Abstract
Objectives
To assess if erythromycin increases gastric emptying and hence improves small intestinal distention during MR enterography.
Methods
Gastric, small intestinal, and large intestinal volumes were assessed with MR after neutral oral contrast (1350 mL in 45 minutes) and balanced randomization to erythromycin (200 mg i.v., age 31 ± 3y, 13 females), or placebo (37 ± 3y, 13 females) in 40 healthy asymptomatic volunteers. Fat-suppressed T2-weighted MR images of the abdomen were acquired on a 1.5T magnet at standard delay times for enterography. Gastric, small, and large intestinal volumes were measured by specialized software. In addition, two radiologists manually measured diameters and percentage distention of jejunal and ileal loops. Treatment effects were evaluated by an ITT analysis based on ANCOVA models.
Results
All subjects tolerated erythromycin. MRI scans of the stomach and intestine were obtained at 62 ± 2 (Mean ± SEM) and 74 ± 2 minutes respectively after starting oral contrast. Gastric volumes were lower (p < 0.0001) after erythromycin (260 ± 49 ml) than placebo (688 ± 63 ml) but jejunal, ileal, and colonic volumes were not significantly different. However, maximum (76–100%) jejunal distention was more frequently observed (p = 0.03) after erythromycin (8/20 subjects [40%]) than placebo (2/20 subjects [10%]). The diameter of a representative ileal loop was greater (p = 0.001) after erythromycin (18.8 ± 4.3 mm) than placebo (17.3 ± 2.8 mm) infusion.
Conclusions
After ingestion of oral contrast, erythromycin accelerated gastric emptying but effects on small intestinal dimensions were variable. In balance, erythromycin did not substantially enhance small intestinal distention during enterography using current standard delay times.
Keywords: distention, enterography, erythromycin, MRI, small intestine
INTRODUCTION
MR and CT enterography are very useful cross-sectional imaging techniques for assessing the bowel wall and perienteric fat in patients with suspected small bowel disease [1, 2]. MRI provides excellent soft-tissue contrast and avoids ionizing radiation. In addition, recent advances in MR imaging techniques have improved spatial and temporal resolution and reduced motion artifacts. Nonetheless, small bowel distension is a prerequisite for an adequate enterography exam [1, 2]. Collapsed intestinal segments may conceal pathological findings, and conversely, may falsely mimic inflammation or a tumor [1, 2].
The bowel can be distended by enteroclysis or enterography. Enteroclysis provides excellent distension but requires placement of a nasoenteric tube, which is not as well tolerated as enterography, which requires ingestion of large volumes of oral contrast. Intravenously administered spasmolytic agents (eg, glucagon or buscopan) can reduce bowel motion artifacts but may not optimize distention [1]. Two primary approaches (i.e. increasing the volume and osmolality of the oral solution) are used to facilitate bowel distention during MR enterography. A comparison of 4 volumes (450, 900, 1,350, and 1,800 mL) of each of four contrast compounds (0.2% locust bean gum plus 2.5% mannitol, VoLumen containing 2.0% sorbitol, VoLumen containing 1.4% sorbitol, and tap water) observed that distention was better with solutions containing a sugar alcohol than water but was not significantly different among various agents containing osmotic additives [3]. A volume of 900 mL achieved sufficient duodenal distention whereas 1350 mL was preferable for visualizing the jejunum and ileum. While adequate distention can be accomplished with a smaller volume of highly concentrated sorbitol solutions (e.g., 450 ml), these solutions are more likely to cause diarrhea [4]. Despite development of these dedicated enteric-distending contrast agents, the small bowel, especially the jejunum, is often poorly distended during routine computed enterography [1]. Adequate jejunal distension and visualization are important in the evaluation of celiac disease, for detecting small bowel masses, and also for Crohn’s disease. While Crohn’s disease has a predilection for the distal ileum, it can skip the terminal ileum [5]. In our experience, Crohn’s disease involving the jejunal loops can be more subtle and overlooked.
Drugs which accelerate gastric motility such as metoclopramide are also used to increase gastric emptying and facilitate intestinal distention in clinical practice [6]. Metoclopramide (10 mg oral) facilitated ileal but not jejunal distention evaluated by computed tomography compared to patients in which it was not used; however, this was not a randomized study [7]. By stimulating motilin receptors, erythromycin can increase antral motility and accelerate gastric emptying [8, 9]. One study evaluated the effects of administering erythromycin before oral contrast during MRI [3]. For a 70 kg person, the dose used in that study i.e., 100 mg i.v. was approximately 50% of the dose (3 mg/kg i.v.) required to increase gastric motility [9]. Because the effects of erythromycin were not compared to placebo, it is not known to our knowledge if erythromycin increased small intestinal distention. Hence, the effects of prokinetic agents on small intestinal distention during MR enterography are unknown.
The objectives of our study were to assess if erythromycin increases gastric emptying, hence improves small intestinal distention during MR enterography in healthy subjects.
MATERIAL AND METHODS
Subjects
Forty three healthy asymptomatic volunteers were recruited from the local community by public advertisement and consented to participate in this study, which was approved by the Institutional Review Board. A gastroenterologist interviewed and examined all subjects to ensure they were healthy and did not have any of the following: symptoms of a functional gastroduodenal or bowel disorders by questionnaire [10], prior gastrointestinal surgery other than appendectomy, cholecystectomy, hysterectomy, tubal ligation, or inguinal hernia repair, medication use (except for stable doses of birth control pill, L-thyroxine, or estrogen replacement therapy), claustrophobia or metal objects in the body. An EKG was obtained to ensure the corrected Q-T interval was ≤ 460 ms because erythromycin can prolong the Q-T interval and rarely induce torsades de pointes [11].
MR Enterography
MR images of the abdomen were acquired with a torso phased-array coil and a 1.5T magnet MRI (GE Healthcare, Waukesha, WI). First, gastric volumes were assessed by an axial 2D half-Fourier acquisition single-shot turbo spin echo (HASTE) sequence (i.e., TR 900 ms, TE 90 ms, 5 mm slices with 0 mm gap, matrix size 256×256, 1 NEX), which imaged the entire stomach in 28 seconds, during two breathholds. Thereafter, small bowel and colonic volumes were evaluated with 5mm thick coronal slices using a fat-suppressed TrueFISP (Fast Imaging Employing Steady State Acquisition, FIESTA) sequence (TE 1.8ms, TR 3.8ms, a fractional field of view of 40×32cm, and an acquisition matrix of 256×192), also during breath-holding. The TrueFISP sequence was not used to evaluate gastric volumes because black band artifacts in the left upper quadrant projected over the stomach and hindered segmentation.
Medication and Oral Contrast
Subjects were instructed to fast for 4 hours prior to the study. Upon arrival, they were randomized to receive either erythromycin lactobionate (Hospira, Lake Forest, Il) at a dose of 3 mg/kg intravenously or matched placebo (sodium chloride [0.9%]). To minimize venous irritation, erythromycin was given as a bolus of 0.5 mg/kg over 10 min followed by an infusion of 2.5 mg/kg in 5% dextrose solution over the next 50 min.
Oral contrast (1350 ml) was ingested over 45 minutes beginning 25 minutes after erythromycin was started. Since erythromycin was given over 60 minutes, the oral contrast was completely ingested 10 minutes after the erythromycin infusion was completed. A low concentration barium solution (VoLumen (1350 ml), Bracco Diagnostics, Monroe Township, NJ), which contains 0.1% weight/volume barium and 2% sorbitol, was used to provide oral contrast. This agent is FDA-approved for use in CT and is widely used for CT and MR enterography in the United States. Its use in MR is considered an off-labeled use. This agent has been shown to provide better small bowel distention than water in the upper and lower abdomen [12]. Sorbitol, available commercially as a sweetening agent, is a sugar alcohol that promotes peristalsis and is a mild osmotic laxative [13].
Data Analysis by Software
Gastric volumes were evaluated using the ANALYZE software system (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) [14]. Where feasible, a validated semi-automated algorithm was used with manual tracing in the remaining subjects. For the manual analysis, the results of which were detailed previously [15], a region of interest (ROI) was manually drawn around gastric contents on each slice. The count of all pixels within each appropriate ROI for all slices was multiplied by voxel size to obtain the volume of gastric contents excluding air. Small intestinal loops were characterized as jejunum or ileum based on their location in the upper and lower abdomen (i.e., above and below the pelvic brim) respectively. Trained technicians segmented images and a radiologist (JF or JH) verified the same. Thereafter, small intestinal and colonic fluid volumes were calculated as described for the stomach. One technician segmented all small intestinal and colonic images and another segmented all gastric images. This analysis was blinded to drug assignment.
Data Analysis by Radiologists
Two radiologists (JF, JEH), who were blinded to drug assignment, simultaneously reviewed and measured the representative and maximum short axis diameter of jejunal and ileal loops on coronal images on a computer workstation (GE Advantage Windows 4.2) by consensus. A representative loop was identified as a loop that was distended to a similar extent as a majority of segmental loops [12]. These radiologists then ranked overall jejunal and ileal distention along a 4-point scale (where 1 = 0–25% segmental distention (non-collapsed), 2 = 26–50% distention, 3 = 51–75% distention, and 4 = 76% – 100% distention).
Statistical Analysis
The effects of erythromycin on gastrointestinal fluid volumes and loop diameters were evaluated by an analysis of covariance (ANCOVA) adjusting for age and gender. The effects of erythromycin on jejunal and ileal distention were assessed by Fisher’s exact test. The relationship among gastric, small intestinal, and colonic fluid volumes was assessed by Spearman’s correlation coefficient. Treatment effects were assessed per an intent-to-treat analysis using all subjects randomized. Missing values were imputed using the corresponding overall mean in all subjects with non-missing data, and an adjustment in the ANCOVA error degrees of freedom (i.e., subtracting one degree of freedom for each missing value imputed), to obtain an appropriate error residual variance. Data are provided as Mean ± SEM.
RESULTS
Clinical Features
Forty three subjects were enrolled. Three subjects declined to participate before MRI scans were performed and 40 subjects completed the study. The distribution of age (37 ± 3y [placebo], 31 ± 3y [erythromycin]), gender (13 females in each group), and BMI (27.7 ± 0.9 [placebo], 26.0 ± 0.7 [erythromycin]) was not significantly different between groups. The time between the onset of the erythromycin bolus and ingestion of oral contrast was 27 ± 2 minutes (placebo) and 24 ± 2 minutes (erythromycin). All subjects tolerated the MR examination and no subjects vomited contrast material.
Effect of Erythromycin on Gastric Volumes
Gastric scans were obtained at 60 ± 3 minutes (placebo) and 61 ± 2 minutes (erythromycin) after subjects started ingesting oral contrast. Except for 1 subject in which imaging artifact precluded gastric segmentation, the gastric contour could be segmented from surrounding organs and gastric volumes could be measured exclusively by a semi-automated process (36 subjects) or by manual segmentation (3 subjects) (Figure 1). Gastric volumes were lower after erythromycin (260 ± 49 ml) than placebo infusions (688 ± 63 ml); treatment effects were significant (P<.0001) (Table 1).
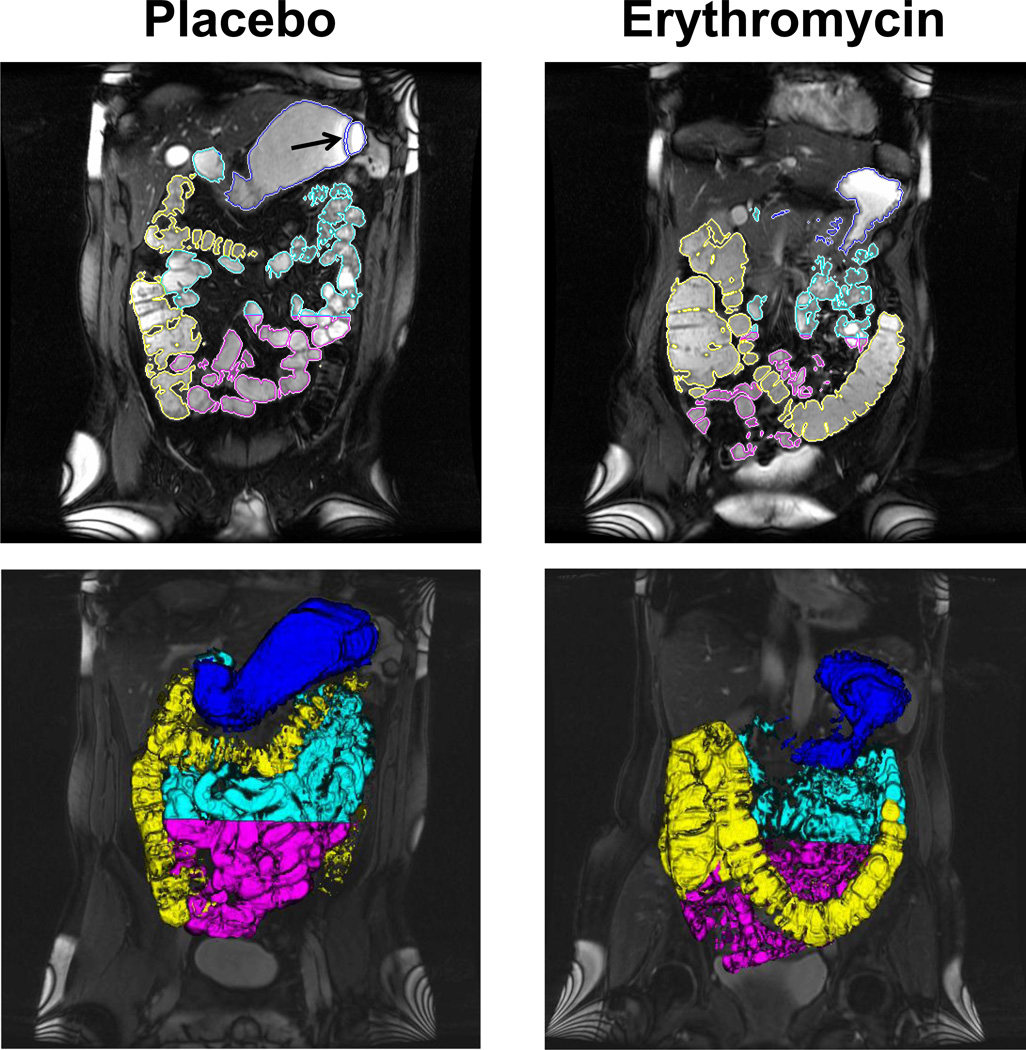
Figure 1. Representative images of gastric, small intestinal, and colonic volumes after intravenous erythromycin and placebo.
The upper panel shows a single coronal slice with ROIs around the stomach, small intestine, and colon. Gastric volumes were calculated using axial HASTE images, which are not shown. The lower panel depicts a 3-D representation of segmented gastric, small intestinal, and colonic volumes. Note greater gastric emptying and colonic filling with erythromycin. Also note the black band artifact (arrow) projecting over the stomach; hence this sequence was not used for semi-automated assessment of gastric volumes.
Table 1.
Effects of Erythromycin and Placebo on Gastrointestinal Fluid Volumes Measured by Software
| Site | Placebo | Erythromycin | P value* |
|---|---|---|---|
| Gastric | 688 (63) | 260 (49) | <.0001 |
| Jejunal | 359 (43) | 451 (74) | 0.27 |
| Ileal | 248 (26) | 248 (30) | 0.96 |
| Colonic | 180 (36) | 190 (52) | 0.71 |
| Small intestine | 607 (56) | 698 (88) | 0.37 |
| Small intestine and colon | 781 (65) | 910 (66) | 0.12 |
| Total gastrointestinal fluid volume | 1269 (65) | 1363 (98) | 0.22 |
Values are Mean (SEM) in ml
p value for treatment effects in ANCOVA, adjusted for age and gender
Effect of Erythromycin on Small Intestinal and Colonic Fluid Volume
The time between the onset of oral contrast administration and the MRI examination used to measure intestinal and colonic volumes was 72 ± 2 minutes (placebo) and 76 ± 2 minutes (erythromycin). Small intestinal and colonic fluid volumes were measured in all 40 subjects (Figure 1). Jejunal, ileal, small intestinal, and colonic fluid volumes were not significantly different between erythromycin and placebo (Table 1). Colonic fluid volumes were inversely correlated with gastric (r = −0.37, P=.02) and small intestinal volumes (r = −0.34, P =.04), which suggests that intraluminal contents were displaced distally. The total gastrointestinal fluid volume was not significantly different (P=.22) between erythromycin (1363 ± 98 ml) and placebo (1269 ± 65 ml) groups. In 4 subjects, this total volume exceeded 1800 ml.
The diameter of a representative jejunal loop was greater among patients who received erythromycin (18.8 ± 4.3 mm) than placebo (17.3 ± 2.8 mm) infusion; however, differences were not significant (P=.25) (Table 2). Maximum overall jejunal distention (i.e., 76–100%) was more frequently (P=.03) observed in subjects who were randomized to erythromycin (8/20 [40%] subjects) than placebo (2/20 [10%] subjects).
Table 2.
Effects of Erythromycin and Placebo on Stomach and Intestinal Diameters Measured by a Radiologist
| Site | Placebo | Erythromycin | p value |
|---|---|---|---|
| Jejunum | |||
| Representative loop diameter (mm) | 17.3 ± 0.6 | 18.8 ± 1.0 | 0.25* |
| % Loop distention | |||
| 0–25% | 2 | 2 | |
| 26–50% | 9 | 6 | |
| 51–75% | 8 | 3 | |
| 76–100% | 2 | 8 | 0.03§ |
| Ileum | |||
| Representative loop diameter (mm) | 16.0 ± 0.4 | 18.8 ± 0.6 | 0.001* |
| % Loop distention | |||
| 0–25% | 0 | 0 | |
| 26–50% | 3 | 1 | |
| 51–75% | 4 | 3 | |
| 76–100% | 14 | 15 | NS§ |
p value for treatment effects in ANCOVA (Loop diameters), adjusted for age and gender
p value from Fisher’s Exact Test
The diameter of a representative ileal loop was larger (P=.001) among patients who received erythromycin (18.8 ± 2.7 mm) than placebo (16.0 ± 1.9 mm) infusion (Figure 2). However, the proportion of subjects who had a grade 4 ileal distention in the erythromycin and placebo groups was not significantly different (P=.35).
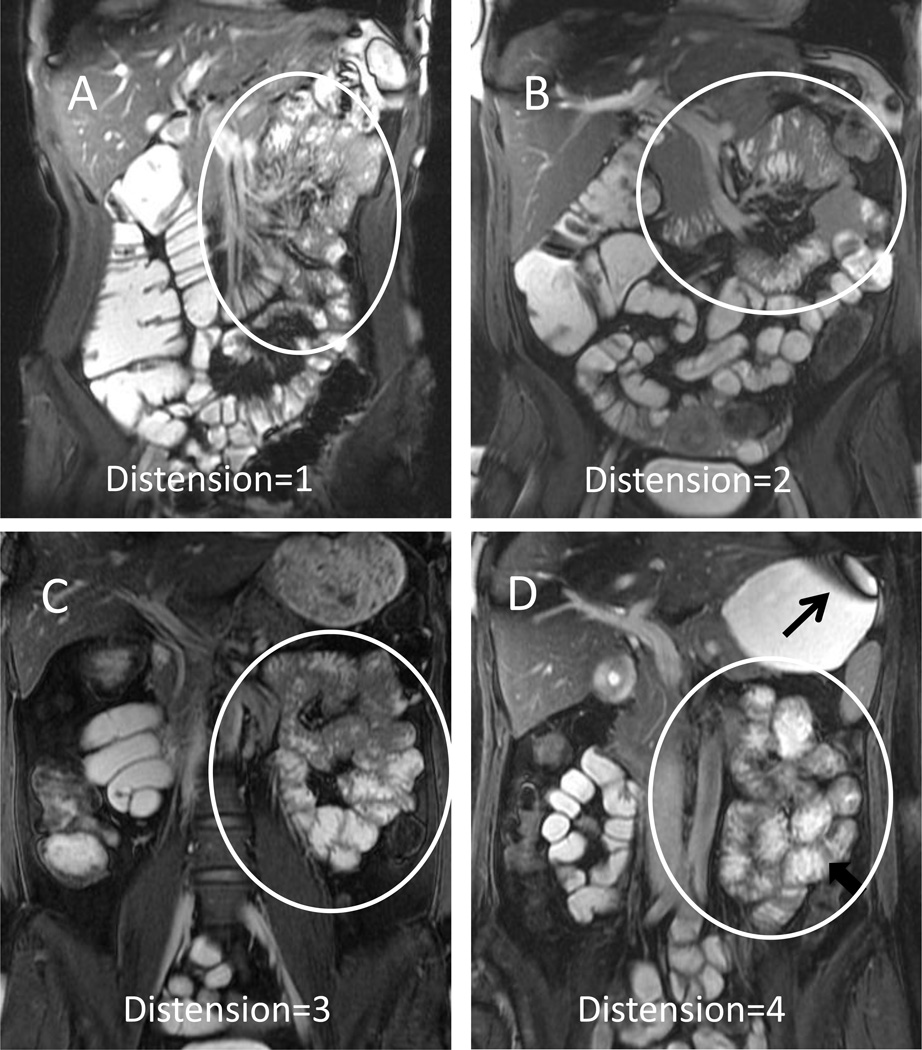
Figure 2. Representative images of jejunal distention.
Coronal True-FISP images with fat suppression show representative jejunal distension (oval marker) scores of 1 (0–25%, Panel A) 2 (25–50%, Panel B), 3 (50–75%, Panel C) and 4 (75–100%, Panel D)
DISCUSSION
MR enterography is becoming more widely utilized to evaluate small bowel abnormalities. Initially utilized for inflammatory bowel disease, other indications now include the evaluation of celiac disease and small bowel masses which can occur in the jejunum and other portions of the small bowel. Therefore adequate distension of the entire small bowel n is necessary for MR enterography. Spasmolytics are routinely administered to reduce bowel peristalsis and decrease motion artifacts during MR enterography. In our vast experience of performing over 1000 MR enterography exams per year, small bowel distention is often suboptimal despite the utilization of the agents. In this study, gastric volumes after ingestion of oral contrast were lower with erythromycin, which suggests that erythromycin accelerated gastric emptying. While the sum of small intestinal and colonic fluid volume was higher after erythromycin than placebo, differences were not statistically significant. However, a larger proportion of subjects who received erythromycin had maximally distended (i.e., > 75% distention) jejunal loops. Also, the diameter of a representative ileal loop was significantly greater for erythromycin than placebo.
This dose of erythromycin (i.e., 200 mg or approximately 3 mg/kg) predominantly increases antral motility (i.e., by inducing a burst of phase 3-like antral contractions), which explains the observed acceleration of gastric emptying [9]. There are four possible explanations as to why erythromycin significantly increased gastric emptying but had lesser effects on small intestinal volumes. First, a type 2 error is possible; based on the observed variability in jejunal and ileal fluid volumes in the placebo group, we had 80% power to detect a difference of 176 ml (i.e., 49% relative to the observed mean in placebo) in jejunal volumes and a difference of 157 ml (i.e., 63% relative to the mean in placebo) in ileal fluid volume after erythromycin infusion. Second, while small intestinal images were acquired at approximately 70 minutes, which is within the 30–80 minute range for optimal bowel distention after this oral contrast agent in a prior study [12], it is conceivable that because erythromycin rapidly and markedly accelerates gastric emptying, jejunal volumes were, by then, on the decline. Third, erythromycin may have also increased small intestinal motility [8, 9] and displaced fluid to the colon, thereby limiting the increase in small bowel volume. Four, even during placebo infusions, a high proportion (i.e., 14 of 20 [70%]) subjects had maximal ileal distention. Hence, a “ceiling” effect might have limited the ability to detect an effect of erythromycin on this parameter. Indeed, during clinical MR enterography, the ileum is usually better distended than the jejunum.
Jejunal and ileal dimensions were evaluated separately by a program that measured volumes and by two radiologists who measured small intestinal diameter. When detecting small bowel lesions, the diameter is a more meaningful parameter of small bowel distention [12]. However, volume assessments, which have been used less frequently, may be more appropriate in physiological studies, for example to assess the effects of perturbations on small bowel water content [16]. It is worth noting that the average difference in small intestinal fluid volume between erythromycin and placebo groups (i.e., approximately 700 – 600 = 100 ml) is distributed over approximately 20 ft of the small intestine.
Erythromycin infusions were well tolerated by all subjects. The risk of erythromycin-induced prolonged Q-T interval is very low, particularly in the absence of host-related risk factors such as electrolyte derangements, organ dysfunction, structural heart disease or other medications which can prolong the Q-T interval [11]. Most case reports of erythromycin-related torsades de pointes describe patients who received intravenous doses of 3–4 gm daily. Q-T prolongation alone rarely leads to torsades de pointes [11].
While this was a prospective study with blinded assessment of stomach, small intestinal and colonic dimensions by several techniques, there were a few other limitations. This study was limited to healthy subjects. A time series of volumes was not obtained. Perhaps earlier scans and/or a time series of scans would have detected drug effects. In order to standardize the objective assessment, small intestinal loops in the upper and lower abdomen were categorized as jejunum and ileum respectively without considering the mucosal fold pattern. While this is probably accurate, it is conceivable that some ileal loops were in the upper abdomen and vice versa. However, these discrepancies should not affect the total small intestinal volume.
CONCLUSIONS
Erythromycin accelerated gastric emptying but effects on jejunal and ileal distention were inconsistent. In balance, this protocol of intravenous erythromycin and scan times did not substantially enhance small bowel distention during MR enterography. However, since erythromycin markedly accelerated gastric emptying, future studies should evaluate a time series of small bowel volumes to identify the optimal time for small bowel imaging. Perhaps earlier scans may identify improved jejunal distention after erythromycin.
Highlights.
Suboptimal small intestinal distention limits jejunal visualization during MRI
In this controlled study, erythromycin increased gastric emptying measured with MRI
However, effects on small intestinal dimensions were variable
Acknowledgements
This study was supported by USPHS Grant P01 DK068055 from the Institutes of Health (NIH). The authors would like to acknowledge Ms. Ann (Annie) E. Almazaar for excellent support with study coordination and the staff of the Biomedical Imaging Resource Core Facility (specifically Philip Edwards and Xi Ge) for their efforts in segmenting and measuring the image data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Mayo Clinic has a significant financial interest in technologies that were developed and used in this research. This development and research have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are being conducted in compliance with Mayo Clinic Conflict of Interest policies.
REFERENCES
- 1.Lauenstein TC, Umutlu L, Kloeters C, Aschoff AJ, Ladd ME, Kinner S. Small bowel imaging with MRI. Acad Radiol. 2012;19(11):1424–1433. doi: 10.1016/j.acra.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn's disease. AJR Am J Roentgenol. 2009;193(1):113–121. doi: 10.2214/AJR.08.2027. [DOI] [PubMed] [Google Scholar]
- 3.Kuehle CA, Ajaj W, Ladd SC, Massing S, Barkhausen J, Lauenstein TC. Hydro-MRI of the small bowel: effect of contrast volume, timing of contrast administration, and data acquisition on bowel distention. AJR Am J Roentgenol. 2006;187(4):W375–W385. doi: 10.2214/AJR.05.1079. [DOI] [PubMed] [Google Scholar]
- 4.Kinner S, Kuehle CA, Herbig S, et al. MRI of the small bowel: can sufficient bowel distension be achieved with small volumes of oral contrast? Eur Radiol. 2008;18(11):2542–2548. doi: 10.1007/s00330-008-1041-7. [DOI] [PubMed] [Google Scholar]
- 5.Samuel S, Bruining DH, Loftus EV, Jr, et al. Endoscopic skipping of the distal terminal ileum in Crohn's disease can lead to negative results from ileocolonoscopy. Clin Gastroenterol Hepatol. 2012;10(11):1253–1259. doi: 10.1016/j.cgh.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Media ACoDaC. ACR Manual on Contrast Media. 2013 [Google Scholar]
- 7.Thoeni RF, Filson RG. Abdominal and pelvic CT: use of oral metoclopramide to enhance bowel opacification. Radiology. 1988;169(2):391–393. doi: 10.1148/radiology.169.2.3174985. [DOI] [PubMed] [Google Scholar]
- 8.Tack J, Janssens J, Vantrappen G, et al. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology. 1992;103(1):72–79. doi: 10.1016/0016-5085(92)91097-n. [DOI] [PubMed] [Google Scholar]
- 9.Annese V, Janssens J, Vantrappen G, et al. Erythromycin accelerates gastric emptying by inducing antral contractions and improved gastroduodenal coordination. Gastroenterology. 1992;102(3):823–828. doi: 10.1016/0016-5085(92)90164-t. [DOI] [PubMed] [Google Scholar]
- 10.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130(5):1466–1479. doi: 10.1053/j.gastro.2005.11.059. [erratum appears in Gastroenterology. 2006 Jul;131(1):336]. [DOI] [PubMed] [Google Scholar]
- 11.Owens RC., Jr QT prolongation with antimicrobial agents: understanding the significance. Drugs. 2004;64(10):1091–1124. doi: 10.2165/00003495-200464100-00005. [DOI] [PubMed] [Google Scholar]
- 12.Young BM, Fletcher JG, Booya F, et al. Head-to-head comparison of oral contrast agents for cross-sectional enterography: small bowel distention, timing, and side effects. J Comput Assist Tomogr. 2008;32(1):32–38. doi: 10.1097/RCT.0b013e318061961d. [DOI] [PubMed] [Google Scholar]
- 13.Skoog SM, Bharucha AE, Camilleri M, Burton DD, Zinsmeister AR. Effects of an osmotically active agent on colonic transit. Neurogastroenterol Motil. 2006;18(4):300–306. doi: 10.1111/j.1365-2982.2006.00757.x. [DOI] [PubMed] [Google Scholar]
- 14.Robb RA. Biomedical Imaging, Visualization and Analysis. New York, NY: John Wiley & Sons, Inc; 1999. [Google Scholar]
- 15.Fidler J, Bharucha AE, Camilleri M, et al. Application of magnetic resonance imaging to measure fasting and postprandial volumes in humans. Neurogastroenterol Motil. 2009;21:42–51. doi: 10.1111/j.1365-2982.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marciani L, Cox EF, Foley JE, et al. Factors influencing fasting small bowel water content as assessed by MRI: increased by ondansetron and reduced by intubation but unaltered by dietary fibre. Neurogastroenterol Motil. 2008;20(Suppl 2) Sat 162. [Google Scholar]