Background: Trefoil factor 2 (TFF2) forms complexes with MUC6 and is concertedly secreted by deep gastric glands.
Results: TFF2 is a lectin that binds to α-GlcNAc-capped MUC6 O-glycans with antibiotic activity against Helicobacter pylori.
Conclusion: There may be a functional link between mucin glycans and TFF2 in H. pylori defense.
Significance: The findings impact in development of defense strategies against H. pylori and in TFF2 receptor-mediated cell signaling.
Keywords: Glycomics, Glycosylation, Helicobacter pylori, Lectin, Mucin, Mucin-type O-Glycans, Trefoil Factor Family 2 (TFF2)
Abstract
Helicobacter pylori infection is the major cause of gastric cancer and remains an important health care challenge. The trefoil factor peptides are a family of small highly conserved proteins that are claimed to play essential roles in cytoprotection and epithelial repair within the gastrointestinal tract. H. pylori colocalizes with MUC5AC at the gastric surface epithelium, but not with MUC6 secreted in concert with TFF2 by deep gastric glands. Both components of the gastric gland secretome associate non-covalently and show increased expression upon H. pylori infection. Although blood group active O-glycans of the Lewis-type form the basis of H. pylori adhesion to the surface mucin layer and to epithelial cells, α1,4-GlcNAc-capped O-glycans on gastric mucins were proposed to inhibit H. pylori growth as a natural antibiotic. We show here that the gastric glycoform of TFF2 is a calcium-independent lectin, which binds with high specificity to O-linked α1,4-GlcNAc-capped hexasaccharides on human and porcine stomach mucin. The structural assignments of two hexasaccharide isomers and the binding active glycotope were based on mass spectrometry, linkage analysis, 1H nuclear magnetic resonance spectroscopy, glycan inhibition, and lectin competition of TFF2-mucin binding. Neoglycolipids derived from the C3/C6-linked branches of the two isomers revealed highly specific TFF2 binding to the 6-linked trisaccharide in GlcNAcα1-4Galβ1-4GlcNAcβ1-6(Fucα1-2Galβ1-3)GalNAc-ol(Structure 1). Supposedly, lectin TFF2 is involved in protection of gastric epithelia via a functional relationship to defense against H. pylori launched by antibiotic α1,4-GlcNAc-capped mucin glycans. Lectin-carbohydrate interaction may have also an impact on more general functional aspects of TFF members by mediating their binding to cell signaling receptors.
Introduction
Human trefoil factor 2 (hTFF2 or TFF2)2 (UniProt Q03403, chromosome 21) is a secretory peptide with a molecular mass of 14.284 Da that comprises 106 amino acid residues and belongs to the trefoil factor family peptides characterized by characteristic cysteine patterns (1–4). It was discovered originally in the porcine pancreas where it was termed “pancreatic spasmolytic polypeptide” (5). However, in humans the major expression site of TFF2 is the stomach (1) where it is secreted together with the mucin MUC6 by cardiac glands, mucous neck cells, and antral gland cells (6, 7). Peptides of the trefoil factor family are characterized by at least one TFF domain, a 40-mer peptide with three conserved disulfide bridges. TFF2, in particular, is composed of two TFF domains (P1, P2), and modified mostly by N-linked glycans within the P1 domain. The stomach-associated glycoform was shown to carry a biantennary complex-type N-glycan with terminal monofucosylated N,N′-diacetyl-lactosediamine antennae (8). It has been postulated that the hydrophobic groove formed by loops 2 and 3 of the TFF domains could exert specific protein and/or carbohydrate binding capacities (9).
Different functional aspects of TFF2 have been reported in the past. On the one hand, gastric TFF2 is strongly associated with mucins (7, 10) where it changes the rheological properties of mucus (11). It is a characteristic constituent of the alternating laminated structure of the gastric surface mucous gel layer where it co-localizes with MUC6 (7, 12).
TFF2 has recently been identified as a gastric stem/progenitor cell marker in Helicobacter pylori infection (13). Further evidence for a link between trefoil factor peptides and H. pylori infection was revealed by demonstrating aberrant increased epithelial expression of TFF2 and MUC6 in H. pylori-infected gastric antrum, incisura, and body (14). Mucin-type O-linked glycans on gastric mucins expressing Lewis-type blood group antigens are long known to play key roles in the colonization (Lewis (b)) and initial inflammatory processes (sialyl-Lewis(x)) of the gastric surface epithelium (15). The surface mucous cell-derived mucin layer is dominated by MUC5AC, whereas the other major gastric mucin, MUC6, is secreted by gland mucous cells, including cardiac gland cells, mucous neck cells, and pyloric gland cells (16). MUC6 appears to be negligible in the surface mucus gel layer (17). Interestingly, H. pylori is exclusively associated with surface mucous cell-derived mucins and only rarely colonizes deeper portions (18). These deeper portions of the mucosa are dominated by MUC6, and its mucin-type O-glycans are distinct from other mucins at organ sites outside of the gastrointestinal tract as they show a characteristic capping with α1-4-linked GlcNAc (18, 19). Strikingly, these gastric MUC6-specific glycans were demonstrated to inhibit H. pylori growth and to function in this way as a natural antibiotic (20, 21). Mechanistically this effect was claimed to be mediated by decreased formation of the essential cell wall component cholesteryl-α-d-glucopyranoside. We here report on a potential link between the H. pylori growth inhibitory potency of MUC6 glycans capped with α1-4-linked GlcNAc and gastric TFF2 by demonstrating that the two TFF domains of TFF2 have metal ion-independent lectin-like properties and bind even under low pH conditions with high specificity to αGlcNAc-capped gastric mucin glycans with previously shown antibiotic activity against H. pylori.
EXPERIMENTAL PROCEDURES
Materials
Gastric Mucins
Mucin from porcine stomach type III (Sigma) was digested extensively with Pronase E to generate Pronase-stable glycopeptides, which were isolated as described (22). Mucins from human meconium or amniotic fluid (22) and MUC6-positive mucins from gastric juice donated by blood group Lewis-defined individuals (23) (MUC6 core protein identification by mass spectrometric proteomics not shown) were extracted with phenol/saline and isolated by size exclusion chromatography.
Other Mucins
Submaxillary mucins from bovine glands were purchased from Sigma.
Glycoproteins
Neoglycoproteins with linked oligosaccharides of blood groups A, B, H, or LSTc structure were from BioCarb (Lund, Sweden) or prepared by Dr. Kolar (Tα-BSA) from Behringwerke (Marburg, Germany). Sialyl-Lewis(x) and sialyl-Lewis(a) neoglycoproteins were purchased from Dextra Laboratories (Reading, UK), and aliquots were enzymatically desialinated with neuraminidase from Clostridium perfringens (BioLabs) prior the binding assays.
Lectins
The lectins from Helix pomatia (Sigma), Triticum vulgare (Sigma), Dolichos biflorus (Sigma), Griffonia simplicifolia II (EY Laboratories Inc., San Matteo, CA), Arachis hypogaea (Sigma), Vicia villosa (Sigma), and Ulex europaeus (Sigma) were used in binding competition analyses.
Synthetic Glycopeptides
GlcNAcβ1-3GalNAcα (core 3)-threonine glycopeptide was a synthetic product supplied by Sussex Research Laboratories (Ottawa, Ontario, Canada).
STRUCTURE 1.
Sugars, Synthetic Oligosaccharides, and Glycoconjugates
Monosaccharides and 4-nitrophenyl α- or β-linked N-acetylglucosaminide were purchased from Sigma, lacto-N-triose from Carbosynth Ltd. (Berkshire, UK), GlcNAcβ1-3Gal from Elicityl (Crolles, France), and a series of milk oligosaccharides from Oxford GlycoSystems Ltd. (Oxford, UK).
Carbohydrate-specific Antibodies
Mouse monoclonal antibodies 2B5 to GlcNAcβ1-3Galβ1-4GlcNAcβ1-6(GalNAc-ol) (kindly provided by Dr. Borchard, University Clinic of Düsseldorf), a series of ISOBM workshop TD-4 anti-MUC1 mouse monoclonal antibodies anti-Lewis(x) DH-1, anti-sialyl-Lewis(x) M26, anti-Lewis(a) CC2, anti-blood group H2 clone 43, and antibodies with blood-group H2 specificity A46-A/B10 and A51-B/A6 (kindly provided by Dr. Karsten, Max Delbrück Center, Berlin, Germany), were used as binding competitors.
Exoglycosidases
Mucin-bound glycans and mucin-derived glycan alditols were treated with the exoglycosidases sialidase from C. perfringens (New England Biolabs, Frankfurt, Germany), blood group H2-specific α-fucosidase was from Xanthomonas manihotis (New England Biolabs), α-N-acetylglucosaminidase, recombinant from RD Systems (Wiesbaden, Germany), β-hexosaminidase from Glyco/Prozyme (Cambridge, UK), α-N-acetylgalactosaminidase from chicken liver (Sigma), the endo-O-glycosidase, recombinant from New England Biolabs, and with the sulfatase from H. pomatia (Sigma). All chemicals (acetonitrile, DMSO, chloroform, methanol, ethanol, and deuterium oxide) were of the highest available grade and purchased from Sigma, if not stated otherwise.
Generation of Human TFF2 Probes
Template for the generation of TFF2 constructs was a plasmid containing the cDNA encoding the 129-amino acid TFF2 precursor protein (GenBankTM BC032820.1). The full-length construct (hTFF2-Fl, p24-p129), the single domain constructs N-P1 (p24-p78) and C-P2 (p74-p129), the full-length construct hTFF2-Fl-(C52/G), and the single domain double mutant N-P1-(W68F59/Q), were generated as secretory fusion proteins with C-terminal oligo-His and Strep-tags, but without their endogenous N-terminal signal peptide (p1-p23). Primers for full-length and single-domain N-P1 and C-P2 constructs were: Fwd1, aatTCTAGAagagaaaccctccccctgccag; Fwd2, aatTCTAGAaccaaagcaagagtcggatcag; Rev1, tgtAGATCTgtaatggcagtcttccacaga; Rev2, tgtAGATCTcgactcttgctttgggagggg. PCR amplification was conducted by utilizing overhang primers with a non-complimentary 3′-extension including recognition sequences for restriction enzymes XbaI and BglII. The digested PCR products were cloned into the eukaryotic pCEP-Pu vector V59 designed for the expression of the C terminally tagged (octa-His and STREP) secretory fusion proteins. Site-directed mutations were introduced with the QuikChange II Site-directed Mutagenesis Kit. Cys42 and Cys52, respectively, were exchanged for Gly in hTFF2-Fl, and a novel N-glycosylation site was generated in the otherwise N-glycosylation-negative P2 domain by mutation of Asp87 to Asn87 and Arg89 to Thr89 in one step using the following primers: Fl-(Cys42/Gly), Fwd, cataacaggacgaacggcggcttccct; Fl(Cys52/Gly), Fwd, atcaccagtgaccaggtgtttgacaat. C-P2 (Asp87/Asn, Arg89/Thr), Fwd, tgcgtcatggaggtctcaaaccgaacaaactgtggctacc. The construct N-P1-(W68F59/Q) was synthesized by Geneart-Life Technolgies (Regensburg, Germany), and ligated into V59 vector as above.
Cells and Cell Culture
Culture media and cell culture flasks were purchased from Biochrom (Berlin, Germany). The human embryonic kidney cell line HEK-293 (Invitrogen) was cultivated and transfected, and recombinant TFF2 probes were isolated via their oligo-His tag as described (24).
Isolation of Gastric Mucin-derived O-Glycans
The glycan chains were released from the mucin core protein by reductive β-elimination (24) or by hydrazinolysis to retain the pyranosidic ring structure of the reducing core-GalNAc (25). After desalting on graphitized carbon, glycan alditols or reducing glycans were chromatographed by polar amino-phase HPLC (System Gold, Beckman-Coulter, Germany) as described (22, 25).
ELISA Binding, Competition, and Inhibition Analyses
Mucin or mucin-derived glycopeptides (100 μg or 10 μg/ml) were immobilized on polystyrene microtitration plates (Nunc, Wiesbaden, Germany). After blocking with 200 μl of 5% BSA/PBS (37 °C, 1 h) the Strep-tagged TFF2 probe (1 μg/ml of calcium- and magnesium-free phosphate-buffered saline) was added in the presence or absence of inhibitors (37 °C, 2 h), and bound TFF2 was detected with Strep-Tactin/alkaline phosphatase conjugate (IBA GmbH, Goettingen, Germany, 1:1000, 37 °C, 1 h) followed by development with p-nitrophenyl phosphate. Plates were read in a Tecan ELISA reader at 405 nm. Statistical evaluation of triplicate assays was based on independent sample t tests and calculation of t and p values. The t quantile (degrees of freedom 4, probability level 0.05) was 2.78.
Structural Analyses
Profiling and Oligosaccharide Sequencing of Permethylated Glycan Alditols by MALDI-MS
Glycan alditols were permethylated (26) and analyzed by mass spectrometry on an UltrafleXtreme MALDI-TOF-TOF instrument (Bruker Daltonics, Bremen, Germany) as described (24). MS/MS was performed by analysis of post-source decay fragments in the laser-induced dissociation mode. Fragment annotation of glycans was assisted by application of the GlycoWorkbench tool (27). T3 sequencing of in-source decay B-type fragment ions was performed under the same MS/MS conditions.
Oligosaccharide sequencing by LC-ESI-MS/MS
Methylated glycan alditols were analyzed by LC-MS/MS on an ESI iontrap, the HCT ultra ETDII PTMDiscovery System (Bruker-Daltonics), coupled with an online easy-nano-LC system (Proxeon, Odense, Denmark) as described (26).
GC-MS of Partially Methylated Alditol Acetates for Linkage Analysis
Partially methylated alditol acetates (PMAA) were prepared from permethylated glycan alditols according to Albersheim et al. (28) and were analyzed by GC-MS on a 15-m RTX5-SILMS capillary column (Restek, Bad Homburg, Germany) (24).
1H Nuclear Resonance Spectroscopy of TFF2 Binding Hexasaccharide Alditol
All NMR spectra were recorded at 303 K on a Bruker Avance II+ 600 spectrometer (1H frequency 600.2 MHz) using a triple resonance z gradient probe (H,P,BB) for inverse detection. They were processed using TopSpin 3.1 software (Bruker). The hexasaccharide analyte (approximately 100 μg) dissolved in water was repeatedly dried by vacuum rotation and resolubilized in deuterated solvent (D2O), which was purchased from Aldrich. The final volume was 450 μl (0.2 mm).
For all experiments, suppression of the residual water signal was achieved by presaturation. One-dimensional high-resolution experiments were recorded with 64k complex data points and 64 scans were collected at a spectral width of 12,000 Hz. The original free induction decay was zero filled to 128k and Fourier transformation using an exponential window function was applied. Two-dimensional total correlation spectroscopy (TOCSY) spectra were recorded using the MLEV-17 pulse sequence with mixing times (spin-lock) in the range of 80 ms. Usually 4k data points were collected for the direct domain (t2) with 32 scans each, and 512 t1 transients were collected.
Preparation and Analysis of Glycan Alditol-derived Neoglycolipids
Glycan alditols (10 μg) were converted to neoglycolipids after mild periodate oxidation by reductive amination to l-1,2-dipalminoyl-sn-glycero-3-phosphoethanolamine according to a previously published protocol for microscale applications (29). Neoglycolipids were eluted from silica gel with chloroform/methanol/water (10:10:1). Duplicate ELISAs were performed on polystyrene microtitration plates (approximately 400 pmol of neoglycolipid per well were applied by drying a 50 μl solution in methanol). About 100–200 pmol of the neoglycolipids in 0.5 μl of methanol (TLC-fractions 1–4) were mixed with an equal aliquot of matrix (saturated solution of 4-hydroxy-α-cyanocinnamic acid in acetonitrile, 0.1% aqueous trifluoroacetic acid, 1:1) and MALDI mass spectrometry was performed in the negative ion mode.
RESULTS
The Human Gastric Glycoform of TFF2 Binds Calcium Independent to Gastric Mucin
Human TFF2 had long been proposed to exhibit lectin-like activities, although strong evidence was never provided. As trefoil factor peptides in general are found in association with mucins of the gastrointestinal tract we tested first whether TFF2 (expressed in HEK-293 cells as a strep-tagged TFF2-Fl probe) was binding active to a series of mucin preparations from human gastric juice. For this purpose we performed binding and binding inhibition studies in a standardized enzyme-linked immunosorbent assay by immobilizing mucins or mucin-derived Pronase-stable glycopeptides onto polystyrene microplates. We could demonstrate a high binding activity of full-length TFF2 to these mucin samples, which was independent of the blood group Lewis status of the gastric juice donors (Figs. 1 and 2A). Similar high binding was found on mucins from human meconium, but no binding above background to bovine submaxillary mucin (Fig. 1A). Further evidence was revealed in binding studies on gastric mucins from porcine stomach (PSM) and derived Pronase-stable PSM glycopeptides, which showed the highest TFF2 binding capacities compared with all other mucins under study (Fig. 1). Binding of TFF2 to mucins revealed concentration-dependent logarithmic characteristics (Fig. 1B). As all binding assays were performed in calcium and magnesium-free PBS, the lectin activity of TFF2 was apparently independent of calcium or other divalent metal ions. Over 2-h time periods at 37 °C the binding activity showed a partial resistance to low pH conditions (Fig. 1A).
FIGURE 1.
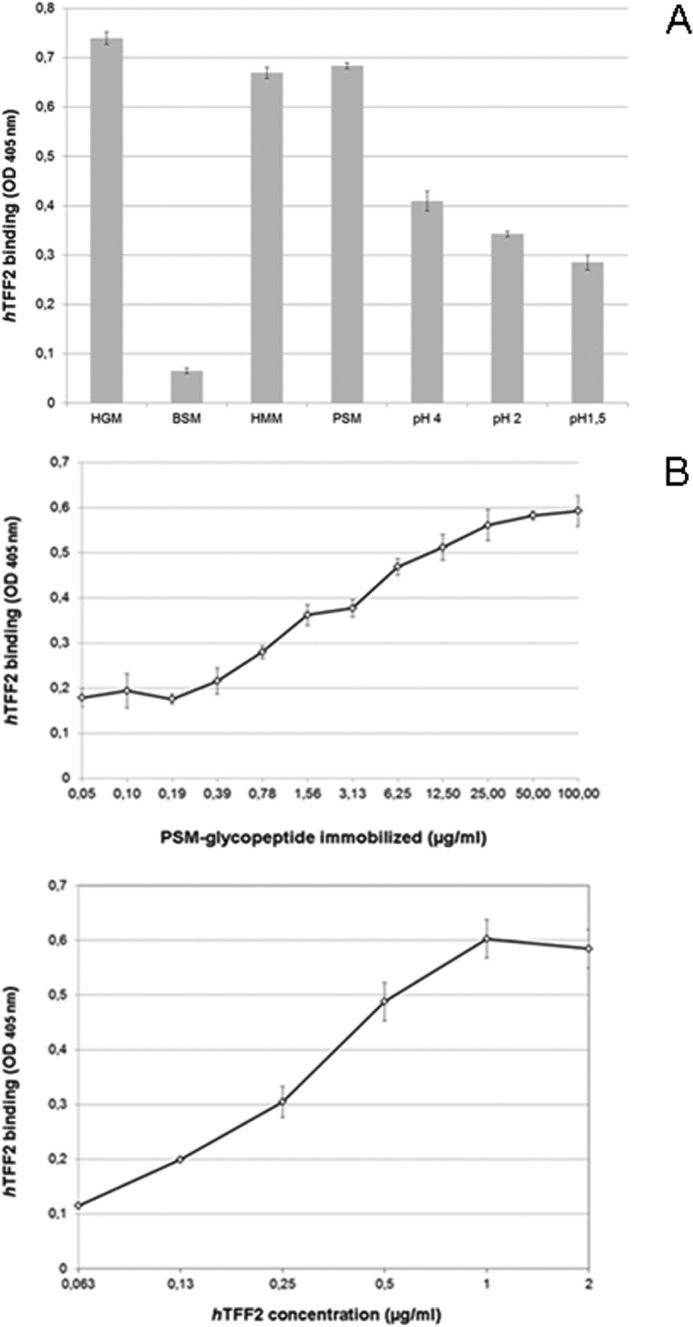
Binding of TFF2 (hTFF2-Fl) to human gastric mucin and porcine gastric mucin glycopeptides. A, triplicate assay (with S.D.) of TFF2 binding to human mucins from gastric juice (HGM, pooled sample), and meconium (HMM), bovine submaxillary mucin (BSM), 5 μg/well, and porcine stomach mucin (PSM) glycopeptides (0.5 μg/well) in calcium- and magnesium-free Dulbecco's phosphate-buffered saline at pH 7.0 (PBS). Binding to PSM glycopeptides was also tested in acidified PBS at pH 4.0, 2.0, and 1.5. Average background binding was at 0.06 (A405 nm). B, triplicate assays (with S.D.) of TFF2 binding to PSM-derived glycopeptides; variation of immobilized glycopeptide amounts tested with hTFF2-Fl at 1 μg/ml (upper part) or variation of hTFF2-Fl concentration tested on 0.5 μg/well of PSM glycopeptides (lower part). Average background binding was at 0.06 (A405 nm).
FIGURE 2.
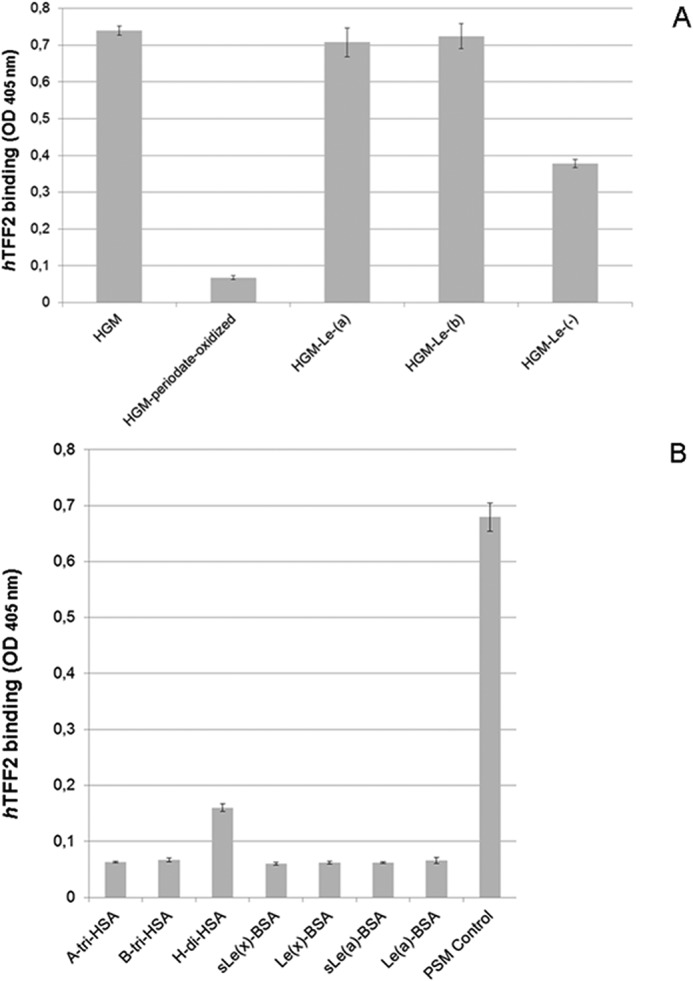
Evidence for blood group Lewis-independent carbohydrate specificity of TFF2 (hTFF2-Fl) binding to mucins. A, triplicate assay (with S.D.) of TFF2 binding to blood group Lewis-defined human gastric mucin (HGM, 5 μg/well) and to a blood group undefined sample (5 μg/well) tested prior to and after mild periodate oxidation of carbohydrates (10 mm sodium metaperiodate in 0.1 m sodium acetate, pH 4.0, overnight incubation at 4 °C in the dark). Average background binding was at 0.06 (A405 nm). B, triplicate assay (with S.D.) of TFF2 binding to neoglycoproteins (0.5 μg/well) carrying blood group-related carbohydrate antigens. Average background binding was at 0.06 (A405 nm).
TFF2 Binds Carbohydrate-dependent, but Blood Group AB and Lewis-independent to Mucin Glycans
Mild selective periodate oxidation of mucin-bound sugars completely abrogated TFF2 binding activity to the background level (p value 2.6 × 10−9), indicating a carbohydrate-dependent interaction with the immobilized mucins (Fig. 2A). A contribution of the mucin core proteins to the binding epitope can be largely ruled out, as binding activity was retained on Pronase-stable glycopeptides derived from porcine stomach mucin (Fig. 1B, refer also to neoglycolipid binding reported below). Treatments with exoglycosidases (sialidase, α2-fucosidase, β-galactosidase, β-hexosaminidase, α-N-acetylglucosaminidase, and α-N-acetylgalactosaminidase) and endo-N-acetylgalactosaminidase did not destroy the binding active epitope (data not shown). Moreover, none of the tested neoglycoproteins carrying blood group-related glycans were found binding positive, with the exception of blood group H disaccharide-HSA, which showed weak, but significant binding activity (p value 1.34 × 10−6) compared with blood group A active neoglycoprotein (Fig. 2B). These initial findings indicated a carbohydrate dependence of TFF2 binding to mucins and demonstrated that blood group A/B or Lewis-related context of this binding can be largely ruled out.
Structural Requirements of TFF2 Lectin Activity
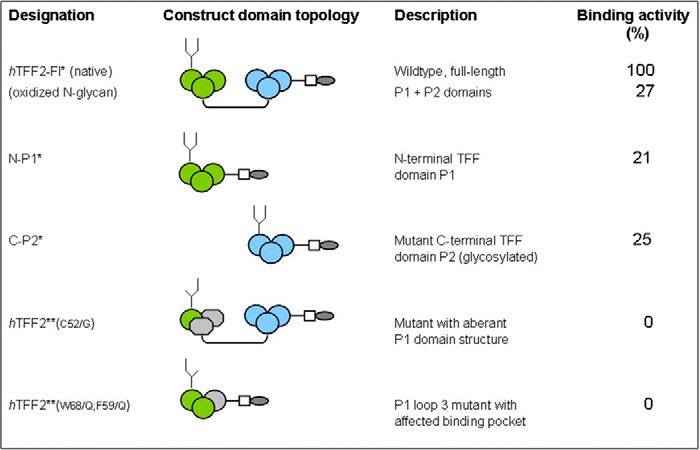
The lectin-like binding capacity of TFF2 on gastric mucins was retained using the single trefoil domain constructs N-P1 and C-P2* (the latter with a designed N-glycosylation site), however, both were at lower avidity compared with the wild type full-length construct (Table 1). Mutant forms of TFF2 (Cys52/Gly) with distorted disulfide bridge formation lacked any binding activity (Table 1). Strikingly, a mild periodate oxidation of the N-linked glycan that modifies the native full-length TFF2 probe and the natural gastric glycoform resulted in strong reduction of lectin activity (Table 1; see also below). This latter finding indicates a potential contribution of N-glycosylation at Asn38 to the active conformation of TFF2. Artificial amino acid sequences introduced as tags (oligo-His and Strep-tag) apparently do not contribute to the binding activity as the double point-mutated construct N-P1-(W68F59/Q) with the affected hydrophobic pocket was binding inactive (Table 1).
TABLE 1.
Survey of hTFF2 probes generated and tested for mucin glycopeptide binding
In the schematic presentation of constructs generated green circles refer to loops 1–3 in the TFF domain P1, blue circles to the corresponding P2 domain (grey shaded areas indicate mutant versions of loops 2 and/or 3). Quantitative data of the probes' binding activities are given in % relative to the native full-length construct hTFF2 (triplicate assays). * Probes were shown to carry N-linked glycans with LacdiNAc modification (authentic gastric glycoform, Ref. 8). **probes to carry aberrant complex-type or high-mannose-type glycans (Bonar, Hanisch; unpublished data, manuscript in preparation).
Human TFF2 Carbohydrate Binding Specificity as Revealed by Inhibition and Competition-based Immunoassays
Lectins
Among a panel of lectins tested as potential competitors of TFF2 the isolectin II from G. simplicifolia (GSA II) showed significant strong competitive effects (p value 1.21 × 10−6) and logarithmic binding inhibition curves with ID50 values in the range of 15 μg/ml (Fig. 3A). Other lectins, like WGA and UEA I, showed a less inhibitory potential at concentrations ranging above 100 μg/ml (Fig. 3A, upper panel). GSA II is known to bind terminal GlcNAc residues, however, it does not discriminate between α- and β-anomeric GlcNAc, whereas wheat germ agglutinin is proposed to bind preferentially to the β-anomer. In conclusion, a terminal GlcNAc residue can be assumed to play a role in the TFF2 binding mucin glycan. Competitive effects were also seen with UEA I (p value 0.18 × 10−6), which might correspond to TFF2 binding activity on blood group H-disaccharide. A very weak, but still significant inhibition was found with PNA (p value 0.018).
FIGURE 3.
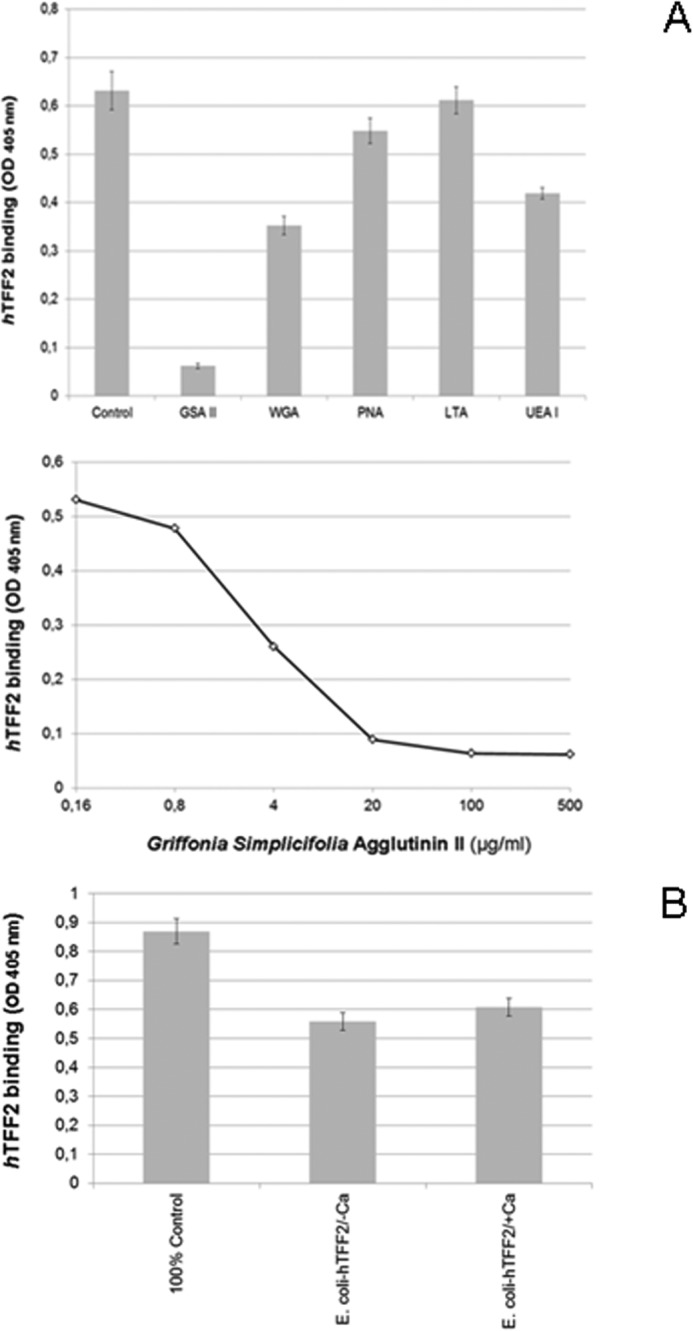
TFF2 (hTFF2-Fl)-binding competition with GSA II and other lectins. A, upper panel, triplicate assays (with S.D.) of TFF2 binding to immobilized PSM glycopeptides (0.5 μg/well) and inhibition assays using lectin competitors (500 μg/ml); lower panel, duplicate binding inhibition assays with 5-fold dilution series of G. simplicifolia agglutinin isolectin II. Average background binding was at 0.06 (A405 nm). B, triplicate assay (with S.D.) of TFF2 (expressed in HEK-293 cells) binding to PSM glycopeptides (0.5 μg/well) and inhibition by human TFF2 expressed in E. coli (10-fold molar excess) in the presence (1 mm) or absence of calcium ions. Average background binding was at 0.06 (A405 nm).
Control experiments with non-glycosylated human TFF2 expressed in E. coli revealed partial competitive inhibition of the gastric glycoform of TFF2 expressed in HEK-293 cells (Fig. 3B). This finding may indicate a dependence of lectin activity on particular TFF2 glycoforms (see above). The latter assumption would be in accordance with the observation that porcine pancreatic TFF2 is not associated with high-molecular mass mucins expressing α1,4-GlcNAc-capped GSA II binding glycans (30). The presence of calcium ions had no significant effect on binding and binding competition (p value 0.068).
Carbohydrate-specific Antibodies
Monoclonal antibodies with a defined carbohydrate specificity were used to unravel the structural features of the TFF2 glycotope. The panel covered a series of carbohydrate blood group specificities, including H2, Lewis(y), Lewis(x), sialyl-Lewis(x), and blood group A/B. No significant binding competition was revealed for any of these antibodies indicating again that no direct blood group dependence of the TFF2 binding to gastric mucins was involved (Table 2).
TABLE 2.
List of monoclonal antibodies with carbohydrate specificity tested in ELISA competition of hTFF2 binding to porcine stomach mucins as negative
Assays were performed in duplicate.
| Designation | Specificity | Concentration range tested |
|---|---|---|
| 19-OLE | Blood group H2 | Hybridoma supernatant |
| FW6 | Fucosylated polylactosamines | Hybridoma supernatant |
| A46-B10 | Blood group H2 | Hybridoma supernatant |
| A51-B/A6 | Blood group Lewis y/H2 | Hybridoma supernatant |
| 2B5 | GlcNAcβ1-3Galβ1-4GlcNAcβ1-6(GalNAc-ol) | Hybridoma supernatant |
| ISOBM128 (DH-1) | Blood group Lewis x | 50 μg/ml |
| ISOBM143 (M26) | Blood group sialyl-Lewis x | 50 μg/ml |
| ISOBM164 (CC2) | Blood group Lewis a | 50 μg/ml |
Mucin Glycans, Oligosaccharides, Glycopeptides, and Other Glycoconjugates
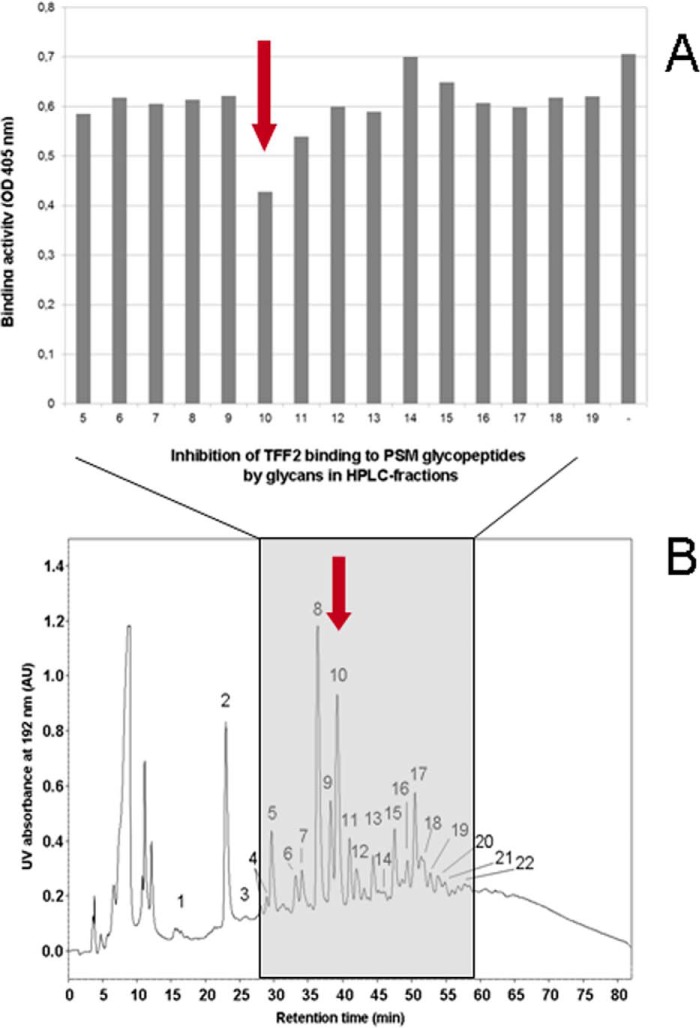
To define the structural aspects of the TFF2 glycotope we cleaved the O-linked glycans from binding active human and porcine gastric mucins and tested HPLC-purified fractions for their inhibitory potency in TFF2/mucin binding assays (Fig. 4). A prior fractionation of the glycan alditols by anion exchange chromatography revealed that all inhibitory potency was confined to the neutral fraction representing the quantitatively dominating portion (Fig. 5A). The inhibitory potential of glycans with intact reducing termini was similar to that of the reductively eliminated glycan alditols in binding inhibition assays (data not shown). Neutral glycan alditols were hence further fractionated by polar-phase HPLC (Fig. 4B). In the over 20 fractions more than 30 glycan species were detected on the basis of their molecular masses by MALDI mass spectrometry. Binding inhibition assays with these HPLC fractions revealed a strong inhibitory power in fraction PSM-N10 (Fig. 4A). No other fractions were found positive pointing to high specific binding of TFF2 to distinct mucin oligosaccharides.
FIGURE 4.
Gastric mucin-derived hexasaccharides inhibit TFF2 (hTFF2-Fl) binding. A, duplicate ELISA inhibition assay of hTFF2-Fl binding to PSM-glycopeptides (0.5 μg/well) by dried aliquots from polar-phase HPLC-separated PSM-derived glycan alditols (fractions 5–19, estimated concentration range of glycan alditols applied: 50–500 μm). Average background binding was at 0.06 (A405 nm). B, representative chromatogram of neutral glycan alditols (250 μg) from PSM separated by polar-phase HPLC.
FIGURE 5.
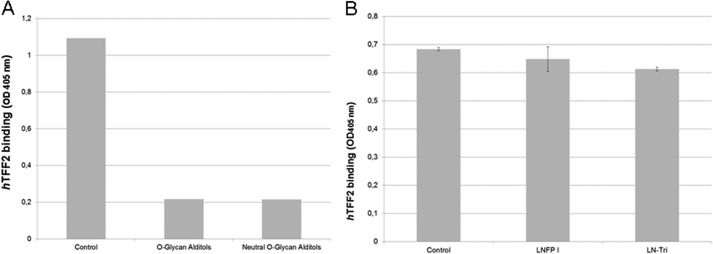
TFF2 binding inhibition by mucin-derived glycan alditols and synthetic oligosaccharides. A, triplicate assays of hTFF2-Fl binding to PSM-glycopeptides (0.5 μg/well; see control) and binding inhibition by PSM-derived glycan alditols (unfractionated O-glycan alditols and neutral fraction prepared by anion exchange chromatography on DEAE-Sephadex A25). Average background binding was at 0.06 (A405 nm). B, triplicate assays of hTFF2-Fl binding to PSM-glycopeptides (0.5 μg/well; see control) and binding inhibition by synthetic oligosaccharides lacto-N-fucopentaose I (LNFPI) and lacto-N-triose (LN-Tri). Average background binding was at 0.06 (A405 nm).
All other oligosaccharides from different sources (milk oligosaccharides, like lacto-N-fucopentaose I (p value 0.142), lacked inhibitory binding activity (Fig. 5B and Table 3). Exceptionally, lacto-N-triose (GlcNAcβ1-3Galβ1-4Glc) significantly reduced binding by about 10% (p value 2.8 × 10−5) (Fig. 5B).
TABLE 3.
List of oligosaccharides and glycoconjugates tested by ELISA inhibition of hTFF2 binding
Assays were performed in duplicate, except those marked with an asterisk. All listed oligosaccharides were tested negative in the respective concentration ranges, with the exception of lacto-N-triose, which showed weak inhibitory potential.
| Designation | Structure of glycan | Concentration range tested |
|---|---|---|
| 3-Sialyllactosamin | NeuAcα2-3Galβ1-4GlcNAc | 1 mm |
| Lacto-N-difucohexaose | Fucα1-2Galβ1-3(Fucα1-4)GlcNAcβ1-3Galβ1-4Glc | 1 mm |
| Lacto-N-fucopentaose I* | Fucα1-2Galβ1-3GlcNAcβ1-3Galβ1-4Glc | 1 mm |
| Lacto-N-fucopentaose II | Galβ1-3(Fucα1-4)GlcNAcβ1-3Galβ1-4Glc | 1 mm |
| Lacto-N-fucopentaose III | Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4Glc | 1 mm |
| Lacto-N-fucopentaose V | Galβ1-3GlcNAcβ1-3Galβ1-4(Fucα1-3)Glc | 1 mm |
| Lacto-difucotetraose | Fucα1-2Galβ1-4(Fucα1-3)Glc | 1 mm |
| Lacto-N-tetraose | Galβ1-3GlcNAcβ1-3Galβ1-4Glc | 1 mm |
| Lacto-N-triose* | GlcNAcβ1-3Galβ1-4Glc | 1 mm |
| Lacto-N-biose | GlcNAcβ1-3Gal | 10 mm |
| α-GlcNAc-pNP | α-GlcNAc | 60 mm |
| β-GlcNAc-pNP | β-GlcNAc | 60 mm |
| Core 3-Threonin | GlcNAcβ1-3GlcNAc | 1 mm |
Structural Analysis of Porcine Stomach Mucin-derived Oligosaccharide Alditol PSM-N10
Mass Spectrometry
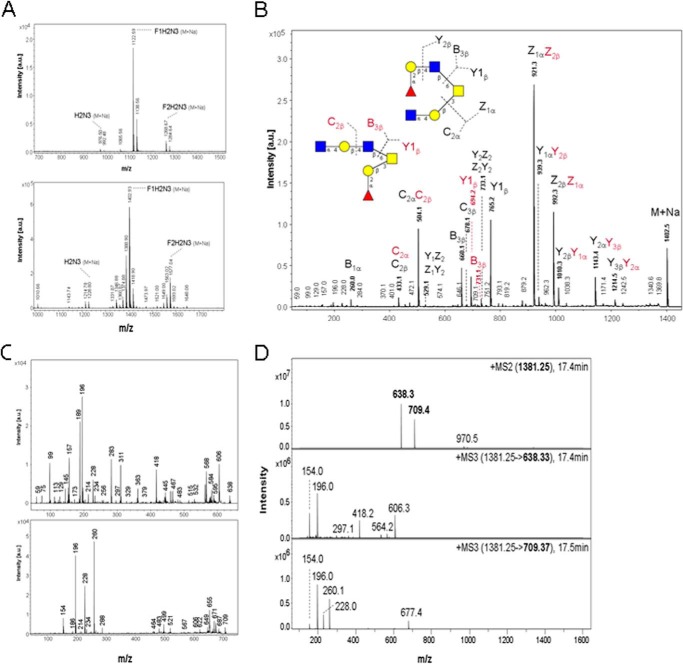
Molecular size and monosaccharide composition of the major glycan alditols in fraction PSM-N10 were derived from pseudomolecular ion masses registered in MALDI-TOF-MS at m/z 1122 (native Na adduct) and m/z 1380 or 1402 (methylated H or Na adducts), respectively (Fig. 6A). These relative masses correspond to hexasaccharides composed of one deoxyhexose (dHex), two hexoses (Hex), two N-acetylhexosamines (HexNAc), and one N-acetylhexosaminitol (HexNAc-ol). Sequencing by MALDI-MS2 (Fig. 6B), post-source decay MALDI-MS3 analysis of in-source B-ion fragments (Fig. 6C), and ESI MS2 and MS3 (Fig. 6D) revealed a core 2-based glycan alditol forming two branched isomers, one with a HexNAc-Hex-HexNAc branch linked to C6 of the core-GalNAcol (indicated by B1 and B3 ions at m/z 260 and 709) and one with a C6-linked dHex-Hex-HexNAc branch (indicated by B1 and B3 ions at m/z 189 and 638) (Fig. 6B). The B3 ions yielded secondary fragments by loss of methanol (m/z at 606 and 677), which indicate that HexNAc in trisaccharide sequences HexNAc-Hex-HexNAc and dHex-Hex-HexNAc is substituted at C4. The core 2-based structure and C6-linkage of the two alternative branches were corroborated by a series of B, Y, C, and Z ions as indicated in Fig. 6B.
FIGURE 6.
Mass spectrometric analysis of TFF2 (hTFF2-Fl) binding hexasaccharides in fraction PSM-N10. A, positive ion-MALDI mass spectrometry of native (upper panel) and permethylated oligosaccharide alditols (lower panel); B, post-source decay MALDI mass spectrum of permethylated glycan alditols corresponding to the molecular ion M+Na at m/z 1402.5; C, Post-source decay MALDI-MS3 analysis of in-source B-ion fragments; D, ESI-MS2 and MS3 of permethylated glycan alditols corresponding to the molecular ion M+H at m/z 1380.5. Graphical symbols of sugars according to the recommendations of the Consortium for Functional Glycomics were used: yellow squares (GalNAc); yellow circles (Gal); blue squares (GlcNAc); red triangles (Fuc).
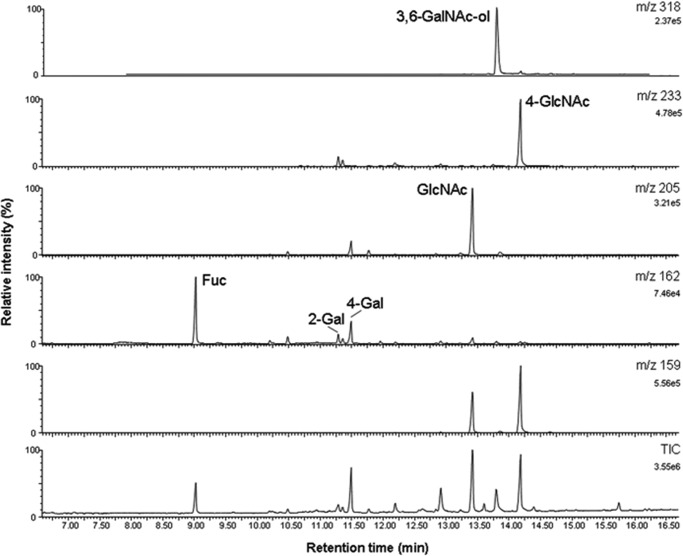
Linkage Analysis
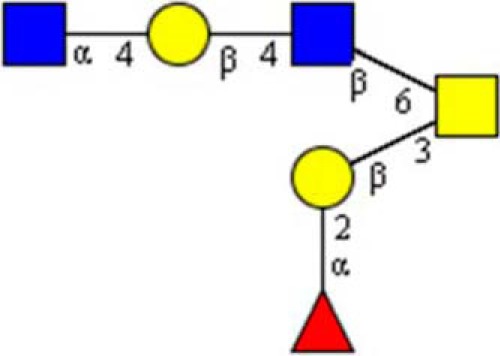
Methylation analysis of the glycan alditol gave further insight into the linkage patterns of the isomeric structures (Fig. 7). The branches of the core 2-based isomers terminate with Fuc and GlcNAc at the non-reducing ends. The proposed core 2 structure is in agreement with the exclusive presence of 3,6-GalNAc-ol (absence of 3-GalNAc-ol). A type 2-lactosamine branch can be inferred from 4-GlcNAc as the only monosubstituted GlcNAc derivative. Two PMAA of monosubstituted galactose were found: 2-Gal and 4-Gal. These two derivatives are in agreement with structural elements Fucα1-2Gal and GlcNAcα1-4Gal. Trace amounts of terminal Gal were detectable presumably derived from a minor admixture of the structurally related afucosylated compound. As no other monosubstituted Gal or GlcNAc derivatives were found, the structural elements revealed by methylation analysis can be matched with data from MS/MS fragmentation analysis of the glycan by proposing two isomeric structures: structure A, GlcNAc1-4Gal1-4GlcNAc1-6(Fuc1-2Gal1-3)GalNAc-ol and structure B, GlcNAc1-4Gal1-3(Fuc1-2Gal1-4GlcNAc1-6)GalNAc-ol.
FIGURE 7.
Methylation analysis of linkages by gas chromatography-mass spectrometry of partially methylated alditol acetates. PMAA were identified via their GC retention times and specific MS fragmentation spectra registered in electron-impact ionization at 70 eV. The total ion chromatogram (TIC) and mass selective ion traces are shown for the major PMAA: m/z 159 (1-O-acetylated N-acetylhexosamines deuterated at C1: terminal and 4-substituted GlcNAc), m/z 162 (1-O-acetylated deoxyhexoses and hexoses deuterated at C1: terminal fucose, 4-substituted Gal; note that 2-substituted Gal is detected as the 13C-isotope of the fragment at m/z 161), m/z 205 (PMAA derived from terminal dHex, Hex, and HexNAc), m/z 233 (preferential fragment of PMAA derived from 4-substituted HexNAc), and m/z 318 (fragment PMAA derived from 3,6-disubstituted HexNAc-ol and 3-substituted HexNAc).
Definition of Anomeric Linkages
To define the anomeric configuration of terminal HexNAc in fraction PSM-N10, a series of exoglycosidases (β-hexosaminidase, α-N-acetylgalactosaminidase, and α-N-acetylglucosaminidase) was used in combination with mass spectrometric inspection of the reaction products. All three enzymes, although highly active on paranitrophenyl conjugates as control substrates, were inactive both, on the free (MALDI-MS) and the mucin-linked hexasaccharide tested by ELISA (data not shown). In light of the above reported results from methylation analysis, this finding is in accordance with lack of terminal α-GalNAc. The negative result with human lysosomal α1-4-N-acetylglucosaminidase is explained by the highly restricted substrate specificity of the enzyme, which cleaves GlcNAcα1-4GlcA/IdoA as in heparan sulfate. The β-hexosaminidase, on the contrary, has a broad substrate specificity and its inability to cleave terminal GlcNAc can be explained by assuming an α-configuration of the sugar. This assumption is further supported by the linkage analysis of subterminal Hex (Gal), as a 1-4-linkage has only been observed so far for terminal α-GlcNAc residues, whereas β-GlcNAc is generally linked 1–3 to Gal.
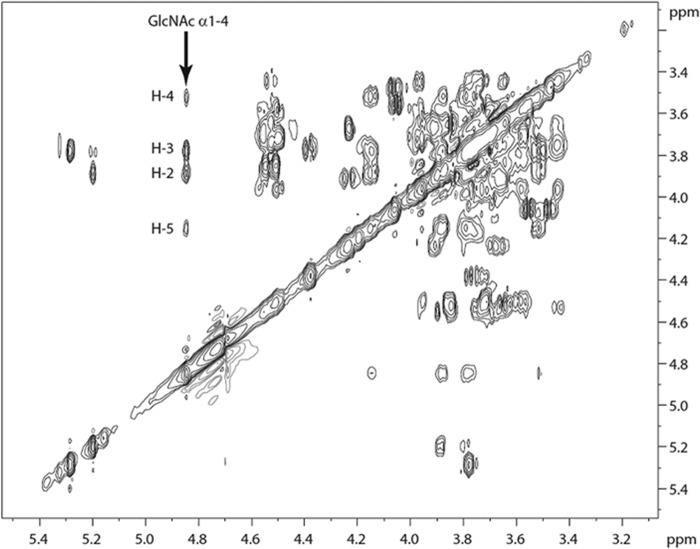
Two-dimensional 1H NMR experiments confirmed the above made structural assumptions, in particular with respect to the anomeric configuration of terminal GlcNAc (Fig. 8). The expression of α-N-acetylglucosamine was corroborated by shifts in the TOCSY spectrum corresponding to the anomeric signal of α-GlcNAc (H-1) at 4.85 ppm (19).
FIGURE 8.
H,H-TOCSY NMR spectrum of oligosaccharides in fraction PSM-N10 (expansion), obtained at 600 MHz (303 K, 80 ms spin lock). Spin system assigned for terminal GlcNAcα1-4. The hexasaccharide analyte (approximately 100 μg) dissolved in water was repeatedly dried by vacuum rotation and resolubilization in deuterated solvent (D2O). The final concentration was 0.2 mm.
Human TFF2 Glycotope Characterization
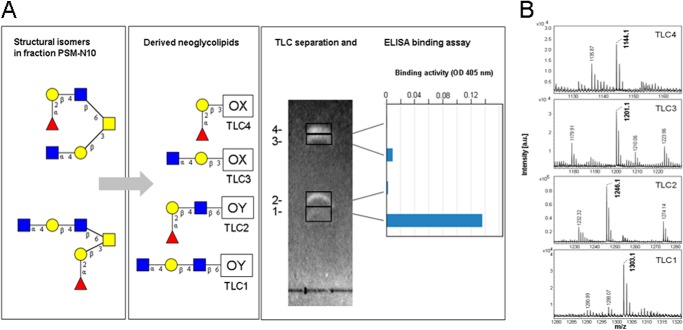
To determine which of the C3- or C6-linked branches in the two isomeric hexasaccharides is binding active in solid phase immunoassays with TFF2, we followed a neoglycolipid approach (29). This technique allows a selective oxidative cleavage of the core GalNAc-ol between carbons C4 and C5 and coupling of the C3/C6 branches to dipalmitoylphosphatidylethanolamine to form neoglycolipids. Application of this technique on glycans in fraction PSM-N10 resulted in four fluorescently labeled TLC bands that were cut for extraction of the neoglycolipids and their testing in ELISA binding assays (Fig. 9A). Of the four bands only one (TLC1) revealed strong binding to TFF2, which was structurally identified by MALDI-MS as HexNAc-Hex-HexAc-6OY (M-H 1303.1) (Fig. 9B). Much weaker binding of TFF2 was observed on the more prominent neoglycolipid contained in TLC3 and corresponding to HexNAc-Hex-3OX (M-H 1201.1) suggesting that the GlcNAcα1-4Galβ1-4GlcNAcβ trisaccharide forms the glycotope of TFF2. Staining intensities of neoglycolipids in TLC1–4 revealed structure A as minor and structure B as major components in fraction PSM-N10. Preferential binding of TFF2 to structure A agrees with the exclusive presence of this isomer in human gastric mucin (19). Binding to the lipid conjugate further supports the exclusive carbohydrate specificity of TFF2. Neoglycolipids prepared from the unfractionated neutral glycan alditols confirmed that only binding active components were isographic with those in TLC1 and -3 (data not shown).
FIGURE 9.
Mass spectrometric identification of TFF2 binding active core 2-hexasaccharide branches as their neoglycolipids. A, thin layer chromatographic separation and duplicate ELISA binding assay of 400 pmol of neoglycolipids derived from glycan alditols in fraction PSM-N10 (for further details refer to the ”Experimental Procedures“). Graphical symbols of sugars according to the recommendations of the Consortium for Functional Glycomics were used: yellow squares (GalNAc); yellow circles (Gal); blue squares (GlcNAc); red triangles (Fuc). B, MALDI mass spectra of TLC1–4 recorded in the negative-ion mode. A saturated solution of matrix (HCCA) in 50% acetonitrile/aqueous trifluoroacetic acid was mixed 1:1 with 100–200 pmol of the neoglycolipids in 0.5 μl of methanol. Sections of mass spectra between m/z 1120 and 1320 are shown. Prominent signals corresponding to M-H ions of the neoglycolipids are annotated in boldface.
DISCUSSION
We provide for the first time unequivocal experimental evidence for a lectin activity of human TFF2 by demonstrating a periodate-sensitive binding to gastric mucins and Pronase-stable mucin glycopeptides, moreover by showing competitive effects with other lectins (GSA II), an inhibition of binding by mucin-derived oligosaccharides, and a binding to mucin glycan-derived neoglycolipids. Strikingly, not all TFF2 preparations bound with similar high avidity (Escherichia coli expressed TFF2). Some evidence could be provided for glycoform dependence of TFF2 lectin activity, as in particular the gastric glycoform was found highly active. The gastric TFF2 is distinct by the characteristic expression of an N-linked complex-type glycan with monofucosylated N,N′-diacetyl-lactosediamine antennae and absence of considerable microheterogeneity (8), a structural feature that is simulated by the recombinant probe expressed in HEK-293 cells.3 TFF2 isoforms expressed at other organ sites, like pancreatic TFF2, are known to lack association with the mucin MUC6 specifically expressing the α-GlcNAc-capped glycans, whereas gastric TFF2 is not only co-expressed with MUC6 but also shows strict association with the high-molecular mass mucin.
The carbohydrate specificity of TFF2 could be narrowed down to a structural part of the PSM-hexasaccharide isomer A by demonstrating binding to the C6-linked trisaccharide GlcNAcα1-4Galβ1-4GlcNAcβ characterized by terminal α4-GlcNAc. There was only a minor cross-reactivity to the C3-linked disaccharide branch GlcNAcα1-4Galβ on isomer B, which points to an extended glycotrope that comprises more than the common disaccharide. In human gastric mucins hexasaccharide isomer A is the only glycan species with α-GlcNAc end capping found in the so far most comprehensive study by Rossez et al. (19).
Epithelial mucins and mucin-bound O-glycans play a dual role in bacterial infection as they mediate pathogen adhesion to epithelial cells via carbohydrate-lectin interactions on the one hand, whereas they act also cytoprotective by entrapping the pathogen in a visco-elastic mucin gel via the same mechanism. This holds true for gastric infections by H. pylori that infects more than half of the world's population in particular. Most H. pylori reside within the mucous gel layer of the stomach covering the apical surface, where they bind to MUC5AC (31), whereas about 20% of the bacteria bind directly to gastric epithelial cells (32). Two adhesins were found to be involved in H. pylori binding under non-inflammatory (BabA) and inflammatory conditions (SabA). The switch from preferential BabA expression (binding to Lewis b/H1 glycans) to the expression of SabA (binding to sialylated Lewis glycans) reflects a corresponding gastritis-associated change in mucin glycosylation (15). The trefoil factor family members are long known to be closely associated with gastrointestinal mucins, and in particular TFF2 was described to bind to the high-molecular mass MUC6 produced by deep gastric glands (30). However, neither the mode of its interaction with mucins nor its principle function were clearly defined. In H. pylori infection both, MUC6 and TFF2, are concertedly up-regulated (14). The mucin produced in deep gastric glands appears to express the majority, if not all of the organ-specific carbohydrates, which have been shown to exert antibiotic effects on H. pylori (20). The co-expressed lectin, TFF2, has been claimed to control epithelial repair (33), but also to regulate mononuclear cell inflammatory responses in H. pylori infection (34). In support of its potential role in H. pylori defense mechanisms, an accelerated progression of gastritis to dysplasia in the pyloric antrum was reported in TFF2−/− mice infected with H. pylori (35). In later stages of gastric cancer development the putative tumor suppressor TFF2 was reported to be down-regulated via promotor hypermethylation (36).
The functional relationship between members of the triad α-GlcNAc-capped MUC6 mucin glycans, TFF2, and H. pylori remains speculative. The specific structural features of the TFF2 glycotope suggest that TFF2 may function as a mediator of the growth inhibitory effect exerted by α-GlcNAc-capped mucin glycans on enzymatic formation of an essential cell wall component of H. pylori, cholesteryl α-d-glucopyranoside (20).
The reported finding may also impact on more general functional aspects of trefoil factor family members. Although not yet defined, it has been proposed that several of the many cellular responses to TFF peptides, including apoptosis, morphogenesis, angiogenesis, and transcriptional regulation may be triggered by a receptor-mediated process (37). In light of the above reported findings, this TFF2 receptor binding could be based on lectin-carbohydrate interaction. This would imply that undefined membrane-bound mucins or non-mucin-type glycoproteins expressing the TFF2 binding glycotope represent candidates as trefoil factor receptors.
Acknowledgments
We thank Dr. Stefan Müller (Bioanalytics, Center for Molecular Medicine Cologne, Germany) for the performance of LC-MS analyses of methylated glycans. We also acknowledge the kind provision of template DNA for generation of hTFF2 constructs by Prof. Werner Hoffmann (University of Magdeburg, Germany).
This work was supported by Deutsche Forschungsgemeinschaft Grant HA 2092/21-1 (to F. G. H.).
D. Bonar and F. G. Hanisch, unpublished data.
- hTFF2
- human trefoil factor family 2
- PSM
- porcine stomach mucin
- PMAA
- Partially methylated alditol acetates
- TOCSY
- Two-dimensional total correlation spectroscopy
- GSA
- isolectin II from G. simplicifolia.
REFERENCES
- 1. Tomasetto C., Rio M. C., Gautier C., Wolf C., Hareuveni M., Chambon P., Lathe R. (1990) hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 9, 407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gött P., Beck S., Machado J. C., Carneiro F., Schmitt H., Blin N. (1996) Human trefoil peptides: genomic structure in 21q22.3 and coordinated expression. Eur. J. Hum. Genet. 4, 308–315 [DOI] [PubMed] [Google Scholar]
- 3. May F. E., Semple J. I., Newton J. L., Westley B. R. (2000) The human two domain trefoil protein, TFF2, is glycosylated in vivo in the stomach. Gut. 46, 454–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann W., Jagla W. (2002) Cell type specific expression of secretory TFF peptides: colocalization with mucins and synthesis in the brain. Int. Rev. Cytol. 213, 147–181 [DOI] [PubMed] [Google Scholar]
- 5. Jørgensen K. H., Thim L., Jacobsen H. E. (1982) Pancreatic spasmolytic polypeptide (PSP): I. preparation and initial chemical characterization of a new polypeptide from porcine pancreas. Regul. Pept. 3, 207–219 [DOI] [PubMed] [Google Scholar]
- 6. Hanby A. M., Poulsom R., Singh S., Elia G., Jeffery R. E., Wright N. A. (1993) Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology 105, 1110–1116 [DOI] [PubMed] [Google Scholar]
- 7. Ota H., Hayama M., Momose M., El-Zimaity H. M., Matsuda K., Sano K., Maruta F., Okumura N., Katsuyama T. (2006) Co-localization of TFF2 with gland mucous cell mucin in gastric mucous cells and in extracellular mucous gel adherent to normal and damaged gastric mucosa. Histochem. Cell Biol. 126, 617–625 [DOI] [PubMed] [Google Scholar]
- 8. Hanisch F. G., Ragge H., Kalinski T., Meyer F., Kalbacher H., Hoffmann W. (2013) Human gastric TFF2 peptide contains an N-linked fucosylated N,N′-diacetyllactosediamine (LacdiNAc) oligosaccharide. Glycobiology 23, 2–11 [DOI] [PubMed] [Google Scholar]
- 9. Gajhede M., Petersen T. N., Henriksen A., Petersen J. F., Dauter Z., Wilson K. S., Thim L. (1993) Pancreatic spasmolytic polypeptide: first three-dimensional structure of a member of the mammalian trefoil family of peptides. Structure 1, 253–262 [DOI] [PubMed] [Google Scholar]
- 10. Kouznetsova I., Laubinger W., Kalbacher H., Kalinski T., Meyer F., Roessner A., Hoffmann W. (2007) Biosynthesis of gastrokine-2 in the human gastric mucosa: restricted spatial expression along the antral gland axis and differential interaction with TFF1, TFF2 and mucins. Cell Physiol. Biochem. 20, 899–908 [DOI] [PubMed] [Google Scholar]
- 11. Thim L., Madsen F., Poulsen S. S. (2002) Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur. J. Clin. Invest. 32, 519–527 [DOI] [PubMed] [Google Scholar]
- 12. Suzuki K., Hayama M., Nakamura M., Yamauchi K., Maruta F., Miyagawa S., Ota H. (2006) Trefoil factor 2 in gland mucous cell mucin in the mucous gel covering normal or damaged gastric mucosa using the Mongolian gerbil model. Scand. J. Gastroenterol. 41, 1390–1397 [DOI] [PubMed] [Google Scholar]
- 13. Ding S. Z., Zheng P. Y. (2012) Helicobacter pylori infection induced gastric cancer: advance in gastric stem cell research and the remaining challenges. Gut Pathog. 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xia H. H., Yang Y., Lam S. K., Wong W. M., Leung S. Y., Yuen S. T., Elia G., Wright N. A., Wong B. C-Y. (2004) Aberrant epithelial expression of trefoil family factor 2 and mucin 6 in Helicobacter pylori infected gastric antrum, incisura, and body and its association with antralisation. J. Clin. Pathol. 57, 861–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobayashi M., Lee H., Nakayama J., Fukuda M. (2009) Roles of gastric mucin-type O-glycans in the pathogenesis of Helicobacter pylori infection. Glycobiology 19, 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferreira B., Marcos N. T., David L., Nakayama J., Reis C. (2006) Terminal a1,4-linked N-acetylglucosamine in Helicobacter pylori-associated intestinal metaplasia of the human stomach and gastric carcinoma cell lines. J. Histochem. Cytochem. 54, 585–591 [DOI] [PubMed] [Google Scholar]
- 17. Phillipson M., Johansson M. E., Henriksnäs J., Petersson J., Gendler S. J., Sandler S., Persson A. E., Hansson G. C., Holm L. (2008) The gastric mucus layers: constituents and regulation of accumulation. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G806–G812 [DOI] [PubMed] [Google Scholar]
- 18. Nakayama J., Yeh J-C., Misra A. K., Ito S., Katsuyama T. (1999) Expression cloning of a human α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1–4Galβ-R, a glycan specifically expressed in the gastric gland mucous cell-type mucin. Proc. Natl. Acad. Sci. U.S.A. 96, 8991–8996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rossez Y., Maes E., Lefebvre Darroman T., Gosset P., Ecobichon C., Joncquel Chevalier Curt M., Boneca I. G., Michalski J-C., Robbe-Masselot C. (2012) Almost all human gastric mucin O-glycans harbor blood-group A, B or H antigens and are potential binding sites for Helicobacter pylori. Glycobiology 22, 1193–1206 [DOI] [PubMed] [Google Scholar]
- 20. Kawakubo M., Ito Y., Okimura Y., Kobayashi M., Sakura K., Kasama S., Fukuda M. N., Fukuda M., Katsuyama T., Nakayama J. (2004) Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305, 1003–1006 [DOI] [PubMed] [Google Scholar]
- 21. Karasawa F., Shiota A., Goso Y., Kobayashi M., Sato Y., Masumoto J., Fujiwara M., Yokosawa S., Muraki T., Miyagawa S., Ueda M., Fukuda M. N., Ishihara K., Nakayama J. (2012) Essential role of gastric gland mucin in preventing gastric cancer in mice. J. Clin. Invest. 122, 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hanisch F-G., Peter-Katalinic J. (1992) Structural studies on fetal mucins from human amniotic fluid: core-typing of short-chain O-linked glycans. Eur. J. Biochem. 205, 527–535 [DOI] [PubMed] [Google Scholar]
- 23. Hanisch F-G., Chai W., Rosankiewicz J. R., Lawson A. M., Stoll M. S., Feizi T. (1993) Core-typing of O-linked glycans from human gastric mucins: lack of evidence for the occurrence of the core sequence Gal1–6GalNAc. Eur. J. Biochem. 217, 645–655 [DOI] [PubMed] [Google Scholar]
- 24. Breloy I., Pacharra S., Ottis P., Bonar D., Grahn A., Hanisch F-G. (2012) O-Linked N,N′-diacetyllactosediamine (LacdiNAc)-modified glycans in extracellular matrix glycoproteins are specifically phosphorylated at subterminal N-acetylglucosamine. J. Biol. Chem. 287, 18275–18286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Müller S., Hanisch F-G. (2002) Recombinant MUC1 probe authentically reflects cell-specific O-glycosylation profiles of endogenous breast cancer mucin: high density and prevalent core 2-based glycosylation. J. Biol. Chem. 277, 26103–26112 [DOI] [PubMed] [Google Scholar]
- 26. Hanisch F-G., Müller S. (2009) Analysis of methylated O-glycan alditols by reversed-phase nano-LC coupled CAD-ESI mass spectrometry. Methods Mol. Biol. 534, 107–115 [DOI] [PubMed] [Google Scholar]
- 27. Ceroni A., Maass K., Geyer H., Geyer R., Dell A., Haslam S. M. (2008) Glycoworkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 7, 1650–1659 [DOI] [PubMed] [Google Scholar]
- 28. Albersheim P., Nevins D. J., English P. D., Karr A. (1967) A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 5, 340–345 [Google Scholar]
- 29. Stoll M. S., Hounsell E. F., Lawson A. M., Chai W. G., Ten F. (1990) Microscale sequencing of O-linked oligosaccharides using mild periodate oxidation of alditols, coupling to phospholipid and TLC-MS analysis of the resulting neoglycolipids. Eur. J. Biochem. 189, 499–507 [DOI] [PubMed] [Google Scholar]
- 30. Stürmer R., Müller S., Hanisch F-G., Hoffmann W. (2014) Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell. Physiol. Biochem. 33, 895–904 [DOI] [PubMed] [Google Scholar]
- 31. Van den Brink G. R., Tytgat K. M., Van der Hulst R. W., Van der Loos C. M., Einerhand A. W., Büller H. A., Dekker J. (2000) H. pylori colocalises with MUC5AC in the human stomach. Gut 46, 601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Colomb F., Robbe-Masselot C., Groux-Degroote S., Boukaert J., Delannoy P., Michalski J-C. (2014) Epithelial mucins and bacterial adhesion. Carbohydr. Chem. 40, 596–623 [Google Scholar]
- 33. Playford R. J., Marchbank T., Chinery R., Evison R., Pignatelli M., Boulton R. A., Thim L., Hanby A. M. (1995) Human spasmolytic polypeptide is a cytoptotective agent that stimulates cell migration. Gastroenterology 108, 108–116 [DOI] [PubMed] [Google Scholar]
- 34. Kurt-Jones E. A., Cao L., Sandor F., Rogers A. B., Whary M. T., Nambiar P. R., Cerny A., Bowen G., Yan J., Takaishi S., Chi A. L., Reed G., Houghton J., Fox J. G., Wang T. C. (2007) Trefoil family factor 2 is expressed in murine gastric and immune cells and controls both gastrointestinal inflammation and systemic immune responses. Infect. Immun. 75, 471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fox J. G., Rogers A. B., Whary M. T., Ge Z., Ohtani M., Jones K. E., Wang T. C. (2007) Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2−/− C57BL6 x Sv129 Helicobacter pylori-infected mice. Am. J. Pathol. 171, 1520–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang P., Yu G., Zhang Y., Xiang Y., Zhu Z., Feng W., Lee W., Zhang Y. (2014) Promoter hypermethylation and downregulation of trefoil factor 2 in human gastric cancer. Oncol. Lett. 7, 1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Otto W. R., Thim L. (2005) Trefoil factor family-interacting proteins. Cell. Mol. Life Sci. 62, 2939–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]