Background: Regulation of dentin mineralization at the gene expression level is poorly understood.
Results: Trps1 supports expression of osteogenic genes Alpl, Phospho1, Runx2, and Sp7 in preodontoblastic cells, and in mature cells Trps1 represses phosphate metabolism genes Phex and Vdr.
Conclusion: The role of Trps1 in mineralization depends on odontoblastic differentiation stage.
Significance: These findings provide insights into regulation of odontoblastic maturation and function.
Keywords: Biomineralization, Dentin, Differentiation, Extracellular Matrix, Gene Expression, Trps1, Odontoblast
Abstract
TRPS1 (tricho-rhino-phalangeal syndrome) is a unique GATA-type transcription factor that acts as a transcriptional repressor. TRPS1 deficiency and dysregulated TRPS1 expression result in skeletal and dental abnormalities implicating TRPS1 in endochondral bone formation and tooth development. Moreover, patients with tricho-rhino-phalangeal syndrome frequently present with low bone mass indicating TRPS1 involvement in bone homeostasis. In addition, our previous data demonstrated accelerated mineralization of the perichondrium in Trps1 mutant mice and impaired dentin mineralization in Col1a1-Trps1 transgenic mice, implicating Trps1 in the mineralization process. To understand the role of Trps1 in the differentiation and function of cells producing mineralized matrix, we used a preodontoblastic cell line as a model of dentin mineralization. We generated both Trps1-deficient and Trps1-overexpressing stable cell lines and analyzed the progression of mineralization by alkaline phosphatase and alizarin red staining. As predicted, based on our previous in vivo data, delayed and decreased mineralization of Trps1-overexpressing odontoblastic cells was observed when compared with control cells. This was associated with down-regulation of genes regulating phosphate homeostasis. Interestingly, Trps1-deficient cells lost the ability to mineralize and demonstrated decreased expression of several genes critical for initiating the mineralization process, including Alpl and Phospho1. Based on these data, we have concluded that Trps1 serves two critical and context-dependent functions in odontoblast-regulated mineralization as follows: 1) Trps1 is required for odontoblast maturation by supporting expression of genes crucial for initiating the mineralization process, and 2) Trps1 represses the function of mature cells and, consequently, restricts the extent of extracellular matrix mineralization.
Introduction
Mineralization is an orchestrated process in which crystals of calcium phosphate, termed hydroxyapatite (HA),2 are laid down in precise amounts within the fibrous extracellular matrix (ECM) (1). Physiological mineralization occurs in skeletal tissues (bone and hypertrophic cartilage) and dental tissues (dentin, cementum, and enamel). Dentin, the most abundant component of the tooth, is very similar to bone in its matrix protein composition. However, unlike bone, dentin does not undergo remodeling and does not participate in calcium homeostasis (2). Cells that produce dentin, odontoblasts, differentiate from cranial neural crest-derived mesenchyme in a process that requires sequential and reciprocal mesenchymal-epithelial interactions. Mature odontoblasts secrete organic and mineral components of the dentin ECM; however, the transcriptional regulation of this process is not well understood (2).
Initiation of mineralization takes place within matrix vesicles (MVs), which bud off from the plasma membrane of cells producing the mineralizing matrix (3–5). The unique protein and lipid composition of MVs supports accumulation of high concentrations of Ca2+ and PO43− (Pi) ions facilitating initial HA crystal formation (6). In particular, mineralization-competent MVs are enriched in tissue-nonspecific alkaline phosphatase (TNAP) and PHOSPHO1 phosphatase that provide Pi (7, 8). A series of genetic experiments with knock-out and transgenic mice demonstrated that TNAP and PHOSPHO1 have nonredundant functions in supporting mineralization (9, 10). PHOSPHO1 acts inside MVs to initiate deposition of HA in this compartment, whereas TNAP regulates mineralization by modifying the ECM environment (9, 11–13). The release of HA from MVs to the ECM supports further tissue mineralization. The extent of tissue mineralization depends on the composition and modifications of ECM proteins, the availability of Ca2+ and Pi, and concentration of mineralization inhibitors, such as osteopontin (Opn), matrix Gla protein (MGP), and inorganic pyrophosphate (PPi) (14–16). Activity of TNAP and transporters of Pi (PiT1 and PiT2) and PPi (Ank) establishes a Pi/PPi ratio that either supports or represses mineralization (11). Although phosphate homeostasis is controlled at the systemic level, many of the proteins involved in this process are highly expressed in cells producing mineralizing matrix, suggesting that they participate in the locally regulated phosphate availability at the sites of mineralization (17–21). In humans, mutations in genes coding for major matrix proteins as well as in genes involved in phosphate metabolism result in skeletal and dental defects, underscoring the critical role of matrix composition and phosphate homeostasis for tissue mineralization (17, 22–26). For example, a genetically heterogeneous disorder, hypophosphatemic rickets, manifests as defective mineralization of skeletal and dental tissues due to dysregulated phosphate homeostasis (27, 28). TNAP deficiency (hypophosphatasia) also results in impaired mineralization associated with increased levels of mineralization inhibitors (29, 30).
A growing body of evidence implicates the Trps1 transcription factor in mineralization, although its role in this process and the mechanism whereby Trps1 regulates mineralization are not well understood. Trps1 is a unique zinc finger protein that belongs to the GATA family of transcription factors (31). Although a majority of studies have demonstrated that Trps1 is a transcriptional repressor, recently it has been shown that during hair formation Trps1 can activate expression of Wnt pathway genes (32–36). Mutations of the TRPS1 gene in humans cause the craniofacial and skeletal dysplasia tricho-rhino-phalangeal syndrome (TRPS) and Ambras syndrome (37, 38). Although these two diseases have distinct clinical presentations, abnormalities observed in patients with TRPS and Ambras indicate that TRPS1 is involved in the development of endochondral bones and teeth. We and others have shown that in perichondrial cells of endochondral bones, as well as in developing odontoblasts, Trps1 is highly expressed prior to mineralization, and the onset of mineralization coincides with down-regulation of Trps1 (32, 39, 40). This expression pattern suggests that Trps1 is involved in the maturation of cells destined to produce mineralizing matrix or that it prevents premature mineralization. The latter function has been demonstrated in our previous studies of a mouse model of TRPS (Trps1ΔGT mice), where we uncovered that Trps1 deficiency leads to premature mineralization of the perichondrium of developing endochondral bones (32). In those studies, we did not address mineralization of dentin, because this occurs postnatally and Trps1ΔGT/ΔGT mice die at birth. To determine whether Trps1 is sufficient to inhibit osteoblast and/or odontoblast-driven mineralization, we generated Col1a1-Trps1 transgenic mice expressing Trps1 from a cell type-specific 2.3-kb fragment of the Col1a1 promoter. Analyses of Col1a1-Trps1 mice demonstrated that Trps1 has a strong dominant negative effect on dentin but little effect on bone mineralization. The impairment in dentin formation in Col1a1-Trps1 mice is associated with repression of the Dspp gene, coding for major dentin matrix proteins required for dentin formation (41). Collectively, results of the studies of Trps1-deficient mice and mice overexpressing Trps1 in osteoblasts and odontoblasts suggest a context-dependent function of Trps1 in the mineralization process. This context may be determined by the type of cell that is driving mineralization or by the cell differentiation stage.
The dental phenotype of TRPS and Ambras patients clearly indicates that TRPS1 is involved in tooth development. On the molecular level, the dynamic and specific expression pattern of Trps1 in developing odontoblasts suggests its role in dentinogenesis. In these studies, we address the role of Trps1 in odontoblast-driven mineralization. We analyzed the consequences of both Trps1 deficiency and up-regulation on the mineralization process and the expression of genes involved in it. Results of these studies demonstrate for the first time that Trps1 regulates mineralization through different mechanisms in preodontoblasts and mature odontoblasts, and thus the role of Trps1 in the mineralization process depends on the odontoblast differentiation stage.
EXPERIMENTAL PROCEDURES
Cell Culture
Preodontoblastic 17IIA11 cells (42, 43) were maintained in standard DMEM (Invitrogen) with 5% FBS (Thermo Fisher Scientific, Logan, UT) and 100 units/ml penicillin and 100 μg/ml streptomycin (Cellgro, Manassas, VA) at 37 °C and 8% CO2. For the osteo-odontogenic differentiation experiments, cells were plated at 5 × 105 cells per well of a 6-well plate. Once cells reached 85–95% confluency, osteo-odontogenic differentiation was induced by osteogenic medium (standard medium supplemented with 7 mm β-glycerophosphate and 50 μg/ml ascorbic acid). Osteogenic medium was changed every 48 h. Trps1-deficient, Trps1-overexpressing, and control stable cell lines were generated as described previously (41).
RNA Extraction, cDNA Synthesis, and Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using GenElute Mammalian Total RNA miniprep kit (Sigma). Total RNA (1 μg), after DNase I treatment (Invitrogen), was converted to cDNA with SuperScript III reverse transcriptase kit (Invitrogen). Gene expression analyses were performed using AB Biosystems 7500 fast real time PCR system and Fast SYBR Green reaction mix (Roche Applied Science). Primer sequences are as follows: Gapdh F, GCAAGAGAGGCCCTATCCCAA, and R, CTCCCTAGGCCCCTCCTGTTATT; Actb F, GACGTTGACATCCGTAAAGACC, and R, CAGGAGGAGCAATGATCTTGATC; Trps1 F, ACAACGGCGAGCAGATTATTAG, and R, TAGTCAATGAACCCTGGGCTTCGTA; Phex F, CAGAAAGCCAAAATCCTCTACTCA, and R, TCCAGTCTAAGCACCGACTTCA; Vdr F, GCCTCCAATTCGTGCAGACGTAAGTACA, and R, GAGCCTTCTTCATTCAGATCCATCGTG; Fam20C F, AACCCATGAAGCAGACGAGAG, and R, GGAGGGACTCTGCGGAAATC; Alpl F, CAGTGGGAGTGAGCGCAGCC, and R, GCACTGGGTGTGGCGTGGTT; Phospho1 F, CCTGGGAAACAGCCGCCGATGTG, and R, CCCGGAGGAGCATAGCAAAGCGAAG; Runx2 F, TGGCCGGGAATGATGAGAAC, and R, TGAAACTCTTGCCTCGTCCG; Sp7 F, GGGCGTTCTACCTGCGACTG, and R, ATCGGGGCGGCTGATTG; ATF4 F, GTGGCCAAGCACTTGAAACC, and R, GGAAAAGGCATCCTCCTTGC; MGP F, AAGCCCAAAAGAGAGTCCAGG, and R, AAGTAGCGGTTGTAGGCAGC; Opn F, ATGAGGCTGCAGTTCTCCTGG, and R, AAAGCTTCTTCTCCTCTGAGCTGCC; Ank F, CACCCTGATAGCCTACAGTGAC, and R, GGAAGGCAGCGAGATACAGG; PiT1 F, AGCACCGTTGCTGGGCTTT, and R, GGCCCAGTGGCACACACTACC; and PiT2 F, CGCGGTTTCCGGAGGGAACG, and R, AGGTTGCTAACTTCGGGAGGCCA. Dspp primer sequences are described in Ref. 44.
Microarray and Data Processing
RNA was isolated as described above, and its purity was assessed by gel electrophoresis (Agilent 2100 Bioanalyzer). Transcriptional profiling was carried out using the Affymetrix Mouse Gene ST 1.0 array at the University of Alabama at Birmingham Heflin Center for Genomic Science using standard methods (Affymetrix GeneChip Expression Technical Manual). The Mouse Gene ST 1.0 chip consists of 24,582 well annotated genes. Briefly, 300 ng of total RNA from each sample was used to generate double strand cDNA by linear amplification using T7-linked random primers and reverse transcriptase. Subsequently, cRNA was generated by standard methods (Affymetrix) followed by single-stranded DNA fragmentation, end label biotinylation, and preparation of hybridization mixture. The arrays were hybridized overnight at 45 °C and then washed, stained, and scanned the next day. Data acquisition software (Affymetrix GeneChip Command Console Software) was used to generate a cell intensity (CEL) file from the stored images containing a single intensity value for each probe cell on the array. The CEL files were imported into GeneSpring GX 11.5.1 (Agilent Technologies, Santa Clara, CA). Using the RMA16 summarization algorithm, GeneSpring GX summarized the CEL data files. Expression values for each mRNA were obtained by the robust multiarray analysis method of Irizarry et al. (45). This protocol adjusts for the background on the raw intensity scale, carries out a nonlinear quantile normalization of the perfect match values, log-transforms the background-adjusted perfect match values, and carries out a robust multi-chip analysis of the quantile normalized log-transformed values (45). Each sample underwent baseline transformation to the mean of the control samples. Entities were filtered based on their signal intensity values by satisfying the upper and lower percentile cutoffs 20–100%. Filtered data were further processed in GeneSpring GX by using a one-way analysis of variance and the multiple testing correction method of Benjamini Hochberg. A fold change cutoff of ≥ ±2 was used to generate downstream datasets (45). To control for the occurrence of false discoveries in the datasets, a corrected p value (q value) ≤ 0.05 was used.
Western Blot
Whole protein extracts were prepared by cell lysis in RIPA buffer supplemented with 1 mm NaF, 2 mm Na2VO4, 2 mm leupeptin, 2 mm pepstatin, and 2 mm PMSF. Protein concentration was determined by micro BCA protein assay kit (Thermo Scientific, Rockford, IL). Protein (15 μg) was subjected to electrophoresis on 4–12% precast BisTris gels (Invitrogen) and transferred onto a nitrocellulose membrane. Specific proteins were detected by fluorescence (Li-Cor Odyssey Infrared Imaging System, LI-COR Biosciences, Lincoln, NE). Primary antibodies against Trps1 (ProteinTech, Chicago) were used at 1:1500 dilution; Vdr (Thermo Scientific, Rockford, IL) and Gapdh (Cell Signaling, Danvers, MA) were used at 1:1000; Runx2 (MBL International, Woburn, MA) was used at 1:250 dilution; Sp7 (Abcam, Cambridge, MA) was used at 1:200; and tubulin (Sigma) was used at 1:10,000. All fluorescent secondary antibodies (LI-COR Biosciences, Lincoln, NE) were used at 1:20,000.
Alizarin Red Staining and Quantification of Mineralization
Cells were fixed in 4% paraformaldehyde and stained with 40 mm alizarin red-S (Sigma) for 10 min. Excess dye was removed by washing with deionized water. To quantify calcium deposits, alizarin red was extracted from stained cells with 10% acetic acid, neutralized with 10% NH4OH to pH 4.1–4.3, and quantified by colorimetric detection by spectrophotometry at 405 nm.
TNAP Activity Assay
To detect TNAP activity, cells were fixed in 4% paraformaldehyde, and the alkaline phosphatase substrate naphthol AS-MX phosphate (0.1 mg/ml) was added in reaction buffer containing 0.5% N,N-dimethylformamide, 2 mm MgCl2, 0.6 mg/ml Fast blue BB salt, and 0.1 m Tris-HCl, pH 8.5. The reaction was stopped by washing in water, and stained cells were imaged. Densitometry of TNAP activity staining was performed using ImageJ software. Images were formatted to the same size; image background was subtracted, a circular area was defined to ensure measurements were taken of the same location and size, and the selected area was analyzed for each image. Measurement values were normalized by taking the inverse and multiplying by 50,000 so that the larger numbers correspond to darker staining (46). Statistics were calculated using the raw, untransformed values.
Isolation of Matrix Vesicles
MVs were isolated by collagenase digestion as described previously (13). Briefly, cells were plated at a density of 7 × 105 cells per 10-cm plate in 10 ml of osteogenic differentiation medium. An equal number of cells was used for each cell line. Plates were incubated at 37 °C with 5% CO2 for 9 days with media change every 3 days. On day 9, cells were washed and digested for 1.5 h with 2.5 mg/ml collagenase (Worthington). After collagenase digestion, cells were centrifuged at 3000 rpm for 10 min. Supernatant was collected and centrifuged at 19,000 rpm for 10 min. Supernatant was again centrifuged at 42,000 rpm for 45 min to get the MV pellet. The pellet was then resuspended in Tris-buffered saline (20 mm Tris, 0.15 mm NaCl).
AFM Images of MVs
Five microliters of MV solution was dropped onto a freshly cleaved mica substrate (Ted Pella, Redding, CA) and allowed to stand for a couple of minutes. Next, 5 μl of glutaraldehyde solution (8% in H2O) (Sigma) was spotted onto the sample drop and dried at room temperature overnight. Samples were imaged by noncontact (AAC) mode in air using a 5500 AFM (Agilent Technologies). Silicon-nitride cantilevers with a nominal resonance frequency of ∼190 kHz (NanosensorsTM, Neuchatel, Switzerland) were employed. Tridimensional AFM images were generated by PicoView software (Agilent Technologies).
Calculation of MV Diameter and Number
AFM imaging was used to investigate the morphology (diameter) and number of MVs isolated from cells. MV diameters were calculated as the peak value of the cross-sections of n = 100 vesicles for each cell line. Mean and standard deviation values for the diameter distributions were obtained through Gaussian fit. The number of MVs isolated from cells was calculated by counting the globular features in AFM images (scan size 5 × 5 μm).
Statistics
All experiments were performed on three control and three Trps1-modified stable cell lines. Data are presented as the mean ± S.D. Probability values were calculated using the Student's t test. p < 0.05 (*) and 0.005 (**) were considered to be statistically significant and highly significant, respectively.
RESULTS
Trps1 Expression during Osteo-odontogenic Differentiation of 17IIA11 Cells
To gain mechanistic insights into the role of Trps1 in the physiology and pathology of odontoblast-regulated mineralization, we employed the 17IIA11 cell line (17A cells) derived from mouse odontoblasts (42, 43). 17A cells are a good model to study molecular mechanisms of odontoblast-mediated mineralization, because they express key transcription factors as well as enzymes and matrix proteins required for mineralization (42). Importantly, when induced by osteogenic medium, 17A cells secrete the ECM that rapidly undergoes mineralization (Fig. 1A). Thus, 17A cells maintained in standard cell culture medium represent preodontoblasts, and when cultured in osteogenic medium, they differentiate into odontoblast-like cells (42, 43). However, because in in vitro systems odontoblastic cells do not polarize, the ECM produced by these cells lacks the characteristic tubular structure of dentin and resembles osteodentin. To indicate this difference between in vivo and in vitro odontoblast differentiation, we use the term “osteo-odontogenic differentiation” for the in vitro differentiation of 17A cells.
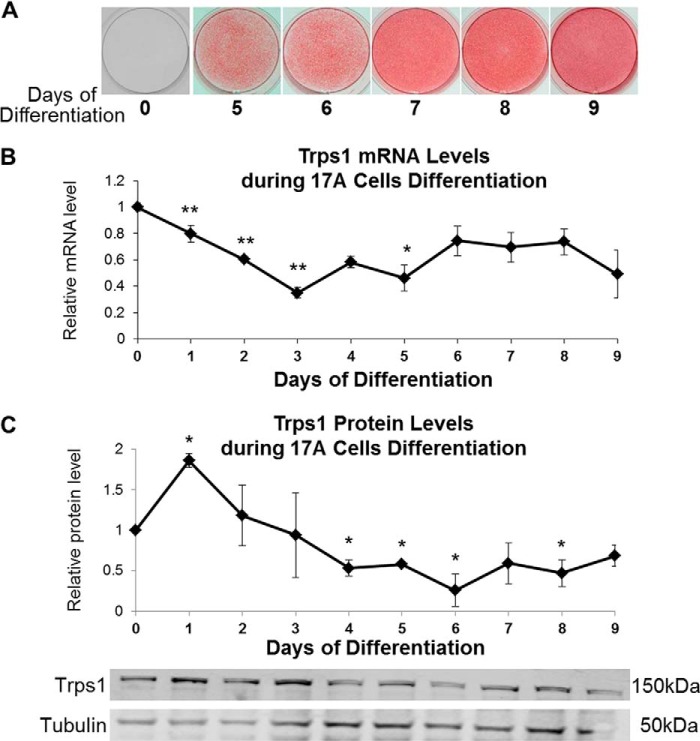
FIGURE 1.
Trps1 expression during osteo-odontogenic differentiation of 17A cells. A, representative images of alizarin red staining showing the progression of 17A-regulated mineralization. B, qRT-PCR results demonstrating Trps1 expression in 17A cells. Data are presented as the mean relative levels of Trps1 mRNA (normalized to Gapdh) ±S.D. from three independent differentiation experiments. Trps1 levels in undifferentiated 17A cells (day 0 of differentiation) were arbitrarily set at 1 and used as a reference for the remaining time points. C, Western blot analyses of Trps1 protein levels. Results of densitometric analyses (top) of Western blots (bottom) demonstrating relative Trps1 protein levels. Data are represented as the mean relative density of Trps1 normalized to tubulin (a protein loading control) ±S.D. from three independent differentiation experiments. Normalized Trps1 levels in undifferentiated 17A cells were arbitrarily set at 1 and used as a reference for the remaining time points. Asterisks denote statistically significant differences compared with day 0 of differentiation (*, p ≤ 0.05; **, p ≤ 0.005).
Trps1 expression was compared in 17A cells at the preodontoblastic stage and upon induction of osteo-odontogenic differentiation by analyzing mRNA and protein levels every 24 h starting from day 0 through day 9 of differentiation, when the mineralization of 17A cells plateaus. Trps1 mRNA and protein were detected in preodontoblastic cells as well as during osteo-odontogenic differentiation and mineralization of 17A cells. At the mRNA level, the highest Trps1 expression was observed in 17A preodontoblastic cells (Fig. 1B). Upon induction of differentiation with osteogenic medium, Trps1 expression transiently decreases, reaching the lowest level prior to formation of mineralization nodules. From days 6 to 9, when differentiated 17A cells support the growth of mineralization nodules, Trps1 expression is maintained at a steady level. At the protein level, the changes of Trps1 in general follow the mRNA, except from day 1 of differentiation, when transient up-regulation of Trps1 is detected (Fig. 1, B and C). Although the changes of Trps1 expression during osteo-odontogenic differentiation of 17A cells are not as dramatic as during odontoblast differentiation in vivo (39), the overall Trps1 expression pattern in 17A odontoblastic cells and odontoblasts in vivo are similar, with the highest Trps1 expression in progenitor cells and the lowest expression in newly differentiated cells.
Up-regulation of Trps1 in Differentiated Odontoblastic Cells Impairs Mineralization
We have recently demonstrated that increased Trps1 expression in mature odontoblasts in vivo results in severe impairment in dentin formation and repression of the Dspp gene (41). Although there is a significant overlap in dental phenotypes of Col1a1-Trps1 and Dspp−/− mice, the onset of dentin abnormalities and extent of mineralization defects in Col1a1-Trps1 mice are more severe than the phenotype of Dspp−/− mice (41, 47). This suggests that Trps1-dependent repression of dentin formation reflects dysregulation of a number of genes involved in mineralization. 17A cells were used as a model of odontoblast-regulated mineralization to identify additional genes that contribute to impaired mineralization caused by Trps1 up-regulation in mature odontoblasts.
To generate a cellular model of Trps1 up-regulation throughout odontoblastic differentiation, a transposon-mediated genomic integration approach was used. We generated three clonal stable cell lines with 3.0-, 3.5-, and 4.5-fold overexpression of V5-tagged Trps1 (Trps1-OE cells) and three control clonal cell lines generated with a “no insert” transposon (Cntr cells). Trps1 overexpression in clonal cell lines was confirmed on both the mRNA and protein level (Fig. 2A). The progress of mineralization in Trps1-OE and Cntr cells was compared using quantification of alizarin red-stained calcium deposits. These analyses demonstrated that mineralization nodules are first detected at day 5 of osteo-odontogenic differentiation of Cntr cells and 2 days later in Trps1-OE cells. Moreover, overexpression of Trps1 in odontoblastic cells results in decreased mineral formation by Trps1-OE cells in comparison with Cntr (Fig. 2B). All three clonal Trps1-OE cell lines demonstrated delayed and decreased mineralization in comparison with control cell lines. This mineralization defect of Trps1-OE cells is consistent with our previous data from Col1a1-Trps1 transgenic mice (41), indicating that overexpression of Trps1 in odontoblasts impairs odontoblast-regulated mineralization in vitro and in vivo. This impaired mineralization is not caused by TNAP deficiency, because no significant differences in TNAP activity were detected in preodontoblastic and odontoblastic Trps1-OE and control cells (Fig. 2C).
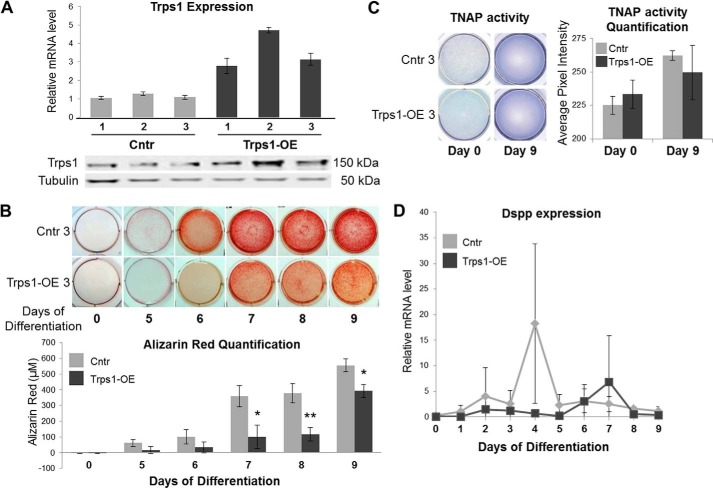
FIGURE 2.
Delayed and decreased mineralization of 17A odontoblastic cells overexpressing Trps1. A, qRT-PCR (top panel) and Western blot (bottom panel) results demonstrating overexpression of Trps1 in three clonal stable cell lines (Trps1-OE) and controls (Cntr, undifferentiated cells). qRT-PCR data are presented as the mean relative levels of Trps1 mRNA normalized to Gapdh ±S.D. from three independent RNA preparations per cell line. Relative Trps1 levels in one of the Cntr analyses were arbitrarily set at 1 and used as a reference for the remaining control and Trps1-OE cell lines. On the Western blot analyses, tubulin was used as a protein loading control. B, representative images of alizarin red staining (top panel) and quantification (bottom panel) of Trps1-OE and Cntr cell lines during osteo-odontogenic differentiation. Quantification of alizarin red staining is presented as the mean ± S.D. from differentiation of three stable cell lines. Asterisks denote statistically significant differences (*, p ≤ 0.05; **, p ≤ 0.005). C, representative images of TNAP activity assay in Trps1-OE and Cntr undifferentiated (day 0) and differentiated (day 9) cells (left panel) and results of densitometric quantification of TNAP activity (right panel). Data are presented as the mean ± S.D. from differentiation of three stable cell lines. No statistically significant difference was detected. D, qRT-PCR results demonstrating dynamic Dspp expression during osteogenic differentiation of Trps1-OE and Cntr cell lines. Data are presented as the mean relative levels of Dspp mRNA (normalized to Gapdh) ±S.D. from three stable cell lines. Dspp expression was not detected in undifferentiated controls; therefore, values for day 1 of Cntr cells were arbitrarily set at 1 and used as a reference for the remaining time points.
In Col1a1-Trps1 transgenic mice, dentin mineralization defects are associated with repression of the Dspp gene; therefore, expression of Dspp was compared during the differentiation of Trps1-OE and control cell lines. qRT-PCR analyses did not detect Dspp expression in undifferentiated cells. Upon initiation of osteogenic differentiation, Dspp is transiently up-regulated shortly before formation of mineralization nodules in control cells. Consistent with the mineralization defects caused by Trps1 overexpression, Dspp up-regulation is delayed and less profound in Trps1-OE cells in comparison with controls (Fig. 2D). However, it is important to note that even at the peak of expression in control cell lines, the levels of Dspp mRNA are very low. Furthermore, we were unable to detect Dsp protein by Western blot analyses (data not shown). This suggests that other mineralization-related genes are involved in the mineralization defects observed in Trps1-OE cells.
Trps1 Represses Phex and Vdr during Later Stages of Mineralization
After confirming that Trps1 up-regulation in 17A odontoblastic cells results in similar mineralization defects to those observed in the dentin of Col1a1-Trps1 mice, we used Trps1-OE cells to identify mineralization-related genes that are dysregulated by Trps1-overexpression in mature odontoblasts. The Affymetrix Mouse Gene ST 1.0 array was used to compare global gene expression in Trps1-OE and Cntr cells at day 6 of differentiation. At this time point, mineralization nodules are clearly visible in Cntr but not in Trps1-OE cells, indicating delayed propagation of HA in the ECM. These analyses identified 158 genes significantly (over 2-fold) down-regulated and 188 genes significantly up-regulated in Trps1-OE cells in comparison with Cntr. Interestingly, the group of most down-regulated genes in Trps1-OE cells is enriched in mineralization-related genes that are associated with hypophosphatemic rickets and mineralization defects in dentin (Table 1). Therefore, the subsequent analyses were focused on determining the changes in Enpp1, Phex, and Vdr expression upon up-regulation of Trps1.
TABLE 1.
Ten most up-regulated and down-regulated genes in 17A odontoblastic cells overexpressing Trps1 (day 6 of osteogenic differentiation)
Microarray (Affymetrix Mouse Exon 1.0 ST array) was performed with total RNA isolated from Trps1-OE and Cntr clonal cell lines.
| Gene name | Entrez ID | Accession | Log fold change (Trps1-OE vs. Cntr) |
|---|---|---|---|
| Il1rl1 | 17082 | NM_001025602 | 3.91379 |
| Maob | 109731 | NM_172778 | 3.13405 |
| Tmem47 | 192216 | NM_138751 | 2.99411 |
| Cd200 | 17470 | NM_010818 | 2.46653 |
| Galnt13 | 271786 | NM_173030 | 2.09059 |
| Tfrc | 22042 | NM_011638 | 2.07298 |
| Serpina3g | 20715 | NM_009251 | 1.84464 |
| Mpa2l | 100702 | NM_194336 | 1.77374 |
| Zcchc5 | 213436 | NM_199468 | 1.74184 |
| Il18rap | 16174 | NM_010553 | 1.69067 |
| Mmp13 | 17386 | NM_008607 | −3.59059 |
| Enpp1 | 18605 | NM_008813 | −3.58652 |
| Dkk1 | 13380 | NM_010051 | −3.16697 |
| Dpep2 | 319446 | NM_176913 | −2.76464 |
| Gabra3 | 14396 | NM_008067 | −2.68961 |
| Ranbp3l | 223332 | NM_198024 | −2.60713 |
| Phex | 18675 | NM_011077 | −2.35128 |
| Enpp6 | 320981 | NM_177304 | −2.34854 |
| Serping1 | 12258 | NM_009776 | −2.06617 |
| Vdr | 22337 | NM_009504 | −2.01732 |
Results of qRT-PCR analyses demonstrated that Phex and Vdr are expressed at low levels in Cntr preodontoblastic cells and during the initiation phase of mineralization (Fig. 3A). Phex expression increases shortly before mineralization nodules are detectable by alizarin red staining and reaches the highest level at day 6 of osteo-odontogenic differentiation, when mineralization nodules are clearly visible (Fig. 3A). As in Cntr cells, the highest Phex mRNA levels are detected on day 6 of differentiation of Trps1-OE cells; however, the magnitude of Phex up-regulation is significantly lower in Trps1-OE cells then in Cntr cells. Western blot analyses of protein extracts isolated on day 8 of differentiation confirmed decreased levels of Phex in Trps1-OE cells (Fig. 3B). The effects of Trps1 overexpression on Vdr were similar to those observed for Phex. In Cntr cells, Vdr expression increases after day 2 of osteo-odontogenic differentiation and is further up-regulated during the mineralization phase. In contrast to up-regulation of Vdr during osteo-odontogenic differentiation of Cntr cells, Trps1-OE cells express Vdr at a steady level. As a result, Vdr is down-regulated during the mineralization nodule formation phase in Trps1-overexpressing cells (Fig. 3A). Although the differences between expression of Vdr in Cntr and Trps1-OE cells were statistically significant on the mRNA level, we did not detect a difference on the protein level by Western blot analyses (Fig. 3B).
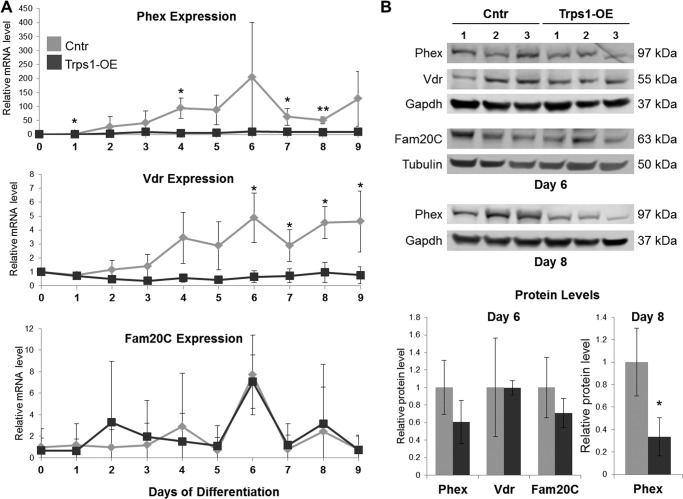
FIGURE 3.
Decreased expression of genes involved in phosphate homeostasis in Trps1-OE cells. A, qRT-PCR graphs depicting Phex, Vdr, and Fam20C mRNA expression during osteo-odontogenic differentiation in Trps1-OE cells in comparison with Cntr cells. Data are presented as the mean relative levels of Phex, Vdr, and Fam20C mRNA (normalized to β-actin) ±S.D. from three stable cell lines. Day 0 values for each cell line were arbitrarily set to 1 and used as a reference for remainder of days of differentiation. B, top panel, Western blots of Phex, Vdr, and Fam20C expression on day 6 and Phex expression on day 8 of differentiation on protein extracts isolated from Trps1-OE and Cntr cell lines. Bottom panel, results of densitometric quantification of the Western blot images. Protein levels were normalized to tubulin or Gapdh as depicted in the top panel. Asterisks denote statistically significant differences (*, p ≤ 0.05; **, p ≤ 0.005).
During osteo-odontogenic differentiation of Cntr cells, Enpp1 expression follows the same pattern as Phex and Vdr; however, Enpp1 is expressed at very low levels. Overexpression of Trps1 resulted in a further decrease of Enpp1 expression, and we were unable to detect Enpp1 mRNA at any day of osteo-odontogenic differentiation in Trps1-OE cells (data not shown).
For comparison, we performed similar expression analyses for the Fam20C gene which, like Phex, Vdr, and Enpp1, is associated with hypophosphatemia and abnormal mineralization in humans (48). Microarray analyses did not detect significant differences in Fam20C expression between Cntr and Trps1-OE cells (data not shown), and this was confirmed by qRT-PCR and Western blot on day 6 of osteo-odontogenic differentiation (Fig. 3). Interestingly, the pattern of Fam20C expression during differentiation and mineralization of Cntr cells is different from the pattern observed for Phex and Vdr (Fig. 3A). Unlike Phex and Vdr, which are strongly up-regulated during mineralization nodule formation, expression of Fam20C increases only transiently on day 6 of osteo-odontogenic differentiation, suggesting different roles of Fam20C versus Phex and Vdr in the mineralization process. In summary, overexpression of Trps1 in odontoblastic cells results in decreased expression of Phex and Vdr specifically during the later phase of mineralization when the growth of nodules is observed.
Trps1-deficient Odontoblastic Cells Do Not Support Mineralization
As shown, overexpression of Trps1 results in decreased and delayed mineralization. Therefore, it would be expected that Trps1-deficient cells have enhanced and/or accelerated mineralization. To test this hypothesis, we generated stable cell lines deficient for Trps1 (Trps1-KD) using lentivirus-delivered shRNAs targeting the Trps1 transcript. Control cell lines were generated using lentivirus-delivered scrambled shRNA (shScr cells). Trps1 knockdown was confirmed at the mRNA level by qRT-PCR and the protein level by Western blot (Fig. 4A). To characterize the consequences of Trps1 deficiency on mineralization, we selected three stable cell lines with the most efficient knockdown of Trps1 as follows: 70, 80, and 85% decreased Trps1 mRNA levels in comparison with shScr cell lines.
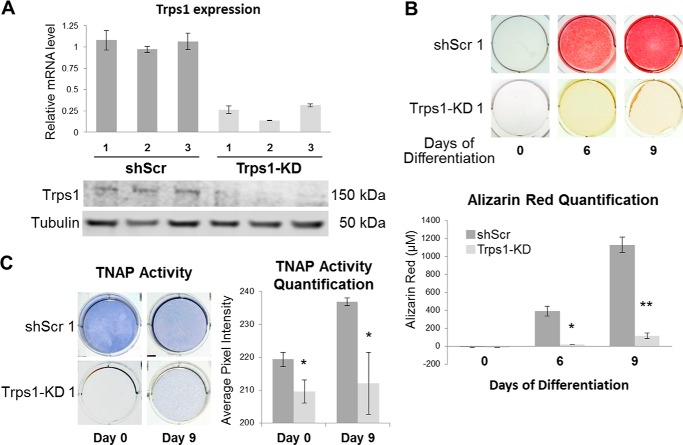
FIGURE 4.
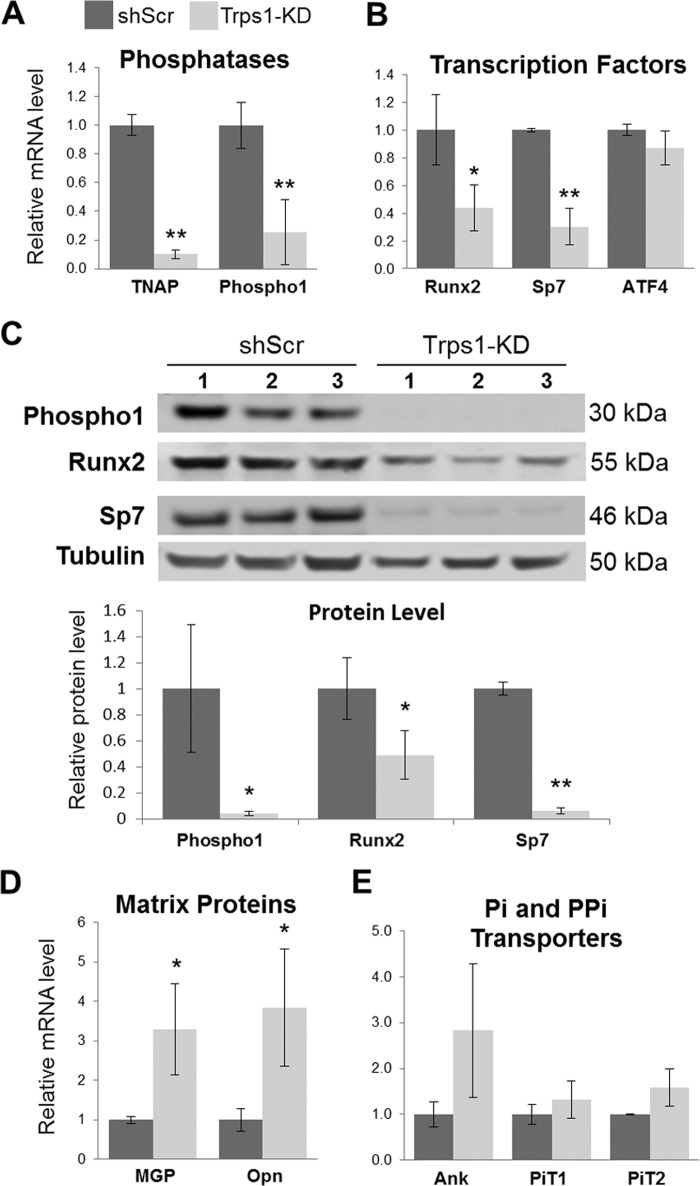
Loss of mineralization potential in Trps1-deficient cells. A, qRT-PCR (top panel) and Western blot (bottom panel) results demonstrating depletion of Trps1 in 3 clonal stable cell lines (Trps1-KD) in comparison with controls (shScr). qRT-PCR data are presented as the mean relative levels of Trps1 mRNA normalized to Gapdh ±S.D. from 3 independent RNA preparations per cell line. Relative Trps1 levels in one of the shScr analyses was arbitrarily set to 1 and used as a reference for the remaining control and Trps1-KD cell lines. On the Western blot analyses of Trps1 protein, tubulin was used as a protein loading control. B, representative images of alizarin red staining (top panel) and quantification (bottom panel) of undifferentiated (day 0) Trps1-KD and control cells, and on day 6 and 9 of osteo-odontogenic differentiation. Quantification of alizarin red staining is presented as the mean ± S.D. from the differentiation experiments of three stable cell lines. C, representative images of TNAP activity staining (left panel) and quantification of the staining (right panel) in Trps1-KD and shScr cells demonstrate decreased TNAP activity in Trps1-deficient cells. Asterisks denote statistically significant differences (*, p ≤ 0.05; **, p ≤ 0.005).
The osteo-odontogenic differentiation of Trps1-KD and shScr cell lines was carried out as before, and progression of mineralization was monitored by alizarin red staining. Surprisingly, even after 9 days of culture in osteogenic medium, there was no evidence of mineralization nodule formation in any of Trps1-KD stable cell lines, whereas in shScr cells abundant mineralization nodules were present already at day 6 of differentiation (Fig. 4B). Considering that 17A cells are already committed to odontoblastic lineage, as demonstrated previously by high TNAP activity and expression of osteogenic markers (42, 43), this indicates that Trps1 deficiency results in loss of the mineralization potential. To identify the underlying cause of this loss, we first analyzed TNAP activity, which is a characteristic feature of mineralizing cells and is required for mineral formation. Comparison of Trps1-KD and shScr cell lines demonstrated that Trps1 deficiency in odontoblastic cells results in significant decrease of TNAP activity (Fig. 4C). Furthermore, undifferentiated Trps1-KD cells had dramatically reduced levels of TNAP mRNA (Alpl) in comparison with controls as demonstrated by qRT-PCR (Fig. 5A). This indicates that the loss of TNAP activity is due to decreased expression of the Alpl gene. Considering that Trps1-KD cells do not form mineralization nodules at all, we also analyzed expression of Phospho1, which codes for a phosphatase involved in the initiation of HA crystal formation. It has been demonstrated that expression of this gene is induced in odontoblasts prior to mineralization of the dentin (12, 13, 49). qRT-PCR and Western analyses show that Phospho1 is also significantly down-regulated in all Trps1-deficient odontoblastic cell lines in comparison with shScr control cells (Fig. 5, A and C).
FIGURE 5.
Down-regulation of mineralization-supporting genes in undifferentiated Trps1-deficient cells. A, qRT-PCR results for TNAP and Phospho1 (phosphatases). B, qRT-PCR results for Runx2, Sp7, and ATF4 (osteogenic transcription factors). C, top panel, Western blot results confirming down-regulation of Phospho1, Sp7, and Runx2 in Trps1-KD cell lines. Tubulin was used as a protein loading control. Bottom panel, results of densitometric analyses of the Western blots. Protein levels were normalized to tubulin. D, qRT-PCR results for MGP and Opn (matrix proteins, mineralization inhibitors). E, qRT-PCR results for Ank, PiT1, and PiT2 (PPi and Pi transporters, respectively). qRT-PCR data were normalized to Gapdh and the average of shScr controls for each gene was arbitrarily set at 1 and used as a reference for all samples. The mean ± S.D. is shown for Trps1-KD and shScr. Asterisks denote statistically significant differences (*, p ≤ 0.05; **, p ≤ 0.005).
Trps1 Is Required for Expression of Genes Initiating Mineralization
To investigate whether the effects of Trps1 deficiency are restricted to down-regulation of phosphatases that support HA formation or whether Trps1 is required for expression of mineralization-related genes in general, we compared global gene expression between preodontoblastic shScr and Trps1-KD cells (day 0 of differentiation). These analyses revealed a total of 204 genes that were significantly (over 2-fold) down-regulated in Trps1-deficient preodontoblastic cells in comparison with controls, including Alpl and Phospho1 genes that were among the most down-regulated genes in Trps1-KD cells (Table 2). The group of 10 most down-regulated genes in Trps1-KD cells also included other mineralization-related genes, such as Ibsp, Smpd3, and Galnt3, suggesting that Trps1 is required for expression of multiple genes that support mineralization.
TABLE 2.
Ten most up-regulated and down-regulated genes in undifferentiated 17A preodontoblastic cells deficient for Trps1
Microarray (Affymetrix Mouse Exon 1.0 ST array) was performed with total RNA isolated from Trps1-KD and shScr clonal cell lines.
| Gene name | Entrez ID | Accession | Log fold change (Trps1-KD vs. shScr) |
|---|---|---|---|
| Prl2c3 | 18812 | NM_011118 | 5.05802 |
| Epha7 | 13841 | NM_010141 | 4.44097 |
| Npr3 | 18162 | NM_001039181 | 4.09527 |
| Vcam1 | 22329 | NM_011693 | 3.91533 |
| Igf1 | 16000 | NM_001111275 | 3.6016 |
| Met | 17295 | NM_008591 | 3.55918 |
| Fbn1 | 14118 | NM_007993 | 3.40414 |
| Atp1b1 | 11931 | NM_009721 | 3.32497 |
| Sema3e | 20349 | NM_011348 | 2.96628 |
| Arhgap29 | 214137 | NM_172525 | 2.94997 |
| Gpr141 | 353346 | NM_181754 | −5.07898 |
| Ibsp | 15891 | NM_008318 | −4.14171 |
| Kcnma1 | 16531 | NM_010610 | −3.48697 |
| Smpd3 | 58994 | NM_021491 | −3.36718 |
| Alpl | 249 | NM_007431 | −3.32149 |
| Ptprd | 19266 | NM_001014288 | −3.16874 |
| Galnt3 | 14425 | NM_015736 | −3.03233 |
| Phospho1 | 237928 | NM_153104 | −2.96953 |
| Sema6a | 20358 | NM_018744 | −2.89978 |
| Ramp1 | 51801 | NM_178401 | −2.88211 |
It has already been demonstrated that undifferentiated 17A cells are positive for osteogenic transcription factors (42, 43). Runx2 and its downstream target Osx (coded by the Sp7 gene) are the key transcription factors that control development of cells producing mineralizing matrix and directly regulate expression of osteogenic genes. From the group of osteogenic transcription factors, only expression of the Sp7 gene was significantly decreased in Trps1-KD cells in the gene array experiment (data not shown). This was confirmed by qRT-PCR on mRNA isolated from three stable Trps1-KD cell lines (Fig. 5B). qRT-PCR analyses also demonstrated significant down-regulation of Runx2 mRNA in Trps1-KD cell lines (Fig. 5B). Western blot analyses demonstrated a more dramatic decrease of both Sp7 and Runx2 at the protein level (Fig. 5C). There was no significant change in expression of the Atf4 gene, which codes for a transcription factor that regulates function of more mature osteogenic cells (Fig. 5B). These results suggest that in preodontoblastic cells Trps1 supports expression of genes involved in the early stages of osteo-odontogenic differentiation.
To follow up on this hypothesis, we analyzed expression of matrix proteins osteopontin (Opn, coded by the Spp1 gene) and matrix Gla protein (MGP) that are activated along with TNAP during early osteo-odontogenic differentiation. However, unlike TNAP, Opn and MGP are inhibitors of mineralization. We found that expression of Opn and Mgp in undifferentiated Trps1-KD cells is higher than in shScr (Fig. 5D). It has been reported that Opn is up-regulated upon TNAP deficiency and by increased PPi levels (14, 50); therefore, we analyzed expression of a PPi transporter Ank but detected no significant difference in Ank expression between Trps1-KD cells and controls (Fig. 5E). Similarly, there were no changes in expression of Pi transporters PiT1 and PiT2 (Fig. 5E). In summary, depletion of Trps1 in preodontoblastic cells results in the loss of the mineralization potential associated with significant down-regulation of phosphatases specifically involved in HA formation, as well as decreased expression of transcription factors essential for the osteogenic program.
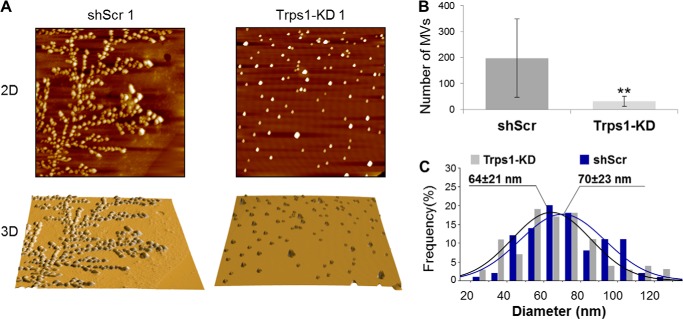
Trps1 Deficiency Affects Matrix Vesicles
The inability of Trps1-deficient cells to initiate the mineralization process and the significant down-regulation of phosphatases associated with MV function suggest defects in MVs. AFM imaging was employed to investigate the size and number of MVs isolated from Trps1-KD cell lines with respect to control cell lines (Fig. 6). MVs from both Trps1-KD and shScr cells had an average diameter of ∼70 nm. However, from the same number of cells, ∼10-fold fewer MVs were isolated from Trps1-KD cell lines in comparison with shScr cells, suggesting that fewer MVs are made by Trps1-KD cells. This implicates Trps1 in MV biogenesis and suggests that Trps1 is required for MV-dependent initiation of the mineralization process.
FIGURE 6.
Trps1 deficiency affects matrix vesicles. A, representative AFM images (scan size 10 × 10 μm) of MVs isolated from Trps1-KD and shScr cell lines. Topography (top panel) and three-dimensional surface rendering (bottom panel) are shown. B, number of MVs (mean ± S.D.) isolated from Trps1-KD and shScr cell lines. Asterisks denote statistically significant differences (**, p < 0.005). C, diameter distributions for MVs (n = 100 for each cell line) isolated from Trps1-KD and shScr cells.
DISCUSSION
Trps1 demonstrates a dynamic and specific expression pattern during odontoblast development. It is highly expressed in preodontoblasts and then is down-regulated upon odontoblast maturation suggesting a Trps1 role in odontoblast differentiation (39). Trps1 expression in secretory odontoblasts is maintained at much lower levels than in progenitors (41). This, in turn, suggests that formation of dentin by mature odontoblasts does not require Trps1 or that Trps1 may have a negative effect on their function. We have recently demonstrated that sustained high Trps1 expression in secretory odontoblasts has deleterious effects on dentin formation (41); however, the role of Trps1 in odontoblast differentiation and function is unknown. In these studies, using the 17A odontoblastic cell line, we delineated the consequences of Trps1 deficiency and up-regulation on the odontoblast-regulated mineralization process. Based on our findings, we propose the following model of the Trps1 role in odontoblasts (Fig. 7). The function of Trps1 in odontoblasts is context-dependent and is specified by the odontoblast differentiation stage. In preodontoblasts, Trps1 is required for MV-dependent initiation of the mineralization process by supporting expression of major MV-associated phosphatases involved in HA formation and for expression of key osteogenic transcription factors. However, in the mature cells, which actively produce mineralizing matrix, Trps1 acts as a repressor by suppressing expression of genes involved in propagating HA within the ECM. We propose that down-regulated expression of the genes involved in the initiation of mineralization is an indirect effect of Trps1 deficiency and may be due to up-regulation of another factor that directly represses these genes. This mechanism incorporates the findings from our in vitro studies into the well established molecular function of Trps1 as a transcriptional repressor (31–35). We cannot definitively rule out the possibility that in preodontoblasts Trps1 directly activates a subset of mineralization genes, as it has been demonstrated for Wnt pathway genes in hair follicle progenitor cells (36). However, thus far, there is no evidence that Trps1 can act as a transcriptional activator in cells other than hair follicle progenitors.
FIGURE 7.
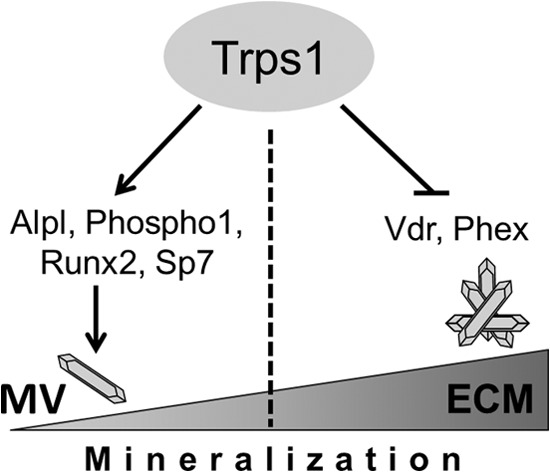
Proposed model of the Trps1 role in the odontoblast-regulated mineralization process. The role of Trps1 in mineralization is context-dependent and differs at the initiation and propagation stages. Trps1 is required for the MV-dependent initiation of mineralization by supporting expression of major phosphatases and transcription factors required for this process. During the later stages of the mineralization process, Trps1 acts as a mineralization inhibitor by repressing expression of genes involved in the mineral propagation within the ECM.
In these studies, we determined that in preodontoblastic cells expression of major osteogenic transcription factors Runx2 and Sp7 is decreased upon Trps1 depletion. This contradicts our earlier data from analyses of Trps1ΔGT/ΔGT endochondral bones where we demonstrated up-regulation of Runx2 in perichondrium and cartilage of Trps1 mutant mice (32). This may reflect a combination of cell autonomous and nonautonomous mechanisms regulating mineralization of the perichondrium versus effects of only cell autonomous mechanisms in the in vitro system. For example, Ihh signaling, which is known to up-regulate Runx2 in endochondral bones, is increased in the perichondrium of Trps1ΔGT/ΔGT mice, implicating cell nonautonomous mechanisms in the accelerated perichondrial mineralization in Trps1-deficient mice (32). Alternatively, this may indicate that different molecular networks regulate mineralization of dentin and perichondrium. This possibility is supported by studies, which discovered distinct pools of Runx2-dependent genes in bone and dental mesenchyme (51). Similarly, the upstream regulation of Runx2 may be different in bones and teeth. In developing endochondral bones, the loss of Trps1 is sufficient to up-regulate Runx2 (32), although in preodontoblastic cells this effect may be counteracted by simultaneous up-regulation of another repressor of Runx2. The same mechanism involving another repressor may underlie the observed down-regulation of Alpl and Phospho1 in Trps1-KD cells. Therefore, we propose that the observed down-regulation of Alpl, Phospho1, Runx2, and Sp7 in Trps1-KD cells is an indirect consequence of Trps1 deficiency and results from the released inhibition of a yet unidentified repressor.
Results of our in vitro studies, considered together with the Trps1 expression pattern in odontoblasts, provide first insights into the Trps1 function in dentinogenesis in vivo. We have shown here that Trps1 depletion from preodontoblastic cells results in the loss of the mineralization potential and down-regulation of multiple mineralization-related genes. This supports the hypothesis that Trps1 is required for odontoblast maturation. Interestingly, the genes most affected by Trps1 deficiency are those associated with MVs, cellular structures that initiate the mineralization process (Table 2). In vivo, a combined deficiency of TNAP and PHOSPHO1 (Phospho1−/−;Alpl+/− mice), two phosphatases that are significantly down-regulated in Trps1-KD cells, resulted in a significant decrease of dentin mineralization and was sufficient to almost completely deplete MVs from the mantle dentin (49). Consistent with these data from animal models, loss of TNAP and PHOSPHO1 in Trps1-deficient odontoblastic cells is associated with a decreased number of MVs. Considering that MVs are abundant in mantle dentin, the first dentin layer formed by newly differentiated odontoblasts, but not in the remaining (circumpulpal) dentin formed by mature odontoblasts (2, 52–56), this suggests that Trps1 is involved in the formation of mantle dentin. The role of Trps1 in the biogenesis of mineralization-competent MVs remains to be determined.
Mineralization of circumpulpal dentin relies on molecular mechanisms different from those that drive mantle dentin formation (57), as underscored by analyses of human conditions associated with dentin defects. In hypophosphatemia caused by either PHEX or VDR mutations, formation of circumpulpal dentin is severely disrupted, although mantle dentin is unaffected, demonstrating the crucial role of phosphate homeostasis genes in the later stages of dentin formation (28, 58–62). Consistent with their role in the function of mature odontoblasts, expression of Phex and Vdr is low in 17A cells prior to mineralization nodule formation and then increases during the propagation of mineralization. This up-regulation of Phex and Vdr in the later phase of mineralization is hindered by Trps1 overexpression, whereas Trps1 has no effect on expression of these genes during the mineralization initiation phase. Our studies demonstrate that Trps1 specifically represses genes that are required for circumpulpal dentin mineralization, and this function is restricted to mature cells that actively support propagation of mineralization in the ECM. This agrees with our recent in vivo studies demonstrating that sustained high Trps1 expression in secretory odontoblasts results in severely abnormal dentin and direct repression of the Dspp gene. However, TNAP levels remain unchanged.
In summary, our studies of an in vitro model of odontoblast differentiation and mineralization provide an understanding of the biological significance of the dynamic changes of Trps1 expression during dentinogenesis. We uncovered that although Trps1 is required for the initiation of odontoblast-regulated mineralization, down-regulation of Trps1 in secretory odontoblasts is necessary to allow for the formation of circumpulpal dentin.
Acknowledgments
We thank Dr. Rajiv Kumar (Mayo Clinic) for providing Phex antibodies and Dr. Chunlin Qin (Baylor College of Dentistry) for providing Fam20C antibodies. For microarray analyses, we thank the University of Alabama at Birmingham Heflin Center for Genomic Sciences and Brian Dawson (Baylor College of Medicine). We thank Terry Bertin for the assistance with qRT-PCR optimization.
This work was supported, in whole or in part, by National Institutes of Health Grants R01DE023083 from NIDCR (to D. N.), T90DE022736 (to M. K.), DE12889 and R01AR53102 from NIAMS (to J. L. M.). This work was also supported by a grant from University of Alabama at Birmingham Center for Metabolic Bone Disease (to D. N.).
- HA
- hydroxyapatite
- ECM
- extracellular matrix
- MV
- matrix vesicle
- TNAP
- tissue-nonspecific alkaline phosphatase
- Opn
- osteopontin
- MGP
- matrix Gla protein
- qRT-PCR
- quantitative RT-PCR
- AFM
- atomic force microscopy
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- F
- forward
- R
- reverse.
REFERENCES
- 1. Gorski J. P. (2011) Biomineralization of bone: a fresh view of the roles of noncollagenous proteins. Front. Biosci. 16, 2598–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldberg M., Kulkarni A. B., Young M., Boskey A. (2011) Dentin: structure, composition and mineralization. Front. Biosci. 3, 711–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Golub E. E. (2009) Role of matrix vesicles in biomineralization. Biochim. Biophys. Acta 1790, 1592–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Golub E. E. (2011) Biomineralization and matrix vesicles in biology and pathology. Semin. Immunopathol. 33, 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson H. C., Garimella R., Tague S. E. (2005) The role of matrix vesicles in growth plate development and biomineralization. Front. Biosci. 10, 822–837 [DOI] [PubMed] [Google Scholar]
- 6. Balcerzak M., Malinowska A., Thouverey C., Sekrecka A., Dadlez M., Buchet R., Pikula S. (2008) Proteome analysis of matrix vesicles isolated from femurs of chicken embryo. Proteomics 8, 192–205 [DOI] [PubMed] [Google Scholar]
- 7. Millán J. L. (2013) The role of phosphatases in the initiation of skeletal mineralization. Calcif. Tissue Int. 93, 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirsch T., Nah H. D., Shapiro I. M., Pacifici M. (1997) Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J. Cell Biol. 137, 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yadav M. C., Simão A. M., Narisawa S., Huesa C., McKee M. D., Farquharson C., Millán J. L. (2011) Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification. J. Bone Miner. Res. 26, 286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McKee M. D., Yadav M. C., Foster B. L., Somerman M. J., Farquharson C., Millán J. L. (2013) Compounded PHOSPHO1/ALPL deficiencies reduce dentin mineralization. J. Dent. Res. 92, 721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harmey D., Hessle L., Narisawa S., Johnson K. A., Terkeltaub R., Millán J. L. (2004) Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am. J. Pathol. 164, 1199–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stewart A. J., Roberts S. J., Seawright E., Davey M. G., Fleming R. H., Farquharson C. (2006) The presence of PHOSPHO1 in matrix vesicles and its developmental expression prior to skeletal mineralization. Bone 39, 1000–1007 [DOI] [PubMed] [Google Scholar]
- 13. Ciancaglini P., Yadav M. C., Simão A. M., Narisawa S., Pizauro J. M., Farquharson C., Hoylaerts M. F., Millán J. L. (2010) Kinetic analysis of substrate utilization by native and TNAP-, NPP1-, or PHOSPHO1-deficient matrix vesicles. J. Bone Miner. Res. 25, 716–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Addison W. N., Azari F., Sørensen E. S., Kaartinen M. T., McKee M. D. (2007) Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J. Biol. Chem. 282, 15872–15883 [DOI] [PubMed] [Google Scholar]
- 15. George A., Veis A. (2008) Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 108, 4670–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson K., Goding J., Van Etten D., Sali A., Hu S. I., Farley D., Krug H., Hessle L., Millán J. L., Terkeltaub R. (2003) Linked deficiencies in extracellular PP(i) and osteopontin mediate pathologic calcification associated with defective PC-1 and ANK expression. J. Bone Miner. Res. 18, 994–1004 [DOI] [PubMed] [Google Scholar]
- 17. van den Bos T., Handoko G., Niehof A., Ryan L. M., Coburn S. P., Whyte M. P., Beertsen W. (2005) Cementum and dentin in hypophosphatasia. J. Dent. Res. 84, 1021–1025 [DOI] [PubMed] [Google Scholar]
- 18. Suzuki H., Amizuka N., Oda K., Noda M., Ohshima H., Maeda T. (2008) Involvement of the klotho protein in dentin formation and mineralization. Anat. Rec. 291, 183–190 [DOI] [PubMed] [Google Scholar]
- 19. Thompson D. L., Sabbagh Y., Tenenhouse H. S., Roche P. C., Drezner M. K., Salisbury J. L., Grande J. P., Poeschla E. M., Kumar R. (2002) Ontogeny of Phex/PHEX protein expression in mouse embryo and subcellular localization in osteoblasts. J. Bone Miner. Res. 17, 311–320 [DOI] [PubMed] [Google Scholar]
- 20. Ye L., MacDougall M., Zhang S., Xie Y., Zhang J., Li Z., Lu Y., Mishina Y., Feng J. Q. (2004) Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J. Biol. Chem. 279, 19141–19148 [DOI] [PubMed] [Google Scholar]
- 21. Yoshiko Y., Wang H., Minamizaki T., Ijuin C., Yamamoto R., Suemune S., Kozai K., Tanne K., Aubin J. E., Maeda N. (2007) Mineralized tissue cells are a principal source of FGF23. Bone 40, 1565–1573 [DOI] [PubMed] [Google Scholar]
- 22. Barron M. J., McDonnell S. T., Mackie I., Dixon M. J. (2008) Hereditary dentine disorders: dentinogenesis imperfecta and dentine dysplasia. Orphanet J. Rare Dis. 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim J. W., Simmer J. P. (2007) Hereditary dentin defects. J. Dent. Res. 86, 392–399 [DOI] [PubMed] [Google Scholar]
- 24. Liu H., Li J., Lei H., Zhu T., Gan Y., Ge L. (2010) Genetic etiology and dental pulp cell deficiency of hypophosphatasia. J. Dent. Res. 89, 1373–1377 [DOI] [PubMed] [Google Scholar]
- 25. MacDougall M., Dong J., Acevedo A. C. (2006) Molecular basis of human dentin diseases. Am. J. Med. Genet. 140, 2536–2546 [DOI] [PubMed] [Google Scholar]
- 26. Souza M. A., Soares Junior L. A., Santos M. A., Vaisbich M. H. (2010) Dental abnormalities and oral health in patients with hypophosphatemic rickets. Clinics 65, 1023–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bergwitz C., Jüppner H. (2012) FGF23 and syndromes of abnormal renal phosphate handling. Adv. Exp. Med. Biol. 728, 41–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Opsahl Vital S., Gaucher C., Bardet C., Rowe P. S., George A., Linglart A., Chaussain C. (2012) Tooth dentin defects reflect genetic disorders affecting bone mineralization. Bone 50, 989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hessle L., Johnson K. A., Anderson H. C., Narisawa S., Sali A., Goding J. W., Terkeltaub R., Millan J. L. (2002) Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. U.S.A. 99, 9445–9449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Foster B. L., Nagatomo K. J., Tso H. W., Tran A. B., Nociti F. H., Jr., Narisawa S., Yadav M. C., McKee M. D., Millán J. I., Somerman M. J. (2013) Tooth root dentin mineralization defects in a mouse model of hypophosphatasia. J. Bone Miner. Res. 28, 271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malik T. H., Shoichet S. A., Latham P., Kroll T. G., Peters L. L., Shivdasani R. A. (2001) Transcriptional repression and developmental functions of the atypical vertebrate GATA protein TRPS1. EMBO J. 20, 1715–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Napierala D., Sam K., Morello R., Zheng Q., Munivez E., Shivdasani R. A., Lee B. (2008) Uncoupling of chondrocyte differentiation and perichondrial mineralization underlies the skeletal dysplasia in tricho-rhino-phalangeal syndrome. Hum. Mol. Genet. 17, 2244–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piscopo D. M., Johansen E. B., Derynck R. (2009) Identification of the GATA factor TRPS1 as a repressor of the osteocalcin promoter. J. Biol. Chem. 284, 31690–31703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suemoto H., Muragaki Y., Nishioka K., Sato M., Ooshima A., Itoh S., Hatamura I., Ozaki M., Braun A., Gustafsson E., Fässler R. (2007) Trps1 regulates proliferation and apoptosis of chondrocytes through Stat3 signaling. Dev. Biol. 312, 572–581 [DOI] [PubMed] [Google Scholar]
- 35. Wuelling M., Kaiser F. J., Buelens L. A., Braunholz D., Shivdasani R. A., Depping R., Vortkamp A. (2009) Trps1, a regulator of chondrocyte proliferation and differentiation, interacts with the activator form of Gli3. Dev. Biol. 328, 40–53 [DOI] [PubMed] [Google Scholar]
- 36. Fantauzzo K. A., Christiano A. M. (2012) Trps1 activates a network of secreted Wnt inhibitors and transcription factors crucial to vibrissa follicle morphogenesis. Development 139, 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fantauzzo K. A., Tadin-Strapps M., You Y., Mentzer S. E., Baumeister F. A., Cianfarani S., Van Maldergem L., Warburton D., Sundberg J. P., Christiano A. M. (2008) A position effect on TRPS1 is associated with Ambras syndrome in humans and the Koala phenotype in mice. Hum. Mol. Genet. 17, 3539–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Momeni P., Glöckner G., Schmidt O., von Holtum D., Albrecht B., Gillessen-Kaesbach G., Hennekam R., Meinecke P., Zabel B., Rosenthal A., Horsthemke B., Lüdecke H. J. (2000) Mutations in a new gene, encoding a zinc-finger protein, cause tricho-rhino-phalangeal syndrome type I. Nat. Genet. 24, 71–74 [DOI] [PubMed] [Google Scholar]
- 39. Kantaputra P., Miletich I., Lüdecke H. J., Suzuki E. Y., Praphanphoj V., Shivdasani R., Wuelling M., Vortkamp A., Napierala D., Sharpe P. T. (2008) Tricho-rhino-phalangeal syndrome with supernumerary teeth. J. Dent. Res. 87, 1027–1031 [DOI] [PubMed] [Google Scholar]
- 40. Kunath M., Lüdecke H. J., Vortkamp A. (2002) Expression of Trps1 during mouse embryonic development. Mech. Dev. 119, S117–S120 [DOI] [PubMed] [Google Scholar]
- 41. Napierala D., Sun Y., Maciejewska I., Bertin T. K., Dawson B., D'Souza R., Qin C., Lee B. (2012) Transcriptional repression of the Dspp gene leads to dentinogenesis imperfecta phenotype in Col1a1-Trps1 transgenic mice. J. Bone Miner. Res. 27, 1735–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lacerda-Pinheiro S., Dimitrova-Nakov S., Harichane Y., Souyri M., Petit-Cocault L., Legrès L., Marchadier A., Baudry A., Ribes S., Goldberg M., Kellermann O., Poliard A. (2012) Concomitant multipotent and unipotent dental pulp progenitors and their respective contribution to mineralised tissue formation. Eur. Cell Mater. 23, 371–386 [DOI] [PubMed] [Google Scholar]
- 43. Priam F., Ronco V., Locker M., Bourd K., Bonnefoix M., Duchêne T., Bitard J., Wurtz T., Kellermann O., Goldberg M., Poliard A. (2005) New cellular models for tracking the odontoblast phenotype. Arch. Oral Biol. 50, 271–277 [DOI] [PubMed] [Google Scholar]
- 44. Huang B., Sun Y., Maciejewska I., Qin D., Peng T., McIntyre B., Wygant J., Butler W. T., Qin C. (2008) Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone. Eur. J. Oral Sci. 116, 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Irizarry R. A., Hobbs B., Collin F., Beazer-Barclay Y. D., Antonellis K. J., Scherf U., Speed T. P. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 [DOI] [PubMed] [Google Scholar]
- 46. Street S. E., Walsh P. L., Sowa N. A., Taylor-Blake B., Guillot T. S., Vihko P., Wightman R. M., Zylka M. J. (2011) PAP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine. Mol. Pain 7, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sreenath T., Thyagarajan T., Hall B., Longenecker G., D'Souza R., Hong S., Wright J. T., MacDougall M., Sauk J., Kulkarni A. B. (2003) Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J. Biol. Chem. 278, 24874–24880 [DOI] [PubMed] [Google Scholar]
- 48. Rafaelsen S. H., Raeder H., Fagerheim A. K., Knappskog P., Carpenter T. O., Johansson S., Bjerknes R. (2013) Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23-related hypophosphatemia, dental anomalies, and ectopic calcification. J. Bone Miner. Res. 28, 1378–1385 [DOI] [PubMed] [Google Scholar]
- 49. McKee M. D., Hoac B., Addison W. N., Barros N. M., Millán J. L., Chaussain C. (2013) Extracellular matrix mineralization in periodontal tissues: noncollagenous matrix proteins, enzymes, and relationship to hypophosphatasia and X-linked hypophosphatemia. Periodontology 2000 63, 102–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Polewski M. D., Johnson K. A., Foster M., Millán J. L., Terkeltaub R. (2010) Inorganic pyrophosphatase induces type I collagen in osteoblasts. Bone 46, 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. James M. J., Järvinen E., Wang X. P., Thesleff I. (2006) Different roles of Runx2 during early neural crest-derived bone and tooth development. J. Bone Miner. Res. 21, 1034–1044 [DOI] [PubMed] [Google Scholar]
- 52. Katchburian E. (1973) Membrane-bound bodies as initiators of mineralization of dentine. J. Anat. 116, 285–302 [PMC free article] [PubMed] [Google Scholar]
- 53. Stratmann U., Schaarschmidt K., Wiesmann H. P., Plate U., Höhling H. J. (1996) Mineralization during matrix-vesicle-mediated mantle dentine formation in molars of albino rats: a microanalytical and ultrastructural study. Cell Tissue Res. 284, 223–230 [DOI] [PubMed] [Google Scholar]
- 54. Massa L. F., Ramachandran A., George A., Arana-Chavez V. E. (2005) Developmental appearance of dentin matrix protein 1 during the early dentinogenesis in rat molars as identified by high-resolution immunocytochemistry. Histochem. Cell Biol. 124, 197–205 [DOI] [PubMed] [Google Scholar]
- 55. Takano Y., Sakai H., Baba O., Terashima T. (2000) Differential involvement of matrix vesicles during the initial and appositional mineralization processes in bone, dentin, and cementum. Bone 26, 333–339 [DOI] [PubMed] [Google Scholar]
- 56. Garcés-Ortíz M., Ledesma-Montes C., Reyes-Gasga J. (2013) Presence of matrix vesicles in the body of odontoblasts and in the inner third of dentinal tissue: a scanning electron microscopy study. Med. Oral Patol. Oral Cir. Bucal. 18, e537–e541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Verdelis K., Lukashova L., Wright J. T., Mendelsohn R., Peterson M. G., Doty S., Boskey A. L. (2007) Maturational changes in dentin mineral properties. Bone 40, 1399–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boukpessi T., Septier D., Bagga S., Garabedian M., Goldberg M., Chaussain-Miller C. (2006) Dentin alteration of deciduous teeth in human hypophosphatemic rickets. Calcif. Tissue Int. 79, 294–300 [DOI] [PubMed] [Google Scholar]
- 59. Goldberg M., Septier D., Bourd K., Hall R., Jeanny J. C., Jonet L., Colin S., Tager F., Chaussain-Miller C., Garabédian M., George A., Goldberg H., Menashi S. (2002) The dentino-enamel junction revisited. Connect. Tissue Res. 43, 482–489 [DOI] [PubMed] [Google Scholar]
- 60. Chaussain-Miller C., Sinding C., Septier D., Wolikow M., Goldberg M., Garabedian M. (2007) Dentin structure in familial hypophosphatemic rickets: benefits of vitamin D and phosphate treatment. Oral Dis. 13, 482–489 [DOI] [PubMed] [Google Scholar]
- 61. Salmon B., Bardet C., Khaddam M., Naji J., Coyac B. R., Baroukh B., Letourneur F., Lesieur J., Decup F., Le Denmat D., Nicoletti A., Poliard A., Rowe P. S., Huet E., Vital S. O., Linglart A., McKee M. D., Chaussain C. (2013) MEPE-derived ASARM peptide inhibits odontogenic differentiation of dental pulp stem cells and impairs mineralization in tooth models of X-linked hypophosphatemia. PLoS One 8, e56749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang X., Rahemtulla F. G., MacDougall M. J., Thomas H. F. (2007) Vitamin D receptor deficiency affects dentin maturation in mice. Arch. Oral Biol. 52, 1172–1179 [DOI] [PubMed] [Google Scholar]