Abstract
BACKGROUND
The safety and effectiveness of automated glycemic management have not been tested in multiday studies under unrestricted outpatient conditions.
METHODS
In two random-order, crossover studies with similar but distinct designs, we compared glycemic control with a wearable, bihormonal, automated, “bionic” pancreas (bionic-pancreas period) with glycemic control with an insulin pump (control period) for 5 days in 20 adults and 32 adolescents with type 1 diabetes mellitus. The automatically adaptive algorithm of the bionic pancreas received data from a continuous glucose monitor to control subcutaneous delivery of insulin and glucagon.
RESULTS
Among the adults, the mean plasma glucose level over the 5-day bionic-pancreas period was 138 mg per deciliter (7.7 mmol per liter), and the mean percentage of time with a low glucose level (<70 mg per deciliter [3.9 mmol per liter]) was 4.8%. After 1 day of automatic adaptation by the bionic pancreas, the mean (±SD) glucose level on continuous monitoring was lower than the mean level during the control period (133±13 vs. 159±30 mg per deciliter [7.4±0.7 vs. 8.8±1.7 mmol per liter], P<0.001) and the percentage of time with a low glucose reading was lower (4.1% vs. 7.3%, P = 0.01). Among the adolescents, the mean plasma glucose level was also lower during the bionic-pancreas period than during the control period (138±18 vs. 157±27 mg per deciliter [7.7±1.0 vs. 8.7±1.5 mmol per liter], P = 0.004), but the percentage of time with a low plasma glucose reading was similar during the two periods (6.1% and 7.6%, respectively; P = 0.23). The mean frequency of interventions for hypoglycemia among the adolescents was lower during the bionic-pancreas period than during the control period (one per 1.6 days vs. one per 0.8 days, P<0.001).
CONCLUSIONS
As compared with an insulin pump, a wearable, automated, bihormonal, bionic pancreas improved mean glycemic levels, with less frequent hypoglycemic episodes, among both adults and adolescents with type 1 diabetes mellitus. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; ClinicalTrials.gov numbers, NCT01762059 and NCT01833988.)
Maintaining glycemic values as close to the nondiabetic range as possible is effective in preventing or delaying long-term complications of type 1 diabetes mellitus,1-3 but achieving near normoglycemia is challenging. Most patients are unable to meet glycemic targets4-6 and have frequent episodes of hypoglycemia, which can be life-threatening.7-11
The availability of accurate continuous glucose monitoring has made feasible the development of bionic endocrine pancreatic systems that are designed to improve glycemic control and reduce the burden on patients. Tests of glycemic regulation lasting 1 day or more with the use of such systems have been limited to tightly regulated inpatient settings.12-25 In inpatient studies lasting 48 hours, we found that a single bihormonal control system, initialized only with the patient's weight, could automatically adapt insulin dosing to meet a broad range of insulin needs and effectively regulate glycemia in adults and adolescents with type 1 diabetes mellitus.23,25 However, the outpatient environment is more challenging, because large variations in meals and activity levels influence insulin requirements and increase the risk of hypoglycemia. Previous out-patient studies of single-hormone systems were limited to an overnight period without meals or exercise.26,27
Here, we present the results of two 5-day trials, one involving adults and one involving adolescents, in which we tested an autonomous, wearable, bihormonal, bionic pancreas in two distinct outpatient settings. These studies minimally constrained patients’ behavior but allowed close observation for risk mitigation and high-density data collection.
METHODS
STUDY PATIENTS
All patients had at least a 1-year history of type 1 diabetes mellitus and were receiving insulin-pump therapy. Adults (in the Beacon Hill Study) were at least 21 years of age; adolescents (in the Summer Camp Study) were campers or counselors between the ages of 12 and 21 years. Other eligibility criteria are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
BIONIC-PANCREAS GLYCEMIC-CONTROL SYSTEM
Insulin and glucagon were administered by a fully automated, bihormonal, bionic pancreas (Fig. S3 in the Supplementary Appendix) with the use of control algorithms similar to those described previously.23,25 The device consisted of an iPhone 4S (Apple), which ran the control algorithm, and a G4 Platinum continuous glucose monitor (DexCom) connected by a custom hardware interface. The user interface displayed the continuous-glucose-monitor tracing and insulin and glucagon doses, and allowed announcement of meal size as “typical,” “more than usual,” “less than typical,” or “a small bite” and the meal type as “breakfast,” “lunch,” or “dinner.” This triggered a partial meal-priming bolus, which automatically adapted insulin dosing to meet 75% of the 4-hour postprandial insulin need for that meal size and type. The first meal-priming bolus of each type was based on the patient's weight (0.05 U per kilogram). Insulin and glucagon were administered subcutaneously by t:slim infusion pumps (Tandem Diabetes Care), which were controlled wirelessly by the iPhone. The control algorithm received continuous glucose-monitoring data and commanded dosing every 5 minutes.
The system was initialized with the use of the patient's weight only; no information about the patient's usual insulin regimen was provided to the algorithm, which automatically adapted insulin dosing online. During operation, no input was provided other than meal announcements and continuous glucose-monitoring calibrations. If the sensor of the continuous glucose monitor failed, the bionic pancreas automatically delivered basal insulin on the basis of requirements determined by the control algorithm at that time on previous days and issued automatic correction doses of insulin or glucagon in response to any manually entered plasma glucose level as determined by fingerstick measurement (see the Methods section in the Supplementary Appendix). If there was a technical problem (e.g., a failed sensor or infusion set), the system regulated any glucose excursion automatically after the problem was resolved. (For details regarding the patients’ technical problems with the device, see Fig. S4 to S55 in the Supplementary Appendix.) Patients were prohibited from taking acet- aminophen because of possible interference with the continuous glucose monitor.28
STUDY DESIGN
In a random-order, crossover design, patients received therapy with a bionic pancreas for 5 days and therapy with their own insulin pump for 5 days. The full study protocols are available at NEJM.org.
Adult Study
During the usual-care (control) period, patients in the adult study lived at home, carried out their usual activities, and managed their diabetes with their own insulin pump and, if desired, their own continuous glucose monitor. Patients wore a G4 Platinum continuous glucose monitor with alarms deactivated and glucose levels masked; it was calibrated with a point-of-care glucometer (HemoCue) twice daily. They kept a diary documenting hypoglycemic episodes, carbohydrate interventions, and exercise. Patients were given a food allowance and encouraged to eat restaurant meals.
During the bionic-pancreas period, patients were free to move about within an 8-km2 (3-mi2) area surrounding the Beacon Hill neighborhood in Boston and were accompanied by study staff members. Patients ate whenever and whatever they liked, primarily in restaurants; daily alcohol intake was limited to three drinks for men and two drinks for women. All patients had access to two gyms and could exercise at will. During nighttime hours (from 11 p.m. to 7 a.m.), patients stayed in a hotel and had their venous plasma glucose levels measured every 30 minutes (every 15 minutes when plasma glucose levels were <70 mg per deciliter [3.9 mmol per liter]) with an autosampling glucose monitor (GlucoScout, International Biomedical), which study staff monitored by means of telemetry. From 7 a.m. to 11 p.m., fingerstick plasma glucose levels were measured with the HemoCue every 2 hours, before meals, and every 30 minutes during exercise and if the patient had symptoms during a hypoglycemic episode. Fingerstick plasma glucose values were concealed from the patients except before meals, during exercise, and during hypoglycemic episodes with symptoms.
Patients could consume carbohydrates at will for symptoms of hypoglycemia (with such consumption counted as an intervention if plasma glucose levels were <70 mg per deciliter) and were required to consume 15 g of carbohydrates if they had a plasma glucose value of less than 50 mg per deciliter (2.8 mmol per liter). During the overnight period, patients were awakened and given 15 g of carbohydrates if they had a venous plasma glucose value of less than 50 mg per deciliter for 30 minutes or a one-time value of less than 40 mg per deciliter (2.2 mmol per liter).
Adolescent Study
Patients in the adolescent study participated in the same activities, ate the same meals, and stayed in the same cabins as nonparticipants in the diabetes camp. During the control period, patients wore a masked continuous glucose monitor plus iPhone device. They used their own insulin pumps and, if desired, their own continuous glucose monitors. Fingerstick plasma glucose measurements were obtained with the HemoCue before meals, at bedtime, at midnight, at approximately 3:45 a.m., before swimming or showering, and during hypoglycemic episodes with symptoms. Patients consumed 15 g of carbohydrates if they had a plasma glucose value of less than 60 mg per deciliter (3.3 mmol per liter) or a value of less than 80 mg per deciliter (4.4 mmol per liter) that was accompanied by symptoms. These episodes were counted as interventions if plasma glucose levels were less than 70 mg per deciliter. For plasma glucose values of 60 to 80 mg per deciliter without symptoms, patients consumed 15 g of carbohydrates if a repeat measurement within 15 to 20 minutes was less than 70 mg per deciliter.
During the two study periods, glucose levels obtained from continuous glucose-monitoring devices were telemetrically monitored. If the glucose level was less than 50 mg per deciliter during the day or if a nocturnal hypoglycemia-alert algorithm projected a plasma glucose level of less than 60 mg per deciliter at night, a plasma glucose measurement was obtained. (Details are provided in the Methods section in the Supplementary Appendix.)
STUDY OUTCOMES
The prespecified coprimary outcomes for the adult study were the mean plasma glucose level (obtained every 2 hours) and the mean percentage of time that the patient had a low glucose level (<70 mg per deciliter) during the bionicpancreas period. The prespecified coprimary outcomes for the adolescent study were the average of scheduled plasma glucose levels and the mean percentage of time that these levels were below 70 mg per deciliter during the bionic-pancreas period and the control period.
Prespecified secondary outcomes for both the adult and adolescent studies included the number of carbohydrate interventions for hypoglycemic episodes, the mean glucose level as measured with the use of continuous glucose monitoring, the time in clinically relevant glucose ranges, and the fraction of patients with a mean glucose level that was consistent with the therapeutic goals issued by the American Diabetes Association.29
Owing to the adaptive nature of the bionic pancreas, we anticipated that outcomes on days 2 through 5 would be more representative of system behavior than those on day 1. Therefore, we prespecified analyses that compared day 1 with days 2 through 5 and that compared the bionic-pancreas periods and the control periods on days 2 through 5.
STUDY OVERSIGHT
The protocols were approved by the human research committee at Massachusetts General Hospital and the institutional review board at Boston University. All adult patients provided written informed consent. Adolescent patients provided written assent, with consent provided by a parent or guardian (see the Methods section in the Supplementary Appendix).
The first author holds a pending patent application for a blood-glucose-control system, assigned to Partners HealthCare and Massachusetts General Hospital, and the second and last authors hold a patent related to a fully automated control system for type 1 diabetes and pending patent applications related to a blood-glucose-control system, all assigned to Boston University.
STATISTICAL ANALYSIS
All data were included in the analyses, including data obtained during periods in which technical problems occurred with the bionic pancreas. Comparisons between study groups were performed with the paired-sample, heteroskedastic Student's t-test. The mean of daily differences was calculated as described previously.30,31
RESULTS
PATIENTS
A total of 52 patients participated in the two studies (Fig. S1 and S2 in the Supplementary Appendix). The baseline characteristics of the patients are shown in Table 1.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Adults† | Adolescents |
|---|---|---|
| No. of patients | 20 | 32 |
| Sex — no. | ||
| Male | 8 | 16 |
| Female | 12 | 16 |
| Age (range) — yr | 40±16 (21–75) | 16±3 (12–20) |
| Weight (range) — kg | 74±10 (50–94) | 69±18 (41–128) |
| Body-mass index (range)‡ | 25±3 (18–33) | 24±5 (17–45) |
| Diabetes duration (range) — yr | 24±11 (5–45) | 9±5 (1–18) |
| Daily insulin dose (range) — U/kg | 0.50±0.11 (0.33–0.76) | 0.80±0.18 (0.43–1.25) |
| Glycated hemoglobin (range) — % | 7.1±0.8 (6.0–8.6) | 8.2±1.0 (5.6–11.6) |
| Estimated average glucose level (range) — mg/dl§ | 158±23 (125–200) | 189±30 (114–286) |
Plus–minus values are means ±SD. To convert the values for glucose to milli-moles per liter, multiply by 0.05551.
All adults had a stimulated C-peptide level of less than the assay limit (<0.1 nmol per liter).
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The estimated average glucose level is based on the glycated hemoglobin level at screening, calculated according to the methods of Nathan et al.32
OUTCOMES IN ADULTS
Plasma Glucose Level
The mean plasma glucose level over the entire 5-day period when the adults were wearing the bionic pancreas was 138 mg per deciliter (range, 116 to 166) (7.7 mmol per liter [range, 6.4 to 9.2]). Plasma glucose levels were below 70 mg per deci-liter 4.8% of the time and below 60 mg per deci-liter 2.3% of the time (Table 2). The mean plasma glucose level overnight was 125 mg per deciliter (range, 97 to 169) (6.9 mmol per liter [range, 5.4 to 9.4]), with plasma glucose levels below 70 mg per deciliter 4.0% of the time and below 60 mg per deciliter 1.7% of the time (Table 2). During 100 patient-days (5 days for the 20 patients) in the bionic-pancreas period, there were 43 carbohydrate interventions for hypoglycemia (1 every 2.3 days), as compared with 68 interventions reported by the patients during the control period (1 every 1.5 days) (P = 0.15).
Table 2.
Summary Results of All 5-Day Experiments among Adults and Adolescents.*
| Variable | Adults (N=20) |
Adolescents (N = 32) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bionic Pancreas | Control | P Value | Bionic Pancreas | Control | P Value | |||||
| mean | range | mean | range | mean | range | mean | range | |||
| Day and night | ||||||||||
| Plasma glucose on days 1 through 5 | ||||||||||
| Mean — mg/dl | 138±14 | 116-166 | 138±18 | 101–185 | 157±27 | 103–221 | 0.004 | |||
| <60 mg/dl — % of time | 2.3±3.2 | 0-8.9 | 2.6±2.9 | 0–11.5 | 3.3±5.0 | 0–17.2 | 0.40 | |||
| <70 mg/dl — % of time | 4.8±5.2 | 0–15.0 | 6.1±4.5 | 0–20.0 | 7.6±7.3 | 0–27.6 | 0.23 | |||
| Carbohydrate interventions — no. | 2.2±3.2 | 0–10.0 | 3.4±3.1 | 0–10.0 | 0.15 | 3.0±3.3 | 0–15.0 | 6.6±5.8 | 0–20.0 | <0.001 |
| Glucose level on continuous monitoring on days 2 through 5 | ||||||||||
| Mean — mg/dl | 133±13 | 114–152 | 159±30.4 | 105–225 | <0.001 | 142±12 | 117–179 | 158±27 | 95–222 | 0.004 |
| <60 mg/dl — % of time | 1.5±1.7 | 0-6.0 | 3.7±3.3 | 0–11.5 | 0.02 | 1.3±1.7 | 0-5.6 | 2.2±3.6 | 0–15.7 | 0.19 |
| <70 mg/dl — % of time | 4.1±3.5 | 0–12.4 | 7.3±4.7 | 0–16.0 | 0.01 | 3.1±2.7 | 0-9.6 | 4.9±5.1 | 0–24.4 | 0.05 |
| 70–120 mg/dl — % of time | 47.7±10.5 | 29.4–65.5 | 30.8±15.7 | 4.1–67.4 | <0.001 | 42.0±7.7 | 31.3–63.1 | 30.0±11.8 | 9.6–56.2 | <0.001 |
| 70–180 mg/dl — % of time | 79.5±8.3 | 69.3–98.2 | 58.8±14.6 | 35.1–82.7 | <0.001 | 75.9±7.9 | 61.4–94.1 | 64.5±14.1 | 29.5–89.5 | <0.001 |
| >180 mg/dl — % of time | 16.5±7.9 | 1.8–26.7 | 33.8±16.4 | 5.7–64.9 | <0.001 | 21.0±7.0 | 4.9–36.5 | 30.6±15.4 | 1.7–69.3 | 0.002 |
| >250 mg/dl — % of time | 4.9±3.7 | 0–12.7 | 12.3±9.9 | 0.1–32.7 | 0.004 | 5.9±4.1 | 0–21.4 | 10.8±9.1 | 0–35.6 | 0.01 |
| SD of all individual SDs — mg/dl | 53±14 | 25–73 | 68±18 | 37–102 | 0.001 | 56±13 | 29–95 | 64±16 | 34–101 | 0.03 |
| Coefficient of variation — % | 40±8 | 22–50 | 43±8 | 34–60 | 0.11 | 39±7 | 25–53 | 40±8 | 25–58 | 0.54 |
| Mean of daily differences — mg/dl | 17±12 | 4–53 | 40±22 | 12–97 | <0.001 | 18±18 | 2–81 | 27±13 | 5–61 | 0.03 |
| Nighttime only | ||||||||||
| Plasma glucose on nights 1 through 5 | ||||||||||
| Mean — mg/dl | 125±19 | 97–169 | 141±20 | 98–190 | 162±37 | 96–241 | 0.02 | |||
| <60 mg/dl — % of time | 1.7±5.2 | 0–22.2 | 1.3±3.4 | 0–10.0 | 2.2±6.1 | 0–30.0 | 0.37 | |||
| <70 mg/dl — % of time | 4.0±8.0 | 0–33.3 | 4.1±6.1 | 0–20.0 | 4.4±6.7 | 0–30.0 | 0.82 | |||
| Carbohydrate intervention — no. | 0.3±0.6 | 0-2.0 | 0.6±0.9 | 0-3.0 | 0.17 | 0.8±1.3 | 0-5.0 | 1.6±1.9 | 0–7.0 | 0.03 |
| Level on continuous glucose monitoring on nights 2 through 5 | ||||||||||
| Mean — mg/dl | 126±17 | 97–170 | 169±52 | 95–286 | 0.002 | 124±11 | 108–146 | 157±36 | 94–248 | <0.001 |
| <60 mg/dl — % of time | 0.4±0.6 | 0-1.6 | 3.3±4.9 | 0–15.4 | 0.01 | 1.0±1.4 | 0-4.9 | 1.7±3.5 | 0–17.7 | 0.28 |
| <70 mg/dl — % of time | 1.8±2.0 | 0–8.6 | 6.2±6.7 | 0–21.9 | 0.01 | 2.6±2.5 | 0-9.4 | 4.0±5.3 | 0–23.7 | 0.16 |
| 70–120 mg/dl — % of time | 57.1±15.8 | 28.9–87.0 | 30.5±20.8 | 0–69.8 | <0.001 | 55.3±13.7 | 29.9–79.2 | 28.3±17.3 | 0–63.8 | <0.001 |
| 70–180 mg/dl — % of time | 86.5±10.0 | 58.1–100 | 55.6±21.9 | 7.0–83.3 | <0.001 | 86.9±8.1 | 68.2–99.2 | 66.7±19.9 | 12.8–91.1 | <0.001 |
| >180 mg/dl — % of time | 11.8±9.5 | 0–39.8 | 38.2±25.1 | 1.6–93.0 | <0.001 | 10.5±7.0 | 0–26.8 | 29.3±22.0 | 0–87.2 | <0.001 |
| >250 mg/dl — % of time | 3.6±5.3 | 0–17.4 | 17.9±20.6 | 0–66.4 | 0.01 | 1.8±2.5 | 0-9.4 | 9.5±12.5 | 0–2.4 | 0.002 |
Plus-minus values are means ±SD. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
Glucose Levels on Continuous Monitoring
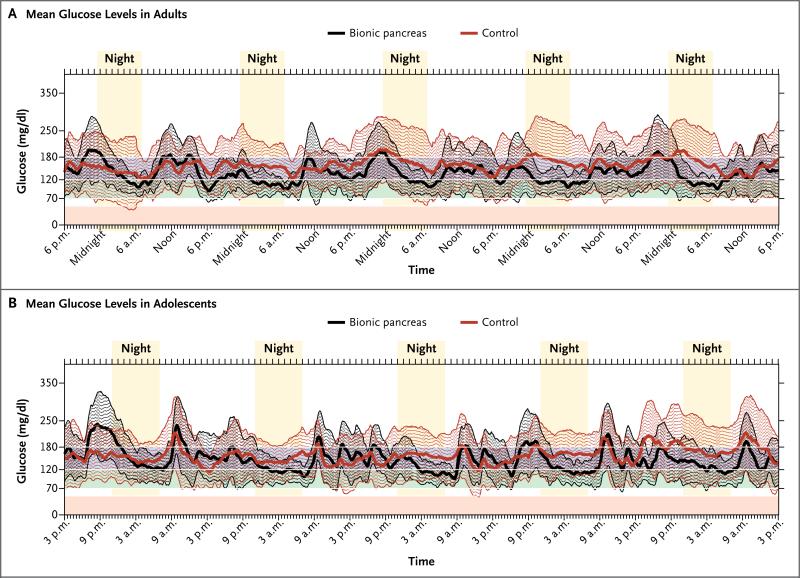
The mean glucose level on continuous monitoring and the time within glucose ranges were similar to the outcomes for plasma glucose levels (Table 2). Because patients performed fewer fingerstick glucose tests during the control period than during the bionic-pancreas period, we also prespecified that comparisons between the bionic-pancreas period and the control period would be based on data from continuous glucose monitoring. There was less variation around the mean glucose level on continuous monitoring during the bionic-pancreas period than during the control period, particularly at night (Fig. 1A).
Figure 1. Variation in the Mean Glucose Level among Adults and Adolescents.
Panel A shows the superimposition of tracings of mean glucose levels on continuous monitoring at all 5-minute steps during the 5-day period in all 20 patients in the adult study during the period when they were wearing the bionic pancreas (black) and during the control period (red). Each tracing is surrounded by an envelope (of corresponding color) that spans 1 SD in either direction around the mean glucose level at each 5-minute step. The mean glucose level during the bionic-pancreas period was 137 mg per deciliter, as compared with 158 mg per deciliter during the control period. Panel B shows tracings for the 32 patients in the adolescent study. The mean glucose level during the bionic-pancreas period was 147 mg per deciliter, as compared with 158 mg per deciliter during the control period. The shaded areas at the bottom of the two panels show clinically significant levels of glucose, including less than 50 mg per deciliter, indicating hypoglycemia (pink); 70 to 120 mg per deciliter, indicating good control (green); and 121 to 180 mg per deciliter, indicating mild hyperglycemia (blue between white lines). To convert the values for glucose to millimoles per liter, multiply by 0.05551.
After automatic adaptation by the bionic pancreas, the mean (±SD) glucose level on continuous monitoring was lower on days 2 through 5 than on day 1 (133±13 vs. 151±21 mg per deciliter [7.4±0.7 vs. 8.4±1.2 mmol per liter], P<0.001) (Fig. 2A), without an increase in the time that glucose levels were lower than 70 mg per deciliter (P = 0.29) or lower than 60 mg per deciliter (P = 0.22). During the control period, there was no significant difference in the mean glucose level on continuous monitoring between day 1 and days 2 through 5.
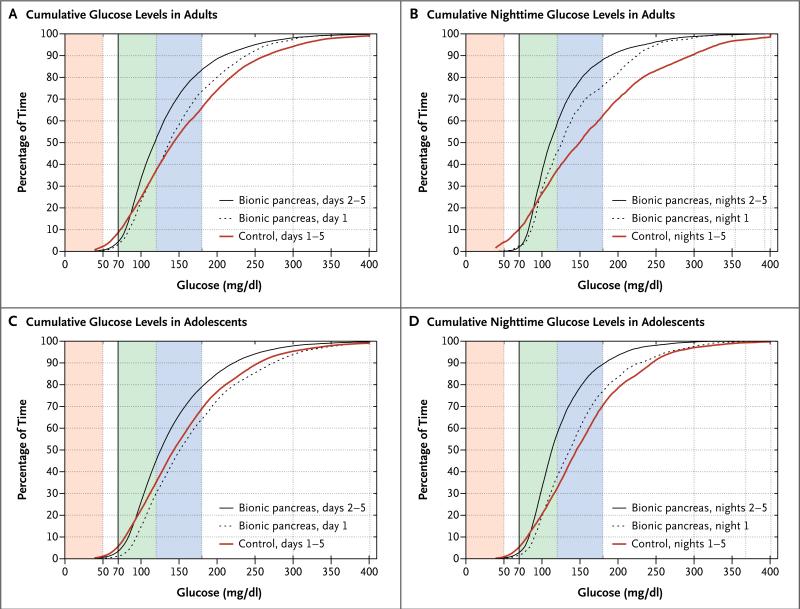
Figure 2. Cumulative Glucose Levels among Adults and Adolescents.
Panel A shows cumulative glucose levels on continuous monitoring during the bionic-pancreas period (day 1 and days 2 through 5) and during the 5-day control period in the 20 patients in the adult study. (During the first 24 hours of the study, the bionic pancreas automatically underwent most of its adaptation.) Panel B shows cumulative nighttime glucose levels in adults. Panels C and D show analogous results for the 32 patients in the adolescent study. In all panels, the shaded regions correspond to a glucose level of less than 50 mg per deciliter (pink), 70 to 120 mg per deciliter (green), and 121 to 180 mg per deciliter (blue). To convert the values for glucose to milli-moles per liter, multiply by 0.05551.
On days 2 through 5 of the bionic-pancreas period, as compared with the control period, the mean glucose level on continuous monitoring was lower (133±13 vs. 159±30 mg per deciliter [7.4±0.7 vs. 8.8±1.7 mmol per liter], P<0.001), the percentage of time with a glucose level between 70 and 180 mg per deciliter (3.9 to 10.0 mmol per liter) was higher (79.5±8.3% vs. 58.8±14.6%, P<0.001), the percentage of time with a glucose level below 70 mg per deciliter was lower (4.1% vs. 7.3%, P = 0.01), and the percentage of time with a glucose level below 60 mg per deciliter was lower (1.5% vs. 3.7%, P = 0.02) (Table 2). At night, there were more pronounced differences between the bionic-pancreas and control periods with respect to the mean glucose level on continuous monitoring (126±17 vs. 169±52 mg per deciliter [7.0±0.9 vs. 9.4±2.9 mmol per liter], P<0.002), the percentage of time with a glucose level between 70 and 180 mg per deciliter (86.5±10.0% vs. 55.6±21.9%, P<0.001), the percentage of time with a glucose level below 70 mg per deciliter (1.8% vs. 6.2%, P = 0.01), and the percentage of time with a glucose level below 60 mg per deciliter (0.4% vs. 3.3%, P = 0.01) (Table 2 and Fig. 2B).
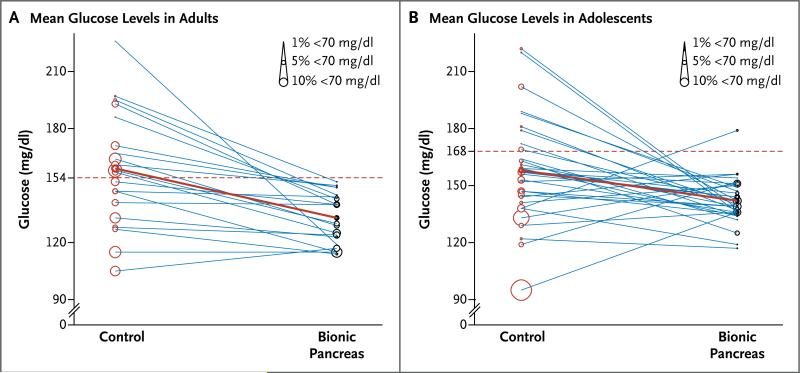
Simultaneous reductions in the mean glucose level on continuous monitoring and the number of hypoglycemic episodes occurred on days 2 through 5 of the bionic-pancreas period, as compared with the control period (Fig. 3A). During the bionic-pancreas period, all the patients had a mean glucose level of less than 154 mg per deciliter (8.5 mmol per liter) on continuous monitoring, a level that corresponds to a glycated hemoglobin level of 7%,32 the therapeutic goal for adults recommended by the American Diabetes Association.29 In contrast, only 9 of 20 patients had a mean glucose level on continuous monitoring that was below this threshold during the control period (Fig. 3A and 4A).
Figure 3. Distributions of Mean Glucose Levels and Hypoglycemia among Adults and Adolescents.
Panel A shows the mean glucose level in each adult on days 2 through 5 of the control period (red circles), which is connected to the corresponding mean glucose level during the bionic-pancreas period (black circles). The diameter of each circle is proportional to the percentage of time that the patient spent with a low glucose value (<70 mg per deciliter) on days 2 through 5. The dashed red line indicates a mean glucose threshold of 154 mg per deciliter, which corresponds to a glycated hemoglobin level of 7%, the upper limit of the therapeutic goal for adults as outlined by the American Diabetes Association. This goal was met in all patients during the bionic-pancreas period. Panel B shows a similar distribution for each of the adolescents, with a cutoff point for the mean glucose level of 168 mg per deci liter, which corresponds to a glycated hemoglobin level of 7.5%, as recommended for adolescents. The one patient in whom this level was not reached on days 2 through 5 had a mean glucose level of 148 mg per deciliter on days 3 through 5. In the two panels, the solid red line indicates the mean for all the patients in the study group. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
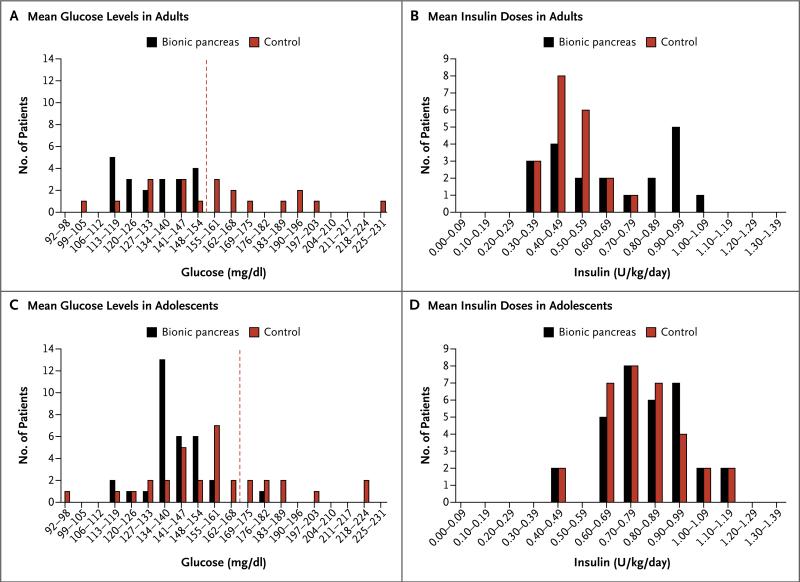
Figure 4. Histogram Distributions of Mean Glucose Levels and Insulin Doses among Adults and Adolescents.
Panel A shows a histogram distribution of the mean glucose levels on continuous monitoring in adults during the bionic-pancreas period and the control period. The distribution shows the number of patients with mean glucose levels divided into intervals of 7 mg per deci-liter on days 2 through 5. The dashed red line indicates a mean glucose level of 154 mg per deciliter, which corresponds to a glycated hemoglobin level of 7%. Panel B shows a similar histogram distribution of total daily doses of insulin in the adults, divided into intervals of 0.1 U per kilogram of body weight per day. Panels C and D show similar histogram distributions for the adolescents, with a cutoff point for the mean glucose level of 168 mg of glucose per deciliter, which corresponds to a glycated hemoglobin level of 7.5%. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
Insulin and Glucagon Doses and Other Outcomes
The total mean daily dose of insulin during the bionic-pancreas period was 0.66±0.23 units per kilogram per day (range, 0.33 to 1.02), which was higher than the total mean daily dose that was determined at screening (0.50±0.11 units per kilogram per day, P = 0.009) (Fig. 4B). Among the 9 patients whose mean glucose level on continuous monitoring was below 154 mg per deci-liter during the control period, there was no significant difference between the bionic- pancreas period and the control period in either the mean total daily dose of insulin (P = 0.23) or the glucose level on continuous monitoring (P = 0.12). Among the other 11 patients, the mean total daily dose of insulin was 50% higher during the bionic-pancreas period than during the control period (P = 0.001); however, the glucose level on continuous monitoring was significantly lower during the bionic-pancreas period than during the control period (139 vs. 180 mg per deciliter [7.7 vs. 10.0 mmol per liter], P<0.001).
The mean carbohydrate consumption during the bionic-pancreas period was 261 g per day. Patients announced approximately two thirds of their meals and snacks to the bionic pancreas. The mean percentage of insulin given as an automated, adaptive, meal-priming bolus was 29% of the daily total and 46% of the nonbasal insulin during days 2 through 5. The mean total daily dose of glucagon during days 2 through 5 of the bionic-pancreas period was 0.82±0.41 mg (range, 0.32 to 1.75).
There were 35 exercise episodes during the bionic-pancreas period and 18 exercise episodes during the control period; 5 of the former episodes and 3 of the latter episodes were associated with carbohydrate interventions. During the control period, 45% of patients used their own unmasked continuous glucose monitor, which is a higher rate of use than that in the general population of patients with type 1 diabetes.32 During the bionic-pancreas period, the patients consumed an average of 1.14 alcoholic drinks per day (range, 0 to 2.4); 12 of the patients consumed an average of between 1 and 2 drinks per day, and 7 consumed an average of between 2 and 3 drinks per day.
OUTCOMES IN ADOLESCENTS
Plasma Glucose Level
On the basis of the six daily scheduled finger-stick measurements of plasma glucose levels, the mean level was 138±18 mg per deciliter (range, 101 to 185) (7.7±1.0 mmol per liter [range, 5.6 to 10.3]) in the bionic-pancreas group and 157±27 mg per deciliter (range, 103 to 221) (8.7±1.5 mmol per liter [range, 5.7 to 12.3]) in the control group (P = 0.004). The percentage of time with glucose levels below 70 mg per deciliter was similar during the two periods (6.1% vs. 7.6%, P = 0.23). During 160 patient-days (5 days for the 32 patients) in the bionic-pancreas period, there were 97 carbohydrate interventions for hypoglycemia (1 every 1.6 days), as compared with 210 interventions during the control period (1 every 0.8 days) (P<0.001).
Glucose Levels on Continuous Monitoring
The mean glucose level on continuous monitoring and the time within ranges were very similar to the outcomes for plasma glucose levels (Table 2). There was less variation around the mean value for the glucose level on continuous monitoring during the bionic-pancreas period than during the control period, particularly at night (Fig. 1B).
After automatic adaptation by the bionic pancreas, the mean glucose level on continuous monitoring was significantly lower on days 2 through 5 than on day 1 (142±12 vs. 169±31 mg per deciliter [7.9±0.7 vs. 9.4±1.7 mmol per liter], P<0.001) (Fig. 2C); there was no significant difference during the control period (P = 0.72).
On days 2 through 5 of the bionic-pancreas period, as compared with the control period, the mean glucose level on continuous monitoring was lower (142±12 vs. 158±27 mg per deciliter [7.9±0.7 vs. 8.8±1.5 mmol per liter], P = 0.004) and the percentage of time with a glucose level between 70 and 180 mg per deciliter was higher (75.9±7.9% vs. 64.5±14.1%, P<0.001); the percentage of time with a glucose level below 70 mg per deciliter was similar (3.1% and 4.9%, respectively; P = 0.05), as was the percentage of time with a glucose level below 60 mg per deciliter (1.3% and 2.2%, respectively; P = 0.19) (Table 2). During the overnight period, there were more pronounced differences between the bionicpancreas period and the control period in the mean glucose level on continuous monitoring (124±11 vs. 157±36 mg per deciliter [6.9±0.6 vs. 8.7±2.0 mmol per liter], P<0.001) and the time with a glucose level between 70 and 180 mg per deciliter (86.9±8.1% vs. 66.7±19.9%, P<0.001), but there was no significant between-period difference in the time with a glucose level below 70 mg per deciliter (2.6% and 4.0%, respectively; P = 0.16) (Table 2 and Fig. 2D).
The American Diabetes Association therapeutic goal for glycemic control in adolescents is a glycated hemoglobin level of 7.5%,29 which corresponds to a mean glucose level of 168 mg per deciliter (9.3 mmol per liter).32 The mean glucose level on continuous monitoring was below this threshold in 31 of 32 patients during days 2 through 5 of the bionic-pancreas period, as compared with 23 of 32 patients during the control period (Fig. 3B and 4C).
Insulin and Glucagon Doses and Other Outcomes
The mean total daily dose of insulin during the bionic-pancreas period was 0.82±0.16 units per kilogram per day (range, 0.41 to 1.13) and was similar to the mean dose during the control period (0.79±0.17 units per kilogram per day, P = 0.27) (Fig. 4D). Also similar were the mean daily values for carbohydrate consumption (247±79 and 264±69 g per day, respectively; P = 0.08). The mean percentage of insulin given as an automated, adaptive, meal-priming bolus was 26% of the daily total and 41% of the non-basal insulin on days 2 through 5. The mean total daily dose of glucagon on days 2 through 5 of the bionic-pancreas period was 0.72±0.26 mg (range, 0.22 to 1.34).
Data on exercise by individual patients were not collected, but the level of activity was very high in the camp environment. During the control period, 9% of patients used their own unmasked continuous glucose monitor.
ADVERSE EVENTS
Among the adults, there were no severe hypoglycemic events. During the bionic-pancreas period, nausea with and without vomiting each occurred once within 2 hours after the last glucagon dose; the vomiting occurred immediately after removal of the intravenous catheter. Three insulin infusion sets and one glucagon infusion set were removed because of pain or inflammation during the bionic-pancreas period (see the Adverse Events section in the Supplementary Appendix).
Among the adolescents, there were no episodes of severe hyoglycemia during the bionicpancreas period. During the control period, there was one episode associated with confusion (plasma glucose level at the time of the episode, 19 mg per deciliter [1.1 mmol per liter]), which was successfully treated with oral carbohydrates. Three patients during each study period had transient hyperketonemia (ketone level, 0.6 to 1.9 mmol per deciliter) that resolved after the infusion set was changed or, in one case, after a technical problem with the bionic pancreas was corrected. One patient reported nausea, and two patients reported vomiting on one occasion each during the bionic-pancreas period. In each case, the last dose of glucagon had been given 2 to 5 hours earlier (see the Adverse Events section in the Supplementary Appendix).
PERFORMANCE OF BIONIC PANCREAS COMPONENTS AND TECHNICAL FAILURES
Among the adults, the mean of the absolute value of the relative difference between glucose levels on continuous monitoring and plasma glucose levels during the bionic-pancreas period was 16.7±5.1% (range, 11.5 to 28.9). Sensor accuracy could not be calculated in the adolescent study because the timing of most plasma glucose measurements was inexact (e.g., “before lunch”). The percentages of time that the insulin and glucagon pumps lost wireless connectivity to the bionic pancreas were 3.5±2.7% and 4.1±2.3%, respectively, among the adults, and 7.0±2.7% and 7.8±2.3%, respectively, among the adolescents. Interruptions were intermittent, reconnection was usually spontaneous, without the need for intervention, and infusions resumed automatically. All data were included in calculations of outcomes, regardless of technical failures.
DISCUSSION
We tested a bihormonal bionic pancreas under conditions that simulated real-world outpatient settings with close monitoring for safety in both adults and adolescents. Meals and physical activity were not regulated, in contrast with previous studies.12-26,33 In the two studies, the bionic pancreas reduced mean levels of plasma glucose and blood glucose on continuous glucose monitoring, as compared with insulin-pump therapy, even though approximately 75% of the patients had better glycemic control at baseline than na tional averages, as reported by the T1D (Type 1 Diabetes) Exchange Clinic Registry.5,6 Among the adolescents, the diabetes care during the control period led to better mean glycemic control than the patients had at home on the basis of their glycated hemoglobin levels at screening. In contrast, the adults received therapy for glycemic control in their usual home and work environments during the control period, so their mean glycemic control during that period was consistent with their baseline glycated hemoglobin levels. Among the adults, the bionic pancreas, as compared with their usual care, reduced the time that glucose levels were below 60 mg per deciliter by 67% (by 94% during the overnight period). Among the adolescents, the extremely close monitoring and rapid intervention for hypoglycemia in the camp setting may explain the lack of a significant between-group difference in the number of hypoglycemic episodes. However, there was a reduction of more than 50% in the amount of carbohydrates given to treat hypoglycemia.
The design of the two studies called for the patients to announce meals to the bionic pancreas through the user interface, although those 18 years of age or older were not reminded if they forgot. Only rough estimates of meal size were provided, eliminating carbohydrate counting and reducing the burden on patients. Less than 30% of the insulin that was delivered in the bionic-pancreas group was in response to meal announcements. In our previous inpatient study, omitting meal announcements increased the mean plasma glucose level by 13 mg per deciliter (0.7 mmol per liter) and did not affect the incidence of hypoglycemia.25 Omission of meal boluses during usual care has more severe consequences for glycemic control.34,35
Our studies had several limitations. Adult patients were limited to moderate alcohol intake; higher alcohol intake might compromise the effectiveness of glucagon. Given the close monitoring by study staff throughout the adolescent study and during the bionic-pancreas period of the adult study, carbohydrate interventions for hypoglycemia may have been given more frequently or earlier than they would have been without supervision. There were intermittent problems with wireless connectivity that caused isolated missed doses of insulin and glucagon by the bionic pancreas; these missed doses may have led to hypoglycemia that could otherwise have been prevented. We did not collect data on nausea during the control period, so we do not know whether the use of glucagon increased its occur-rence.
Limitations of the bionic pancreas include a risk of hypoglycemia if acetaminophen ingestion leads to overestimation of the blood glucose level. As the bionic pancreas improved glycemic control, it delivered more insulin to adult patients who had poor control during the period of usual care. (This was not the case among adults with good glycemic control during usual care or among adolescents.) The balance of risks and benefits associated with delivering more insulin to achieve more physiologic glycemic control will require further study. Finally, the long-term safety of peripheral microdose glucagon administration has not been established. In our previous studies, mean plasma glucagon levels remained in the normal fasting range most of the time,17,23,25 and levels of glucagon are lower than normal in people with type 1 diabetes in the late postprandial period and during exercise36; however, longer-term studies will be necessary to address this issue.
Currently available rapid-acting insulin analogues still have relatively slow absorption after subcutaneous injection, and the poor stability of currently available glucagon formations necessitated daily replacement of the glucagon in the pump with freshly reconstituted material. Since a single device that integrates all the compo nents of a bionic pancreas is not yet available, we had to rely on wireless connectivity to the insulin and glucagon pumps, which was not completely reliable. Despite these challenges associated with currently available technologies, the use of the bihormonal bionic pancreas in our two short-term studies resulted in better glycemic control than is possible with the current standard of care.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK085633, to Dr. Damiano; and R01DK097657, to Drs. Russell and Damiano), the Leona M. and Harry B. Helmsley Charitable Trust (2014PG-T1D006, to Dr. Damiano), and the Earle Charlton Fund for Innovative Research in Diabetes (to Dr. Nathan), and by gifts from the Frederick Banting Foundation, Ralph Faber, and John Whitlock.
Dr. Russell reports receiving consulting fees from Medtronic (through Diabetes Technology Management), lecture fees from Tandem Diabetes, Sanofi Aventis, Eli Lilly, Abbott Diabetes Care, and Biodel, and loaned equipment and technical assistance from International Biomedical, Abbott Diabetes Care, Medtronic, Insulet, and Hospira and holding a pending patent application for a blood glucose control system (PCT/US 13/870,634), assigned to Partners HealthCare and Massachusetts General Hospital; Drs. El-Khatib and Damiano, holding a patent related to a fully automated control system for type 1 diabetes (US 7,806,854) and pending patent applications related to a blood glucose control system (PCT/US 11/058,688 and PCT/US 13/870,634), all assigned to Boston University; and Dr. Damiano, receiving lecture fees from DexCom, Tandem Diabetes Care, Eli Lilly, and Biodel and loaned equipment and technical assistance from DexCom and Tandem Diabetes Care. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients and their families for their participation and enthusiasm. Additional acknowledgments are provided in the Supplementary Appendix.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Writing Team for the Diabetes Control and Complications Trial–Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–9. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr. 2009;155:668–72. doi: 10.1016/j.jpeds.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA. The T1D Exchange clinic registry. J Clin Endocrinol Metab. 2012;97:4383–9. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 6.Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36:2035–7. doi: 10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46:271–86. [PubMed] [Google Scholar]
- 8.Leese GP, Wang J, Broomhall J, et al. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26:1176–80. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- 9.Cengiz E, Xing D, Wong JC, et al. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange clinic registry. Pediatr Diabetes. 2013;14:447–54. doi: 10.1111/pedi.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstock RS, Xing D, Maahs DM, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab. 2013;98:3411–9. doi: 10.1210/jc.2013-1589. [DOI] [PubMed] [Google Scholar]
- 11.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–12. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 12.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55:3344–50. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 13.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934–9. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 14.Renard E, Place J, Cantwell M, Che vassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas. Diabetes Care. 2010;33:121–7. doi: 10.2337/dc09-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-Logic Artificial Pancreas System: a pilot study in adults with type 1 diabetes. Diabetes Care. 2010;33:1072–6. doi: 10.2337/dc09-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33:1282–7. doi: 10.2337/dc09-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Trans Med. 2010;2(27):27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab. 2011;96:1402–8. doi: 10.1210/jc.2010-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Youssef J, Castle JR, Branigan DL, et al. A controlled study of the effectiveness of an adaptive closed-loop algorithm to minimize corticosteroid-induced stress hyperglycemia in type 1 diabetes. J Diabetes Sci Technol. 2011;5:1312–26. doi: 10.1177/193229681100500602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy HR, Kumareswaran K, Elleri D, et al. Safety and efficacy of 24-h closed-loop insulin delivery in well-controlled pregnant women with type 1 diabetes: a randomized crossover case series. Diabetes Care. 2011;34:2527–9. doi: 10.2337/dc11-1430.. [Erratum, Diabetes Care 2012;35:191.]
- 21.Weinzimer SA, Sherr JL, Cengiz E, et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35:1994–9. doi: 10.2337/dc12-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz JL, Sherr JL, Cengiz E, et al. Effect of insulin feedback on closed-loop glucose control: a crossover study. J Diabetes Sci Technol. 2012;6:1123–30. doi: 10.1177/193229681200600517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35:2148–55. doi: 10.2337/dc12-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elleri D, Allen JM, Kumareswaran K, et al. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care. 2013;36:838–44. doi: 10.2337/dc12-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Khatib FH, Russell SJ, Magyar KL, et al. Autonomous and continuous adaptation of a bihormonal bionic pancreas in adults and adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2014;99:1701–11. doi: 10.1210/jc.2013-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368:824–33. doi: 10.1056/NEJMoa1206881. [DOI] [PubMed] [Google Scholar]
- 27.Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37:1204–11. doi: 10.2337/dc13-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dexcom G4 Platinum continuous glucose monitoring system user's guide — chapter 2.3: contraindications. Dexcom; San Diego, CA: 2013. ( http://www.dexcom.com/sites/dexcom.com/files/dexcom-g4-platinum/ifu/dexcom-g4-platinum-ifu.pdf) [Google Scholar]
- 29.American Diabetes Association Standards of medical care in diabetes — 2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molnar GD, Taylor WF, Ho MM. Dayto-day variation of continuously monitored glycaemia: a further measure of diabetic instability. Diabetologia. 1972;8:342–8. doi: 10.1007/BF01218495. [DOI] [PubMed] [Google Scholar]
- 31.Service FJ, Nelson RL. Characteristics of glycemic stability. Diabetes Care. 1980;3:58–62. doi: 10.2337/diacare.3.1.58. [DOI] [PubMed] [Google Scholar]
- 32.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–8. doi: 10.2337/dc08-0545.. [Erratum, Diabetes Care 2009;32:207.]
- 33.Kovatchev BP, Renard E, Cobelli C, et al. Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care. 2013;36:1851–8. doi: 10.2337/dc12-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olinder AL, Kernell A, Smide B. Missed bolus doses: devastating for metabolic control in CSII-treated adolescents with type 1 diabetes. Pediatr Diabetes. 2009;10:142–8. doi: 10.1111/j.1399-5448.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 35.Burdick J, Chase HP, Slover RH, et al. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113(3):e221–e224. doi: 10.1542/peds.113.3.e221. [DOI] [PubMed] [Google Scholar]
- 36.Kudva YC, Carter RE, Cobelli C, Basu R, Basu A. Closed-loop artificial pancreas systems: physiological input to enhance next-generation devices. Diabetes Care. 2014;37:1184–90. doi: 10.2337/dc13-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.