Abstract
Background
Associated with reduced trauma, laparoscopic colon surgery is an alternative to open surgery. Furthermore, complete mesocolic excision (CME) has been shown to provide superior nodal yield and offers the prospect of better oncological outcomes.
Methods
All oncologic laparoscopic right colon resections with CME performed by a single surgeon since the beginning of his surgical practice were retrospectively analyzed for operative duration and perioperative outcomes.
Results
The study included 81 patients. The average duration of surgery was 220.0 (range 206–233) minutes. The initial durations of about 250 minutes gradually decreased to less than 200 minutes in an inverse linear relationship (y = −0.58x × 248). The major complication rate was 3.6% ± 4.2% and the average nodal yield was 31.3 ± 4.1. CumulativeSum analysis showed acceptable complication rates and oncological results from the beginning of surgeon’s laparoscopic career.
Conclusion
Developing laparoscopic skills can provide acceptable outcomes in advanced right hemicolectomy for a surgeon who primarily trained in open colorectal surgery. Operative duration is nearly triple that reported for conventional laparoscopic right hemicolectomy. The slow operative duration learning curve without a plateau reflects complex anatomy and the need for careful dissection around critical structures. Should one wish to adopt this strategy either based on some available evidence of superiority or with intention to participate in research, one has to change the view of right hemicolectomy being a rather simple case to being a complex, lengthy laparoscopic surgery.
Abstract
Contexte
La chirurgie du côlon par laparoscopie, qui réduit les traumatismes, est une solution de rechange à la chirurgie ouverte. De plus, il a été démontré que l’excision mésocolique complète (EMC) optimise le curage ganglionnaire et offre la perspective de meilleurs résultats oncologiques.
Méthodes
On a examiné rétrospectivement la durée de l’opération et les résultats périopératoires de toutes les résections du côlon droit réalisées par laparoscopie avec EMC pratiquées par un seul chirurgien depuis le début de sa carrière.
Résultats
L’étude a été menée auprès de 81 patients. La durée moyenne de l’intervention chirurgicale était de 220 minutes (intervalle de 206 à 233 minutes). Au début, l’intervention durait environ 250 minutes; avec le temps, sa durée a progressivement diminué de sorte qu’à la fin, elle était de moins de 200 minutes, d’après une relation linéaire négative (y = −0,58x × 248). Le taux de complications graves s’est établi à 3,6 % ± 4,2 % et le nombre moyen de nœuds lymphatiques excisés a été de 31,3 ± 4,1. En utilisant la méthode d’analyse des sommes cumulées, on a observé un taux de complications et des résultats oncologiques acceptables depuis le début de la carrière du chirurgien en laparoscopie.
Conclusion
En perfectionnant sa technique laparoscopique, un chirurgien formé principalement en chirurgie colorectale ouverte peut produire des résultats acceptables dans les cas d’hémicolectomie droite avancée. La durée de l’intervention chirurgicale est presque le triple de celle d’une hémicolectomie droite laparoscopique classique. La courbe d’apprentissage lente sans plateau montre bien la complexité des structures anatomiques et la nécessité de faire preuve de prudence lors de la résection autour de structures vitales. Quiconque souhaite adopter cette méthode, soit en raison de données démontrant sa supériorité ou dans le but de participer à une recherche, doit adopter une nouvelle perspective, c’est-à-dire que l’hémicolectomie droite laparoscopique n’est pas une intervention simple, mais une chirurgie complexe qui prend beaucoup de temps.
Laparoscopic colon surgery has been shown to offer clear evidence of benefit when compared to open surgery. These benefits include reduced length of hospital stay, earlier return of bowel function as well as reduced blood loss and pain without compromising quality of oncological resection and nodal yield.1–4 Furthermore, complete mesocolic excision (CME) has been demonstrated to provide superior nodal yield5 and offers prospects of better oncological outcomes than non-CME surgery.6 The purpose of this study is 2-fold: first, to analyze laparoscopic CME right hemicolectomy with respect to operative durations and perioperative outcomes for a novice minimally invasive colorectal surgeon, and second, to draw conclusions with respect to the feasibility of adopting advanced laparoscopic right hemicolectomy by a surgeon who primarily trained in open colon surgery.
Methods
From 2008–2011, prospective data on consecutive laparoscopic right hemicolectomies with CME for colon cancer were entered into a database and were later extracted for retrospective analysis. The surgical approach was approved by departmental committee, and patients provided informed consent.
All surgeries were performed by a single colorectal surgeon (B.S.M.) who was primarily trained in open colorectal surgery with general minimally invasive surgery (MIS) training in laparoscopic appendectomy and cholecystectomy. The surgeon had completed formal laparoscopic and robotic courses and had support from established MIS colorectal staff at his institution. Furthermore, the surgeon’s prior training involved open CME. The present series covers the beginning of his MIS colorectal practice.
Statistical analysis
Patient-specific data and outcome measures were analyzed using standard statistics. CumulativeSum (CUSUM) plots were used to track major complications and failures to harvest at least 12 lymph nodes. The CUSUM plots are commonly used to assess learning progress and proficiency in the medical field across many specialties, including colorectal surgery.7–9 They can be a valuable tool to rapidly depict unfavourable trends. The CUSUM plots are a visual representation of cumulative failures and successes; the plot starts at zero and goes down with success or up with failure. In its simplest form, as used in this study, the plot will decrease by a fraction consistent with established acceptable failure rate and up by a fraction consistent with a success rate. For example, if an acceptable rate of major complication is 10%, the graph will go down only by 0.1 units with a success and up by 0.9 units with a failure. Failure is therefore depicted more dramatically than success. While a plot centred on the zero line indicates a rate consistent with an established acceptable failure rate, upward and downward sloping plots indicate less and more favourable rates, respectively. A V-mask is an overlay shape in the form of a V on its side that is superimposed on the graph of the CUSUM in order to determine an out of control process. Minitab 15 statistical software was used to generate the CUSUM plots with the V-mask centred on the last case using standard settings, which assume Montgomery approximation.10 In the present study, for the purpose of generating a CUSUM plot, conversion to open surgery, perioperative death, hemorrhage, need for reoperation within 1 month and any leak or any type of intra-abdominal abscess were tracked and were considered operative failures. Conversion to open surgery was added to this category in order to simplify tracking operative failures for the CUSUM plots. Although, rates of these complications vary greatly, we chose an acceptable failure rate of 5%, as this rate would pass the bar set by all large trials.11–13 The American Joint Committee on Cancer (AJCC) recommends assessment of 12 or more nodes for accurate staging.14 Despite available reports on average yields of various right hemicolectomy procedures, to our knowledge no studies report the rate of failure to retrieve at least 12 nodes in right hemicolectomy. In their study of learning curves in laparoscopic sigmoidectomy, Choi and colleagues15 suggested a 10% lymph node retrieval failure rate for creating CUSUM plots.15 We set the failure rate to retrieve at least 12 lymph nodes at 10%, with the caveat that our actual rate could serve as the first reported benchmark for this parameter.
CME laparoscopic right hemicolectomy
Although some adjustments were made during the initial period of about 15 cases, the following is a general description of the surgical technique used.
A 10 mm transumbilical camera port is placed through a 1 cm midline incision. This incision is later extended supra- and infraumbilically for specimen extraction. The surgeon works mostly between the patient’s legs, using bilateral lower abdominal ports. An assistant applies traction as needed through a left upper quadrant (LUQ) port.
The root of the small bowel mesentery is lifted and the ileocecal junction is displayed. Adhesions and the peritoneum lateral to the ascending colon are incised past the hepatic flexure, staying between the embryological planes just anterior to the Gerota fascia, duodenum and ureter. The lateral mobilization proceeds carefully over the duodenum and pancreas with the landmark for the completion of lateral mobilization being the superior mesenteric vein.
The medial dissection begins by incising the base of the mesentery. The assistant’s grasper is used on the bloodless fold of Treves at the ileocecal junction to stretch up the mesentery toward the right lower quadrant port, lifting up the vessels from the retroperitoneum. This brings out a sulcus between the medial side of the ileocolic pedicle and the retroperitoneum. An advanced energy device can eventually be used to further open the peritoneal opening and to isolate the vessels.
The preferred dissecting and sealing device is the curved EnSeal device with cutting blade (Ethicon Endo-Surgery). This is mostly for its ergonomics and ability to retain control over the length of a burn, allowing for faster work with avascular tissue.
When completely dissected, the ileocolic vessels are clipped at the root and transected. Having left a piece of gauze over the duodenum and pancreas from the lateral side at the conclusion of lateral dissection helps guide the depth of dissection and further assists in maintaining the correct dissection plain. The mesenteric dissection line is then extended further cephalad up to the origin of the right colic artery, if present, and toward the middle colic artery, which is always present.
The middle colic artery is always dissected to its origin from the superior mesenteric artery for maximal nodal yield. In limited resections involving very proximal lesions, dissection is often carried out further along the middle colic artery up to its bifurcation and only the right branch of the middle colic artery is transected. Lateral to the middle colic artery, the right colic vein, coursing anteriorly from the Henle trunk, can usually be identified and transected. All the central nodal tissue is then swept with the specimen.
With traction on the colon, the lesser sac is entered and the gastrocolic tissue is divided. The progress is continued along the mobilization plane, drawing the hepatic flexure inferiorly and medially. Generous distal resection margin is transected through the LUQ port using an endostapler. The remaining mesentery can then be transected using an advanced energy-based device, and the terminal ileum can be transected intracorporeally using an endostapler.
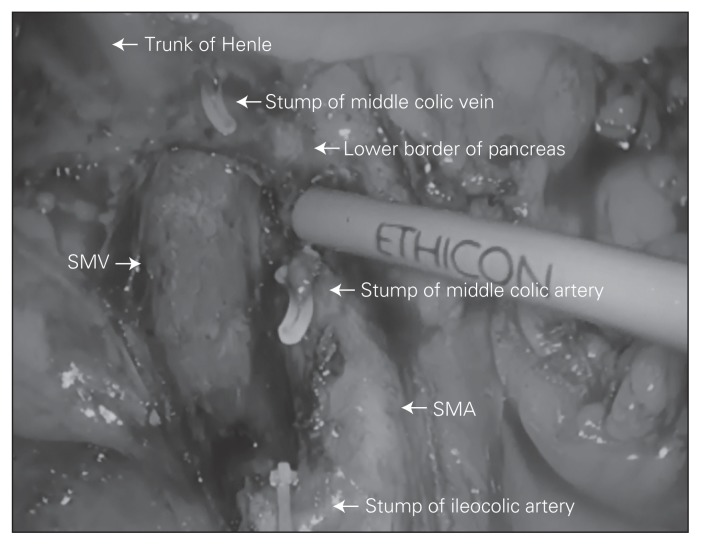
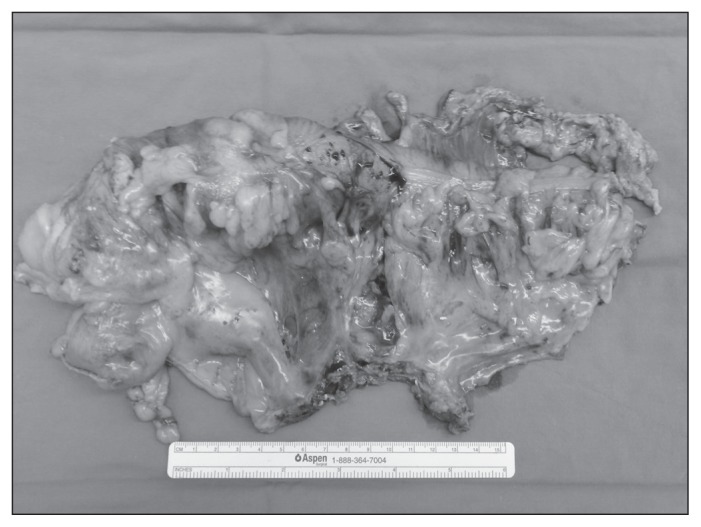
The specimen is put into an endo-bag, which is closed tight and parked over the liver for later retrieval through a 3 cm midline transumbilical incision. An intracorporeal anastomosis is performed, eliminating problems with extra-corporeal delivery and unnecessary traction on the mesentery. The terminal ileum and transverse colon are aligned in side-to-side isoperistaltic fashion, and enterotomies are made for the insertion of an endostapler. Firing an endostapler through these enterotomies creates the crotch of the final anastomosis. The resulting common enterotomy is then put under tension using 3 traction sutures, and the enterotomy is closed with another load of endostapler completing the side-to-side, functional end-to-end anastomosis. Figure 1 shows an intracorporeal view of dissection extent, and Figure 2 shows a typical retrieved specimen.
Fig. 1.
Intracorporeal view of dissection extent featuring smooth superior mesenteric artery (SMA) and superior mesenteric vein (SMV) skeletonized all the way up to the pancreas and showing the individual branches ligated at their origin.
Fig. 2.
Example of a retrieved specimen featuring clean peritoneal surfaces and maximal distance from the central tie to the tumour.
Results
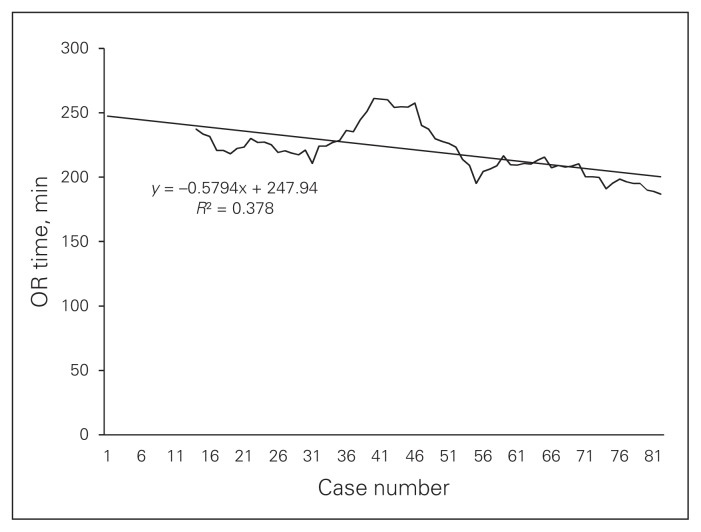
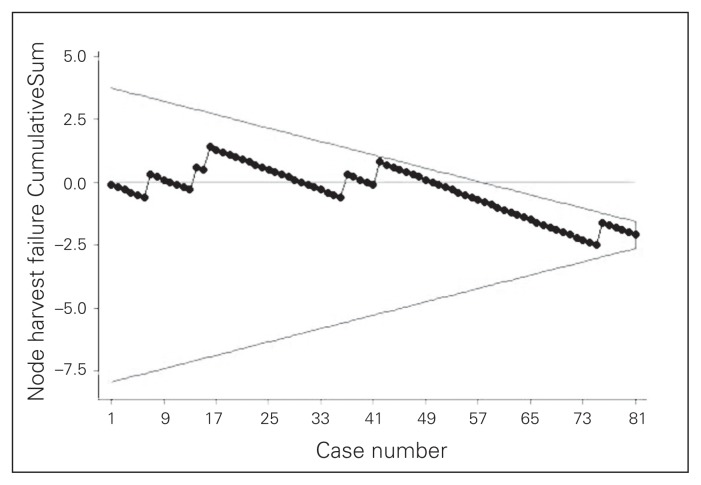
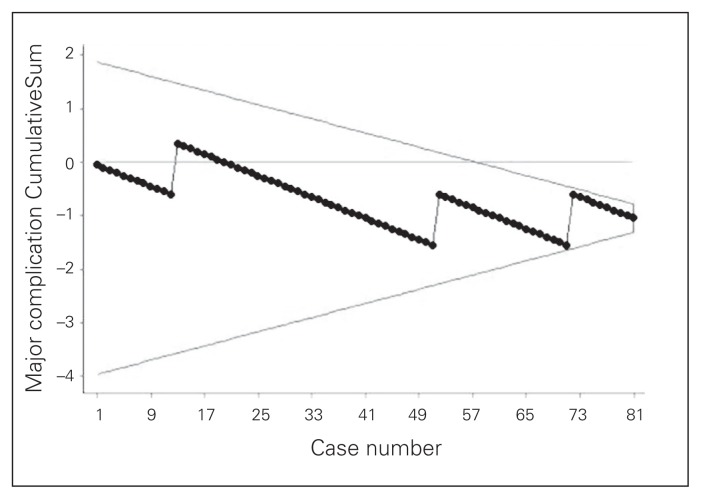
The study included 81 patients. Data summarizing preoperative parameters and operative outcomes are shown in Table 1. Outcome results feature a major complication rate of 3.6%, which includes conversions, leaks/intra-abdominal abscesses and a need for reoperation for an early trocar hernia. The average nodal yield was 31.3. The average duration of surgery was 220.0 minutes. The duration of surgery curve (Fig. 3), shows that the initial duration of surgery of about 250 minutes gradually decreased to less than 200 minutes by the 81st case in an inverse linear relationship with successive cases (y = −0.58 × 248). Finally, CUSUM plots (Fig. 4 and Fig. 5) indicate acceptable nodal yields and complication rates from the beginning of surgeon’s laparoscopic practice.
Table 1.
Preoperative parameters and perioperative outcomes (n = 81)
| Factor | Mean (95% CI)* |
|---|---|
| Preoperative parameters | |
| Age, yr | 63.7 (60.7–66.76) |
| Sex, % male | 58.0 (47.3–68.8) |
| BMI | 23.3 (22.60–23.92) |
| Stage, % | |
| 0 | 1.2 (0.0–3.6) |
| I | 25.9 (16.4–35.5) |
| II | 33.3 (23.1–43.6) |
| III | 35.8 (25.4–46.2) |
| IV | 3.7 (0.0–7.8) |
| Anesthesia ASA score, median (min–max) | 2 (1–3) |
| Operative outcomes | |
| OR time, min | 220 (206–233) |
| EBL, mL | 116 (83–148) |
| No. of lymph nodes harvested | 31.3 (27.2–35.4) |
| Lymph node yield < 12, % | 7.4 (1.7–13.10) |
| Major complications, % | 3.7 (0.0–7.8) |
| Conversion to open† | 0.0 |
| Death/hemorrhage | 0.0 |
| Leak or any intra-abdominal abcess | 2.5 (0.0–5.9) |
| Need for reoperation within 1 mo (incarcerated trochar site hernia) | 1.2 (0.0–3.6) |
| Positive resection margin | 0.0 |
ASA = American Society of Anesthesiologists; BMI = body mass index; CUSUSM = CummulativeSum; CI = confidence interval; EBL = estimated blood loss; OR = operating room.
Unless otherwise indicated.
Conversion to open was added to this category in order to track operative failures, as defined for the CUSUM plots.
Fig. 3.
Total duration of surgery. Ten-case moving average with best fit curve. OR = operating room.
Fig. 4.
Outcome CummulativeSum curve with V-mask tracking failure to harvest at least 12 nodes, assuming an acceptable failure rate of 10%.
Fig. 5.
Outcome CummulativeSum (CUSUM) curve with V-mask tracking major complications, as featured in Table 1, assuming an acceptable failure rate of 5%.
Discussion
While adoption of total mesorectal excision in the management of rectal cancer has led to significant improvement in survival, the survival rates for colon cancer have improved only slightly in the last 2 decades; disease-free survival rates for rectal cancer have even surpassed those for colon cancer in some studies.16–20 The reported survival rates for colon cancer vary widely among centres but seem higher in centres that routinely perform standardized, extensive colonic resections6,21,22 — a fact that may be subject to selection bias. Some of the most intriguing results come from Erlangen, Germany, reporting an 18% improvement in 5-year survival following the standardization of colonic resection to include CME.23 This result seems to be mostly attributable to surgery, as their data originate from a period before instigating adjuvant chemotherapy.23,24 Although most of these results are based only on regional, non-comparative studies and a multitude of other contributing factors, such as patient characteristics, regional diet and type of adjuvant care received, play a role, the published outcomes suggest probable room for optimizing surgical management. To our knowledge, no readily available studies have been published that specifically address laparoscopic colectomies with CME in the context of long-term survival.
Future research is necessary to quantify the effect CME has on survival rates for right-sided colon cancer. However, this was not the purpose of the present study. The intent of this study was to provide guidance to surgeons wishing to adopt this strategy based on the previously published evidence by showing that laparoscopic CME is possible with acceptable perioperative outcomes and outstanding nodal yields when adhering to good surgical principles. The results presented in this report suggest that a laparoscopic right-sided hemicolectomy with CME can be performed safely by a colorectal surgeon who primarily trained in open colorectal surgery in a setting where the surgeon attended a formal laparoscopic course and where adequate laparoscopic proctoring is available. This finding is demonstrated by CUSUM curves showing that perioperative safety and nodal yields were not compromised even early in the surgeon’s practice.
The average nodal yield of 31.3 is consistent with previously published reports of 30 lymph nodes with right hemicolectomy and CME and is superior to average yields of fewer than 20 lymph nodes with conventional surgery.5 We report a failure rate to retrieve at least 12 lymph nodes of 7.4% to serve as, to our knowledge, a first reported reference rate for this type of surgery. Although our results offer insight into the adoption of MIS among surgeons trained primarily in open surgery, they serve only as a guide because they are not necessarily generalizable as they originate from a single surgeon’s experience operating on a specific patient population.
A lateral to medial approach was used in our study. Not only is such an approach familiar from open surgery, it also helps ensure a correct dissection plane, which is often lost when performing CME from a medial approach. To surgeons who prefer high ligation of the ileocolic vessels early during the operation for further theoretical oncological reasons, we recommend simple ligation of the vessels using the medial approach followed by lateral mobilization to ensure a correct dissection plane before completing the medial dissection.
Duration of surgery remains one of the largest obstacles for laparoscopic CME. The operative duration learning curve reveals initial durations of about 250 minutes, which is more than double the durations reported for conventional laparoscopic right hemicolectomy performed by experienced laparoscopic surgeons.25 However, the duration decreased to below 200 minutes by the end of the study. Reflecting complex anatomy and the need for careful dissection around critical anatomic structures, especially during central vessel ligation, the operative duration learning curve reveals a rather slow linear inverse relationship between duration of surgery and successive operative cases. This relationship continues being linear without reaching a plateau, even at the end of the study, indicating potential for further improvement beyond the initial 81 cases.
Conclusion
In the current knowledge milieu where laparoscopic colon surgery provides superior perioperative outcomes and CME further offers prospects of better oncological outcomes, this data set offers encouragement in terms of feasibility to surgeons primarily trained in open colon surgery who wish to expand their skill set and can justify the additional time required. The question remaining is whether surgeons in North America should first adopt open CME or whether it is feasible to learn laparoscopic CME directly.
Footnotes
The data presented in this study have been presented as a podium presentation during the Canadian Association of General Surgeons annual conference in Calgary, Alta., Sept. 13–16, 2012.
Competing interests: None declared.
Contributors: G. Melich, H. Hur, S.H. Baik, J. Faria, N.K. Kim and B.S. Min designed the study. D.H. Jeong and B.S. Min acquired the data, which G. Melich, D.H. Jeong, J. Faria and B.S. Min analyzed. G. Melich and B.S. Min wrote the article, which all authors reviewed and approved for publication.
References
- 1.Reza MM, Blasco JA, Andradas E, et al. Systematic review of laparoscopic versus open surgery for colorectal cancer. Br J Surg. 2006;93:921–8. doi: 10.1002/bjs.5430. [DOI] [PubMed] [Google Scholar]
- 2.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–9. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 3.Guller U, Jain N, Hervey S, et al. Laparoscopic vs open colectomy: outcomes comparison based on large nationwide databases. Arch Surg. 2003;138:1179–86. doi: 10.1001/archsurg.138.11.1179. [DOI] [PubMed] [Google Scholar]
- 4.Aly EH. Laparoscopic colorectal surgery: summary of the current evidence. Ann R Coll Surg Engl. 2009;91:541–4. doi: 10.1308/003588409X464757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West NP, Hohenberger W, Weber K, et al. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010;28:272–8. doi: 10.1200/JCO.2009.24.1448. [DOI] [PubMed] [Google Scholar]
- 6.Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation — technical notes and outcome. Colorectal Dis. 2009;11:354–64. doi: 10.1111/j.1463-1318.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- 7.Lim TO, Soraya A, Ding LM, et al. Assessing doctors’ competence: application of CUSUM technique in monitoring doctor’s performance. Int J Qual Health Care. 2002;14:251–8. doi: 10.1093/oxfordjournals.intqhc.a002616. [DOI] [PubMed] [Google Scholar]
- 8.Colquhoun PHD. CUSUM analysis of J-pouch surgery reflects no learning curve after board certification. Can J Surg. 2008;51:296–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Bokhari MB, Patel CB, Ramos-Valadez DI, et al. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc. 2011;25:855–60. doi: 10.1007/s00464-010-1281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery DC. Introduction to Statistical Quality Control. 4th ed. New York (NY): Wiley; 2000. [Google Scholar]
- 11.Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–9. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 12.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–26. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 13.Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–84. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 14.Greene FLP, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. Philadelphia (PA): Lippincott Raven; 2002. pp. 107–17. [Google Scholar]
- 15.Choi DH, Jeong WK, Lim SW, et al. Learning curves for laparoscopic sigmoidectomy used to manage curable sigmoid colon cancer: single-institute, three-surgeon experience. Surg Endosc. 2009;23:622–8. doi: 10.1007/s00464-008-9753-y. [DOI] [PubMed] [Google Scholar]
- 16.Martling AL, Holm T, Rutqvist LE, et al. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Lancet. 2000;356:93–6. doi: 10.1016/s0140-6736(00)02469-7. [DOI] [PubMed] [Google Scholar]
- 17.Kapiteijn E, Putter H, van de Velde CJH, et al. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in the Netherlands. Br J Surg. 2002;89:1142–9. doi: 10.1046/j.1365-2168.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- 18.Wibe A, Moller B, Norstein J, et al. A national strategic change in treatment policy for rectal cancer — implementation of total mesorectal excision as routine treatment in Norway: a national audit. Dis Colon Rectum. 2002;45:857–66. doi: 10.1007/s10350-004-6317-7. [DOI] [PubMed] [Google Scholar]
- 19.Birgisson H, Talback M, Gunnarsson U, et al. Improved survival in cancer of the colon and rectum in Sweden. Eur J Surg Oncol. 2005;31:845–53. doi: 10.1016/j.ejso.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Iversen LH, Norgaard M, Jepsen P, et al. Trends in colorectal cancer survival in northern Denmark: 1985–2004. Colorectal Dis. 2007;9:210–7. doi: 10.1111/j.1463-1318.2006.01130.x. [DOI] [PubMed] [Google Scholar]
- 21.Pramateftakis MG. Optimizing colonic cancer surgery: high ligation and complete mesocolic excision during right hemicolectomy. Tech Coloproctol. 2010;14:S49–51. doi: 10.1007/s10151-010-0609-9. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Enomoto M, Higuchi T, et al. Clinical significance of lymph node ratio and location of nodal involvement in patients with right colon cancer. Dig Surg. 2011;28:190–7. doi: 10.1159/000323966. [DOI] [PubMed] [Google Scholar]
- 23.Hohenberger W, Reingruber B, Merkel S. Surgery for colon cancer. Scand J Surg. 2003;92:45–52. doi: 10.1177/145749690309200107. [DOI] [PubMed] [Google Scholar]
- 24.Gall FP, Hermanek P. Wandel und derzeitiger Stand der Behand-lung des colorectalen Carcinoms. Chirurg. 1992;63:227–34. [PubMed] [Google Scholar]
- 25.Senagore AJ, Delaney CP, Brady KM, et al. Standardized approach to laparoscopic right colectomy: outcomes in 70 consecutive cases. J Am Coll Surg. 2004;199:675–9. doi: 10.1016/j.jamcollsurg.2004.06.021. [DOI] [PubMed] [Google Scholar]