaining to each study patient were extracted from the medical records and entered into a computerized data set. Data points of interest included patient demographics, preoperative features (serology, imaging, medical therapy), operative features (type of surgery, precautions specific to hydatid disease, cholangiography, complications, blood loss, duration) and postoperative features (length of stay, complications, pathology, recurrence, serology). No imaging classification of hydatid liver cysts was used at our centre during the study period, as such cysts are too infrequent in North America.
Indications for surgical treatment were based on World Health Organization recommendations.12 Briefly, surgery was indicated for large liver cysts; symptomatic cysts; cysts at risk of rupture; infected cysts; and cysts demonstrating or at risk for fistulization into the biliary tree, diaphragm/ pleural cavity or other vital organs. Completely calcified cysts were not routinely treated. In general, complete surgical resection was the preferred treatment option at our institution. Pericystectomy was not performed owing to our lack of experience with this procedure. Combinations of resection and marsupialization/drainage were used when the entire burden of hepatic cysts could not be safely resected owing to anatomic location or concerns regarding the volume of the future liver remnant. Associated surgical procedures were performed based on the anatomic location of individual cysts and evidence of fistulizing disease on preoperative imaging. Diaphragmatic resection and primary repair was carried out whenever the cyst appeared to have invaded or be adherent to the diaphragm. Percutaneous puncture-aspiration-injection-reinjection (PAIR) was not performed at our institution except in patients who were deemed not to be surgical candidates.
Based on the Brisbane 2000 terminology,13 formal liver resection was defined as the complete surgical excision of all hydatid cysts within the liver. In practice, this approach involved resective principles similar to those used in oncologic liver surgery, with resection of the entire cyst, including at a minimum its laminated membrane and typically including the pericyst into healthy parenchyma to achieve a negative resection margin. When the cyst was initially too bulky to allow for safe liver resection, the cyst was incised and drained using a large-bore gynecologic suction aspirator. The cyst cavity was then filled with hypertonic saline and allowed to sit undisturbed for 10–15 minutes. In all cases, we took precautions, including isolating the liver with laparotomy pads soaked in hypertonic saline and suturing a plastic sterile drape to the apex of any cyst before suction aspiration, to avoid intra-abdominal spillage of cyst contents. Marsupialization involved the drainage of the cyst contents, unroofing of the cyst wall as much as possible and oversewing of the remaining wall circumference to avoid its reaccumulation. Operative drainage consisted of evacuation of the cyst contents in a manner analogous to PAIR.
Preoperative or postoperative antiparasitic agents were routinely administered after consultation with an infectious diseases specialist. All postoperative complications were recorded and graded according to the Clavien–Dindo classification.14 Follow-up after surgery was at the surgeon’s discretion and was typically carried out in conjunction with the infectious diseases consultant. Disease recurrence was defined on the basis of evidence of 1 or more novel hydatid cyst on high-quality imaging, as interpreted by experienced abdominal radiologists.
Statistical analysis
We compared patients treated between 2003 and 2012 with those treated between 1988 and 2002. We generated summary statistics as proportions for categorical variables and as medians with interquartile ranges for continuous variables. Cyst recurrence was examined using the Kaplan–Meier and actuarial life tables methods, using the time from definitive surgery to recurrence in months. Dichotomous outcomes were compared using the Fisher exact test, and time-to-event data were compared using the log-rank test. All statistical analyses were performed using SAS software version 9.2 (SAS Institute Inc.).
Results
Forty patients with hydatid liver cysts underwent surgery, 20 of which took place in the last 10 years (Table 1). Most patients were men and were of Mediterranean, Middle-Eastern and Canadian origin. Most patients presented with nondifferentiated abdominal pain and underwent a combination of ultrasonography and computed tomography of the abdomen to make the diagnosis of hydatid liver cyst. Preoperative ecchinococcal serology was available for 37 patients and was positive in 68%.
Table 1.
Demographic and clinical characteristics of study participants
| Characteristic | Group; no. (%)* | ||
|---|---|---|---|
| Whole cohort, n = 40 | 1988–2002, n = 20 | 2003–2012, n = 20 | |
| Sex, male/female | 24 (60)/16 (40) | 10 (50)/10 (50) | 14 (70)/6 (30) |
| Age, median (IQR) yr | 40.5 (32.5–55.5) | 43 (31–57.5) | 39.5 (33.5–50.5) |
| Country of birth† | |||
| Canada‡ | 9 (23) | 4 (20) | 5 (25) |
| Algeria | 9 (23) | 3 (15) | 6 (30) |
| Lebanon | 5 (13) | 2 (10) | 3 (15) |
| Morocco | 4 (10) | 3 (15) | 1 (5) |
| Italy | 3 (8) | 3 (15) | 0 |
| Bulgaria | 2 (5) | 0 | 2 (10) |
| Greece | 2 (5) | 2 (10) | 0 |
| Other§ | 5 (13) | 3 (15) | 2 (10) |
| Presenting symptoms/signs | |||
| Abdominal pain | 28 (70) | 14 (70) | 14 (70) |
| No symptoms/incidental | 11 (28) | 5 (25) | 6 (30) |
| Nausea/vomiting | 6 (15) | 3 (15) | 3 (15) |
| Palpable mass | 3 (8) | 2 (10) | 1 (5) |
| Jaundice and/or fever | 3 (8) | 1 (5) | 2 (10) |
| Echinoccocal serology† | |||
| Positive | 25 (68) | 12 (60) | 13 (76) |
| Negative | 12 (32) | 8 (40) | 4 (24) |
| Imaging modalities | |||
| Ultrasonography | 35 (88) | 19 (95) | 16 (80) |
| CT, abdomen | 38 (95) | 20 (100) | 18 (90) |
| CT, chest | 6 (15) | 3 (15) | 3 (15) |
| MRI | 20 (50) | 7 (35) | 13 (65)¶ |
CT = computed tomography; IQR = interquartile range; MRI = magnetic resonance imaging.
Unless otherwise indicated.
Data missing for some patients.
Four of the 9 Canadian patients were from the First Nations.
Afghanistan, Armenia, Romania, Spain, Turkey, each with n = 1.
p = 0.06.
The characteristics of all hydatid cysts are presented in Table 2. The majority of patients had a single hydatid cyst, whereas 10% had 4 or more concomitant cysts. Bilobar disease was found in 11 (28%) patients, 73% of whom had 2 or more cysts.
Table 2.
Hydatid cyst features
| Cyst features | Group; no. (%)* | ||
|---|---|---|---|
| Whole cohort n = 40 | 1988–2002 n = 20 | 2003–2012 n = 20 | |
| Liver location† | |||
| Right hemiliver | 18 (46) | 10 (50) | 8 (40) |
| Left hemiliver | 10 (26) | 5 (25) | 5 (5) |
| Bilobar | 11 (28) | 5 (25) | 6 (30) |
| Number of cysts | |||
| 1 | 27 (68) | 14 (70) | 13 (65) |
| 2 | 6 (15) | 2 (10) | 4 (20) |
| 3 | 3 (8) | 3 (15) | 0 |
| ≥ 4 | 4 (10) | 1 (5) | 3 (15) |
| Maximal cyst diameter‡ | |||
| All cysts, median (IQR), cm | 7.9 (5–12) | 7 (5–10.1) | 8.9 (5.3–12.1) |
| Per patient, median (IQR), cm | 9.9 (6–13) | 10 (6–13.5) | 11 (7.6–12.8) |
IQR = interquartile range.
Unless otherwise indicated.
Data missing for some patients.
Fifty-three cysts in 37 patients.
In total, 95% of patients underwent medical therapy: 67% (n = 26) in the preoperative phase, 87% (n = 34) in the postoperative phase and 60% (n = 24) in both the pre- and postoperative phases. All but 1 patient were treated with albendazole (1 patient was treated with mebendazole). The 3 patients who did not receive medical therapy were treated before 1992.
All patients included in this series underwent surgical therapy (Table 3); 33 (83%) patients underwent liver resection with or without marsupialization/drainage. Conservative therapy (drainage or marsupialization) was carried out as the sole procedure in 7 (18%) patients. Radical liver resection was the sole form of surgical therapy in 60% of patients. Of the 24 patients who underwent radical liver resections, 19 had major and 5 had minor hepatectomies. The 9 patients who underwent combination liver resection and marsupialization/drainage had an additional 3 major and 6 minor liver resections. All but 1 patient who underwent resection and marsupialization had bilobar disease. Hypertonic saline was administered in 32 (80%) patients.
Table 3.
Medical and surgical therapies of hydatid liver cysts
| Surgical therapy | Group; no. (%) | ||
|---|---|---|---|
| Whole cohort n = 40 | 1988–2002 n = 20 | 2003–2012 n = 20 | |
| Main procedures | |||
| Radical liver resection | 24 (60) | 13 (65) | 11 (55) |
| Liver resection + marsupialization | 6 (15) | 1 (5) | 5 (25) |
| Marsupialization | 4 (10) | 1 (5) | 3 (15) |
| Liver resection + drainage | 3 (8) | 3 (15) | 0 |
| Drainage | 3 (8) | 2 (10) | 1 (5) |
| Associated procedures | |||
| Intraoperative ultrasonography | 20 (50) | 5 (25) | 15 (75)† |
| Diaphragmatic resection/repair | 8 (20) | 3 (15) | 5 (25) |
| Cholangiography | 7 (18) | 7 (35) | 0† |
| Closure of cyst-biliary fistula | 3 (8) | 3 (15) | 0 |
| CBD exploration | 3 (8) | 3 (15) | 0 |
| IVC resection/repair | 2 (5) | 2 (10) | 0 |
| Right adrenalectomy | 2 (5) | 2 (10) | 0 |
| Intra-abdominal abscess drainage | 2 (5) | 0 | 2 (2) |
| Other* | 7 (3% each) | 3 (15) | 4 (20) |
CBD = common bile duct; ERCP = endoscopic retrograde cholangiopancreatography; IVC = inferior vena cava.
Gastric lesser curve resection, pericardial resection, sphincteroplasty, thoracotomy, ERCP, pelvic hydatid cysts excision, bilateral lung hydatid cysts excision (wedges at second operation 2 months later).
p < 0.05.
In 20 (50%) patients, an additional 34 procedures (excluding intraoperative ultrasonography) were performed during the same anesthetic (Table 3). These procedures were uniformly associated with anatomic or technical challenges associated with individual cysts, including fusion with the diaphragm (n = 8, 20%), paracaval/posterior location (n = 3, 8%) and concern over possible biliary fistulization (n = 12, 30%). The median duration of surgery was 295 (IQR 245–375, range 165–515) minutes. Intraoperative complications occurred in 7 (18%) patients; complications included hemorrhage (n = 2), minimal leakage of cyst contents controlled with hypertonic saline-soaked sponges (n = 3), hypotension suspected of being related to anaphylaxis (n = 2) and anesthesia-induced hypotension (n = 1). There was no difference in duration of surgery (p = 0.69) or operative complications (p = 0.63) between the study periods.
The median length of stay in hospital was 9.5 (IQR 6–16.5, range 4–93) days. Postoperative stay was 15 (IQR 10–22) days in 1988–2002 and 7 (IQR 6–8.5) days in 2003–2012 (p = 0.023). A total of 29 postoperative complications were identified among 19 (48%) patients. Based on the Clavien–Dindo classification, all postoperative complications were at most grade 3a in severity (Table 4). Of the complications directly related to the liver surgery, wound infection was the most common, followed by bile leakage. One patient underwent percutaneous drainage of an infected liver bed collection that was attributable to a bile leak (grade 3a) and also experienced delirium following right hepatectomy (grade 1). Four other patients had bile leaks identified within closed suction drains. Of these, 1 patient was managed conservatively (grade 1), 1 required the addition of antibiotics (grade 2) and 2 required endoscopic retrograde cholangiopancreatography for definitive control (grade 3a). Finally, 1 patient required percutaneous drainage of a residual liver abscess (grade 3a) and another had a delayed percutaneous drainage of a large pleural effusion at 3 weeks (grade 3a). There was no significant difference in overall postoperative complications between radical and other liver procedures (54% v. 38%, p = 0.30). There was no significant difference in overall postoperative complication rates between study periods (50% v. 45%, p = 0.75) except for postoperative bile leakage (25% v. 0%, p = 0.047).
Table 4.
Postoperative complications, by Clavien–Dindo classification
| Group; no. (%) | ||||
|---|---|---|---|---|
| Complications | Grade 1 | Grade 2 | Grade 3a | Total |
| Wound infection | 6 | 1 | 7 (18) | |
| Bile leak | 1 | 1 | 3 | 5 (13) |
| Gastroparesis/ileus | 2 | 2 | 4 (10) | |
| Pleural effusion | 2 | 1 | 3 (8) | |
| Clostridium difficile colitis | 2 | 2 (5) | ||
| Pneumonia | 2 | 2 (5) | ||
| Residual abscess | 1 | 1 (3) | ||
| Pulmonary edema | 1 | 1 (3) | ||
| Urinary tract infection | 1 | 1 (3) | ||
| Vocal cord trauma | 1 | 1 (3) | ||
| Delirium | 1 | 1 (3) | ||
| Pancreatitis | 1 | 1 (3) | ||
| Total, % of complications | 11 (38) | 11 (38) | 7 (24) | 29 |
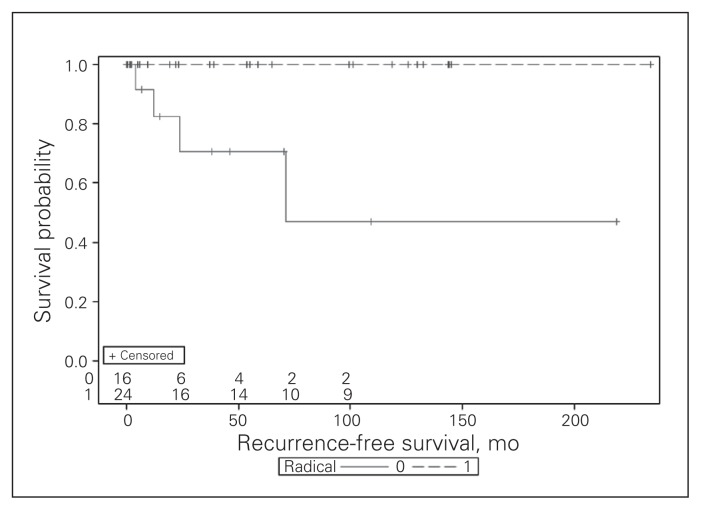
The preoperative diagnosis of hydatid liver cysts was confirmed at pathology and microscopic examination of cyst contents in all patients. The median follow-up after surgery was 39 (IQR 10–105, range 0.2–234) months. The Kaplan–Meier curve for recurrence-free survival is presented in Figure 1, comparing radical resection and other procedures (log-rank test p = 0.002). The 3-year recurrence-free survival was 89% for the whole cohort, 100% for radical resection and 71% for other procedure types. Four (10%) patients experienced hydatid cyst recurrence at 4, 12, 24 and 71 months, respectively, after undergoing simple drainage (n = 1), liver resection/drainage (n = 1), and liver resection/marsupialization (n = 2). No case of recurrence occurred in patients who underwent radical resection (25% v. 0%, p = 0.020). There was no significant difference in the crude recurrence rate at maximal follow-up (p = 0.61) or in disease-free recurrence between the study time periods (p = 0.11).
Fig. 1.
Kaplan–Meier curve for recurrence-free survival following hydatid cyst surgery, comparing radical resections (dashed line) and other procedures (solid line), log-rank test p = 0.002.
Discussion
This study has addressed the surgical management of hydatid liver cysts in the context of a Western tertiary hepatobiliary referral centre. This work has documented the relative rarity of liver surgery for ecchinococcal disease in North America and has also documented a high proportion of complicated cysts. Importantly, we have shown that long-term successful management of hydatid liver cysts can be achieved with radical liver surgery, demonstrating an acceptably low level of major postoperative morbidities and a low recurrence rate.
The literature on hydatid liver disease is intensely driven by investigators from Mediterranean centres as a consequence of their extensive experience with this pathology.3–6 Over the years, a few contributions from Western centres have appeared, originating mostly in Europe.7,9,10 In contrast, the North American experience with hydatid disease remains extremely limited owing to the rarity of this pathology and is restricted to rare series and occasional case reports.8,11 To our knowledge, the present series represents the largest combined surgical experience with hydatid disease of the liver in North America. Although the sample size presented in this work is small in comparison to large series from endemic areas, it is nevertheless highly important, as it portrays the work of trained liver surgeons with a wealth of experience in resective oncologic liver surgery but with limited experience with liver infestations. Moreover, the present series is unique in that it depicts a province-wide tertiary referral pattern within a city with steady immigration from Mediterranean and North African countries.
Despite the rarity of hydatid liver disease in nonendemic areas as well as the complexity of the procedures performed in this series, an acceptable rate of postoperative morbidity was achieved. Other series of liver surgery for hydatid disease have reported postoperative complication rates of 20%–49%.3,4,7–9,15–17 While the overall complication rate identified in this study (48%) was on the higher end of the previously published range, we argue that this is an acceptable morbidity rate. All recorded complications were classified as Clavien–Dindo grade 3a or less, with 76% of complications classified as grade 1 or 2. All surgical and medical complications were accounted for in our series in contrast to other studies that sometimes included only surgical or cyst cavity–related complications. In our series, the most notable complications were postoperative bile leaks in 5 patients, gastroparesis and/or ileus in 4 patients and wound infections in 7 patients — findings that are likely to be related to the complicated nature of cysts treated in this cohort.
Roughly half the patients included in this series required additional operative procedures related to complicated hydatosis. Additional procedures associated with complicated cysts were broadly classified as biliary fistulization (30%), diaphragmatic fistulization (20%) and paracaval fusion (8%). Biliary fistulization is a well-known complication of hydatid disease that is thought to occur in 6.6%–26% of patients.18 The presence of a biliary fistula is independently associated with deep abdominal complications following hydatid surgery (odds ratio 2.27, 95% confidence interval 1.38–3.72).15 Our results are consistent with those of other series. For instance, Secchi and colleagues16 reported carrying out additional biliary procedures among 146 of 268 (54%) patients with complicated hydatid cysts, including bile duct exploration (52%), endoscopic therapy (17%) and enterobiliary anastomoses (31%). Kilic and colleagues19 also reported a direct association between maximal cyst size and the likelihood of biliary–cyst communication, with 7.5 cm being the cutoff with the best predictive value (sensitivity 79%, specificity 73%). Chautems and colleagues7 further reported that 71% of patients with complicated cysts were found at surgery to have a cyst–biliary fistula, as defined by the visualization of an open bile duct following removal of a cyst. Furthermore, this group also identified daughter cysts within the common bile duct in 50% of patients, who were treated by choledochotomy, clearance and T-tube placement. Diaphragmatic invasion was reported in 14% of complicated cysts within this same study.7 Findings from the present study, as well as comparison to the literature, lend further support to the use of radical surgery, particularly formal liver resection, as a primary mode of therapy for complicated hydatid cysts. When this approach is deemed technically feasible and safe by experienced liver surgeons, it allows for the resection of both the cystic cavity and any area of fistulization.
This work has documented an excellent recurrence-free survival rate among patients treated with radical surgery as well as among those treated with a combination of surgery and drainage procedures. These data compare favourably with those reported by Tagliacozzo and colleagues,3 who reviewed their experience with the surgical management of 454 patients with hepatic hydatosis. This group compared conservative (drainage, marsupialization, omentoplasty; n = 214) and radical surgery (resection, pericystectomy; n = 240), documenting recurrence rates of 30.4% and 1.2% (p < 0.001), respectively. Radical surgery was also associated with a much lower rate of postoperative morbidity (79.9% v. 16.2%, p < 0.001) and comparable mortality (6.5% v. 9.2%, p = 0.30).3 In another study, Chautems and colleagues7 reported on the surgical management of 35 patients with complicated cysts, achieving complete cyst removal in 69% of patients via hepatectomy (n = 12) or pericystectomy (n = 12). This strategy was comparable to our own, although it relied less heavily on formal hepatectomy, and achieved a 0% recurrence rate at a median of 103 months. More recently, Birnbaum and colleagues9 reported on 97 patients with hydatid disease, 85 of whom underwent radical surgery (52 liver resections). While this group presented data in favour of radical resection, the median follow-up among those undergoing resection was only 1.5 months, thus limiting their conclusions on cyst recurrence. In contrast to these studies, other groups3–5,17,20,21 have documented variable but generally higher recurrence rates with conservative surgical procedures (11%–30%), highlighting the superiority of radical surgery in preventing recurrence of liver hydatosis. It should be noted that at least 1 group did not find any difference in recurrence rates between conservative and radical surgery after 10 years in a large cohort of 672 patients who underwent primarily conservative surgery (80%).5
Limitations
This study has several limitations. First, it was a retrospective review of medical charts and surgeon records. As with all retrospective series, there is a risk of bias related to the information quality available in medical records as well as its extraction. Second, length of follow-up was unequal, with a small number of patients having follow-up at secondary institutions and being lost early to follow-up. Although it is possible that additional cases of disease recurrence may have occurred among these patients, we argue that this is unlikely given the specialized nature of therapy for ecchinococcal disease and the likelihood that these patients would have been referred back to our centre had recurrence occurred.
Conclusion
The surgical management of hydatid liver cysts in North America remains rare and challenging. Associated complications of hydatid liver cysts, such as biliary fistulization, diaphragmatic fistulization, or vascular abutment, are relatively common and must be anticipated. In the North American context, it is recommended that complicated hydatid cysts of the liver be managed whenever possible using principles of radical liver resection by experienced hepatic surgeons.
Footnotes
Presented in part at the 9th World Congress of the International Hepato-Pancreato-Biliary Association, Apr. 20, 2010, Buenos Aires, Argentina, and the Canadian Surgery Forum, Sept. 3–4, 2010, Québec, Que.
Funding: G. Martel and the HPB Surgery and Liver Transplantation Unit (Université de Montréal) are supported in part by a clinical and research bursary from Covidien Canada.
Competing interests: None declared.
Contributors: All authors designed the study and acquired the data, which G. Martel, A. Bégin and R. Lapointe analyzed. G. Martel wrote the article, which all authors reviewed and approved for publication.
References
- 1.Nunnari G, Pinzone MR, Gruttadauria S, et al. Hepatic echinococcosis: clinical and therapeutic aspects. World J Gastroenterol. 2012;18:1448–58. doi: 10.3748/wjg.v18.i13.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kayaalp C. Hydatid cyst of the liver. In: Blumgart LH, Belghiti J, Jarnagin WR, et al., editors. Surgery of the liver, biliary tract, and pancreas. 4th ed. Philadelphia (PA): Saunders; 2006. pp. 952–970. [Google Scholar]
- 3.Tagliacozzo S, Miccini M, Bonapasta SA, et al. Surgical treatment of hydatid disease of the liver: 25 years of experience. Am J Surg. 2011;201:797–804. doi: 10.1016/j.amjsurg.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Safioleas MC, Misiakos EP, Kouvaraki M, et al. Hydatid disease of the liver: a continuing surgical problem. Arch Surg. 2006;141:1101–8. doi: 10.1001/archsurg.141.11.1101. [DOI] [PubMed] [Google Scholar]
- 5.El Malki HO, El Mejdoubi Y, Souadka A, et al. Does primary surgical management of liver hydatid cyst influence recurrence? J Gastrointest Surg. 2010;14:1121–7. doi: 10.1007/s11605-010-1220-0. [DOI] [PubMed] [Google Scholar]
- 6.Balik AA, Basoglu M, Celebi F, et al. Surgical treatment of hydatid disease of the liver: review of 304 cases. Arch Surg. 1999;134:166–9. doi: 10.1001/archsurg.134.2.166. [DOI] [PubMed] [Google Scholar]
- 7.Chautems R, Buhler LH, Gold B, et al. Surgical management and long-term outcome of complicated liver hydatid cysts caused by Echinococcus granulosus. Surgery. 2005;137:312–6. doi: 10.1016/j.surg.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Langer JC, Rose DB, Keystone JS, et al. Diagnosis and management of hydatid disease of the liver: a 15-year North American experience. Ann Surg. 1984;199:412–7. doi: 10.1097/00000658-198404000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birnbaum DJ, Hardwigsen J, Barbier L, et al. Is hepatic resection the best treatment for hydatid cyst? J Gastrointest Surg. 2012;16:2086–93. doi: 10.1007/s11605-012-1993-4. [DOI] [PubMed] [Google Scholar]
- 10.Stoot JH, Jongsma CK, Limantoro I, et al. More than 25 years of surgical treatment of hydatid cysts in a nonendemic area using the “frozen seal” method. World J Surg. 2010;34:106–13. doi: 10.1007/s00268-009-0267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mekeel KL, Hemming AW. Combined resection of the liver and the inferior vena cava for hydatid disease. J Gastrointest Surg. 2007;11:1741–3. doi: 10.1007/s11605-007-0292-y. [DOI] [PubMed] [Google Scholar]
- 12.WHO Informal Working Group on Echinococcosis. Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull World Health Organ. 1996;74:231–42. [PMC free article] [PubMed] [Google Scholar]
- 13.Strasberg SA, Belghiti J, Clavien PA, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2:333–9. doi: 10.1080/136518202760378489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dindo D, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Malki HO, El Mejdoubi Y, Souadka A, et al. Predictive factors of deep abdominal complications after operation for hydatid cyst of the liver: 15 years of experience with 672 patients. J Am Coll Surg. 2008;206:629–37. doi: 10.1016/j.jamcollsurg.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Secchi MA, Pettinari R, Mercapide C, et al. Surgical management of liver hydatosis: a multicentre series of 1412 patients. Liver Int. 2010;30:85–93. doi: 10.1111/j.1478-3231.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- 17.Yorganci K, Sayek I. Surgical treatment of hydatid cysts of the liver in the era of percutaneous treatment. Am J Surg. 2002;184:63–9. doi: 10.1016/s0002-9610(02)00877-2. [DOI] [PubMed] [Google Scholar]
- 18.El Malki HO, El Mejdoubi Y, Souadka A, et al. Predictive model of biliocystic communication in liver hydatid cysts using classification and regression tree analysis. BMC Surg. 2010;10:16. doi: 10.1186/1471-2482-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilic M, Yoldas O, Koc M, et al. Can biliary-cyst communication be predicted before surgery for hepatic hydatid disease: Does size matter? Am J Surg. 2008;196:732–5. doi: 10.1016/j.amjsurg.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 20.Magistrelli P, Masetti R, Coppola R, et al. Surgical treatment of hydatid disease of the liver: a 20-year experience. Arch Surg. 1991;126:518–23. doi: 10.1001/archsurg.1991.01410280122020. [DOI] [PubMed] [Google Scholar]
- 21.Akbulut S, Senol A, Sezgin A, et al. Radical vs conservative surgery for hydatid liver cysts: experience from single center. World J Gastroenterol. 2010;16:953–9. doi: 10.3748/wjg.v16.i8.953. [DOI] [PMC free article] [PubMed] [Google Scholar]