Abstract
Long-chain omega-3 polyunsaturated fatty acids (LC-O3PUFAs) exhibit therapeutic potential for the treatment and prevention of the neurological deficits associated with spinal cord injury (SCI). However, the mechanisms implicated in these protective responses remain unclear. The objective of the present functional metabolomics study was to identify and define the dominant metabolic pathways targeted by dietary LC-O3PUFAs. Sprague-Dawley rats were fed rodent purified chows containing menhaden fish oil-derived LC-O3PUFAs for 8 weeks before being subjected to sham or spinal cord contusion surgeries. We show, through untargeted metabolomics, that dietary LC-O3PUFAs regulate important biochemical signatures associated with amino acid metabolism and free radical scavenging in both the injured and sham-operated spinal cord. Of particular significance, the spinal cord metabolome of animals fed with LC-O3PUFAs exhibited reduced glucose levels (−48%) and polar uncharged/hydrophobic amino acids (<−20%) while showing significant increases in the levels of antioxidant/anti-inflammatory amino acids and peptides metabolites, including β-alanine (+24%), carnosine (+33%), homocarnosine (+27%), kynurenine (+88%), when compared to animals receiving control diets (p < 0.05). Further, we found that dietary LC-O3PUFAs impacted the levels of neurotransmitters and the mitochondrial metabolism, as evidenced by significant increases in the levels of N-acetylglutamate (+43%) and acetyl-CoA levels (+27%), respectively. Interestingly, this dietary intervention resulted in a global correction of the pro-oxidant metabolic profile that characterized the SCI-mediated sensorimotor dysfunction. In summary, the significant benefits of metabolic homeostasis and increased antioxidant defenses unlock important neurorestorative pathways of dietary LC-O3PUFAs against SCI.
Keywords: Metabolomics, DHA metabolome, Spinal cord injury, ROS, Neuroprotection, Pain
Introduction
Spinal cord injury (SCI) is a major debilitating neurological condition that affects several million people worldwide. The initial physical insult sustained in SCI triggers a longer secondary damage that leads to inflammation, demyelination, and apoptosis ultimately leading to dysfunction [1]. This secondary injury phase is characterized by metabolic alterations, glutamate-induced excitotoxicity, and oxidative stress [2–5]. The levels of these reactive oxygen (ROS) and nitrogen species (NOS) increase considerably when the metabolism is compromised, which can result in irreversible damage to cell membrane lipids, proteins and nucleic acids. ROS scavengers, including catalase, glutathione (GSH), and superoxide dismutase (SOD), are endogenous defense mechanisms that combat oxidative damage. Although there is no current cure for SCI, accumulating clinical and experimental evidence support interventions that target these restorative pathways and hold tremendous promise in ameliorating neurological dysfunction [6].
Long-chain omega-3 polyunsaturated fatty acids (LC-O3PUFAs) modulate multiple pathways that contribute to secondary damage following SCI [7–19]. We recently demonstrated that the administration of LC-O3PUFAs restores the cord lipid homeostasis, confers neuroprotection, prevents sensorimotor dysfunction and neuropathic pain, and facilitates locomotor recovery following acute and chronic SCI when administered before the injury [17–19]. However, there is very limited understanding of the pathways activated by dietary LC-O3PUFAs in the injured central nervous system.
Dietary fatty acids exert potent effects on cellular metabolism through tightly regulated mechanisms at the transcriptional, posttranscriptional, translational, or posttranslational levels. This study investigates the global non-lipid targets of nutritional LC-O3PUFAs, which offers the advantage of linking dietary LC-O3PUFA-gene interactions to distinctive metabolites and small molecules. This study represents the first unbiased attempt to identify the biologically meaningful metabolic networks that are influenced by dietary LC-O3PUFAs during the acute and chronic injury phases following SCI. In addition, we define the putative biochemical signatures associated with resiliency against SCI.
Materials and Methods
Animals
All animal studies were performed in compliance with the Loma Linda University School of Medicine regulations and institutional guidelines consistent with the NIH Guide for the Care and Use of Laboratory Animals. Female Sprague-Dawley rats were received from Charles River Laboratories (Portage, MI) and single housed in environmentally enriched cages on alternating 12 h light/dark cycles after being acclimated to the new environment for one week.
Experimental design and diets
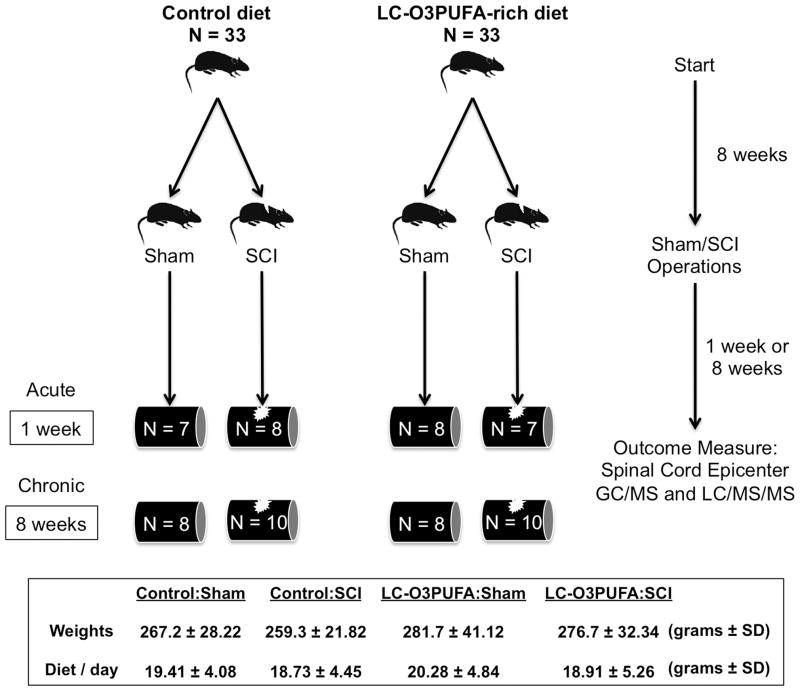
Young adult rats (185–200 grams) were fed control or fish oil-enriched diets for 8 weeks were subjected to sham injury or spinal cord injury and subsequently allowed to recover for 1 or 8 weeks after trauma. Spinal cord tissue was collected for global metabolic profiling (n = 7–10 samples per group). This study used two independent cohorts (cohort 1: at least 7 animals per diet group, allowed to survive until 1 weeks post-operation; cohort 2: at least 8 animals per diet group, allowed to survive 8 weeks post-operation). Behavioral data from rats in cohort 2 was previously reported [18,19]. Figure 1 summarizes the timeline outlining the experimental design. For each group, the average animal weight and daily food consumption is expressed in grams ± standard deviation.
Figure 1. Timeline outlining experimental design and animal groups.
Rats were fed control or LC-O3PUFA-enriched chows for 8 weeks before being subjected to sham or SCI operations. Rats were removed for terminal global metabolomics analyses during acute and chronic injury stages. Values for weights and food consumption are average grams (g) ± S.D; n = at least 16 rats per group.
Custom AIN-93G-based diets were prepared with modifications to the omega-3 fatty acid source as described previously [18,19]. Typical analysis of the AIN-93G formulation reveals 7.1% fat, containing cholesterol (0 ppm), linoleic acid (3.58%), linolenic acid (0.55%), arachidonic acid (0%), omega-3 fatty acids (0.55%), total saturated fatty acids (1.05%), total monosaturated fatty acids (1.54%), and polyunsaturated fatty acids (3.78%). In our study, the dietary omega-3 fatty acids were supplied as either soybean oil (control chow) or menhaden fish oil (DHA = 12.82-gm and EPA = 6.91-gm per 100 gm of fish oil). Because the fish oil-based diet contained 6.23 grams of fish oil per 100 grams of diet, we estimate that feeding a 270-gram rat with approximately 20 grams of diets (or 1.25 grams of fish oil per day) should result in a daily intake of approximately 60 mg of DHA and 32 mg of EPA per 100 grams of body weight. The total absolute amount of ingested LC-O3PUFAs may vary when additional sources of omega-3 in the AIN-93G diet are considered. Mass-spec analysis further revealed that the level of cholesterol in the menhaden fish oil was 0.582-g/100 g. Cholesterol was added to control diets to match this level. The diets were stored at 4 C and used fresh. The amount of food ingested was recorded daily during weekdays and averaged during weekends. Table 1 summarizes the composition of the diets.
Table 1. Detailed compositional analysis of AIN-93G-based diets.
The level of dietary fat was approximately 6% of dry weight supplied as either soybean oil (control chow) or menhaden fish oil (LC-O3PUFA-enriched chow). Gas chromatography coupled with mass spectrometry demonstrated that the level of DHA and eicosapentaenoic acid (EPA) in the menhaden fish oil was 12.82-g and 6.91-g, respectively, per 100g of diet. The level of cholesterol was 0.582-g/100g. This amount was added to control diets to make the levels consistent with that of the fish oil diet.
| Ingredient | AIN-93G Control diet (%) | AIN-93G Fish oil-enriched diet (%) |
|---|---|---|
| Casein | 20 | 20 |
| L-Cystine | 0.3 | 0.3 |
| Corn starch | 39.7 | 39.7 |
| Maltodextrin | 13.2 | 13.2 |
| Sucrose | 10 | 10 |
| Fiber | 5 | 5 |
| Vitamin mix | 1 | 1 |
| Mineral mix | 3.5 | 3.5 |
| Choline bitartrate | 0.25 | 0.25 |
| tBHQ | 0.0014 | 0.0014 |
| Soybean oil | 7 | 0.77 |
| Fish oil (DHA + EPA + cholesterol) | 0 | 6.23 |
| Cholesterol (added to match fish oil levels) | 0.0121 | 0 |
| Omega-3 Fatty Acids in Oils | ||
| DHA | <1 g/100 g soybean oil | 12.82 g/100 g fish oil |
| EPA | <1 g/100 g soybean oil | 6.91 g/100 g fish oil |
| omega-6:omega-3 | 7.5:1 | 1:3.7 |
Surgical and Post-Operative Procedures
Eight weeks after the dietary intervention, animals were deeply anesthetized with a mixture of ketamine/xylazine (80 mg/kg and 10 mg/kg, respectively). The New York University (NYU) Impactor was used to generate a contusive lesion to the thoracic 10 level of the spinal cord [20]. The spinal cord was subjected to weight drop impact using a 10-g rod released from a height of 12.5-mm. Sham animals received only a laminectomy surgery. The animals body temperature was maintained at 37°C during surgery. The muscle layers were then sutured and the skin layers closed. Postoperative care of SCI rats included manual bladder expression at least two times a day until the return of spontaneous urination. Cefazolin (Bristol Myers Squibb, New York, NY; 25 mg/kg, s.q.) and Buprenex® (buprenorphine; Reckett and Colman Pharmaceuticals, Inc. Richmond, VA; 0.05 mg/kg, s.c.) were also given to all rats for 5 and 3 consecutive days, respectively. Animals were allowed to survive for 1 or 8 weeks post-operation and the spinal cord tissue dissected for metabolomics analysis.
Metabolomic Profiling
Global metabolic profiling was performed as previously described [19,18]. Animals were deeply anesthetized and perfused with ice-cold PBS. The spinal cord tissue (75–100 mg) was dissected and put into liquid nitrogen and then stored at −80°C until use. The tissue samples were homogenized in water and the protein precipitated with methanol containing four standards to report on extraction efficiency. The resulting supernatant was split into equal aliquots for analysis on the three independent platforms: ultrahigh performance liquid chromatography/tandem mass spectrometry (UHPLC/MS/MS2) optimized for basic species, UHPLC/MS/MS2 optimized for acidic species, and gas chromatography/mass spectrometry (GC/MS). The metabolites were identified by comparing the ion features in the experimental samples and to a reference library of chemical standards that includes retention time, molecular weight (m/z), preferred adducts, and in-source fragments as well as associated MS spectra. The biochemical features were curated by visual inspection for quality control using the software developed at Metabolon [21].
Metabolomics Analyses
The false discovery rate (FDR) was calculated as previously reported [18,22,23]. The q-value describes the false discovery rate and takes into account the multiple comparisons.
The partial least square-discriminant analysis (PLS-DA) was used to identify predictors between groups as previously described [19,18]. This regression method provides information that can predict the class membership (Y) via linear combination of original variables (X). We performed the separation distance permutation to assess the significance of class discriminations between groups. The variable importance in projection (VIP) measures the impact of each metabolite in the model. VIP is a weighted sum of squares of the PLS loadings and takes into account the amount of explained Y-variation in each dimension. Biochemicals with values above 1 are considered important contributors to the group discriminations.
The metabolomic functional analyses were generated through the use of Ingenuity Pathways Analysis (IPA) (www.ingenuity.com). The relative metabolite ratios obtained from metabolomics analyses were converted to fold change values by the IPA software. The data set was filtered to include metabolites that were associated with biological functions in the Ingenuity Pathways Knowledge Base. The IPA algorithm uses the p-value (p < 0.05) to determine the probability that each biological function assigned to that data set is due to random chance alone. This value is calculated by considering the number of metabolites in the dataset that participate in that function and the total number of features that are known to be associated with that function in the IPA knowledge database. The level of statistical significance was set at a p-value less than 0.05, suggesting non-random associations. We also used established analytical approaches to aid in the interpretation of our large data set [24]. For a biochemical reaction in which (A + B) substrates yield (C + D) products, then low levels of A and/or B with concomitant increases in C and/or D suggest increased metabolism towards formation of products. The inverse scenario was interpreted as accumulation of substrates.
Statistical Analysis
Statistical analyses were performed using SPSS version 20.0 (IBM: SPSS, Armonk, New York), Prism 5 software v5d (GraphPad Software Inc., San Diego, CA), the “R” program (http://cran.r-project.org/), metaboanalyst [25,26], and IPA. ANOVA contrasts were used to identify features that differed significantly between groups. Associations were made with the Spearman’s rank correlation tests in order to explore relationships between the oxidative profile and the functional phenotype. Data are presented as mean ± SD, unless otherwise specified. The differences were considered statistically significant at p < 0.05.
Results
Our previous reports showed that preventive administration of docosahexaenoic acid (DHA) or consumption of a diet rich in long-chain omega-3 polyunsaturated fatty acids (LC-O3PUFAs) confers potent prophylaxis against multiple SCI co-morbidities and improves functional recovery [17,19,18]. However, the mechanisms underlying these beneficial effects remain largely unknown. The goal of this study was to characterize the impact of LC-O3PUFA dietary supplementation on the spinal cord non-lipid metabolic responses during the acute and chronic phases of SCI recovery as well as in the sham-operated spinal cord. The study design is summarized in Figure 1.
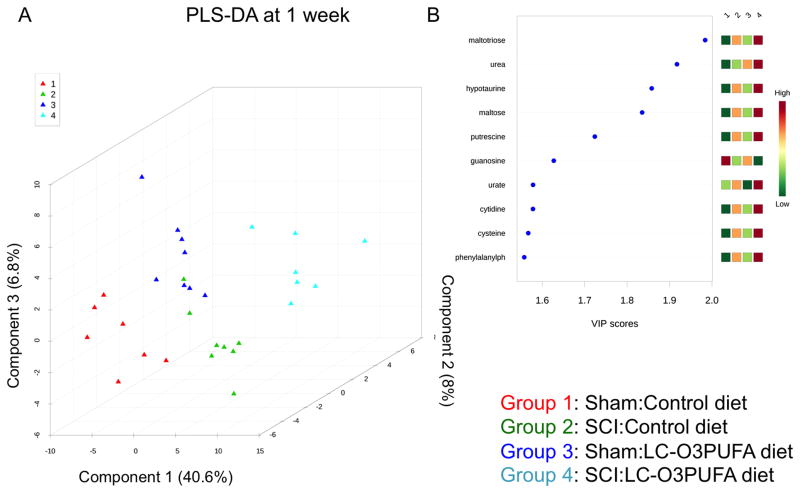
The ability to measure and study dietary LC-O3PUFA’s targets and derivatives has been facilitated by the availability of untargeted metabolomics. Because the neurometabolome is tightly regulated, this technique allows for detection of very subtle alterations in biochemical pathways [27]. The partial least square-discriminant analysis (PLS-DA) score plot was obtained using the variation scores of the first three principal components. In the generated regression models, these components explained more than 50% of the differences between groups at 1 and 8 weeks post-surgery (Figure 2A and Figure 3A). Each plot mark corresponds to a rat in the study and the variability in metabolite levels that were detected for that animal. Permutation analyses validated the class discrimination (observed test statistic p < 0.05 in both models). PLS analyses revealed that the diet enriched in LC-O3PUFAs had a significant impact in the levels of selective carbohydrates, amino acids, and small peptides with antioxidant capabilities. These small molecules showed the strongest influence to the observed metabolomics differences between groups. This was evidenced by variable importance in projection (VIP) values above 1 (Figure 2B and Figure 3B).
Figure 2. Dietary LC-O3PUFAs significantly modulate the non-lipid spinal cord metabolome during acute injury stages.
(A) Partial least square discriminant analysis (PLS-DA) distinguished subgroups based on operation and dietary intake at 1 week post-operation. This model was constructed using scaled intensity peaks of the global non-lipid detected features. Permutation provided statistically significant separations between subgroups (p < 0.05; not shown). (B) The variable influence on projection (VIP) reflects the importance of amino acids and antioxidant peptides in the generated PLS model.
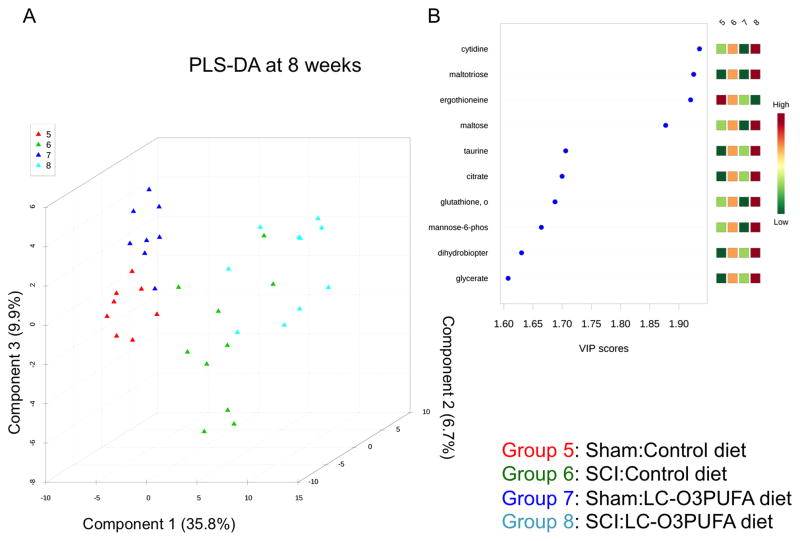
Figure 3. Dietary LC-O3PUFAs significantly modulate the non-lipid spinal cord metabolome during chronic injury stages.
(A) Partial least square discriminant analysis (PLS-DA) discriminated between groups based on dietary intake at 8 weeks post-operation. The model was generated using scaled intensity peaks of the global non-lipid detected features. Permutation provided statistically significant separations between subgroups (p < 0.05; data not shown). (B) The variable influence on projection (VIP) indicates the importance of carbohydrates, amino acids, and antioxidant peptides at 8 week-post operations.
Here, LC-MS/MS data was analyzed using Ingenuity Pathway Analysis software, which as expected revealed that dietary LC-O3PUFAs preferentially target pathways associated with cellular homeostasis and neurological function (Figure 4). In particular, we found that the most significant regulated functions in animals fed diets with enriched in LC-O3PUFAs were related to the transport and metabolism of amino acids and neurotransmitter systems and Ca2+-mediated cell signaling, supporting the role of LC-O3PUFAs as crucial regulators of the neuron-glia signaling network [28]. In addition, the dietary LC-O3PUFAs had a robust impact on the metabolism of peptides and amino acids implicated in the regulation of reactive oxygen and nitrogen species. Table 2 summarizes the most significant functions and the number of molecules that were altered by the LC-O3PUFA-rich diet in sham and injured animals.
Figure 4. Results of LC-MS/MS data analysis using Ingenuity Pathways Analysis (IPA) software.
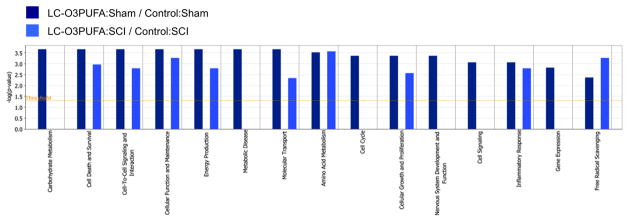
The data set was filtered for non-lipid small-molecules that met the 1.5 fold cut-off criteria. These metabolites were associated with biological functions in the IPA Knowledge Base. The p-value was calculated using right-tailed Fischer’s exact test and represents the probability that each biological function assigned to that data set is due to random chance alone. P-value < 0.05 were considered statistically significant.
Table 2.
Major pathways and functions associated with dietary consumption of LC-O3PUFAs in rats
| Diet Effect in Sham Rats | |||
|---|---|---|---|
| Category | Diseases or Functions Annotation | p-Value | # Molecules |
| Cellular Function and Maintenance | cellular homeostasis | 1.94E-04 | 17 |
| Molecular Transport | transport of molecule | 8.00E-04 | 16 |
| Small Molecule Biochemistry | synthesis of nitric oxide | 1.19E-09 | 15 |
| Cell Signaling | synthesis of nitric oxide | 1.19E-09 | 15 |
| Free Radical Scavenging | metabolism of reactive oxygen species | 1.68E-06 | 15 |
| Amino Acid Metabolism | transport of amino acids | 2.84E-11 | 12 |
| Molecular Transport | transport of amino acids | 2.84E-11 | 12 |
| Small Molecule Biochemistry | transport of amino acids | 2.84E-11 | 12 |
| Molecular Transport | quantity of Ca2+ | 5.72E-06 | 12 |
| Cell Signaling | quantity of Ca2+ | 5.72E-06 | 12 |
| Diet Effect in SCI Rats | |||
|---|---|---|---|
| Category | Diseases or Functions Annotation | p-Value | # Molecules |
| Cellular Function and Maintenance | cellular homeostasis | 2.93E-05 | 14 |
| Free Radical Scavenging | synthesis of reactive oxygen species | 9.76E-07 | 12 |
| Protein Synthesis | metabolism of protein | 1.02E-06 | 12 |
| Molecular Transport | transport of molecule | 6.82E-04 | 12 |
| Small Molecule Biochemistry | synthesis of nitric oxide | 3.62E-08 | 11 |
| Cell Signaling | synthesis of nitric oxide | 3.62E-08 | 11 |
| Cell-To-Cell Signaling and Interaction | activation of blood cells | 4.15E-06 | 11 |
| Cell Death and Survival | cell survival | 1.64E-03 | 11 |
| Cellular Growth and Proliferation | growth of bacteria | 7.29E-11 | 10 |
| Cell Signaling | quantity of Ca2+ | 2.45E-06 | 10 |
Dietary LC-O3PUFAs Improve Cellular Bioenergetics and Antioxidant Metabolism in Sham Animals
To gain insight into the potential biochemical targets implicated in the neuroprophylactic responses, we investigated the neurometabolome of sham-operated animals. We found that the diet rich in LC-O3PUFAs significantly altered the metabolism of distinctive amino acids and carbohydrates when compared to sham animals receiving control diets (p < 0.05). For instance, ANOVA contrasts revealed that the LC-O3PUFA-rich diet increased the spinal cord levels of carnosine (+33%), homocarnosine (+27%), and the major protective precursor, β-alanine (+24%). The levels of 4-guanidinobutanoate (+34%), which is a common byproduct of arginine metabolism and distant precursor of homocarnosine, were increased in the spinal cord of sham-operated rats fed with LC-O3PUFAs when compared to control animals. Recent evidence suggests that the biochemical pathways implicated in the metabolism of these neuroprotective peptides may protect the spinal cord from inflammation and tissue damage after SCI [29,30]. The diet slightly decreased the levels of the precursor/metabolite amino acid glutamine (−14%) while glutamate levels remained stable and led to significantly higher Glu/Gln ratios (data not shown), suggesting reduced glutamine synthesis or accelerated recycling/catabolism. We found that the dietary intervention regulated the tryptophan metabolism, as evidenced by increased levels of kynurenine (88%) in the spinal cord of animals fed LC-O3PUFA-rich diets when compared to controls. Kynurenines are important modulators of oxidative stress and neurodegeneration [31,32].
Our results demonstrate a significant reduction in key metabolites implicated in the pentose phosphate pathway, including glucose (−48%), glucose-6-phosphate (−24%) and sedoheptulose-7-phosphate (−46%). Further, the diet increased the levels of acetyl CoA (27%) when compared to the control diet fed animals. Together with the findings showing a reduction in the levels of polar uncharged and hydrophobic side chain amino acid derivatives, including threonine levels (−28% from control), phosphoserine (−33%), betaine (−23%), and 3-hydroxyisobutyrate (−31%), these results suggest an increased protein turnover and/or amino acid shunting to energetic pathways.
We found that the diet rich in LC-O3PUFA had significantly lower levels of ergothioneine (−30%) at 8 weeks post-operation, suggesting differences in the levels of this naturally occurring amino acid between the dietary oils. The major metabolomic changes observed in sham-operated rats consuming LC-O3PUFAs are summarized in Table 3. Both IPA and metaboanalyst bioinformatics software were employed to gain insights into the major metabolic targets of dietary LC-O3PUFAs in the sham-operated spinal cord (Figure 5).
Table 3. Significant metabolites targeted by dietary LC-O3PUFAs in sham rats.
Fold changes when compared to sham animals receiving control diets. The additional columns depict the individual effects of diet and SCI in the modulating the levels of each feature. Up arrow, upregulation; down arrow, downregulation; =, not significant change.
| One Week Post-Sham Operation (LC-O3PUFA Diet/Control Diet) | |||
|---|---|---|---|
| Metabolite | Fold-chage | Diet Effect | SCI Effect |
| threonine | 0.72 |

|
= |
| beta-alanine | 1.24 |

|
= |
| glutamine | 0.86 |

|
= |
| 2-aminoadipate | 0.61 |

|
= |
| kynurenine | 1.88 |

|
= |
| urea | 1.44 |

|
= |
| carnosine | 1.33 |

|

|
| homocarnosine | 1.27 |

|

|
| Eight Weeks Post-Sham Operation (LC-O3PUFA Diet/Control Diet) | |||
|---|---|---|---|
| Metabolite | Fold-chage | Diet Effect | SCI Effect |
| amino acids with polar uncharged and hydrophobic side chains: serine, threonine and valine, leucine, isoleucine metabolism | <0.80 |

|
= |
| 4-guanidinobutanoate | 1.34 |

|
= |
| fructose | 0.71 |

|
= |
| glucose-6P | 0.76 |

|

|
| glucose | 0.52 |

|

|
| sedoheptulose-7-phosphate | 0.54 |

|

|
| N1-methyladenosine | 0.74 |

|
= |
| adenosine 2′-monophosphate (2′-AMP) | 0.79 |

|

|
| acetyl CoA | 1.27 |

|

|
| ergothioneine | 0.70 |

|
= |
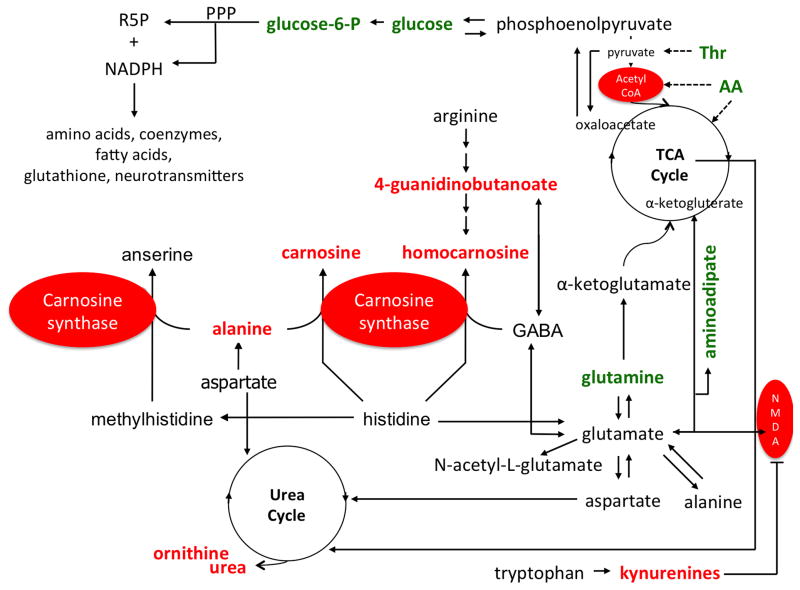
Figure 5. Metabolic pathways targeted by dietary LC-O3PUFA in the sham rat spinal cord.
Dietary LC-O3PUFAs target the metabolism of carnosine and homocarnosine, as evidenced by increased levels of alanine, carnosine, homocarnosine, and 4-guanidinobutanoate in the spinal cord of animals fed with LC-O3PUFAs. The diet rich in LC-O3PUFAs also altered the glutamine-glutamate cycling. A distinctive group of amino acid systems were affected by the diet, including threonine and tryptophan. In particular, animals fed with LC-O3PUFAs showed dramatic increases in the levels of kynurenines, which can regulate mitochondrial homeostasis and oxidative stress, inflammation, and glutamate excitotoxicity through NMDA receptor inhibition. Notably, the diet increased the levels of ornithine and urea, while decreasing glucose and glucose-6-P levels, showing selective alterations in the spinal cord cell bioenergetics. This support that LC-O3PUFAs fuel energy production largely by oxidative phosphorylation via the tricarboxylic acid (TCA) cycle and pentose phosphate pathway (PPP) rather than glycolysis, which are essential pathways for the synthesis of necessary macromolecules (i.e. amino acids, neurotransmitters, glutathione, nucleosides and lipids required for assembling new cells). These pathways may represent important mechanisms by which dietary LC-O3PUFAs confer prophylaxis against neurodegeneration and dysfunction in SCI. This reservoir of protective molecules and antioxidant bioavailability is expected to make neurons and glia more resistant against calcium overload, glutamate toxicity, and cell death following SCI. Metabolites in red increased with the dietary intervention. Features in green decreased with the LC-O3PUFA diet when compared to controls. Putative enzymatic/receptor targets are highlighted in red ovals. Abbreviations: AA, amino acid (polar); GABA, gamma-aminobutyrate; NADPH, nicotinamide adenine dinucleotide phosphate; NMDA, N-methyl-D-aspartate; PPP, pentose phosphate pathway; R5P, ribose 5-phosphate; Thr, threonine.
Dietary LC-O3PUFAs Target Amino Acid Systems and Complex Carbohydrates at One-Week Post-SCI
We found that the diet rich in LC-O3PUFAs selectively targeted the metabolism of molecules implicated in oxidative protection and amino acid turnover at one week post-injury. In particular, the rats fed with LC-O3PUFAs showed increased levels of cystathionine (+43%), ornithine (+76%), urea (+36%), and hippurate (+219) when compared to injured animals fed control diets.
Another novel finding of this study is that dietary LC-O3PUFAs dramatically upregulated the levels of heme (+292%) in the spinal cord of injured rats at 1 week post-injury (wpi), suggesting increased levels of proteins containing protective heme groups.
Similar to the diet effects in sham-operated rats, we found that the dietary intervention resulted in selective modulation of carbohydrates and the glutamate neurotransmitter system. For instance, we found that dietary LC-O3PUFAs increased the levels of the glucose-containing saccharides maltose (+62%) and maltoriose (+218%). The diet rich in LC-O3PUFAs increased the levels of N-acetylglutamate (+43%) when compared to injured animals fed control diets.
The dietary intervention slightly decreased the levels of methionine (−9%), arginine (−10%), hypoxanthine (−10%), and phosphopantetheine (−16%) when compared to controls at one-week post-SCI (p < 0.05). The metabolomic alterations at 1 wpi are summarized in Table 4.
Table 4. Important small-molecule targets of LC-O3PUFAs in spinal cord injured rats.
Fold changes when compared to injured rats receiving control diets. The additional columns illustrate the individual effects of diet and SCI in the regulating the levels of each biochemical. Up arrow, upregulation; down arrow, downregulation; =, not significant change.
| One Week Post-SCI (LC-O3PUFA Diet/Control Diet) | |||
|---|---|---|---|
| Metabolite | Fold-chage | Diet Effect | SCI Effect |
| N-acetylglutamate | 1.43 |

|

|
| cystathionine | 1.43 |

|
= |
| Methionine and acetylmethionine | 0.91, 0.88 |

|

|
| arginine | 0.90 |

|

|
| ornithine | 1.76 |

|

|
| urea | 1.36 |

|
= |
| maltose and maltotriose | 1.62, 2.18 |

|

|
| hypoxanthine | 0.90 |

|
= |
| heme | 2.92 |

|
= |
| phosphopantetheine | 0.84 |

|

|
| hippurate | 2.19 |

|
= |
| Eight Weeks Post-SCI (LC-O3PUFA Diet/Control Diet) | |||
|---|---|---|---|
| Metabolite | Fold-chage | Diet Effect | SCI Effect |
| asparagine | 0.75 |

|
= |
| N2-acetyllysine | 1.49 |

|
= |
| 2-aminobutyrate | 0.54 |

|
= |
| 5-methylthioadenosine (MTA) | 1.19 |

|
= |
| 4-guanidinobutanoate | 1.27 |

|
= |
| glutathione, reduced (GSH) | 1.42 |

|
= |
| glutathione, oxidized (GSSG) | 1.34 |

|

|
| anserine | 0.50 |

|
= |
| gamma-glutamylglutamine | 1.15 |

|

|
| maltose | 1.71 |

|

|
| maltriose | 1.17 |

|
= |
| citrate | 1.30 |

|

|
| cytidine | 1.20 |

|

|
| uridine | 1.15 |

|
= |
| methylphosphate | 1.26 |

|
= |
| ergothioneine | 0.52 |

|
= |
Dietary LC-O3PUFAs Increase Antioxidant Defenses and Prevent GSH Depletion in the Chronically Injured Spinal Cord
Of particular significance, the animals receiving the LC-O3PUFA-rich diet showed increased glutathione turnover (GSH, +42% and GSSH, +34%) at 8 wpi, suggesting improved antioxidant defenses. The diet slightly increased the levels of γ-glutamylglutamine (+15). This finding provides further evidence of the impact of dietary LC-O3PUFAs in the modulation of the glutamatergic system.
Interestingly, the LC-O3PUFA-rich diet increased the levels of biomarkers associated with cell proliferation and epigenetic mechanism, including cytidine (+20%), uridine (+15%), and N-acetyllisine (+49%) when compared to control fed rats at 8 wpi. We also detected decreased levels of asparagine (−25%), 2-aminobutyrate (−46%), anserine (−50%), and ergothioneine (−48%) in rats consuming LC-O3PUFAs at 8 weeks post-SCI. The metabolic signatures of dietary LC-O3PUFAs at 8 wpi are summarized in Table 4. IPA-assisted metabolic maps were generated to facilitate the understanding of the complex pathways regulated by dietary LC-O3PUFAs (Figure 6). The complete non-lipid metabolomic profile detected in the spinal cord of the studied groups is represented in Table 5 and Table 6.
Figure 6. Metabolic pathways targeted by dietary LC-O3PUFA in the injured spinal cord.
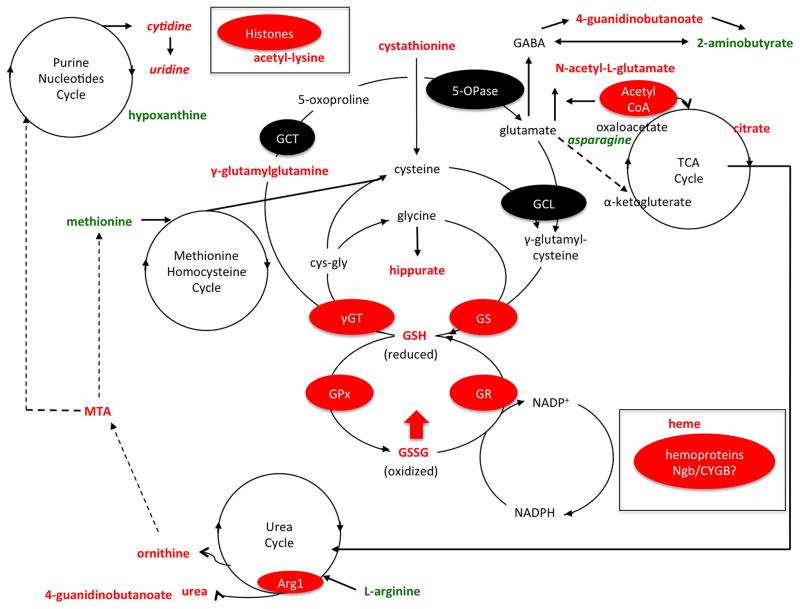
Although we did not characterize the specific sources of ROS in the present study, our metabolomics dataset supports the role of mitochondrial dysfunction as a major source during both acute and chronic injury stages. Interestingly, we identified glutathione (GSH) metabolism as a molecular target of dietary LC-O3PUFAs. For instance, the animals receiving the dietary intervention showed increased spinal cord levels of γ-glutamylglutamine, cystathione, hippurate, GSH, and GSSH, suggesting increased production and/or reduced depletion of antioxidant pools after SCI. Notably, the levels of heme were increased in the spinal cord of rats exposed to the LC-O3PUFA-rich diet, proposing a novel protective mechanism for LC-O3PUFAs. Similar to the findings observed in sham rats, LC-O3PUFAs altered the TCA and Urea cycle. The increased levels of purine nucleotides and acetyl-lysine suggests a mechanism for which chronic dietary supplementation with LC-O3PUFAs modulates plasticity, growth, and gene expression. Metabolites in red increased with the dietary intervention. Features in green decreased with the LC-O3PUFA diet when compared to controls. Putative enzymatic and protein targets are highlighted in red ovals. Abbreviations: 5-OPase, 5-oxoprolinase; Arg1, arginase; CYGB, cytoglobin; cys-gly, cysteine-glycine; GSH, glutathione, reduced; GSSH, glutathione disulfide, oxidized; GCL, γ-glutamylcysteine ligase; GS, glutathione synthase; GPx, glutathione peroxidase; GR, glutathione reductase; GST, glutathione-S-transferase; γGT, γ-glutamyltransferase; GCT, γ-glutamylcylotransferase; MTA, 5′-methylthioadenosine; Ngb, neuroglobin; TCA, tricarboxylic acid.
Table 5. Complete non-lipid metabolomic profile at one-week post-operation.
Data represents averages of scaled metabolite amount ± standard deviation. For each detected metabolite, the raw area counts were re-scaled to set the median metabolite relative amount equal to 1. The Human Metabolome Database (HMDB) identifier has been provided. The green color in the heat map indicates averaged median values equal or lower than 1.5-fold from the median metabolite amount of 1, whereas red indicates averaged median values equal or higher than 1.5-fold from the median metabolite amount.
| Metabolite 1 week |
HMDB | Subpathway | Control: SCI | Control: Sham | O3PUFA: SCI | O3PUFA: Sham | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Median Metabolite Levels |
Standard Deviation |
Average Median Metabolite Levels |
Standard Deviation |
Average Median Metabolite Levels |
Standard Deviation |
Average Median Metabolite Levels |
Standard Deviation |
||||
| Amino Acids | 2-aminoadipate | HMDB00510 | Lysine Metabolism | 1.18 | 0.28 | 1.07 | 0.25 | 1.07 | 0.25 | 0.65 | 0.27 |
| 2-aminobutyrate | HMDB00650 | Butanoate metabolism | 1.66 | 0.90 | 0.79 | 0.29 | 0.79 | 0.29 | 0.69 | 0.35 | |
| 2-methylbutyroylcarnitine (C5) | HMDB00378 | Valine, leucine and isoleucine metabolism | 1.14 | 0.36 | 0.38 | 0.03 | 0.38 | 0.03 | 0.38 | 0.04 | |
| 3-(4-hydroxyphenyl)lactate (HPLA) | HMDB00755 | Phenylalanine & tyrosine metabolism | 1.41 | 0.36 | 0.74 | 0.21 | 0.74 | 0.21 | 0.75 | 0.08 | |
| 3-hydroxyisobutyrate | HMDB00336 | Valine, leucine and isoleucine metabolism | 1.04 | 0.26 | 0.84 | 0.36 | 0.84 | 0.36 | 0.79 | 0.29 | |
| 3-phosphoserine | HMDB00272 | Glycine, serine and threonine metabolism | 0.94 | 0.37 | 1.01 | 0.33 | 1.01 | 0.33 | 1.01 | 0.28 | |
| 4-guanidinobutanoate | HMDB03464 | Guanidino and acetamido metabolism | 1.22 | 0.18 | 0.80 | 0.26 | 0.80 | 0.26 | 0.82 | 0.20 | |
| 5-aminovalerate | HMDB03355 | Urea cycle; arginine-, proline-, metabolism | 1.12 | 0.12 | 0.80 | 0.15 | 0.80 | 0.15 | 0.79 | 0.16 | |
| 5-methylthioadenosine (MTA) | HMDB01173 | Polyamine metabolism | 0.88 | 0.16 | 1.08 | 0.17 | 1.08 | 0.17 | 1.11 | 0.14 | |
| 5-oxoproline | HMDB00267 | Glutathione metabolism | 0.93 | 0.07 | 1.08 | 0.09 | 1.08 | 0.09 | 1.09 | 0.13 | |
| alanine | HMDB00161 | Alanine and aspartate metabolism | 1.17 | 0.16 | 0.78 | 0.10 | 0.78 | 0.10 | 0.82 | 0.10 | |
| arginine | HMDB00517 | Urea cycle; arginine-, proline-, metabolism | 1.08 | 0.10 | 0.97 | 0.08 | 0.97 | 0.08 | 0.96 | 0.07 | |
| asparagine | HMDB00168 | Alanine and aspartate metabolism | 1.34 | 0.21 | 0.77 | 0.19 | 0.77 | 0.19 | 0.77 | 0.12 | |
| aspartate | HMDB00191 | Alanine and aspartate metabolism | 0.81 | 0.06 | 1.26 | 0.17 | 1.26 | 0.17 | 1.39 | 0.13 | |
| beta-alanine | HMDB00056 | Alanine and aspartate metabolism | 0.92 | 0.10 | 0.90 | 0.23 | 0.90 | 0.23 | 1.12 | 0.24 | |
| betaine | HMDB0043 | Glycine, serine and threonine metebolism | 1.42 | 0.11 | 0.65 | 0.15 | 0.65 | 0.15 | 0.70 | 0.11 | |
| C-glycosyltryptophan* | Tryptophan metabolism | 1.41 | 0.59 | 0.93 | 0.23 | 0.93 | 0.23 | 0.94 | 0.18 | ||
| citrulline | HMDB00904 | Urea cycle; arginine-, proline-, metabolism | 1.64 | 0.35 | 0.61 | 0.15 | 0.61 | 0.15 | 0.53 | 0.10 | |
| creatine | HMDB00064 | Creatine metabolism | 0.88 | 0.12 | 1.05 | 0.17 | 1.05 | 0.17 | 1.06 | 0.21 | |
| creatinine | HMDB00562 | Creatine metabolism | 0.95 | 0.18 | 1.10 | 0.22 | 1.10 | 0.22 | 1.14 | 0.19 | |
| cystathionine | HMDB00099 | Cysteine, methionine, SAM, taurine metabolism | 0.94 | 0.26 | 1.06 | 0.24 | 1.06 | 0.24 | 1.01 | 0.17 | |
| cysteine | HMDB00574 | Cysteine, methionine, SAM, taurine metabolism | 1.04 | 0.15 | 0.90 | 0.15 | 0.90 | 0.15 | 0.95 | 0.09 | |
| cysteine-glutathione disulfide | HMDB00656 | Glutathione metabolism | 1.49 | 0.43 | 0.81 | 0.12 | 0.81 | 0.12 | 0.79 | 0.23 | |
| cystine | HMDB00192 | Cysteine, methionine, SAM, taurine metabolism | 0.99 | 0.35 | 0.99 | 0.32 | 0.99 | 0.32 | 0.72 | 0.15 | |
| gamma-aminobutyrate (GABA) | HMDB00112 | Glutamate metabolism | 0.72 | 0.09 | 1.29 | 0.15 | 1.29 | 0.15 | 1.40 | 0.23 | |
| glutamate | HMDB03339 | Glutamate metabolism | 0.86 | 0.07 | 1.10 | 0.07 | 1.10 | 0.07 | 1.14 | 0.11 | |
| glutamine | HMDB00641 | Glutamate metabolism | 0.96 | 0.11 | 1.05 | 0.11 | 1.05 | 0.11 | 0.90 | 0.14 | |
| glutathione, oxidized (GSSG) | HMDB03337 | Glutathione metabolism | 1.80 | 1.87 | 0.95 | 0.27 | 0.95 | 0.27 | 0.97 | 0.35 | |
| glutathione, reduced (GSH) | HMDB00125 | Glutathione metabolism | 1.06 | 0.34 | 1.07 | 0.26 | 1.07 | 0.26 | 1.15 | 0.23 | |
| glycine | HMDB00123 | Glycine, serine and threonine metabolism | 0.95 | 0.12 | 1.08 | 0.14 | 1.08 | 0.14 | 1.15 | 0.13 | |
| histidine | HMDB00177 | Histidine metabolism | 1.45 | 0.26 | 0.72 | 0.08 | 0.72 | 0.08 | 0.75 | 0.09 | |
| hydroxyisovaleroylcarnitine (C5) | Valine, leucine and isoleucine metabolism | 1.68 | 0.32 | 0.67 | 0.14 | 0.67 | 0.14 | 0.67 | 0.16 | ||
| hypotaurine | HMDB00965 | Cysteine, methionine, SAM, taurine metabolism | 1.74 | 0.28 | 0.54 | 0.14 | 0.54 | 0.14 | 0.62 | 0.11 | |
| isoleucine | HMDB00172 | Valine, leucine and isoleucine metabolism | 1.43 | 0.15 | 0.82 | 0.07 | 0.82 | 0.07 | 0.82 | 0.07 | |
| isovalerylcarnitine (C5) | HMDB00688 | Valine, leucine and isoleucine metabolism | 1.41 | 0.55 | 0.23 | 0.11 | 0.23 | 0.11 | 0.19 | 0.10 | |
| kynurenine | HMDB00684 | Tryptophan metabolism | 2.15 | 3.86 | 2.58 | 5.01 | 2.58 | 5.01 | 4.84 | 4.10 | |
| leucine | HMDB00687 | Valine, leucine and isoleucine metabolism | 1.43 | 0.10 | 0.79 | 0.05 | 0.79 | 0.05 | 0.80 | 0.07 | |
| lysine | HMDB00182 | Lysine metabolism | 1.22 | 0.31 | 0.89 | 0.11 | 0.89 | 0.11 | 0.93 | 0.05 | |
| methionine | HMDB00696 | Cysteine, methionine, SAM, taurine metabolism | 1.28 | 0.11 | 0.86 | 0.06 | 0.86 | 0.06 | 0.88 | 0.06 | |
| N-acetyl-aspartyl-glutamate (NAAG) | HMDB01067 | Glutamate metabolism | 0.71 | 0.05 | 1.18 | 0.06 | 1.18 | 0.06 | 1.14 | 0.05 | |
| N-acetylalanine | HMDB00766 | Alanine and aspartate metabolism | 1.25 | 0.15 | 0.88 | 0.10 | 0.88 | 0.10 | 0.95 | 0.05 | |
| N-acetylaspartate (NAA) | HMDB00812 | Alanine and aspartate metabolism | 0.49 | 0.16 | 1.46 | 0.16 | 1.46 | 0.16 | 1.31 | 0.23 | |
| N-acetylglutamate | HMDB01138 | Glutamate metabolism | 0.61 | 0.20 | 1.23 | 0.35 | 1.23 | 0.35 | 1.34 | 0.28 | |
| N-acetylmethionine | HMDB11745 | Cysteine, methionine, SAM, taurine metabolism | 1.58 | 0.16 | 0.62 | 0.04 | 0.62 | 0.04 | 0.67 | 0.09 | |
| N-acetylthreonine | Glycine, serine and threonine metabolism | 1.27 | 0.29 | 0.78 | 0.10 | 0.78 | 0.10 | 0.77 | 0.11 | ||
| N2-acetyllysine | HMDB00446 | Lysine metabolism | 1.04 | 0.28 | 1.18 | 0.22 | 1.18 | 0.22 | 1.08 | 0.24 | |
| N6-acetyllysine | HMDB00206 | Lysine metabolism | 1.12 | 0.18 | 0.91 | 0.24 | 0.91 | 0.24 | 0.93 | 0.13 | |
| ornithine | HMDB03374 | Urea cycle; arginine-, proline-, metabolism | 1.82 | 0.65 | 0.84 | 0.11 | 0.84 | 0.11 | 0.85 | 0.08 | |
| phenylalanine | HMDB00159 | Phenylalanine & tyrosine metabolism | 1.44 | 0.15 | 0.83 | 0.09 | 0.83 | 0.09 | 0.83 | 0.08 | |
| pipecolate | HMDB00070 | Lysine metabolism | 0.99 | 0.29 | 0.72 | 0.36 | 0.72 | 0.36 | 0.66 | 0.28 | |
| proline | HMDB00162 | Urea cycle; arginine-, proline-, metabolism | 1.27 | 0.12 | 0.88 | 0.08 | 0.88 | 0.08 | 0.83 | 0.06 | |
| putrescine | HMDB01414 | Polyamine metabolism | 1.42 | 0.24 | 0.42 | 0.05 | 0.42 | 0.05 | 0.52 | 0.17 | |
| S-adenosylhomocysteine (SAH) | HMDB00939 | Cysteine, methionine, SAM, taurine metabolism | 0.84 | 0.12 | 1.14 | 0.12 | 1.14 | 0.12 | 1.10 | 0.10 | |
| sarcosine (N-Methylglycine) | HMDB00271 | Glycine, serine and threonine metabolism | 0.97 | 0.13 | 1.06 | 0.15 | 1.06 | 0.15 | 1.01 | 0.23 | |
| serine | HMDB00187 | Glycine, serine and threonine metabolism | 1.21 | 0.19 | 0.77 | 0.13 | 0.77 | 0.13 | 0.80 | 0.10 | |
| spermidine | HMDB01257 | Polyamine metabolism | 1.18 | 0.74 | 0.95 | 0.11 | 0.95 | 0.11 | 1.14 | 0.30 | |
| taurine | HMDB00251 | Cysteine, methionine, SAM, taurine metabolism | 1.70 | 0.51 | 0.72 | 0.22 | 0.72 | 0.22 | 0.78 | 0.18 | |
| threonine | HMDB00167 | Glycine, serine and threonine metabolism | 1.11 | 0.13 | 0.98 | 0.21 | 0.98 | 0.21 | 0.71 | 0.16 | |
| hydroxyproline | HMDB00725 | Urea cycle; arginine-, proline-, metabolism | 1.48 | 0.67 | 0.64 | 0.23 | 0.64 | 0.23 | 0.48 | 0.15 | |
| tryptophan | HMDB00929 | Tryptophan metabolism | 1.23 | 0.10 | 0.82 | 0.11 | 0.82 | 0.11 | 0.83 | 0.13 | |
| tyrosine | HMDB00158 | Phenylalanine & tyrosine metabolism | 1.37 | 0.13 | 0.75 | 0.08 | 0.75 | 0.08 | 0.77 | 0.07 | |
| urea | HMDB00294 | Urea cycle; arginine-, proline-, metabolism | 0.85 | 0.28 | 0.75 | 0.21 | 0.75 | 0.21 | 1.07 | 0.26 | |
| valine | HMDB00883 | Valine, leucine and isoleucine metabolism | 1.42 | 0.11 | 0.84 | 0.06 | 0.84 | 0.06 | 0.85 | 0.06 | |
| Carbohydrates | dihydroxyacetone | HMDB01882 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.18 | 0.41 | 0.84 | 0.51 | 0.84 | 0.51 | 1.22 | 0.59 |
| 1, 5-anhydroglucitol (1, 5-AG) | HMDB02712 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.24 | 0.23 | 0.74 | 0.22 | 0.74 | 0.22 | 0.81 | 0.29 | |
| 3-phosphoglycerate | HMDB00807 | Glycolysis, gluconeogenesis, pyruvate metabolism | 0.99 | 0.20 | 1.03 | 0.14 | 1.03 | 0.14 | 0.93 | 0.16 | |
| arabinose | HMDB00646 | Nucleotide sugars, pentose metabolism | 0.98 | 0.15 | 0.98 | 0.14 | 0.98 | 0.14 | 1.08 | 0.18 | |
| arabitol | HMDB01851 | Nucleotide sugars, pentose metabolism | 1.05 | 0.52 | 1.21 | 0.36 | 1.21 | 0.36 | 0.99 | 0.29 | |
| dihydroxyacetone phosphate (DHAP) | HMDB01473 | Glycolysis, gluconeogenesis, pyruvate metabolism | 0.92 | 0.22 | 0.94 | 0.19 | 0.94 | 0.19 | 1.00 | 0.22 | |
| erythronate* | HMDB00613 | Aminosugars metabolism | 1.26 | 0.29 | 1.02 | 0.14 | 1.02 | 0.14 | 0.80 | 0.11 | |
| fructose | HMDB00660 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.24 | 0.40 | 0.79 | 0.13 | 0.79 | 0.13 | 0.91 | 0.34 | |
| fructose 6-phosphate | HMDB00124 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.62 | 0.47 | 0.80 | 0.10 | 0.80 | 0.10 | 0.82 | 0.26 | |
| glucose | HMDB00122 | Glycolysis, gluconeogenesis, pyruvate metabolism | 2.26 | 1.26 | 0.22 | 0.06 | 0.22 | 0.06 | 0.38 | 0.36 | |
| glucose 6-phosphate | HMDB01401 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.54 | 0.47 | 0.68 | 0.10 | 0.68 | 0.10 | 0.72 | 0.24 | |
| glycerate | HMDB00139 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.28 | 0.31 | 0.95 | 0.32 | 0.95 | 0.32 | 0.93 | 0.20 | |
| Isobar: hexose diphosphates | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.00 | 0.39 | 0.86 | 0.26 | 0.86 | 0.26 | 1.00 | 0.32 | ||
| Isobar: ribulose 5-phosphate, xylulose 5-phosphate | Nucleotide sugars, pentose metabolism | 1.05 | 0.24 | 0.94 | 0.09 | 0.94 | 0.09 | 0.96 | 0.18 | ||
| lactate | HMDB00190 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.01 | 0.12 | 0.99 | 0.11 | 0.99 | 0.11 | 1.04 | 0.13 | |
| maltose | HMDB00163 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.53 | 0.72 | 0.44 | 0.07 | 0.44 | 0.07 | 0.47 | 0.08 | |
| maltotriose | HMDB01262 | Fructose, mannose, galactose, starch, and sucrose metabolism | 0.84 | 0.55 | 0.26 | 0.01 | 0.26 | 0.01 | 0.28 | 0.04 | |
| mannitol | HMDB00765 | Fructose, mannose, galactose, starch, and sucrose metabolism | 0.84 | 0.50 | 0.62 | 0.22 | 0.62 | 0.22 | 0.80 | 0.38 | |
| mannose | HMDB00169 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.65 | 0.45 | 0.57 | 0.14 | 0.57 | 0.14 | 0.61 | 0.16 | |
| mannose 6-phosphate | HMDB01078 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.61 | 0.47 | 0.72 | 0.14 | 0.72 | 0.14 | 0.74 | 0.26 | |
| N-acetylneuraminate | HMDB00230 | Aminosugars metabolism | 1.03 | 0.11 | 1.04 | 0.20 | 1.04 | 0.20 | 0.87 | 0.18 | |
| ribitol | HMDB00508 | Nucleotide sugars, pentose metabolism | 1.02 | 0.37 | 1.05 | 0.33 | 1.05 | 0.33 | 0.96 | 0.24 | |
| ribose | HMDB00283 | Nucleotide sugars, pentose metabolism | 0.99 | 0.17 | 1.16 | 0.31 | 1.16 | 0.31 | 1.45 | 0.60 | |
| ribulose | HMDB00621 | Nucleotide sugars, pentose metabolism | 1.11 | 0.37 | 0.76 | 0.31 | 0.76 | 0.31 | 0.81 | 0.33 | |
| sedoheptulose-7-phosphate | HMDB01068 | Nucleotide sugars, pentose metabolism | 1.37 | 0.55 | 1.61 | 0.36 | 0.61 | 0.36 | 0.94 | 0.41 | |
| sorbitol | HMDB00247 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.13 | 0.40 | 0.86 | 0.25 | 0.86 | 0.25 | 0.93 | 0.27 | |
| sucrose | HMDB00258 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.76 | 0.15 | 0.72 | 0.03 | 0.72 | 0.03 | 1.02 | 0.82 | |
| Cofactors and vitamins | 3′-dephosphocoenzyme A | HMDB01373 | Pantothenate and CoA metabolism | 0.79 | 0.34 | 1.20 | 0.41 | 1.20 | 0.41 | 1.52 | 0.66 |
| alpha-tocopherol | HMDB01893 | Tocopherol metabolism | 1.23 | 1.15 | 0.91 | 0.08 | 0.91 | 0.08 | 0.93 | 0.24 | |
| ascorbate (Vitamin C) | HMDB00044 | Ascorbate and aldarate metabolism | 1.23 | 0.23 | 0.77 | 0.14 | 0.77 | 0.14 | 0.85 | 0.15 | |
| CoA | HMDB01423 | Pantothenate and CoA metabolism | 0.76 | 0.32 | 1.58 | 0.77 | 1.58 | 0.77 | 1.74 | 0.87 | |
| dehydroascorbate | HMDB01264 | Ascorbate and aldarate metabolism | 1.03 | 0.48 | 0.92 | 0.22 | 0.92 | 0.22 | 0.90 | 0.35 | |
| dihydrobiopterin | HMDB00038 | Tetrahydrobiopterin metabolism | 1.11 | 0.28 | 0.71 | 0.15 | 0.71 | 0.15 | 0.62 | 0.00 | |
| FAD | HMDB01248 | Riboflavin metabolism | 1.00 | 0.10 | 0.95 | 0.08 | 0.95 | 0.08 | 1.02 | 0.08 | |
| heme | HMDB03178 | Hemoglobin and porphyrin metabolism | 0.71 | 0.12 | 0.68 | 0.05 | 0.68 | 0.05 | 0.80 | 0.27 | |
| nicotinamide | HMDB01406 | Nicotinate and nicotinamide metabolism | 0.88 | 0.07 | 1.06 | 0.07 | 1.06 | 0.07 | 1.09 | 0.08 | |
| NAD+ | HMDB00902 | Nicotinate and nicotinamide metabolism | 0.88 | 0.13 | 1.07 | 0.21 | 1.07 | 0.21 | 1.07 | 0.17 | |
| pantothenate (Vitamin B5) | HMDB00210 | Pantothenate and CoA metabolism | 1.00 | 0.16 | 1.00 | 0.16 | 1.00 | 0.16 | 1.08 | 0.20 | |
| phosphopantetheine | HMDB01416 | Pantothenate and CoA metabolism | 0.91 | 0.10 | 1.12 | 0.13 | 1.12 | 0.13 | 1.18 | 0.14 | |
| pyridoxal | HMDB01545 | Pyridoxal metabolism | 1.02 | 0.17 | 0.97 | 0.31 | 0.97 | 0.31 | 1.08 | 0.29 | |
| riboflavin (Vitamin B2) | HMDB00244 | Riboflavin metabolism | 1.11 | 0.12 | 0.84 | 0.12 | 0.84 | 0.12 | 0.92 | 0.16 | |
| Energy | cis-aconitate | HMDB00072 | Krebs cycle | 1.49 | 0.18 | 0.61 | 0.09 | 0.61 | 0.09 | 0.66 | 0.10 |
| citrate | HMDB00094 | Krebs cycle | 1.66 | 0.38 | 0.65 | 0.09 | 0.65 | 0.09 | 0.69 | 0.10 | |
| fumarate | HMDB00134 | Krebs cycle | 1.01 | 0.22 | 1.04 | 0.17 | 1.04 | 0.17 | 1.15 | 0.23 | |
| malate | HMDB00153 | Krebs cycle | 1.10 | 0.16 | 0.96 | 0.07 | 0.96 | 0.07 | 1.01 | 0.14 | |
| phosphate | HMDB01429 | Oxidative phosphorylation | 0.97 | 0.04 | 1.02 | 0.03 | 1.02 | 0.03 | 1.07 | 0.05 | |
| pyrophosphate (PPi) | HMDB00250 | Oxidative phosphorylation | 0.85 | 0.50 | 0.75 | 0.41 | 0.75 | 0.41 | 1.03 | 0.66 | |
| succinylcarnitine (C4) | Krebs cycle | 1.20 | 0.30 | 0.76 | 0.19 | 0.76 | 0.19 | 0.92 | 0.13 | ||
| Nucleotides | 2′-deoxycytidine | HMDB00014 | Pyrimidine metabolism, cytidine containing | 1.45 | 0.20 | 0.64 | 0.09 | 0.64 | 0.09 | 0.67 | 0.13 |
| 2′-deoxyinosine | HMDB00071 | Purine metabolism, (hypo)xanthine/inosine containing | 1.15 | 0.44 | 0.46 | 0.00 | 0.46 | 0.00 | 0.46 | 0.00 | |
| 5,6-dihydrouracil | HMDB00076 | Purine metabolism, adenine containing | 1.04 | 0.39 | 1.08 | 0.18 | 1.08 | 0.18 | 0.95 | 0.23 | |
| adenine | HMDB00034 | Purine metabolism, adenine containing | 0.78 | 0.15 | 1.29 | 0.15 | 1.29 | 0.15 | 1.23 | 0.22 | |
| adenosine | HMDB00050 | Purine metabolism, adenine containing | 0.33 | 0.16 | 1.56 | 0.43 | 1.56 | 0.43 | 1.75 | 0.39 | |
| 2′-AMP | HMDB11617 | Purine metabolism, adenine containing | 1.13 | 0.48 | 0.85 | 0.22 | 0.85 | 0.22 | 0.82 | 0.28 | |
| 3′-AMP | HMDB03540 | Purine metabolism, adenine containing | 1.18 | 0.14 | 0.76 | 0.16 | 0.76 | 0.16 | 0.75 | 0.18 | |
| AMP | HMDB00045 | Purine metabolism, urate metabolism | 0.80 | 0.15 | 1.17 | 0.36 | 1.17 | 0.36 | 1.34 | 0.23 | |
| allantoin | HMDB00462 | Pyrimidine metabolism, cytidine containing | 1.16 | 0.58 | 0.87 | 0.35 | 0.87 | 0.35 | 1.00 | 0.56 | |
| cytidine | HMDB00089 | Pyrimidine metabolism, cytidine containing | 1.09 | 0.07 | 0.94 | 0.04 | 0.94 | 0.04 | 0.96 | 0.06 | |
| 3′-CMP | Purine metabolism, guanine containing | 0.92 | 0.15 | 0.98 | 0.15 | 0.98 | 0.15 | 1.09 | 0.18 | ||
| guanosine | HMDB00133 | Purine metabolism, (hypo)xanthine/inosine containing | 0.54 | 0.09 | 1.33 | 0.10 | 1.33 | 0.10 | 1.25 | 0.18 | |
| hypoxanthine | HMDB00157 | Purine metabolism, (hypo)xanthine/inosine containing | 1.02 | 0.07 | 1.00 | 0.09 | 1.00 | 0.09 | 1.02 | 0.10 | |
| inosine | HMDB00195 | Purine and pyrimidine metabolism | 0.79 | 0.07 | 1.05 | 0.06 | 1.05 | 0.06 | 1.09 | 0.04 | |
| methylphosphate | Purine metabolism, adenine containing | 0.94 | 0.14 | 1.07 | 0.11 | 1.07 | 0.11 | 1.09 | 0.13 | ||
| 1-methyladenosine | HMDB03331 | Pyrimidine metabolism, uracil containing | 1.21 | 0.31 | 0.86 | 0.18 | 0.86 | 0.18 | 0.90 | 0.35 | |
| pseudouridine | HMDB00767 | Pyrimidine metabolism, uracil containing | 1.36 | 0.31 | 0.76 | 0.19 | 0.76 | 0.19 | 0.81 | 0.08 | |
| uracil | HMDB00300 | Purine metabolism, urate metabolism | 1.30 | 0.17 | 0.76 | 0.14 | 0.76 | 0.14 | 0.78 | 0.12 | |
| urate | HMDB00289 | Pyrimidine metabolism, uracil containing | 1.64 | 0.37 | 0.54 | 0.24 | 0.54 | 0.24 | 0.48 | 0.12 | |
| uridine | HMDB00296 | Purine metabolism, (hypo)xanthine/inosine containing | 0.77 | 0.10 | 1.17 | 0.12 | 1.17 | 0.12 | 1.10 | 0.16 | |
| xanthine | HMDB00292 | Purine metabolism, (hypo)xanthine/inosine containing | 1.12 | 0.16 | 0.94 | 0.09 | 0.94 | 0.09 | 0.94 | 0.09 | |
| xanthosine | HMDB00299 | Dipeptide derivative | 1.28 | 0.25 | 0.78 | 0.14 | 0.78 | 0.14 | 0.72 | 0.15 | |
| Peptides | anserine | HMDB00194 | Dipeptide derivative | 0.70 | 0.31 | 1.61 | 1.53 | 1.61 | 1.53 | 2.15 | 1.47 |
| carnosine | HMDB00033 | gamma-glutamyl | 0.58 | 0.04 | 0.99 | 0.31 | 0.99 | 0.31 | 1.31 | 0.23 | |
| gamma-glutamylalanine | gamma-glutamyl | 1.37 | 0.23 | 0.80 | 0.15 | 0.80 | 0.15 | 0.80 | 0.22 | ||
| gamma-glutamylglutamate | gamma-glutamyl | 0.85 | 0.11 | 1.15 | 0.15 | 1.15 | 0.15 | 1.19 | 0.19 | ||
| gamma-glutamylglutamine | HMDB11738 | gamma-glutamyl | 1.02 | 0.21 | 0.92 | 0.10 | 0.92 | 0.10 | 0.94 | 0.14 | |
| gamma-glutamylglycine | HMDB11667 | gamma-glutamyl | 0.71 | 0.21 | 1.11 | 0.25 | 1.11 | 0.25 | 1.31 | 0.31 | |
| gamma-glutamylleucine | HMDB11171 | gamma-glutamyl | 1.20 | 0.15 | 0.75 | 0.21 | 0.75 | 0.21 | 0.80 | 0.10 | |
| gamma-glutamylmethionine | gamma-glutamyl | 1.18 | 0.16 | 0.92 | 0.18 | 0.92 | 0.18 | 0.81 | 0.20 | ||
| gamma-glutamylphenylalanine | HMDB00594 | gamma-glutamyl | 1.11 | 0.23 | 0.89 | 0.16 | 0.89 | 0.16 | 0.89 | 0.16 | |
| gamma-glutamylthreonine* | gamma-glutamyl | 1.09 | 0.17 | 0.97 | 0.11 | 0.97 | 0.11 | 0.89 | 0.20 | ||
| gamma-glutamyltyrosine | Dipeptide | 1.08 | 0.11 | 0.82 | 0.33 | 0.82 | 0.33 | 0.91 | 0.29 | ||
| glycylglycine | HMDB11733 | Dipeptide | 1.26 | 0.34 | 0.93 | 0.18 | 0.93 | 0.18 | 0.90 | 0.07 | |
| glycylleucine | HMDB00759 | Dipeptide | 1.17 | 0.21 | 0.90 | 0.09 | 0.90 | 0.09 | 0.97 | 0.15 | |
| glycylphenylalanine | Dipeptide derivative | 1.08 | 0.11 | 0.79 | 0.22 | 0.79 | 0.22 | 0.77 | 0.23 | ||
| homocarnosine | HMDB00745 | Dipeptide | 0.82 | 0.17 | 1.07 | 0.13 | 1.07 | 0.13 | 1.36 | 0.17 | |
| isoleucylglycine | Dipeptide | 1.15 | 0.27 | 0.95 | 0.12 | 0.95 | 0.12 | 0.93 | 0.13 | ||
| leucylglycine | Dipeptide | 1.00 | 0.29 | 1.08 | 0.28 | 1.08 | 0.28 | 1.25 | 0.37 | ||
| leucylserine | Dipeptide | 1.56 | 0.24 | 0.76 | 0.19 | 0.76 | 0.19 | 0.62 | 0.22 | ||
| phenylalanylglycine | Dipeptide | 1.23 | 0.42 | 0.28 | 0.06 | 0.28 | 0.06 | 0.28 | 0.05 | ||
| phenylalanylphenylalanine | Dipeptide | 1.30 | 0.31 | 0.31 | 0.08 | 0.31 | 0.08 | 0.46 | 0.23 | ||
| phenylalanylserine | Dipeptide | 2.04 | 0.57 | 0.82 | 0.16 | 0.82 | 0.16 | 0.90 | 0.08 | ||
| prolylmethionine | Dipeptide | 1.35 | 0.34 | 0.85 | 0.14 | 0.85 | 0.14 | 0.86 | 0.19 | ||
| threonylalanine | Dipeptide | 0.98 | 0.27 | 0.98 | 0.30 | 0.98 | 0.30 | 1.11 | 0.37 | ||
| tryptophylglycine | Dipeptide | 1.37 | 0.33 | 0.91 | 0.13 | 0.91 | 0.13 | 0.81 | 0.21 | ||
| tyrosylglycine | Dipeptide | 1.77 | 0.29 | 0.62 | 0.12 | 0.62 | 0.12 | 0.64 | 0.14 | ||
| tyrosylleucine | 1.55 | 0.29 | 0.63 | 0.09 | 0.63 | 0.09 | 0.68 | 0.17 | |||
| Xenobiotics | 2-pyrrolidinone | HMDB02039 | Chemical | 1.10 | 0.50 | 1.46 | 0.68 | 1.46 | 0.68 | 1.45 | 1.17 |
| dihydrokaempferol | Food component/Plant | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | ||
| ergothioneine | HMDB03045 | Food component/Plant | 1.30 | 0.23 | 0.89 | 0.25 | 0.89 | 0.25 | 0.91 | 0.30 | |
| erythritol | HMDB02994 | Sugar, sugar substitute, starch | 0.95 | 0.23 | 1.13 | 0.19 | 1.13 | 0.19 | 1.06 | 0.21 | |
| glycerol 2-phosphate | HMDB02520 | Chemical | 1.12 | 0.35 | 0.90 | 0.24 | 0.90 | 0.24 | 0.87 | 0.32 | |
| hippurate | HMDB00714 | Benzoate metabolism | 0.74 | 0.44 | 0.90 | 0.32 | 0.90 | 0.32 | 1.08 | 0.69 | |
| ketamine | Drug | 1.05 | 1.14 | 0.72 | 0.69 | 0.72 | 0.69 | 0.80 | 0.92 | ||
| pentobarbital | Drug | 0.71 | 0.61 | 1.02 | 0.62 | 1.02 | 0.62 | 1.33 | 0.92 | ||
Table 6. Complete non-lipid metabolomic profile at eight weeks post-operation.
Data represents averages of scaled metabolite amount ± standard deviation. For each small molecule, the raw area counts were re-scaled to set the median metabolite amount equal to 1. The Human Metabolome Database (HMDB) identifier has been provided. The green color in the heat map indicates averaged median values equal or lower than 1.5-fold from the median metabolite amount of 1, whereas red indicates averaged median values equal or higher than 1.5-fold from the median metabolite amount.
| Metabolite 8 weeks |
HMDB | Subpathway | Control: SCI | Control: Sham | O3PUFA: SCI | O3PUFA: Sham | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Median Metabolite Levels |
Standard Deviation |
Average Median Metabolite Levels |
Standard Deviation |
Average Median Metabolite Levels |
Standard Deviation |
Average Median Metabolite Levels |
Standard Deviation |
||||
| Amino Acids | 2-aminoadipate | HMDB00510 | Lysine metabolism | 1.06 | 0.44 | 1.20 | 0.46 | 1.20 | 0.46 | 1.08 | 0.50 |
| 2-aminobutyrate | HMDB00650 | Butanoate metabolism | 1.64 | 0.80 | 0.89 | 0.68 | 0.89 | 0.68 | 0.79 | 0.48 | |
| 2-hydroxybutyrate (AHB) | HMDB00008 | Cysteine, methionine, SAM, taurine metabolism | 1.14 | 0.32 | 0.96 | 0.31 | 0.96 | 0.31 | 0.68 | 0.30 | |
| 2-methylbutyroylcarnitine | HMDB00378 | Valine, leucine and isoleucine metabolism | 0.92 | 0.41 | 1.04 | 0.30 | 1.04 | 0.30 | 0.60 | 0.22 | |
| 3-(4-hydroxyphenyl)lactate | HMDB00755 | Phenylalanine & tyrosine metabolism | 0.91 | 0.22 | 0.92 | 0.23 | 0.92 | 0.23 | 0.90 | 0.26 | |
| 3-hydroxyisobutyrate | HMDB00336 | Valine, leucine and isoleucine metabolism | 0.87 | 0.27 | 0.93 | 0.29 | 0.93 | 0.29 | 0.76 | 0.21 | |
| 3-phosphoserine | HMDB00272 | Glycine, serine and threonine metabolism | 0.95 | 0.25 | 1.17 | 0.42 | 1.17 | 0.42 | 0.61 | 0.29 | |
| 4-guanidinobutanoate | HMDB03464 | Guanidino and acetamido metabolism | 0.84 | 0.23 | 1.06 | 0.19 | 1.06 | 0.19 | 1.29 | 0.39 | |
| 5-methylthioadenosine (MTA) | HMDB01173 | Polyamine metabolism | 0.88 | 0.13 | 1.05 | 0.19 | 1.05 | 0.19 | 1.04 | 0.14 | |
| 5-oxopriline | HMDB00267 | Glutathione metabolism | 1.10 | 0.32 | 1.18 | 0.12 | 1.18 | 0.12 | 0.90 | 0.11 | |
| agmatine | HMDB01432 | Polyamine metabolism | 1.16 | 0.40 | 1.47 | 0.58 | 1.47 | 0.58 | 0.87 | 0.27 | |
| alanine | HMDB00161 | Alanine and aspartate metabolism | 1.21 | 0.22 | 1.29 | 0.21 | 1.29 | 0.21 | 0.85 | 0.08 | |
| arginine | HMDB00517 | Urea cycle; arginine-, proline-, metabolism | 1.01 | 0.16 | 0.95 | 0.18 | 0.95 | 0.18 | 1.00 | 0.07 | |
| asparagine | HMDB00168 | Alanine and aspartate metabolism | 1.00 | 0.22 | 0.75 | 0.26 | 0.75 | 0.26 | 1.10 | 0.15 | |
| aspartate | HMDB00191 | Alanine and aspartate metabolism | 0.82 | 0.23 | 0.79 | 0.12 | 0.79 | 0.12 | 1.27 | 0.15 | |
| beta-alanine | HMDB00056 | Alanine and aspartate metabolism | 1.08 | 0.42 | 0.92 | 0.37 | 0.92 | 0.37 | 1.02 | 0.52 | |
| betaine | HMDB00043 | Glycine, serine and threonine metabolism | 1.20 | 0.33 | 1.37 | 0.28 | 1.37 | 0.28 | 0.58 | 0.14 | |
| C-glycosyltryptophan* | Tryptophan metabolism | 1.09 | 0.32 | 1.19 | 0.20 | 1.19 | 0.20 | 0.96 | 0.12 | ||
| citrulline | HMDB00904 | Urea cycle; arginine-, proline-, metabolism | 1.19 | 0.26 | 1.33 | 0.25 | 1.33 | 0.25 | 0.30 | 0.03 | |
| creatine | HMDB00064 | Creatine metabolism | 0.90 | 0.16 | 0.95 | 0.07 | 0.95 | 0.07 | 1.07 | 0.07 | |
| creatinine | HMDB00562 | Creatine metabolism | 0.95 | 0.21 | 1.03 | 0.14 | 1.03 | 0.14 | 1.01 | 0.22 | |
| cystathionine | HMDB00099 | Cysteine, methionine, SAM, taurine metabolism | 0.91 | 0.16 | 1.01 | 0.25 | 1.01 | 0.25 | 1.10 | 0.21 | |
| cysteine | HMDB00574 | Cysteine, methionine, SAM, taurine metabolism | 1.11 | 0.40 | 1.38 | 0.39 | 1.38 | 0.39 | 0.83 | 0.15 | |
| cysteine-glutathione disulfide | HMDB00656 | Glutathione metabolism | 1.33 | 0.39 | 1.40 | 0.16 | 1.40 | 0.16 | 0.83 | 0.08 | |
| cystine | HMDB00192 | Cysteine, methionine, SAM, taurine metabolism | 1.34 | 0.63 | 1.33 | 0.35 | 1.33 | 0.35 | 0.75 | 0.12 | |
| gamma-aminobutyrate (GABA) | HMDB00112 | Glutamate metabolism | 0.87 | 0.32 | 0.73 | 0.22 | 0.73 | 0.22 | 1.44 | 0.46 | |
| glutamate | HMDB03339 | Glutamate metabolism | 0.80 | 0.14 | 0.86 | 0.09 | 0.86 | 0.09 | 1.19 | 0.10 | |
| glutamine | HMDB00641 | Glutamate metabolism | 0.96 | 0.21 | 1.08 | 0.16 | 1.08 | 0.16 | 1.00 | 0.12 | |
| glutathione, oxidized (GSSG) | HMDB03337 | Glutathione metabolism | 1.14 | 0.39 | 1.54 | 0.21 | 1.54 | 0.21 | 0.70 | 0.15 | |
| glutathione, reduced (GSH) | HMDB00125 | Glutathione metabolism | 0.89 | 0.14 | 1.25 | 0.30 | 1.25 | 0.30 | 0.99 | 0.17 | |
| glycine | HMDB00123 | Glycine, serine and threonine metabolism | 0.77 | 0.22 | 0.81 | 0.14 | 0.81 | 0.14 | 1.23 | 0.14 | |
| histidine | HMDB00177 | Histidine metabolism | 1.14 | 0.16 | 1.13 | 0.13 | 1.13 | 0.13 | 0.73 | 0.04 | |
| hydroxyisovaleroyl carnitine | Valine, leucine and isoleucine metabolism | 1.12 | 0.41 | 1.37 | 0.24 | 1.37 | 0.24 | 0.85 | 0.22 | ||
| hypotaurine | HMDB00965 | Cysteine, methionine, SAM, taurine metabolism | 1.50 | 0.67 | 1.58 | 0.61 | 1.58 | 0.61 | 0.84 | 0.35 | |
| isoleucine | HMDB00172 | Valine, leucine and isoleucine metabolism | 1.23 | 0.24 | 1.13 | 0.13 | 1.13 | 0.13 | 0.69 | 0.06 | |
| isovalerylcarnitine | HMDB00688 | Valine, leucine and isoleucine metabolism | 1.04 | 0.42 | 1.20 | 0.39 | 1.20 | 0.39 | 0.89 | 0.28 | |
| kynurenine | HMDB00684 | Tryptophan metabolism | 0.87 | 0.66 | 0.74 | 0.57 | 0.74 | 0.57 | 0.96 | 0.58 | |
| leucine | HMDB00687 | Valine, leucine and isoleucine metabolism | 1.21 | 0.24 | 1.14 | 0.13 | 1.14 | 0.13 | 0.71 | 0.08 | |
| lysine | HMDB00182 | Lysine metabolism | 1.22 | 0.30 | 1.34 | 0.15 | 1.34 | 0.15 | 0.91 | 0.09 | |
| methionine | HMDB00696 | Cysteine, methionine, SAM, taurine metabolism | 1.14 | 0.18 | 1.11 | 0.12 | 1.11 | 0.12 | 0.76 | 0.08 | |
| N-acetyl-aspartyl-glutamate (NAAG) | HMDB01067 | Glutamate metabolism | 0.79 | 0.19 | 0.81 | 0.10 | 0.81 | 0.10 | 1.77 | 0.17 | |
| N-acetylalanine | HMDB00766 | Alanine and aspartate metabolism | 1.05 | 0.32 | 0.92 | 0.14 | 0.92 | 0.14 | 1.10 | 0.16 | |
| N-acetylaspartate (NAA) | HMDB00812 | Alanine and aspartate metabolism | 0.77 | 0.22 | 0.82 | 0.09 | 0.82 | 0.09 | 1.55 | 0.07 | |
| N-acetylglutamate | HMDB01138 | Glutamate metabolism | 0.77 | 0.21 | 0.73 | 0.15 | 0.73 | 0.15 | 1.47 | 1.19 | |
| N-acetylmethionine | HMDB11745 | Cysteine, methionine, SAM, taurine metabolism | 1.40 | 0.31 | 1.22 | 0.17 | 1.22 | 0.17 | 0.49 | 0.08 | |
| N-acetyltryptophan | Tryptophan metabolism | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | ||
| N2-acetyllysine | HMDB00446 | Lysine metabolism | 0.93 | 0.25 | 1.38 | 0.38 | 1.38 | 0.38 | 1.03 | 0.46 | |
| ophthalmate | HMDB05765 | Glutathione metabolism | 1.21 | 0.61 | 0.95 | 0.58 | 0.95 | 0.58 | 0.93 | 0.41 | |
| ornithine | HMDB03374 | Urea cycle; arginine-, proline-, metabolism | 1.07 | 0.31 | 1.19 | 0.20 | 1.19 | 0.20 | 0.87 | 0.22 | |
| phenylalanine | HMDB00159 | Phenylalanine & tyrosine metabolism | 1.21 | 0.22 | 1.13 | 0.14 | 1.13 | 0.14 | 0.86 | 0.09 | |
| pipecolate | HMDB00070 | Lysine metabolism | 1.25 | 0.38 | 1.28 | 0.25 | 1.28 | 0.25 | 0.93 | 0.24 | |
| proline | HMDB00162 | Urea cycle; arginine-, proline-, metabolism | 1.13 | 0.11 | 1.15 | 0.15 | 1.15 | 0.15 | 0.79 | 0.05 | |
| putrescine | HMDB01414 | Polymine metabolism | 1.90 | 0.71 | 1.99 | 0.73 | 1.99 | 0.73 | 0.62 | 0.19 | |
| S-adenosylhomocysteine (SAH) | HMDB00939 | Cysteine, methionine, SAM, taurine metabolism | 0.90 | 0.23 | 0.88 | 0.15 | 0.88 | 0.15 | 1.10 | 0.15 | |
| S-methylcysteine | HMDB02108 | Cysteine, methionine, SAM, taurine metabolism | 0.86 | 0.42 | 0.96 | 0.33 | 0.96 | 0.33 | 0.69 | 0.16 | |
| saccharopine | HMDB00279 | Lysine metabolism | 0.65 | 0.01 | 0.75 | 0.21 | 0.75 | 0.21 | 0.91 | 0.40 | |
| sarcosine (N-Methylglycine) | HMDB00271 | Glycine, serine and threonine metabolism | 1.00 | 0.37 | 1.05 | 0.32 | 1.05 | 0.32 | 1.07 | 0.13 | |
| serine | HMDB03406 | Glycine, serine and threonine metabolism | 1.16 | 0.23 | 1.22 | 0.19 | 1.22 | 0.19 | 0.87 | 0.10 | |
| spermidine | HMDB01257 | Polyamine metabolism | 0.88 | 0.25 | 0.97 | 0.19 | 0.97 | 0.19 | 1.00 | 0.12 | |
| taurine | HMDB00251 | Cysteine, methionine, SAM, taurine metabolism | 1.24 | 0.30 | 1.47 | 0.28 | 1.47 | 0.28 | 0.76 | 0.16 | |
| threonine | HMDB00167 | Glycine, serine and threonine metabolism | 1.04 | 0.19 | 1.04 | 0.15 | 1.04 | 0.15 | 0.86 | 0.14 | |
| trans-4-hydroxyproline | HMDB00725 | Urea cycle; arginine-, proline-, metabolism | 0.87 | 0.61 | 0.75 | 0.59 | 0.75 | 0.59 | 0.29 | 0.11 | |
| tryptophan | HMDB00929 | Tryptophan metabolism | 1.10 | 0.19 | 1.10 | 0.13 | 1.10 | 0.13 | 0.97 | 0.07 | |
| tyrosine | HMDB00158 | Phenylalanine & tyrosine metabolism | 1.17 | 0.21 | 1.18 | 0.14 | 1.18 | 0.14 | 0.64 | 0.06 | |
| urea | HMDB00294 | Urea cycle; arginine-, proline-, metabolism | 0.76 | 0.49 | 0.98 | 0.54 | 0.98 | 0.54 | 1.24 | 0.47 | |
| valine | HMDB00883 | Valine, leucine and isoleucine metabolism | 1.21 | 0.27 | 1.16 | 0.15 | 1.16 | 0.15 | 0.69 | 0.07 | |
| Carbohydrates | 1,5-anhydroglucitol (1,5-AG) | HMDB02712 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.14 | 0.52 | 1.04 | 0.28 | 1.04 | 0.28 | 0.83 | 0.21 |
| 1,6-anhydroglucose | HMDB00640 | Glycolysis, gluconeogenesis, pyruvate metabolism | 0.57 | 0.27 | 0.90 | 0.55 | 0.90 | 0.55 | 0.64 | 0.34 | |
| arabinose | HMDB00646 | Nucleotide sugars, pentose metabolism | 0.52 | 0.29 | 0.69 | 0.53 | 0.69 | 0.53 | 0.66 | 0.46 | |
| arabitol | HMDB01851 | Nucleotide sugars, pentose metabolism | 0.91 | 0.26 | 1.02 | 0.36 | 1.02 | 0.36 | 0.97 | 0.31 | |
| dihydroxyacetone phosphate (DHAP) | HMDB01473 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.08 | 0.29 | 1.07 | 0.28 | 1.07 | 0.28 | 0.88 | 0.17 | |
| erythronate* | HMDB00613 | Aminosugars metabolism | 1.12 | 0.36 | 1.15 | 0.23 | 1.15 | 0.23 | 0.92 | 0.17 | |
| fructose | HMDB00660 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.28 | 0.47 | 1.38 | 0.31 | 1.38 | 0.31 | 0.69 | 0.15 | |
| fructose 1-phosphate | HMDB01076 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.24 | 0.63 | 1.45 | 0.61 | 1.45 | 0.61 | 0.67 | 0.22 | |
| fructose-6-phosphate | HMDB00124 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.46 | 0.49 | 1.72 | 0.43 | 1.72 | 0.43 | 0.25 | 0.08 | |
| galactose | HMDB00143 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.31 | 0.37 | 1.39 | 0.27 | 1.39 | 0.27 | 0.29 | 0.09 | |
| glucose | HMDB00122 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.61 | 0.72 | 2.07 | 0.70 | 2.07 | 0.70 | 0.13 | 0.04 | |
| glucose-6-phosphate (G6P) | HMDB01401 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.44 | 0.47 | 1.69 | 0.41 | 1.69 | 0.41 | 0.23 | 0.07 | |
| glycerate | HMDB00139 | Glycolysis, gluconeogenesis, pyruvate metabolism | 0.86 | 0.23 | 1.13 | 0.48 | 1.13 | 0.48 | 0.67 | 0.34 | |
| Isobar: fructose 1,6-diphosphate, glucose 1,6-diphosphate, myo-inositol 1,4 or 1 | Glycolysis, gluconeogenesis, pyruvate metabolism | 0.97 | 0.22 | 0.91 | 0.13 | 0.91 | 0.13 | 0.98 | 0.16 | ||
| Isobar: ribulose 5-phosphate, xylulose 5-phosphate | Nucleotide sugars, pantose metabolism | 1.40 | 0.57 | 1.57 | 0.41 | 1.57 | 0.41 | 0.55 | 0.11 | ||
| lactate | HMDB00190 | Glycolysis, gluconeogenesis, pyruvate metabolism | 1.01 | 0.24 | 1.09 | 0.18 | 1.09 | 0.18 | 0.96 | 0.13 | |
| maltose | HMDB00163 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.30 | 0.41 | 2.21 | 1.04 | 2.21 | 1.04 | 0.39 | 0.07 | |
| maltotriose | HMDB01262 | Fructose, mannose, galactose, starch, and sucrose metabolism | 0.64 | 0.18 | 1.40 | 0.70 | 1.40 | 0.70 | 0.52 | 0.00 | |
| mannitol | HMDB00765 | Fructose, mannose, galactose, starch, and sucrose metabolism | 0.98 | 0.46 | 0.88 | 0.49 | 0.88 | 0.49 | 0.75 | 0.26 | |
| mannose | HMDB00169 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.39 | 0.37 | 1.52 | 0.27 | 1.52 | 0.27 | 0.29 | 0.03 | |
| mannose-6-phosphate | HMDB01078 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.43 | 0.45 | 1.76 | 0.43 | 1.76 | 0.43 | 0.25 | 0.07 | |
| N-acetylglucosamine 6-phosphate | HMDB02817 | Aminosugars metabolism | 0.99 | 0.43 | 1.35 | 0.42 | 1.35 | 0.42 | 0.83 | 0.36 | |
| N-acetylneuraminate | HMDB00230 | Aminosugars metabolism | 1.08 | 0.26 | 1.09 | 0.15 | 1.09 | 0.15 | 0.83 | 0.11 | |
| ribose | HMDB00283 | Nucleotide sugars, pentose metabolism | 0.90 | 0.32 | 1.09 | 0.35 | 1.09 | 0.35 | 1.01 | 0.13 | |
| ribulose | HMDB00621,HMDB03371 | Nucleotide sugars, pentose metabolism | 0.31 | 0.55 | 1.34 | 0.70 | 1.34 | 0.70 | 0.62 | 0.32 | |
| sedoheptulose-7-phosphate | HMDB01068 | Nucleotide sugars, pentose metabolism | 1.44 | 0.64 | 1.52 | 0.53 | 1.52 | 0.53 | 0.24 | 0.11 | |
| sorbitol | HMDB00247 | Fructose, mannose, galactose, starch, and sucrose metabolism | 1.19 | 0.43 | 1.23 | 0.36 | 1.23 | 0.36 | 0.78 | 0.23 | |
| Cofactors and vitamins | 3′-dephosphocoenzyme A | HMDB01373 | Pantothenate and CoA metabolism | 0.73 | 0.26 | 0.79 | 0.27 | 0.79 | 0.27 | 1.30 | 0.35 |
| acetyl CoA | HMDB01206 | Pantothenate and CoA metabolism | 0.82 | 0.19 | 0.89 | 0.16 | 0.89 | 0.16 | 1.30 | 0.28 | |
| alpha-tocopherol | HMDB01893 | Tocopherol metabolism | 1.19 | 0.14 | 1.29 | 0.26 | 1.29 | 0.26 | 0.77 | 0.15 | |
| ascorbate (Vitamin C) | HMDB00044 | Ascorbate and aldarate metabolism | 1.19 | 0.24 | 1.32 | 0.28 | 1.32 | 0.28 | 0.85 | 0.11 | |
| coenzyme A | HMDB01423 | Pantothenate and CoA metabolism | 0.73 | 0.20 | 0.83 | 0.16 | 0.83 | 0.16 | 1.48 | 0.27 | |
| dihydrobiopterin | HMDB00038 | Folate metabolism | 1.18 | 0.37 | 1.40 | 0.47 | 1.40 | 0.47 | 0.53 | 0.14 | |
| flavin adenine dinucleotide (FAD) | HMDB01248 | Riboflavin metabolism | 1.09 | 0.20 | 1.13 | 0.16 | 1.13 | 0.16 | 0.83 | 0.11 | |
| heme | Hemoglobin and porphyrin metabolism | 1.01 | 0.37 | 1.07 | 0.29 | 1.07 | 0.29 | 0.96 | 0.42 | ||
| nicotinamide | HMDB01406 | Nicotinate and nicotinamide metabolism | 0.97 | 0.22 | 0.99 | 0.19 | 0.99 | 0.19 | 1.00 | 0.20 | |
| pantothenate | HMDB00210 | Pantothenate and CoA metabolism | 1.24 | 0.24 | 1.36 | 0.28 | 1.36 | 0.28 | 0.82 | 0.17 | |
| phosphopantetheine | HMDB01416 | Pantothenate and CoA metabolism | 1.00 | 0.19 | 1.20 | 0.33 | 1.20 | 0.33 | 0.94 | 0.16 | |
| riboflavin (Vitamin B2) | HMDB00244 | Riboflavin metabolism | 1.07 | 0.05 | 1.08 | 0.23 | 1.08 | 0.23 | 0.80 | 0.15 | |
| Energy | acetylphosphate | HMDB01494 | Oxidative phosphorylation | 0.98 | 0.20 | 1.11 | 0.18 | 1.11 | 0.18 | 0.97 | 0.04 |
| citrate | HMDB00094 | Krebs cycle | 1.15 | 0.32 | 1.49 | 0.43 | 1.49 | 0.43 | 0.79 | 0.18 | |
| fumarate | HMDB00134 | Krebs cycle | 0.95 | 0.33 | 1.14 | 0.35 | 1.14 | 0.35 | 1.08 | 0.31 | |
| malate | HMDB00156 | Krebs cycle | 0.94 | 0.24 | 1.05 | 0.20 | 1.05 | 0.20 | 0.94 | 0.07 | |
| phosphate | HMDB01429 | Oxidative phosphorylation | 0.97 | 0.07 | 0.98 | 0.05 | 0.98 | 0.05 | 1.03 | 0.04 | |
| pyrophosphate (PPi) | HMDB00250 | Oxidative phosphorylations | 1.04 | 0.30 | 1.15 | 0.27 | 1.15 | 0.27 | 425F02;0.89 | 0.20 | |
| Nucleotides | adenine | HMDB00034 | Purine metabolism, adenine containing | 0.81 | 0.18 | 0.86 | 0.13 | 0.86 | 0.13 | 1.35 | 0.28 |
| adenosine | HMDB00050 | Purine metabolism, adenine containing | 0.73 | 0.21 | 0.67 | 0.16 | 0.67 | 0.16 | 2.87 | 0.63 | |
| adenosine 2′-monophosphate (2′-AMP) | HMDB11617 | Purine metabolism, adenine containing | 1.42 | 0.48 | 1.35 | 0.22 | 1.35 | 0.22 | 0.58 | 0.10 | |
| adenosine 3′-monophosphate (3′-AMP) | HMDB03540 | Purine metabolism, adenine containing | 1.03 | 0.20 | 1.09 | 0.18 | 1.09 | 0.18 | 0.87 | 0.19 | |
| adenosine 5′-monophosphate (AMP) | HMDB00045 | Purine metabolism, adenine containing | 1.04 | 0.32 | 1.05 | 0.23 | 1.05 | 0.23 | 0.93 | 0.36 | |
| allantoin | HMDB00462 | Purine metabolism, urate metabolism | 0.70 | 0.39 | 0.92 | 0.51 | 0.92 | 0.51 | 0.99 | 0.95 | |
| arabinosylhypoxanthine | Purine metabolism, (hypo)xanthine/inosine containing | 0.76 | 0.38 | 0.71 | 0.17 | 0.71 | 0.17 | 0.63 | 0.00 | ||
| cytidine | HMDB00089 | Pyrimidine metabolism, cytidine containing | 8F3802;1.12 | 0.16 | 1.35 | 0.16 | 1.35 | 0.16 | 0.86 | 0.06 | |
| cytidine-3′-monophosphate (3′-CMP) | Pyrimidine metabolism, cytidine containing | 8F3802;1.12 | 0.15 | 1.02 | 0.18 | 1.02 | 0.18 | 0.97 | 0.15 | ||
| guanosine | HMDB00133 | Purine metabolism, guanine containing | 0.65 | 0.28 | 0.70 | 0.18 | 0.70 | 0.18 | 1.76 | 0.30 | |
| hypoxanthine | HMDB00157 | Purine metabolism, (hypo)xanthine/inosine containing | 1.13 | 0.32 | 1.14 | 0.19 | 1.14 | 0.19 | 0.94 | 0.10 | |
| inosine | Purine metabolism, (hypo)xanthine/inosine containing | 0.88 | 0.12 | 0.95 | 0.08 | 0.95 | 0.08 | 1.05 | 0.08 | ||
| methylphosphate | Purine and pyrimidine metabolism | 1.00 | 0.33 | 1.26 | 0.20 | 1.26 | 0.20 | 0.95 | 0.09 | ||
| N1-methyladenosine | HMDB03331 | Purine metabolism, adenine containing | 0.94 | 0.26 | 1.02 | 0.18 | 1.02 | 0.18 | 0.71 | 0.23 | |
| pseudouridine | HMDB00767 | Pyrimidine metabolism, uracil containing | 1.06 | 0.17 | 1.02 | 0.15 | 1.02 | 0.15 | 605002;0.99 | 0.14 | |
| uracil | HMDB00300 | Pyrimidine metabolism, uracil containing | D11803;1.35 | 0.38 | 1.26 | 0.24 | 1.26 | 0.24 | 0.85 | 0.08 | |
| urate | HMDB00289 | Purine metabolism, urate metabolism | 1.11 | 0.30 | 1.53 | 0.48 | 1.53 | 0.48 | 0.77 | 0.31 | |
| uridine | HMDB00296 | Pyrimidine metabolism, uracil containing | 1.01 | 0.17 | 1.16 | 0.14 | 1.16 | 0.14 | 0.94 | 0.11 | |
| xanthine | HMDB00292 | Purine metabolism, (hypo)xanthine/inosine containing | 0.91 | 0.18 | 1.02 | 0.18 | 1.02 | 0.18 | 0.96 | 0.08 | |
| xanthosine | HMDB00299 | Purine metabolism, (hypo)xanthine/inosine containing | 1.25 | 0.42 | 1.54 | 0.36 | 1.54 | 0.36 | 0.81 | 0.14 | |
| Peptides | anserine | HMDB00194 | Dipeptide derivative | 1.56 | 1.31 | 0.78 | 0.46 | 0.78 | 0.46 | 0.96 | 0.40 |
| gamma-glutamylalanine | gamma-glutamyl | 0.98 | 0.33 | 1.01 | 0.29 | 1.01 | 0.29 | 1.09 | 0.12 | ||
| gamma-glutamylglutamate | gamma-glutamyl | 0.74 | 0.23 | 0.76 | 0.12 | 0.76 | 0.12 | 1.57 | 0.11 | ||
| gamma-glutamylglutamine | HMDB11738 | gamma-glutamyl | 0.84 | 0.13 | 0.97 | 0.15 | 0.97 | 0.15 | 1.14 | 0.16 | |
| gamma-glutamylglycine | HMDB11667 | gamma-glutamyl | 0.48 | 0.34 | 0.60 | 0.36 | 0.60 | 0.36 | 1.18 | 0.23 | |
| gamma-glutamylleucine | HMDB11171 | gamma-glutamyl | 1.24 | 0.33 | 0.11 | 0.32 | 0.11 | 0.32 | 0.99 | 0.12 | |
| gamma-glutamylmethionine | gamma-glutamyl | 1.07 | 0.28 | 0.96 | 0.25 | 0.96 | 0.25 | 0.92 | 0.14 | ||
| gamma-glutamylphenylalanine | HMDB00594 | gamma-glutamyl | 1.22 | 0.55 | 0.94 | 0.19 | 0.94 | 0.19 | 0.97 | 0.20 | |
| gamma-glutamylthreonine* | gamma-glutamyl | 0.93 | 0.24 | 764502;1.07 | 0.36 | 1.07 | 0.36 | 1.18 | 0.42 | ||
| glycylglycine | HMDB11733 | Dipeptide | 0.95 | 0.38 | 1.08 | 0.30 | 1.08 | 0.30 | 0.86 | 0.50 | |
| glycylleucine | HMDB00759 | Dipeptide | 1.05 | 0.31 | 1.03 | 0.25 | 1.03 | 0.25 | 0.91 | 0.21 | |
| homocarnosine | HMDB00745 | Dipeptide derivative | 0.87 | 0.18 | 1.01 | 0.19 | 1.01 | 0.19 | 1.22 | 0.28 | |
| isoleucylglycine | Dipeptide | 1.09 | 0.35 | 1.23 | 0.31 | 1.23 | 0.31 | 0.93 | 0.33 | ||
| leucylglycine | Dipeptide | 1.22 | 0.64 | 1.23 | 0.38 | 1.23 | 0.38 | 0.73 | 0.27 | ||
| leucylserine | Dipeptide | 1.32 | 0.65 | 1.50 | 0.35 | 1.50 | 0.35 | 0.85 | 0.20 | ||
| Xenobiotics | 2-ethylhexanoate (isobar with 2-propylpentanoate) | Chemical | 0.97 | 0.23 | 0.93 | 0.13 | 0.93 | 0.13 | 1.04 | 0.16 | |
| 2-pyrrolidinone | HMDB02039 | Chemical | 0.85 | 0.42 | 1.07 | 0.34 | 1.07 | 0.34 | 1.28 | 0.47 | |
| ergothioneine | HMDB03045 | Food component/Plant | 1.28 | 0.28 | 0.66 | 0.12 | 0.66 | 0.12 | 0.94 | 0.71 | |
| erythritol | HMDB02994 | Sugar, sugar substitute, starch | 0.87 | 0.32 | 0.96 | 0.22 | 0.96 | 0.22 | 1.00 | 0.19 | |
| glycerol 2-phosphate | HMDB02520 | Chemical | 1.24 | 0.43 | 1.49 | 0.37 | 1.49 | 0.37 | 0.73 | 0.23 | |
| glycolate (hydroxyacetate) | HMDB00115 | Chemical | 1.14 | 0.30 | 0.97 | 0.38 | 0.97 | 0.38 | 0.76 | 0.14 | |
| hippurate | HMDB00714 | Benzoate metabolism | 0.94 | 0.26 | 1.14 | 0.39 | 1.14 | 0.39 | 0.78 | 0.16 | |
| pentobarbital | Drug | 1.12 | 0.29 | 1.15 | 0.49 | 1.15 | 0.49 | 1.01 | 0.21 | ||
Neural Oxidative Status is Significantly Associated with Sensorimotor Function after SCI
To determine the relationship between the spinal cord oxidative status and sensorimotor functional recovery, we performed Spearman correlation analyses. We found that the oxidative status biomarker (GSH:GSSH ratio) was associated with motor (r value = 0.76, p < 0.0001; n = 49 pairs when combining both timepoints and when normalizing the data by removing highest and lowest scores from each group) and sensory function (r value = −0.54, p < 0.01; n = 31 pairs when using rats at 8 weeks post-operation). These findings support that oxidative stress plays an important role in determining functional outcomes after SCI and suggest its potential applicability to evaluate prognosis for neurological improvements in injured patients. The results underscore the importance of dietary LC-O3PUFAs in modulating the oxidative status.
Discussion
Rats fed a diet rich in long-chain omega-3 polyunsaturated fatty acids (LC-O3PUFAs) are more resilient in withstanding the secondary damage events and sensorimotor dysfunction associated with spinal cord injury (SCI) [17,19,18]. The present report defines the major underlying non-lipid targets of dietary LC-O3PUFAs through the unbiased interrogation of spinal cord tissue metabolite levels. We now report that dietary LC-O3PUFAs have a profound impact in the neural bioenergetics and antioxidant metabolomic profile. This was evidenced by marked and selective changes in the levels of carbohydrates, amino acids and nucleic acids.
Injury to the spinal cord leads to a complex cascade of pathophysiological processes that result in neural dysfunction. Multiple acute processes have been proposed that contribute to inflammation, cell death and dysfunction, and include ischemia, edema, cell membrane derangements, neurotransmitter and ionic imbalances, compromised energy metabolism, and production of free radicals. Our central guiding hypothesis is that prophylaxis and very early therapeutic interventions may be required to reduce the physical and psychological burden of disease on individuals at risk or afflicted by SCI.
LC-O3PUFAs confer prophylaxis against SCI
Along these lines, there are several clinical and occupational scenarios that present a significant risk of being affected by SCI. These situations include but are not limited to open repair for ruptured abdominal aortic aneurysm, thoracic endovascular aortic repair (TEVAR), amyotrophic lateral sclerosis, atherosclerosis, cerebral palsy, spina bifida, vitamin B12 deficiency, multiple sclerosis, iatrogenic ischemia, syringomyelia, spondylolysis, disc herniations, radiation toxicity, and tumors, sports, and military conflicts [33–37].
LC-O3PUFAs target antioxidant systems, carbohydrate-amino acid bioenergetics and peptides in the sham-operated spinal cord
The neural metabolism slows considerably in the early phase of SCI, resulting in alterations to the energetic metabolism. Further, the production of reactive oxygen or nitrogen species that accompany the pathophysiology of SCI can lead to deleterious lipid and protein alterations. This increased oxidative and nitrative stress requires metabolic pathways to be redesigned to satisfy large demands for antioxidants. This study demonstrates that dietary LC-O3PUFAs can target the metabolism of carnosine and homocarnosine. These dipeptides are derived from histidine and GABA and represent major endogenous antioxidant/anti-inflammatory chemicals in the spinal cord. For instance, administration of carnosine to SCI animals decreases immune cell infiltration, inducible nitric oxide synthase expression, pro-inflammatory cytokine expression, and apoptosis while improving motor function [30]. Further, carnosine attenuates nociceptive responses in inflammatory pain models [38]. We propose that, these dipeptides could be partly responsible for the locomotor recovery and marked antinociceptive behaviors that are exhibited by animals consuming these long-chain fatty acids.
Glutamine is an important precursor of releasable glutamate and also one of the most effective substrates and modulators of glucose metabolism. The metabolism of glutamine is tightly regulated as it plays major roles in neurotransmission, immunomodulation, and glutathione synthesis [39,40] and in SCI [41]. Further, recent studies suggest that metabolic ratios evaluating chemical changes in glutamine-glutamate cycling may have prognostic value in neurotrauma and could help explain the state of gliosis and hyperexcitability often observed in SCI [42,43,4,44]. This study reveals that dietary LC-O3PUFAs target the glutamine-glutamate cycling even in sham animals. Because the LC-O3PUFA-rich diet did not alter the levels of glutamate in the spinal cord, this effect could reflect a reduced glutamine synthesis or accelerated glutamine recycling/catabolism. These findings imply that dietary LC-O3PUFAs modulate the expression of glutamine synthetase, which has been shown to protect from glutamate-induced excitotoxicity in SCI [45]. The metabolism of tryptophan has also been implicated in the pathophysiology of SCI [46,47]. Here, we provide evidence suggesting that the metabolism of tryptophan is geared towards the generation of kynurenines in the rats receiving LC-O3PUFA-rich diets. These metabolites are important regulators of mitochondrial homeostasis and oxidative stress, inflammation, and glutamate excitotoxicity [31].
The data shows that the levels of metabolic substrates and intermediates of glycolysis such as glucose, glucose-6-P, fructose, and sedoheptulose-7-P are significantly reduced by the LC-O3PUFA diet in the sham-operated animals. These observations together with our previous findings demonstrating that LC-O3PUFAs increase the levels of palmitoleate (a marker of de novo lipogenesis) suggest that these fatty acids reprogram the neural cell glucose bioenergetics [19]. LC-O3PUFAs may stimulate energy production largely by oxidative phosphorylation via the tricarboxylic acid (TCA) cycle and pentose phosphate pathway (PPP) rather than glycolysis. This aneplerotic flux could have the advantage of diverting glycolytic intermediates into various biosynthetic pathways, which are essential for the synthesis of necessary macromolecules (i.e. amino acids, nucleosides and lipids required for assembling new cells). This idea is supported by recent findings showing that dietary LC-O3PUFAs reduce glycolysis by decreasing the expression of glycolytic enzymes [48]. The reduced levels of selective amino acids with concomitant increases in the levels of urea and ornithine further support the strong influence of dietary PUFAs in modulating neural bioenergetics. Interestingly, the carbon skeleton of most of the amino acids that were reduced by the LC-O3PUFA diet can also feed into the TCA cycle and converted to pyruvate, acetyl CoA, acetoacetate and succinyl CoA, suggesting a selective shunting of amino acids into the cycle. This observation indicates that the diet rich in LC-O3PUFAs may increase the protein and amino acid turnover and/or storage as fat and glycogen. Again, this could result in a more efficient system to handle excess amino nitrogen, which could be beneficial in situations in which neurons are starving or exposed to toxic levels of neurotransmitters such as during SCI.
Together, these observations lead us to conclude that the ability to regulate the neural cell bioenergetics and to increase and sustain stable levels of endogenous antioxidants may represent important mechanisms by which dietary LC-O3PUFAs confer prophylaxis against SCI. Through building a reservoir of protective molecules and by improving the cellular energetics and antioxidant availability, neurons and glia are likely to be more resistant against calcium overload, glutamate toxicity, cell death, and dysfunction after SCI.
Administration of LC-O3PUFAs in the diet improves the spinal cord redox potential in the injured spinal cord
Chronic oxidative damage after SCI results in neuroinflammation and maladaptive plasticity leading to sensorimotor abnormalities [49]. The SH-containing tripeptide glutathione is effective in the protection of SH-carrying proteins against oxygen radicals and may thereby be especially important for neuroprotection [50,51]. The glutathione metabolism is rapidly activated following SCI [52] and its experimental depletion in SCI leads to appreciable worsening of neutrophil infiltration, lipid peroxidation, apoptosis, and loss of hind leg movement [53]. Thus, the intracellular glutathione pool could be important for limiting oxidative stress–induced neuronal injury. Our data suggest that glutathione is a molecular target of dietary LC-O3PUFAs and could represent a key mechanism conferring protection in SCI rats and is inline with previous findings in rat hippocampal cultures [54].
Another finding that reflects an enhanced antioxidant system in rats fed with LC-O3PUFAs are the increased levels of sulfur amino acids. The increase in cystathionine and decrease of methionine observed in rats fed diets rich in LC-O3PUFAs suggests an increase in transulfuration pathway. This observation is supported by a study indicating that LC-O3PUFAs are capable of increasing the expression of cystathionine-γ-lyase (CSL), which catalyzes the splitting of cystathionine into cysteine and α-ketobutyrate [55].
A novel finding of the study is the effect of the diet in increasing the levels of heme in the spinal cord, suggesting that dietary LC-O3PUFAs regulate the metabolism of heme-containing proteins such as hemoproteins. Hemoproteins contain a globin fold, the structural hallmark of the tissue hemoglobins [56,57], which enables the binding of oxygen, nitric oxide, or free radicals. The globin fold also enables the tissue hemoglobin to serve as either a facilitator of oxygen transport within a cell, a scavenger of nitric oxide, and/or an enzyme with peroxidase activity. Hemoproteins such as cytoglobin (CYGB) neuroglobin (Ngb) have been implicated in mediating neurorestorative responses in models of hypoxia-ischemia and spinal cord trauma [58–60]. Although LC-O3PUFAs may also prevent the degradation of these key proteins, the effects of dietary LC-O3PUFAs on this important family needs to be clarified and further characterized.
Dietary LC-O3PUFAs increase the levels of glucose-containing oligosaccharides during the acute injury phase
Of particular significance we found that the diet rich in LC-O3PUFAs elevated the levels of oligosaccharides containing only glucose (i.e., maltose and maltotriose). Two potential mechanisms may be involved in this process: (1) the LC-O3PUFA diet facilitates the carbohydrate accumulation through glycogenesis or (2) the LC-O3PUFA diet may prevent the depletion of glycogen, a hallmark of ischemic damage. These mechanisms can facilitate energy production and offer metabolic alternatives to the damaged cord. For instance, LC-O3PUFAs could be important regulators of brain energy metabolism by affecting glucose utilization, the expression of the glucose transporter-1 (GLUT1), and the metabolism of glycans and glycoproteins, supporting the results presented herein [61,62].
LC-O3PUFAs increase the levels of pyrimidines and epigenetic biomarkers
Studies have shown that brain phosphatide synthesis requires three circulating compounds: DHA, uridine, and choline [63]. Interestingly, oral administration of these precursors increases the levels of phosphatides, synaptic proteins and number of dendritic spines in the brain [63]. The increased levels of these precursors in the animals receiving LC-O3PUFAs in the diet support our previous electrophysiological findings showing increased spinal plasticity and repair responses in SCI animals pretreated with DHA injections [17].
This study shows that dietary intake of LC-O3PUFAs increased the levels of acetyllisine. It is thus reasonable to propose that this increased acetylation levels of lysine could reflect potential epigenetic mechanisms mediated by the diet. In fact, DHA has been reported to increase the acetylation of H3 and Bcl-2 levels, promoting gene expression and suggesting a mechanism for the neuroprotective roles of DHA [64]. Our own studies provide further support to this genetic regulation by DHA [65].
In summary, dietary-essential LC-O3PUFAs accrete in the spinal cord cell membranes and alter multiple crucial metabolic pathways implicated in cell bioenergetics, signal transduction, gene expression, synaptic plasticity, and calcium regulation. Although DHA and EPA structure is an excellent target for lipid peroxidation and may function as free-radical scavenger, we demonstrate that LC-O3PUFA accretion in advance of injury may promote neural resilience against SCI by regulating the neuron-glia metabolic network. Accumulated LC-O3PUFAs can be released from cell membranes at the onset of injury and trigger the docosanoid pathway and DHA-derived messengers, including docosatrienes, resolvins, and neuroprotectins, such as neuroprotectin D1 [66–70].
Conclusions
Small-molecule profiling defined the underlying biologic state and distinctive non-lipid metabolic signatures associated with dietary LC-O3PUFA. We demonstrated that the consumption of a diet rich in LC-O3PUFAs has a wide-ranging impact on the spinal cord metabolism. These changes may explain some of the observed differences in the phenotype of animals fed LC-O3PUFA-rich diets after SCI. Global neurometabolomic analyses uncovered the LC-O3PUFA metabolome as quite diverse and defined various metabolic pathways, including amino acid neurotransmitter systems and antioxidant defenses as its principal non-lipid targets in vivo. These changes demonstrate that LC-O3PUFAs are able to influence signaling networks beyond those associated with lipid metabolism. The rapid treatment of SCI animals with DHA and EPA has been shown to ameliorate several of the secondary biological responses that accompany the physical trauma. We have shown that these protective effects can be expanded to prophylactic interventions, which may be a preventive measure to provide critical protection to individuals with known risk for SCI, including surgical patients, contact sports athletes, soldiers, and first responders. Although dietary LC-O3PUFAs may lack target specificity when compared to other therapeutics, diet may be more effective with respect to costs and safety concerns and deserve serious consideration in clinical applications. Through defining the protective mechanism of dietary LC-O3PUFA, we hope that this study serves to inspire future studies on the identification and validation of novel prophylactic and therapeutic targets for nervous system lesions.
Acknowledgments
We thank our lab staff and the attendees of the ISN-ASN Satellite Symposium “Unveiling the Significance of Lipid Signaling in Neurodegeneration and Neuroprotection” in Cancun for providing helpful discussions during the preparation of this manuscript. The authors are indebted to Miguel Serrano-Illan, Kathia Cordero, Manuel Montero and the animal care facility personnel for their excellent support with animal care. We would like to thank Dr. Jaime Lecker from BioServ® for her valuable consultation during the design of the nutritional formulations. We also thank Lorena Salto for her editorial assistance. The research reported in this publication was supported by P20MD006988 and 5R25GM060607.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Advances in physiology education. 2002;26 (1–4):238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- 2.Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997;765 (2):283–290. doi: 10.1016/s0006-8993(97)00573-8. [DOI] [PubMed] [Google Scholar]
- 3.McAdoo DJ, Xu G, Robak G, Hughes MG, Price EM. Evidence that reversed glutamate uptake contributes significantly to glutamate release following experimental injury to the rat spinal cord. Brain Res. 2000;865 (2):283–285. doi: 10.1016/s0006-8993(00)02296-4. [DOI] [PubMed] [Google Scholar]
- 4.Fujieda Y, Ueno S, Ogino R, Kuroda M, Jonsson TJ, Guo L, Bamba T, Fukusaki E. Metabolite profiles correlate closely with neurobehavioral function in experimental spinal cord injury in rats. PLoS One. 2012;7 (8):e43152. doi: 10.1371/journal.pone.0043152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21 (6):754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 6.Kwon BK, Okon E, Hillyer J, Mann C, Baptiste D, Weaver LC, Fehlings MG, Tetzlaff W. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. Journal of Neurotrauma. 2011;28 (8):1545–1588. doi: 10.1089/neu.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci. 2006;26 (17):4672–4680. doi: 10.1523/JNEUROSCI.5539-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang-Lazdunski L, Blondeau N, Jarretou G, Lazdunski M, Heurteaux C. Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J Vasc Surg. 2003;38 (3):564–575. doi: 10.1016/s0741-5214(03)00473-7. [DOI] [PubMed] [Google Scholar]
- 9.Huang WL, King VR, Curran OE, Dyall SC, Ward RE, Lal N, Priestley JV, Michael-Titus AT. A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain. 2007;130 (Pt 11):3004–3019. doi: 10.1093/brain/awm223. [DOI] [PubMed] [Google Scholar]
- 10.Ward RE, Huang W, Curran OE, Priestley JV, Michael-Titus AT. Docosahexaenoic acid prevents white matter damage following spinal cord injury. Journal of Neurotrauma. 2010;27 (10):1769–1780. doi: 10.1089/neu.2010.1348. [DOI] [PubMed] [Google Scholar]
- 11.Lim S-N, Huang W, Hall JCE, Ward RE, Priestley JV, Michael-Titus AT. The acute administration of eicosapentaenoic acid is neuroprotective after spinal cord compression injury in rats. Prostaglandins Leukot Essent Fatty Acids. 2010;83 (4–6):193–201. doi: 10.1016/j.plefa.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Hall JC, Priestley JV, Hugh Perry V, Michael-Titus AT. Docosahexaenoic Acid, but not Eicosapentaenoic Acid, Reduces the Early Inflammatory Response Following Compression Spinal Cord Injury in the Rat. J Neurochem. 2012;121 (5):738–750. doi: 10.1111/j.1471-4159.2012.07726.x. [DOI] [PubMed] [Google Scholar]
- 13.López-Vales R, Redensek A, Skinner TAA, Rathore KI, Ghasemlou N, Wojewodka G, DeSanctis J, Radzioch D, David S. Fenretinide promotes functional recovery and tissue protection after spinal cord contusion injury in mice. J Neurosci. 2010;30 (9):3220–3226. doi: 10.1523/JNEUROSCI.5770-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holly LT, Blaskiewicz D, Wu A, Feng C, Ying Z, Gomez-Pinilla F. Dietary therapy to promote neuroprotection in chronic spinal cord injury. Journal of neurosurgery Spine. 2012;17 (2):134–140. doi: 10.3171/2012.5.SPINE1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim SN, Gladman SJ, Dyall SC, Patel U, Virani N, Kang JX, Priestley JV, Michael-Titus AT. Transgenic mice with high endogenous omega-3 fatty acids are protected from spinal cord injury. Neurobiol Dis. 2013;51:104–112. doi: 10.1016/j.nbd.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Satkunendrarajah K, Fehlings MG. Do omega-3 polyunsaturated fatty acids ameliorate spinal cord injury?: Commentary on: Lim et al., Improved outcome after spinal cord compression injury in mice treated with docosahexaeonic acid. Exp. Neurol. Jan; 239:13–27. Exp Neurol. 2013;249:104–110. doi: 10.1016/j.expneurol.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Figueroa JD, Cordero K, Baldeosingh K, Torrado AI, Walker RL, Miranda JD, Leon MD. Docosahexaenoic acid pretreatment confers protection and functional improvements after acute spinal cord injury in adult rats. J Neurotrauma. 2012;29 (3):551–566. doi: 10.1089/neu.2011.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figueroa JD, Cordero K, Serrano-Illan M, Almeyda A, Baldeosingh K, Almaguel FG, De Leon M. Metabolomics uncovers dietary omega-3 fatty acid-derived metabolites implicated in anti-nociceptive responses after experimental spinal cord injury. Neuroscience. 2013;255C:1–18. doi: 10.1016/j.neuroscience.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueroa JD, Cordero K, Llan MS, De Leon M. Dietary omega-3 polyunsaturated fatty acids improve the neurolipidome and restore the DHA status while promoting functional recovery after experimental spinal cord injury. J Neurotrauma. 2013;30 (10):853–868. doi: 10.1089/neu.2012.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9 (2):123–126. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- 21.Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform. 2010;2 (1):9. doi: 10.1186/1758-2946-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100 (16):9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storey JD, Tibshirani R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol Biol. 2003;224:149–157. doi: 10.1385/1-59259-364-X:149. [DOI] [PubMed] [Google Scholar]
- 24.Deo RC, Hunter L, Lewis GD, Pare G, Vasan RS, Chasman D, Wang TJ, Gerszten RE, Roth FP. Interpreting metabolomic profiles using unbiased pathway models. PLoS Comput Biol. 2010;6 (2):e1000692. doi: 10.1371/journal.pcbi.1000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37 (Web Server issue):W652–660. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2.0--a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012;40 (Web Server issue):W127–133. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41 (Database issue):D801–807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazan NG, Musto AE, Knott EJ. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol Neurobiol. 2011;44 (2):216–222. doi: 10.1007/s12035-011-8200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellia F, Vecchio G, Cuzzocrea S, Calabrese V, Rizzarelli E. Neuroprotective features of carnosine in oxidative driven diseases. Mol Aspects Med. 2011;32 (4–6):258–266. doi: 10.1016/j.mam.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Di Paola R, Impellizzeri D, Salinaro AT, Mazzon E, Bellia F, Cavallaro M, Cornelius C, Vecchio G, Calabrese V, Rizzarelli E, Cuzzocrea S. Administration of carnosine in the treatment of acute spinal cord injury. Biochem Pharmacol. 2011;82 (10):1478–1489. doi: 10.1016/j.bcp.2011.07.074. [DOI] [PubMed] [Google Scholar]
- 31.Zadori D, Klivenyi P, Szalardy L, Fulop F, Toldi J, Vecsei L. Mitochondrial disturbances, excitotoxicity, neuroinflammation and kynurenines: novel therapeutic strategies for neurodegenerative disorders. J Neurol Sci. 2012;322 (1–2):187–191. doi: 10.1016/j.jns.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Szalardy L, Klivenyi P, Zadori D, Fulop F, Toldi J, Vecsei L. Mitochondrial disturbances, tryptophan metabolites and neurodegeneration: medicinal chemistry aspects. Curr Med Chem. 2012;19 (13):1899–1920. doi: 10.2174/092986712800167365. [DOI] [PubMed] [Google Scholar]
- 33.Boden BP, Jarvis CG. Spinal injuries in sports. Phys Med Rehabil Clin N Am. 2009;20 (1):55–68. vii. doi: 10.1016/j.pmr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 34.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359 (9304):417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 35.Weaver FM, Burns SP, Evans CT, Rapacki LM, Goldstein B, Hammond MC. Provider perspectives on soldiers with new spinal cord injuries returning from Iraq and Afghanistan. Archives of physical medicine and rehabilitation. 2009;90 (3):517–521. doi: 10.1016/j.apmr.2008.09.560. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda H, Fukuda T, Iritani O, Nakazawa T, Tanaka H, Sasaki H, Minatoya K, Ogino H. Spinal cord injury is not negligible after TEVAR for lower descending aorta. Eur J Vasc Endovasc Surg. 2010;39 (2):179–186. doi: 10.1016/j.ejvs.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Peppelenbosch AG, Vermeulen Windsant IC, Jacobs MJ, Tordoir JH, Schurink GW. Open repair for ruptured abdominal aortic aneurysm and the risk of spinal cord ischemia: review of the literature and risk-factor analysis. Eur J Vasc Endovasc Surg. 2010;40 (5):589–595. doi: 10.1016/j.ejvs.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Ohsawa M, Mutoh J, Asato M, Yamamoto S, Ono H, Hisa H, Kamei J. Carnosine has antinociceptive properties in the inflammation-induced nociceptive response in mice. Eur J Pharmacol. 2012;682 (1–3):56–61. doi: 10.1016/j.ejphar.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Newsholme P, Curi R, Gordon S, Newsholme EA. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J. 1986;239 (1):121–125. doi: 10.1042/bj2390121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth E. Immune and cell modulation by amino acids. Clin Nutr. 2007;26 (5):535–544. doi: 10.1016/j.clnu.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Diaz-Ruiz A, Salgado-Ceballos H, Montes S, Maldonado V, Tristan L, Alcaraz-Zubeldia M, Rios C. Acute alterations of glutamate, glutamine, GABA, and other amino acids after spinal cord contusion in rats. Neurochem Res. 2007;32 (1):57–63. doi: 10.1007/s11064-006-9225-5. [DOI] [PubMed] [Google Scholar]
- 42.Richards DA, Tolias CM, Sgouros S, Bowery NG. Extracellular glutamine to glutamate ratio may predict outcome in the injured brain: a clinical microdialysis study in children. Pharmacol Res. 2003;48 (1):101–109. [PubMed] [Google Scholar]
- 43.Erschbamer M, Oberg J, Westman E, Sitnikov R, Olson L, Spenger C. 1H-MRS in spinal cord injury: acute and chronic metabolite alterations in rat brain and lumbar spinal cord. Eur J Neurosci. 2011;33 (4):678–688. doi: 10.1111/j.1460-9568.2010.07562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Widerstrom-Noga E, Pattany PM, Cruz-Almeida Y, Felix ER, Perez S, Cardenas DD, Martinez-Arizala A. Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. Pain. 2013;154 (2):204–212. doi: 10.1016/j.pain.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C, Wu W, Zhang B, Xiang J, Zou J. Temporospatial expression and cellular localization of glutamine synthetase following traumatic spinal cord injury in adult rats. Mol Med Rep. 2013;7 (5):1431–1436. doi: 10.3892/mmr.2013.1383. [DOI] [PubMed] [Google Scholar]
- 46.Heyes MP, Achim CL, Wiley CA, Major EO, Saito K, Markey SP. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem J. 1996;320 (Pt 2):595–597. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blight AR, Saito K, Heyes MP. Increased levels of the excitotoxin quinolinic acid in spinal cord following contusion injury. Brain Res. 1993;632 (1–2):314–316. doi: 10.1016/0006-8993(93)91167-q. [DOI] [PubMed] [Google Scholar]
- 48.Andrade-Vieira R, Han JH, Marignani PA. Omega-3 polyunsaturated fatty acid promotes the inhibition of glycolytic enzymes and mTOR signaling by regulating the tumor suppressor LKB1. Cancer Biol Ther. 2013;14 (11):1050–1058. doi: 10.4161/cbt.26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gwak YS, Hassler SE, Hulsebosch CE. Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain. 2013;154 (9):1699–1708. doi: 10.1016/j.pain.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Aoyama K, Nakaki T. Impaired glutathione synthesis in neurodegeneration. Int J Mol Sci. 2013;14 (10):21021–21044. doi: 10.3390/ijms141021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aoyama K, Nakaki T. Neuroprotective properties of the excitatory amino acid carrier 1 (EAAC1) Amino Acids. 2013;45 (1):133–142. doi: 10.1007/s00726-013-1481-5. [DOI] [PubMed] [Google Scholar]
- 52.Diaz-Ruiz A, Alcaraz-Zubeldia M, Maldonado V, Salgado-Ceballos H, Mendez-Armenta M, Rios C. Differential time-course of the increase of antioxidant thiol-defenses in the acute phase after spinal cord injury in rats. Neurosci Lett. 2009;452 (1):56–59. doi: 10.1016/j.neulet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 53.Genovese T, Mazzon E, Esposito E, Muia C, Di Paola R, Di Bella P, Bramanti P, Cuzzocrea S. Role of endogenous glutathione in the secondary damage in experimental spinal cord injury in mice. Neurosci Lett. 2007;423 (1):41–46. doi: 10.1016/j.neulet.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Zhao X, Mao ZY, Wang XM, Liu ZL. Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport. 2003;14 (18):2457–2461. doi: 10.1097/01.wnr.0000093483.65585.86. [DOI] [PubMed] [Google Scholar]
- 55.Huang T, Wahlqvist ML, Li D. Effect of n-3 polyunsaturated fatty acid on gene expression of the critical enzymes involved in homocysteine metabolism. Nutr J. 2012;11:6. doi: 10.1186/1475-2891-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wittenberg BA, Wittenberg JB. Transport of oxygen in muscle. Annu Rev Physiol. 1989;51:857–878. doi: 10.1146/annurev.ph.51.030189.004233. [DOI] [PubMed] [Google Scholar]
- 57.Garry DJ, Mammen PP. Neuroprotection and the role of neuroglobin. Lancet. 2003;362 (9381):342–343. doi: 10.1016/S0140-6736(03)14055-X. [DOI] [PubMed] [Google Scholar]
- 58.Brittain T, Skommer J, Henty K, Birch N, Raychaudhuri S. A role for human neuroglobin in apoptosis. IUBMB Life. 2010;62 (12):878–885. doi: 10.1002/iub.405. [DOI] [PubMed] [Google Scholar]
- 59.Mammen PP, Shelton JM, Ye Q, Kanatous SB, McGrath AJ, Richardson JA, Garry DJ. Cytoglobin is a stress-responsive hemoprotein expressed in the developing and adult brain. J Histochem Cytochem. 2006;54 (12):1349–1361. doi: 10.1369/jhc.6A7008.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian SF, Yang HH, Xiao DP, Huang YJ, He GY, Ma HR, Xia F, Shi XC. Mechanisms of neuroprotection from hypoxia-ischemia (HI) brain injury by up-regulation of cytoglobin (CYGB) in a neonatal rat model. J Biol Chem. 2013;288 (22):15988–16003. doi: 10.1074/jbc.M112.428789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida H, Ikeda I, Tomooka M, Mawatari M, Imaizumi K, Seto A, Tsuji H. Effect of dietary seal and fish oils on lipid metabolism in hamsters. J Nutr Sci Vitaminol (Tokyo) 2001;47 (3):242–247. doi: 10.3177/jnsv.47.242. [DOI] [PubMed] [Google Scholar]
- 62.Pifferi F, Jouin M, Alessandri JM, Roux F, Perriere N, Langelier B, Lavialle M, Cunnane S, Guesnet P. n-3 long-chain fatty acids and regulation of glucose transport in two models of rat brain endothelial cells. Neurochem Int. 2010;56 (5):703–710. doi: 10.1016/j.neuint.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 63.Wurtman RJ, Cansev M, Sakamoto T, Ulus I. Nutritional modifiers of aging brain function: use of uridine and other phosphatide precursors to increase formation of brain synapses. Nutr Rev. 2010;68(Suppl 2):S88–101. doi: 10.1111/j.1753-4887.2010.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sadli N, Ackland ML, De Mel D, Sinclair AJ, Suphioglu C. Effects of zinc and DHA on the epigenetic regulation of human neuronal cells. Cell Physiol Biochem. 2012;29 (1–2):87–98. doi: 10.1159/000337590. [DOI] [PubMed] [Google Scholar]
- 65.Almaguel FG, Liu J-W, Pacheco FJ, Casiano CA, De Leon M. Activation and reversal of lipotoxicity in PC12 and rat cortical cells following exposure to palmitic acid. J Neurosci Res. 2009;87 (5):1207–1218. doi: 10.1002/jnr.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lukiw WJ, Cui J-G, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. Journal of Clinical Investigation. 2005;115 (10):2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bazan NG. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot Essent Fatty Acids. 2009;81 (2–3):205–211. doi: 10.1016/j.plefa.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J Lipid Res. 2009;50(Suppl):S400–405. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bazan NG, Eady TN, Khoutorova L, Atkins KD, Hong S, Lu Y, Zhang C, Jun B, Obenaus A, Fredman G, Zhu M, Winkler JW, Petasis NA, Serhan CN, Belayev L. Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Exp Neurol. 2012;236 (1):122–130. doi: 10.1016/j.expneurol.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belayev L, Khoutorova L, Atkins KD, Eady TN, Hong S, Lu Y, Obenaus A, Bazan NG. Docosahexaenoic Acid Therapy of Experimental Ischemic Stroke. Transl Stroke Res. 2011;2 (1):33–41. doi: 10.1007/s12975-010-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]