Peripheral artery occlusive disease (PAOD) is an emerging adverse event in chronic myelogenous leukemia (CML) patients treated with nilotinib.1, 2, 3, 4 The largest published cohort of CML patients treated with nilotinib comes from the phase III ENESTnd trial (Evaluating Nilotinib Efficacy and Safety in Clinical Trials - newly diagnosed patients), which compared nilotinib (300 or 400 mg BID) to standard therapy with imatinib (400 mg). From the 3-year follow-up analysis published in 2012, 8 patients out of 556 (1.4%) in the nilotinib arm were identified as having PAOD.5 This figure does not take into account three patients reported in the 2-year analysis as having PAOD, but who were removed from the 3-year analysis as a result of changes in the AE search algorithm.6 When these three patients are included, 11 patients out of 556 (2.0%) can be considered as having PAOD compared to no cases among the 280 patients treated in the imatinib arm.
Two recent reports analyzed more closely the relative incidence of PAOD in nilotinib-treated patients.7,8 The first reviewed POAD cases reported in three trials (IRIS, TOPS and ENESTnd) and generated three groups of patients: those not exposed to tyrosine kinase inhibitors (n=553), those exposed to imatinib (n=1301) and those exposed to nilotinib (n=556). PAOD incidence was 0.6%, 0.2% and 1.3% in these groups, respectively.7 In the second academic study, 129 CML patients treated with imatinib or nilotinib were prospectively screened for PAOD using the ankle-brachial index (ABI).8 In this cohort, the incidence of PAOD was far higher than the previously reported data (6.3% with first-line imatinib, 26.0% with first-line nilotinib and 35.7% with second-line nilotinib), with a higher cumulative incidence in patients at high risk for vascular disease than that in low-risk patients. Despite these reports, little is known about PAOD improvement after nilotinib discontinuation. We report a case of severe PAOD observed in a CML patient treated with nilotinib as fourth-line treatment followed by very rapid clinical improvement—within a few weeks after nilotinib discontinuation—despite persistence of severe artery stenosis.
A 64-year-old male was diagnosed with chronic-phase CML in 1996. At diagnosis he had a low Sokal score and a history of smoking, which he had stopped 10 years earlier. No other cardiovascular risks other than age were recorded. The patient received interferon alfa as first-line therapy for 4 years and imatinib 400 mg/day for 5 years as second-line therapy due to a lack of complete cytogenetic response (CCyR). Treatment was switched to dasatinib due to intolerance (muscle pain and myositis) despite CCyR and a major molecular response (MMR). A complete molecular response (CMR4.5) was obtained under dasatinib 100–140 mg/day without adverse events. However, in 2006 he developed grade 3 pleural effusion and dasatinib was discontinued after several attempts to resume failed. Nilotinib was initiated in 2008 at the dose of 800 mg/day (trough level 2035 ng/ml) and then reduced to 600 mg/day (trough level 1560 ng/ml), at which time the patient was in CMR4, achieving CMR4.5 in 2011.
While receiving nilotinib, he developed bilateral xanthoma of the eyelids, which was confirmed histologically. His total cholesterol level was mildly elevated prior to nilotinib (2.5 g/l) and increased rapidly to 3.5 g/l after treatment initiation. Low-density lipoprotein (LDL) cholesterol increased within the same range from 1.5 to 2.5 g/l. The patient started atorvastatin in October 2012 (20 mg/day, subsequently increased to 30 mg/day), after which a dramatic decrease of total and LDL cholesterol values (1.66 and 0.8 g/l, respectively) was observed. Atorvastatin was then discontinued in April 2013 owing to major muscle pain in the upper and lower limbs.
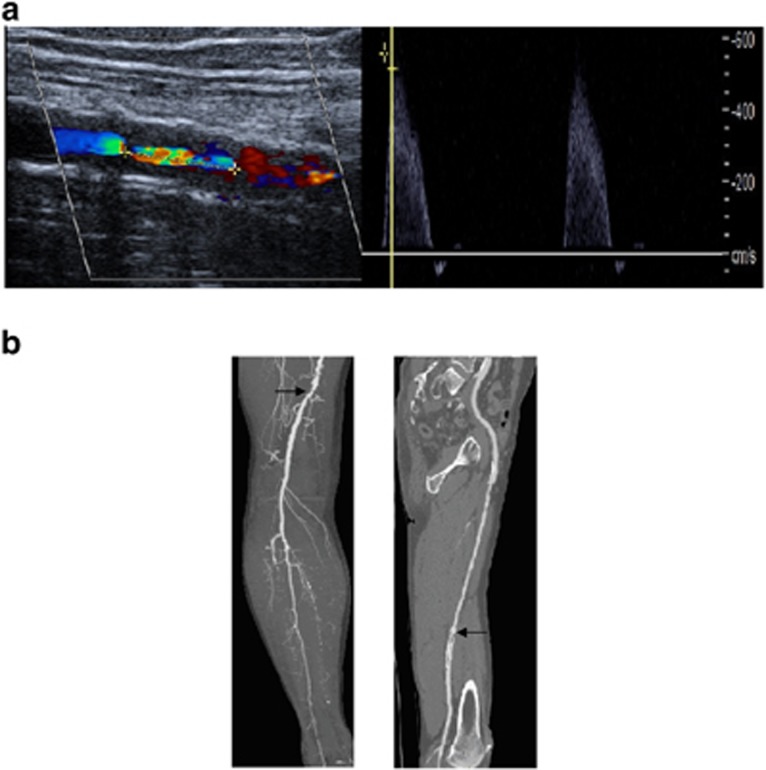
Three years after nilotinib initiation, in September 2011, the patient reported an intermittent calf claudication of the right lower limb (walking distance 50 m). His ABI value was low (0.55) and a Doppler ultrasound revealed a 90% stenosis of the right superficial femoral artery associated with a distal arteriopathy (Figure 1a). Despite implementing daily walking and low-dose aspirin (100 mg daily), the patient continued to suffer from claudication and was limited in his activity. A computerized tomography angiography (CTA) confirmed the conclusion of the Doppler ultrasound (Figure 1b). An angioplasty of the femoral superficial artery with a stent implantation was then performed in May 2012. The nilotinib daily dose was reduced to 400 mg/day (trough level 958 ng/ml). The patient's walking distance improved slightly to 100 m. His ABI value was 0.6 and intra-stent stenosis (more than 90%) of the superficial femoral artery persisted (Figure 1b). Nilotinib was stopped in January 2013. After 2 weeks, clinical symptoms related to PAOD disappeared and his walking distance returned to normal (the patient performed a 10 -km walk without any symptoms). Despite the persistence of a severe hemodynamically relevant stenosis (>90%), his ABI improved to 0.7 and remained stable. A summary of the patient's relevant clinical parameters in relation to the timing of nilotinib treatment is provided in Table 1.
Figure 1.
Imaging of the right femoral superficial artery stenosis by (a) Doppler ultrasound and (b) CTA associated with a distal arteriopathy (left image) and intra-stent stenosis (right image) diagnosed after angioplasty.
Table 1. Evolution of metabolic and vascular parameters during and after nilotinib.
| Parameter | Pre-nilotinib | Nilotinib 800 mg/day | Nilotinib 600 mg/day | Nilotinib 400 mg/day | Post angioplasty | Post-nilotinib discontinuation |
|---|---|---|---|---|---|---|
| Trough level (ng/ml) | — | 2035 | 1560 | 958 | — | — |
| Atorvastatin | No | No | Yes | Yes | No | |
| Total cholesterol (mg/l, NR<2) | 2.4 | 1.8 | 1.6 | 2.6 | ||
| HDL cholesterol (mg/l, NR>0.4) | 0.6 | 1.0 | 0.6 | 1.7 | ||
| LDL cholesterol (mg/L, NR<1.6) | 1.6 | 0.6 | 0.8 | 1.4 | ||
| Glycemia (g/l) | 1.09 | 1.16 | ||||
| Systolic blood pressure (mm Hg) | 120 | 120 | 120 | 155 | ||
| Walking distance (m) | 50 | 50 | 50 | 100 | Normal | |
| ABI | 0.5 | 0.5 | 0.6 | 0.7 | ||
| % Stenosis | 90% | 90% | 90% |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; NR, normal range.
To the best of our knowledge, rapid reversion of clinical symptoms related to severe POAD after nilotinib discontinuation has not been reported previously. This observation is particularly interesting given the context that severe vascular occlusion persisted after nilotinib cessation despite improvement of ABI and normalization of the walking distance. These discrepancies between the level of vascular occlusion and the clinical symptoms may be observed when collateral vessels develop. However, this hypothesis is not in line with the 2 weeks timeframe of improvement of the claudication in our patient.
The widely accepted mechanism accounting for PAOD refers to the formation of atherosclerotic plaques in patients with pre-existing vascular risk factors or a vascular history.1, 2, 3, 4, 5, 6, 7, 8 Metabolic changes induced by nilotinib (increased cholesterol including LDL cholesterol2 and hyperglycemia) may have a negative impact on pre-existing atherosclerosis lesions. Recently, Racil et al.9 studied glucose metabolism in 10 CML patients receiving nilotinib (as first- or subsequent-line therapy). The authors demonstrated that impaired glucose metabolism was related to insulin resistance and compensatory hyperinsulinemia, and suggested that blocking c-ABL may affect the insulin receptor signaling pathway. Dyslipidemia may also be, in part, secondary to this phenomenon. Our observation suggests that mechanisms potentially affecting the vascular endothelium or artery compliance may be involved in the physiopathology of nilotinib-induced PAOD in addition to atherosclerotic plaques. Nonetheless, to date, no direct evidence of an off-target effect of nilotinib on the structure of the vessels has been reported.10
PR received a research grant from Novartis. The remaining authors declare no conflict of interest.
References
- Aichberger KJ, Herndlhofer S, Schernthaner GH, Schillinger M, Mitterbauer-Hohendanner G, Sillaber C, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86:533–539. doi: 10.1002/ajh.22037. [DOI] [PubMed] [Google Scholar]
- Le Coutre P, Rea D, Abruzzese E, Dombret H, Trawinska MM, Herndlhofer S, et al. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst. 2011;103:1347–1348. doi: 10.1093/jnci/djr292. [DOI] [PubMed] [Google Scholar]
- Quintas-Cardama A, Kantarjian H, Cortes J. Nilotinib-associated vascular events. Clin Lymphoma Myeloma Leuk. 2012;12:337–340. doi: 10.1016/j.clml.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Letendre L. Nilotinib treatment-associated peripheral artery disease and sudden death: yet another reason to stick to imatinib as front-line therapy for chronic myelogenous leukemia. Am J Hematol. 2011;86:610–611. doi: 10.1002/ajh.22051. [DOI] [PubMed] [Google Scholar]
- Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12:841–851. doi: 10.1016/S1470-2045(11)70201-7. [DOI] [PubMed] [Google Scholar]
- Giles FJ, Mauro MJ, Hong F, Ortmann CE, McNeill C, Woodman RC, et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia. 2013;27:1310–1315. doi: 10.1038/leu.2013.69. [DOI] [PubMed] [Google Scholar]
- Kim TD, Rea D, Schwarz M, Grille P, Nicolini FE, Rosti G, et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or Imatinib. Leukemia. 2013;27:1316–1321. doi: 10.1038/leu.2013.70. [DOI] [PubMed] [Google Scholar]
- Racil Z, Razga F, Drapalova J, Buresova L, Zackova D, Palackova M, et al. Mechanism of impaired glucose metabolism during nilotinib therapy in patients with chronic myelogenous leukemia. Haematologica. 2013;98:e124–e126. doi: 10.3324/haematol.2013.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]