Blinatumomab has shown efficacy in B-cell malignancies.1, 2, 3 Rapid clearance of peripheral B cells has been demonstrated, resulting in sustained B-cell depletion throughout the treatment period.1, 2, 3 This report describes serum immunoglobulin levels during and after blinatumomab treatment in a phase 2 study in patients with minimal residual disease (MRD) of B-precursor acute lymphoblastic leukemia (ALL).2,3
The study followed an open-label, multicenter, single-arm, phase 2 design. Study details and primary analysis data have been described previously.3 Briefly, patients with positive MRD status (>10−4 detectable blast cells using quantitative PCR) at any time after induction and consolidation therapy according to German multicenter study group for adult ALL (GMALL) protocols were eligible. The primary objective was to determine the efficacy of blinatumomab in patients with MRD-positive B-precursor ALL. Patients received blinatumomab as a continuous intravenous infusion at a dose of 15 μg/m2/day over 4 weeks, followed by a 2-week treatment-free period (6-week cycles). For patients with an allogeneic donor, an allogeneic hematopoietic stem cell transplantation (HSCT) was offered at any time after the first 6-week cycle. Responders could receive three additional consolidation cycles of blinatumomab treatment. Between May 2008 and November 2009, 21 patients with MRD-positive B-precursor ALL were treated.
Serum immunoglobulins IgM, IgG, IgA and IgE have a central role in the humoral immune response by binding to extracellular pathogens, thereby activating the complement system along with effector cells, which ultimately leads to pathogen eradication.4 Whereas serum IgM antibodies are mainly produced during a primary immune response by plasma cells originating from activated naive B cells, IgG, IgA, and IgE are also secreted in large amounts during secondary immune responses by plasma cells originating from activated memory B cells. Most long-lived antibody-based immunity against invading pathogens is provided by serum IgG and mucosal IgA. Hence, therapy-induced depletion of CD19-positive B cells and plasma blasts, and associated subsequent decline of plasma cells can result in a long-term decrease of serum immunoglobulin concentrations, which recover only after regeneration of naive and memory B cells from CD19-negative hematopoietic B-cell progenitors. Patients receiving B-cell–depleting therapies may therefore be susceptible to severe infections during and after treatment.
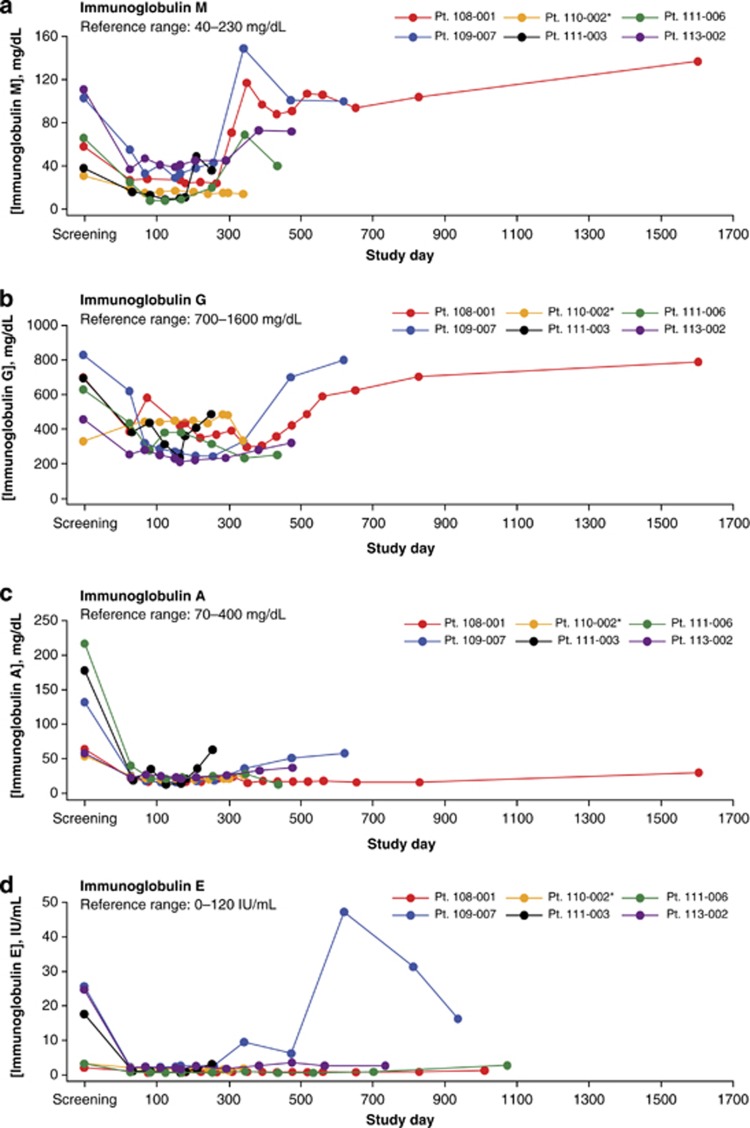
In our phase 2 study, IgM, IgG, IgA and IgE levels were monitored during a follow-up time ranging from 255 to 1605 days (median, 457.5 days) in six patients with MRD-positive B-precursor ALL who did not receive HSCT after blinatumomab treatment. Four of the six patients had Philadelphia chromosome (Ph)-negative ALL; two had Ph-positive ALL. After completion of blinatumomab treatment, the four patients with Ph-negative ALL did not receive any further treatment for their disease, whereas the two patients with Ph-positive ALL received tyrosine kinase inhibitors. One of the two patients with Ph-positive ALL had no MRD response at the end of blinatumomab treatment (Table 1). The five responders received blinatumomab for a median of 154 days (infusion period plus treatment-free period); the nonresponder was treated for 287 days. Three patients entered the study with an IgA level, four with an IgG level, and two with an IgM level below normal range. The most pronounced immunoglobulin decrease was observed for IgA, with a decline to 6% (range, 6–39%) of baseline in response to blinatumomab treatment (Figures 1a–d; Table 1). The lowest levels of IgM and IgG were 12% (range, 12–45%) and 29% (range, 29–101%) of baseline, respectively. In the five responders, the median time to lowest level was 168 days for IgA, 126 days for IgM, and 260 days for IgG. In the nonresponder, this time was 112 days for IgA, and 245 days for IgM. IgG levels in the nonresponder did not decrease in response to blinatumomab treatment. None of the five responders showed a return of serum IgA levels to baseline after blinatumomab treatment, but in two responders the IgA recovery exceeded 50% of baseline. One of the five responders showed a recovery of both serum IgG and IgM levels to above baseline, and IgG and IgM recovery exceeded 50% in three and four of the other responders, respectively. The nonresponder presented with less than 50% recovery of both IgA and IgM, whereas serum IgG levels were not decreased by blinatumomab treatment.
Table 1. Serum immunoglobulin levels of individual patients at various time points during the study.
|
Patient identifier |
Time point of measurement |
IgA mg/dl |
IgG mg/dl |
IgM mg/dl |
Tyrosine kinase inhibitor treatment post blinatumomab |
|---|---|---|---|---|---|
| Reference range | (70–400) | (700–1600) | (40–230) | ||
| 111–006a | Screening | 217 (100%) | 629 (100%) | 66 (100%) | None |
| Lowest value | 13 (6%) | 232 (37%) | 8 (12%) | ||
| Last FU | 13 (6%) | 250 (40%) | 49 (60%) | ||
| 113–002a | Screening | 58 (100%) | 457 (100%) | 111( 100%) | None |
| Lowest value | 21 (36%) | 210 (40%) | 37 (33%) | ||
| Last FU | 37 (64%) | 320 (70%) | 72 (65%) | ||
| 109–007a | Screening | 132 (100%) | 830 (100%) | 103 (100%) | None |
| Lowest value | 16 (13%) | 242 (29%) | 29 (28%) | ||
| Last FU | 58 (44%) | 801 (97%) | 100 (97%) | ||
| 108–001a | Screening | 64 (100%) | 700 (100%) | 58 (100%) | None |
| Lowest value | 15 (33%) | 296 (42%) | 24 (41%) | ||
| Last FU | 30 (47%) | 789 (113%) | 137 (236%) | ||
| 110–002b,c | Screening | 54 (100%) | 330 (100%) | 31 (100%) | Imatinib, dasatinib |
| Lowest value | 20 (39%) | 333 (101%) | 14 (45%) | ||
| Last FU | 26 (48%) | 333 (101%) | 14 (45%) | ||
| 111–003b | Screening | 178 (100%) | 696 (100%) | 38 (100%) | Imatinib |
| Lowest value | 13 (35%) | 234 (34%) | 9 (24%) | ||
| Last FU | 63 (73%) | 487 (70%) | 36 (91%) |
Abbreviation: FU, follow-up.
Percentages are normalized to respective baseline values. ‘Screening' indicates baseline.
Philadelphia chromosome-negative.
Philadelphia-chromosome positive.
nonresponder (no MRD response).
Figure 1.
Serum immunoglobulin levels over time in a phase 2 study of blinatumomab in patients with MRD-positive B-precursor acute lymphoblastic leukemia (ALL). Panels show data for serum IgM (a), IgG (b), IgA (c) and IgE (d) concentrations for five responders and one nonresponder (Patient 110–002, indicated with an asterisk).
Immunoglobulin levels and isotype recovery sequence (IgM>IgG>IgA) in responders correlated with the expected mode of action of blinatumomab, with initial B-cell depletion leading to decreased immunoglobulin levels during and after treatment and a subsequent return of IgM-secreting plasma cells originating from newly developed naive B cells. However, in the nonresponding patient, no reduction of IgG levels and a <50% recovery of IgM levels were observed, suggesting incomplete depletion of plasma blasts during, and diminished return of naive B cells after, blinatumomab therapy. As described above, patients in the current study had hypogammaglobulinemia at study entry, stemming from prior front-line chemotherapy and/or the underlying ALL. Chemotherapy has known suppressive effects on immunoglobulin levels, with IgM and IgG typically showing the greatest decrease after treatment.5 Vincristine and prednisone appear to have a major role specifically in the drop of IgG levels.5 Chemotherapy dose reductions can lessen immunoglobulin level decreases and support recovery.5
As CD19, but not CD20, expression is maintained on plasma blasts, anti-CD19 antibodies are predicted to induce a more profound decrease of immunoglobulin levels than anti-CD20 antibodies.6 However, extensive clinical experience has shown that the anti-CD20 monoclonal antibody rituximab may decrease serum levels of immunoglobulins across various disease settings. For example, in patients with anti-neutrophil cytoplasmic autoantibody-associated vasculitis, rituximab may exacerbate a cyclophosphamide-induced decline of immunoglobulin levels.7 Similarly, data from two patients with idiopathic thrombocytopenic purpura and pre-existing primary antibody deficiency, who presented with recurrent infections immediately following rituximab therapy, suggest exacerbation of the underlying immune deficiency.8 In a study of patients with newly diagnosed aggressive B-cell lymphoma, hypogammaglobulinemia was present at the end of chemotherapy treatment but resolved 12 months later. However, addition of rituximab was associated with a more pronounced decline in immunoglobulin levels than chemotherapy treatment alone, and that decline was sustained 12 months after cessation of therapy.9 A cross-study analysis of data from 211 patients with B-cell lymphoma who received rituximab treatment showed that hypogammaglobulinemia developed in 38.5% and symptomatic hypogammaglobulinemia (after multiple courses of treatment) in 6.6% of patients.10 Two case reports have described hypogammaglobulinemia that lasted for 6–7 years after completion of rituximab-based treatment in patients with follicular lymphoma.11,12 In one patient, who had normal immunoglobulin concentrations at treatment start, the level of IgG dropped to below 100 mg/dl, and both IgA and IgM remained undetectable for 6 years after treatment stop, despite recovery of peripheral B-cell counts.12 A second patient presented with pan-hypogammaglobulinemia and a history of recurrent sinus infections 7 years after the completion of therapy. The symptoms resolved completely in response to monthly intravenous immunoglobulin treatments.11 Finally, rituximab has also been shown to delay immunologic recovery after autologous transplantation, with low IgG levels at 2 years post transplantation.13,14
The data from our phase 2 study show that blinatumomab-mediated depletion of B cells and plasma blasts is reflected in decreased serum concentrations of immunoglobulins during and after treatment. Whereas naive B cells tended to be regenerated soon after treatment, as suggested by recovery of IgM levels, memory B cells and plasma cells might take longer to reappear, based on the observation that recovery of IgG and IgA levels was delayed after treatment. Infections associated with B-cell–depleting therapies may occur during and after treatment until full recovery of IgG levels, despite prior normalization of peripheral B-cell counts owing to regeneration of naive, but not memory, B cells. In the present study, grade 3 infections, regardless of causal relationship to blinatumomab, were reported in four out of the 21 patients (19%), with two (9.5%) cases assessed as related to blinatumomab treatment. No infection-related deaths occurred. Similarly, the rate of grade 3 or 4 infections during rituximab maintenance therapy for the treatment of non-Hodgkin lymphoma was stated as 9.7%.15 A review of blinatumomab data along with rituximab data available in the literature does not suggest notable differences in the long-term recovery of serum immunoglobulin levels. Of note, it appears that rituximab therapy may cause a sustained reduction of immunoglobulin concentrations in serum despite the return of peripheral B cells, suggesting a similar regeneration pattern as that proposed to follow blinatumomab treatment. Additional depletion of plasma blasts by blinatumomab, but not by rituximab, is not expected to be an issue as these cells are comparably short-lived and, therefore, unable to sustain normal immunoglobulin levels throughout and after treatment. These cells, once again, have to differentiate out of regenerated naive and memory B cells after treatment.
In summary, baseline and periodic monitoring of immunoglobulin levels may be considered in patients who receive antibody therapies against B-cell targets, such as blinatumomab. Their suppressive effect on immunoglobulin levels appears to be more pronounced than the suppressive effect of chemotherapy. In patients with severe infections and low serum IgG concentrations, IgG substitution treatment should be considered according to local guidelines.
Acknowledgments
Editorial assistance was provided by Cory Pfeiffenberger, PhD (Complete Healthcare Communications Inc.), whose work was funded by Amgen Inc., and Beate Quednau, PhD (Amgen Inc.). This study was funded by Amgen Inc. and is registered with ClinicalTrials.gov (identifier, NCT00560794).
Author Contributions
G Zugmaier, S Alekar, MS Topp, E Degenhard, N Gökbuget, and M Klinger conceived and/or designed the work described herein; acquired data and/or had an important role in interpreting the results; drafted and/or revised the manuscript and approved the final version. A Viardot, H-A Horst, S Neumann, M Stelljes, RC Bargou, M Goebeler and D Wessiepe acquired data; had an important role in interpreting the results; drafted and/or revised the manuscript; and approved the final version.
G Zugmaier, E Degenhard and M Klinger are employees of Amgen Research (Munich) GmbH and stockholders in Amgen Inc. MS Topp has received honoraria as an invited speaker and consultant from Amgen Inc. S Alekar is an employee of and stockholder in Amgen Inc. A Viardot has consulted for Janssen-Cilag GmbH, Pfizer GmbH, and Gilead Sciences GmbH and received honoraria from Roche Pharmaceuticals and Pfizer GmbH. H-A Horst has received research funding from Amgen Inc. RC Bargou has consulted for and received honoraria from Amgen Inc.; he is listed as an inventor on patents related to blinatumomab but does not receive royalties from them. D Wessiepe is an employee of Metronomia GmbH and is a contractor to Amgen Inc. N Gökbuget has received research support and honoraria as invited speaker and consultant from Amgen Inc. The remaining authors declare no conflict of interest.
Footnotes
Disclaimer
The authors had access to all study data and take responsibility for the final submitted version.
References
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- Topp MS, Gokbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120:5185–5187. doi: 10.1182/blood-2012-07-441030. [DOI] [PubMed] [Google Scholar]
- Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- Murphy K. Janeway's Immunobiology. Vol 1. New York City: Garland Science - Taylor & Francis; 2011. [Google Scholar]
- van Tilburg CM, Bierings MB, Berbers GA, Wolfs TF, Pieters R, Bloem AC, et al. Impact of treatment reduction for childhood acute lymphoblastic leukemia on serum immunoglobulins and antibodies against vaccine-preventable diseases. Pediatr Blood Cancer. 2012;58:701–707. doi: 10.1002/pbc.23258. [DOI] [PubMed] [Google Scholar]
- Venhoff N, Effelsberg NM, Salzer U, Warnatz K, Peter HH, Lebrecht D, et al. Impact of rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitides. PLoS One. 2012;7:e37626. doi: 10.1371/journal.pone.0037626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluml S, McKeever K, Ettinger R, Smolen J, Herbst R. B-cell targeted therapeutics in clinical development. Arthritis Res Ther. 2013;15 (suppl 1:S4. doi: 10.1186/ar3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwakar L, Gorrie S, Richter A, Chapman O, Dhillon P, Al-Ghanmi F, et al. Does rituximab aggravate pre-existing hypogammaglobulinaemia? J Clin Pathol. 2010;63:275–277. doi: 10.1136/jcp.2009.068940. [DOI] [PubMed] [Google Scholar]
- Dunleavy K, Reiter BLW, Grant CM, Shovlin M, Wright GW, Fleisher TA, et al. Rituximab Is associated with prolonged immunoglobulin deficiency in newly diagnosed patients with aggressive B-cell lymphoma receiving immunochemotherapy. Blood. 2010;116:abstr 2881. [Google Scholar]
- Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13:106–111. doi: 10.1016/j.clml.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AR, Kleiner A, Rich L, Conners C, Fisher RI, Anolik J, et al. Profound hypogammaglobulinemia 7 years after treatment for indolent lymphoma. Cancer Invest. 2008;26:431–433. doi: 10.1080/07357900701809068. [DOI] [PubMed] [Google Scholar]
- Irie E, Shirota Y, Suzuki C, Tajima Y, Ishizawa K, Kameoka J, et al. Severe hypogammaglobulinemia persisting for 6 years after treatment with rituximab combined chemotherapy due to arrest of B lymphocyte differentiation together with alteration of T lymphocyte homeostasis. Int J Hematol. 2010;91:501–508. doi: 10.1007/s12185-010-0528-6. [DOI] [PubMed] [Google Scholar]
- Kasamon YL, Jones RJ, Brodsky RA, Fuchs EJ, Matsui W, Luznik L, et al. Immunologic recovery following autologous stem-cell transplantation with pre- and posttransplantation rituximab for low-grade or mantle cell lymphoma. Ann Oncol. 2010;21:1203–1210. doi: 10.1093/annonc/mdp484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio M, Fujimoto K, Yamamoto S, Endo T, Sakai T, Obara M, et al. Delayed redistribution of CD27, CD40 and CD80 positive B cells and the impaired in vitro immunoglobulin production in patients with non-Hodgkin lymphoma after rituximab treatment as an adjuvant to autologous stem cell transplantation. Br J Haematol. 2007;137:349–354. doi: 10.1111/j.1365-2141.2007.06584.x. [DOI] [PubMed] [Google Scholar]
- van Oers MH, Van Glabbeke M, Giurgea L, Klasa R, Marcus RE, Wolf M, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin's lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28:2853–2858. doi: 10.1200/JCO.2009.26.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]