Background: HIV-1 Nef targets the coreceptor CD4 to the multivesicular body (MVB) pathway for degradation via an unknown mechanism.
Results: Nef interacts with Alix in late endosomes, and this is required for efficient lysosomal targeting of CD4.
Conclusion: Nef utilizes Alix as an adaptor to target CD4 for lysosomal degradation.
Significance: The study clarifies the mechanism by which Nef down-regulates expression of specific host-cell proteins.
Keywords: Endosome, Human Immunodeficiency Virus (HIV), Intracellular Trafficking, Lysosome, Protein Sorting, Alix, Multivesicular Body (MVB), Nef
Abstract
Nef is an accessory protein of human immunodeficiency viruses that promotes viral replication and progression to AIDS through interference with various host trafficking and signaling pathways. A key function of Nef is the down-regulation of the coreceptor CD4 from the surface of the host cells. Nef-induced CD4 down-regulation involves at least two independent steps as follows: acceleration of CD4 endocytosis by a clathrin/AP-2-dependent pathway and targeting of internalized CD4 to multivesicular bodies (MVBs) for eventual degradation in lysosomes. In a previous work, we found that CD4 targeting to the MVB pathway was independent of CD4 ubiquitination. Here, we report that this targeting depends on a direct interaction of Nef with Alix/AIP1, a protein associated with the endosomal sorting complexes required for transport (ESCRT) machinery that assists with cargo recruitment and intraluminal vesicle formation in MVBs. We show that Nef interacts with both the Bro1 and V domains of Alix. Depletion of Alix or overexpression of the Alix V domain impairs lysosomal degradation of CD4 induced by Nef. In contrast, the V domain overexpression does not prevent cell surface removal of CD4 by Nef or protein targeting to the canonical ubiquitination-dependent MVB pathway. We also show that the Nef-Alix interaction occurs in late endosomes that are enriched in internalized CD4. Together, our results indicate that Alix functions as an adaptor for the ESCRT-dependent, ubiquitin-independent targeting of CD4 to the MVB pathway induced by Nef.
Introduction
The human immunodeficiency virus (HIV) interferes with the host cell protein sorting machinery to promote viral replication and evade defense mechanisms (1, 2). A key mediator of these actions is Nef, a virally encoded accessory protein that is most abundantly expressed in early stages of the infection cycle. Nef enhances production of infectious virions and promotes disease progression (3–7), in part through modulation of the expression of specific proteins on the surface of infected cells (8). One of the best characterized effects of Nef is the down-regulation of CD4, a type I transmembrane glycoprotein expressed on the surface of helper T-lymphocytes and cells of the macrophage/monocyte lineage. CD4 acts as a coreceptor for the recognition of MHC class II molecules exposed on the surface of antigen-presenting cells and transduces positive signals that trigger adaptive immune responses. CD4 also interacts with the envelope glycoprotein of HIV-1, HIV-2, and simian immunodeficiency virus (SIV),6 serving as a primary receptor for entry of these viruses into the target cells (9).
Down-regulation of CD4 in HIV-1-infected cells is thought to prevent deleterious superinfection (10, 11) and to facilitate release of newly produced viral particles (12). Nef down-regulates CD4 by accelerating its endocytosis from the cell surface and subsequently targeting it for lysosomal degradation (13–15). Only recently has the host molecular machinery used by Nef to interfere with protein trafficking started to be unraveled. Nef concentrates CD4 into nascent clathrin-coated buds at the plasma membrane (16, 17) through the assembly of a tripartite complex with the cytosolic tail of CD4 and the adaptor protein 2 (AP-2) complex (18). Binding to AP-2 requires a dileucine motif (D/E)XXXL(L/I) and a diacidic motif (D/E)D in the C-terminal flexible loop of Nef (19–21). Disruption of either of these motifs compromises the ability of Nef to induce CD4 down-regulation (14, 19, 22, 23).
Unlike typical endocytic recycling receptors, CD4 molecules that are internalized by Nef do not return to the cell surface but are instead delivered to lysosomes for degradation (13, 15, 24). This pathway is similar to that followed by signaling receptors, transporters, and other transmembrane proteins that undergo ubiquitination-mediated incorporation into nascent intraluminal vesicles (ILVs) of multivesicular bodies (MVBs) for eventual transport to lysosomes (25). However, in previous work, we found that sorting of CD4 into ILVs induced by Nef was independent of CD4 ubiquitination (26). Ubiquitination-independent sorting in MVBs has been recently reported for a number of other cargo proteins (27–30).
Biogenesis of MVBs involves the action of endosomal sorting complexes required for transport (ESCRT)-0, -I, -II, and -III proteins, which mediate ubiquitinated cargo concentration and ILV formation (31). Indeed, targeting of CD4 to MVBs by Nef requires TSG101, a component of the ESCRT-I complex (26), but how Nef promotes incorporation of CD4 into the MVB pathway is presently unknown. Nef physically interacts with Alix (also known as AIP1 or PDCD6IP) (32, 33), a multifunctional protein that binds to ESCRT-I (34, 35) and ESCRT-III (34–39) proteins, and mediates cargo recruitment (40–43) and membrane remodeling during endosomal maturation (44, 45). It has been recently shown that Alix mediates ubiquitination-independent, ESCRT-dependent MVB sorting through direct interaction with cargo (41, 42).
To determine whether Alix has a role in Nef-induced targeting of CD4 to the MVB pathway, we further investigated the Alix-Nef interaction. We found that Nef binds to both the Bro1 and V domains of Alix. Depletion of Alix or overexpression of a truncated construct comprising the V domain (VD) impairs lysosomal degradation of CD4 induced by Nef, likely by disturbing interaction with endogenous Alix. Moreover, we found that Nef interacts with Alix in late endosomes that contain internalized CD4. These observations indicate that Nef uses Alix as an adaptor for ubiquitination-independent targeting of transmembrane cargo to the MVB pathway.
EXPERIMENTAL PROCEDURES
Recombinant DNA Constructs, Mammalian Expression Vectors
The pCMV-CD4 vector encoding human CD4 was provided by Klaus Strebel (NIAID, National Institutes of Health). pCIneo-Nef, pNef.IRES.GFP and pCMV-Nef.V5, all encoding HIV-1 Nef (NL4-3 variant) and the respective dileucine mutants, were described previously (26, 32). The cDNA sequence encoding human Alix central (V) domain (VD; residues 360–716) and the Bro1 domain (residues 1–359) were amplified by PCR from HeLa cell cDNA and subcloned as an EcoRI/SalI insert into pCIneo 3×HA-Ubq (26), where HA is hemagglutinin and Ubq is ubiquitin, to obtain pCIneo3×HA Alix VD. Human cDNA encoding full-length HRS was amplified by PCR from HeLa cell cDNA and cloned as an EcoRI/SalI insert into the pCINeo 3×HA-Ubq to generate pCIneo3×HA-HRS. For bimolecular fluorescence complementation (BiFC) experiments, pcDNA3.1/Zeo (Invitrogen)-based vectors encoding the N- and C-terminal halves of Venus fluorescent protein (VNt, residues 1–158, and VCt, residues 159–239, respectively), fused to a leucine zipper sequence (46), were provided by Jurgen Miller (University of Warwick, Coventry, UK). The leucine zipper sequences in these vectors were replaced by full-length NL4-3 Nef and Alix-coding sequences to obtain pNef-VNt and pVCt-Alix.
Escherichia coli Expression Vectors
To express GST-Alix fusion proteins in E. coli, the human cDNA sequence encoding full-length Alix was amplified by PCR and subcloned as an EcoRI/SalI insert into pGEX5.1 (GE Healthcare). This strategy was also used to produce GST-Alix domain constructs (see Fig. 1A), encoding the N-terminal Bro1 domain (residues 1–359), the central V domain (VD; residues 360–716), the C-terminal proline-rich domain (PRD; residues 717–868), and the following Alix C-terminal deletion mutants encoding the following: Bro1 residues 1–167 (Bro1(1–167)); Bro1 plus the first 76 amino acid residues of VD (Bro1VD(1–436)); Bro1 plus the first 245 residues of VD (Bro1VD(1–605)), and the C-terminal PRD deletion (AlixBro1VD(1–717)). The pHis6-Parallel2-Nef vector, described previously by Chaudhuri et al. (19), was used to express NL-4.3 Nef as an N-terminal hexahistidine-tagged protein (His6-Nef) in E. coli. Also, for E. coli expression as His6-tagged proteins, the sequences encoding HIV-1 Nef alleles NA7, DH12-3, and the SIVmac239 Nef allele in pIRES2-eGFP, described previously by Chaudhuri et al. (19), were subcloned into pET28a vector as a EcoRI/SalI insert. All subcloning products were verified by DNA sequencing.
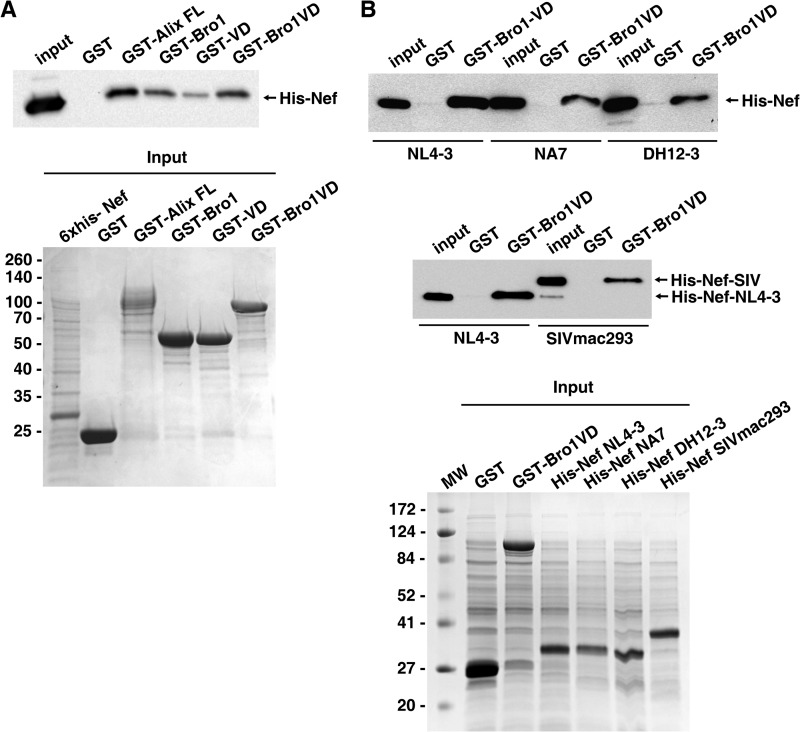
FIGURE 1.
Nef interacts with both Bro1 and V domain of Alix. A, schematic representation of N-terminal GST-tagged Alix domain constructs. Below each of the three domain structures of Alix, Bro1 domain, V domain (VD), and proline-rich domain (PRD), are listed the known interaction partners. B, in vitro analyses of interactions between Nef and Alix domains. Recombinant GST and GST fusion proteins were produced in E. coli and immobilized onto glutathione-Sepharose beads, as described under “Experimental Procedures.” HEK-293T Nef-strep-FLAG cells were cultivated with 1 μg/ml doxycycline to induce expression of Nef-strep-FLAG. Total cell lysates were prepared and incubated with immobilized GST, GST-AlixFL, and truncated Alix-GST proteins as follows: GST-Bro1, GST-VD, or GST-PRD. Binding of Nef to GST fusion proteins was analyzed by immunoblotting with anti-FLAG antibody (top). An aliquot of 10% input of recombinant proteins (Input) was subjected to SDS-PAGE and stained with Coomassie Blue (bottom). C, Nef binds to the N-terminal region of Bro1. Shown is a schematic representation of the C-terminal deletion mutants of Alix used for GST-pulldown analyses (top). Recombinant GST fusion proteins were produced in E. coli and immobilized as in B and incubated with total cell lysate of HEK cells expressing V5 epitope-tagged Nef (Nef-V5). Binding of Nef to GST fusion proteins was analyzed by immunoblotting with anti-V5 (left panel). An aliquot of 10% input of recombinant proteins (Input) was resolved by SDS-PAGE and stained with Coomassie Blue (middle and right panels). The molecular masses are indicated in kilodaltons.
Cell Lines, Transfections, and RNA Interference (RNAi)
HeLa CCL-2 cells were purchased from the American Type Culture Collection (Manassas, VA). These cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen), supplemented with 100 units of penicillin/ml, 0.1 mg of streptomycin/ml, and 10% (v/v) fetal bovine serum (FBS). A stable HEK-293-Nef-strep-FLAG cell line for tetracycline-inducible expression of Nef-strep-FLAG was produced using the Fli-InTM T-RExTM system from Invitrogen following the manufacturer's instructions. Briefly, HIV-1 Nef (NL4-3 variant) coding sequence was amplified by PCR and inserted into the pCDNA5/FRT/TO vector (Invitrogen), which was then used to transfect Flp-InTM 293 T-REx cells (Invitrogen), a cell line derived from HEK-293 (ATCC; CRL-1573) that contains a single integrated Flp recombination Target (FRT) site, allowing position-specific insertion of a single copy of the expression construct into the genome. Cells were transiently transfected with the plasmids indicated in the figure legends by using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. The small interfering RNA (siRNA) targeting human Alix (5′-GCA GUA AUA UGU CUG CUC A-3′) (47) was purchased from Dharmacon (Lafayette, CO), and siRNA transfections were carried out using the Oligofectamine reagent from Invitrogen.
Antibodies
Unconjugated or allophycocyanin-conjugated monoclonal antibodies to human CD4, used for uptake experiments, immunofluorescence, and fluorescence-activated cell sorting (FACS) analysis, were from Invitrogen. Rabbit polyclonal antibody to human CD4 and goat polyclonal antibody to human Alix (N-20) used for immunoblotting were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibody to the human EGF receptor was from Abcam (Cambridge, MA). Monoclonal antibody to CD63 was purchased from BD Biosciences, and monoclonal antibody to HRS (A-5) was from Enzo Life Sciences (Farmingdale, NY). Monoclonal antibodies to His tag and hemagglutinin (HA) tag (16B12) were from Sigma and Covance (Berkeley, CA), respectively. Affinity-purified rabbit antibody to HA was purchased from Thermo Scientific (Rockford, IL). Rabbit antiserum to SNX2 and GFP were generous gifts from C. Haft (NIDDK, National Institutes of Health), and R. Hegde (MRC, Cambridge, UK), respectively. Rabbit antiserum to HIV-1 Nef was obtained from the AIDS Research and Reference Reagent Program, National Institutes of Health, deposited originally by Ronald Swanstrom. Horseradish peroxidase-conjugated donkey anti-mouse IgG and donkey anti-rabbit IgG were from GE Healthcare. Secondary antibodies conjugated to fluorophores a indicated in the figure legends were from Invitrogen.
FACS Analysis
Cells transfected with pCMV-CD4 and pNef.IRES.GFP or pNefLL/AA.IRES.GFP plasmids were incubated for 24 h, harvested, stained with allophycocyanin-conjugated primary antibody against CD4, and fixed as described previously (19, 26). Untransfected cells or cells transfected with pIRES-GFP plasmid only were used as a control for nonspecific antibody labeling. In all experiments, GFP fluorescence was used to identify and select transfected cells. The relative levels of CD4 in the surface of cells expressing GFP were inferred from allophycocyanin fluorescence intensity data acquired using a FACSCanto flow cytometer (BD Biosciences). Data analysis and preparation of histograms were performed using FloJo software (Tree Star, Ashland, OR).
SDS-PAGE and Immunoblot Analysis
Cells were lysed for 20 min on ice using lysis buffer (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 10% (v/v) glycerol, 5 mm EDTA, 1% (v/v) Triton X-100), supplemented with a protease inhibitor mixture (Sigma). Cell lysates were centrifuged for 20 min at 20,000 × g, and supernatants were recovered. Protein levels in supernatants were measured using the protein assay from Bio-Rad to equalize total protein levels. Samples were mixed with sample buffer (4% SDS, 160 mm Tris-HCl (pH 6.8), 20% (v/v) glycerol, 100 mm DTT, and 0.1% bromphenol blue) and boiled. Proteins were resolved by SDS-PAGE and transferred onto a nitrocellulose membrane, which were then blocked with PBS-T (PBS, 0.5%, Tween 20) and 5% nonfat dry milk for 1 h. Primary antibodies were added in PBS, 1% BSA for 1 h at room temperature or overnight at 4 °C. After three washes with PBS-T, the membranes were incubated with HRP-conjugated secondary antibody for 1 h and washed again, and proteins were detected by using enhanced chemiluminescence (ECL) solutions (solution 1: 1 m Tris-HCl (pH 8.5), 250 mm luminol, 90 mm p-coumaric acid; and solution 2: 30%H2O2, 1 m Tris-HCl (pH 8.5)).
Recombinant Protein Expression and GST Pulldown Assays
E. coli BL21-Star cells were transformed with pHis6-Parallel2-Nef (19) or pGEX 5.1 vectors to express GST and GST-Alix fusion proteins (plasmids described above). Expression of recombinant proteins was induced with 1 mm of isopropyl β-d-thiogalactopyranoside, and cells were grown for 3 h at 30 °C. After incubation, cells were harvested and disrupted by sonication in ice-cold lysis buffer (50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 10% glycerol, 2 mm EDTA, 10 mm DTT), supplemented with 500 μg/ml lysozyme and 1 mm 4-(2-aminoethyl)benzenesulfonyl fluoride. Insoluble material was removed by centrifugation, and proteins in supernatant were further solubilized by addition of 1% Nonidet P-40. For pulldown experiments, GST and GST fusion proteins were immobilized onto glutathione-Sepharose 4B beads (GE Healthcare). The beads were extensively washed with ice-cold lysis buffer and subsequently incubated with lysates of isopropyl β-d-thiogalactopyranoside-induced E. coli expressing His6-Nef proteins. Alternatively, beads were incubated with total lysates of HEK-293-Nef-strep-FLAG cells or lysates of HEK293 cells expressing Nef-V5 for 1 h at 4 °C on ice. The beads were centrifuged at 100 × g, washed three times with lysis buffer, supplemented with 1% (v/v) Triton X-100, and subsequently resuspended in SDS-PAGE sample buffer. Beads were boiled, and proteins were resolved by SDS-PAGE.
Epidermal Growth Factor (EGF) Uptake and Degradation Assay
For immunofluorescence experiments, cells grown on coverslips were transfected with plasmids indicated in the figure legends. 24 h after transfection, cells were washed with PBS and starved in serum-reduced Opti-MEM culture media (Invitrogen) for 1 h at 37 °C. Cells were then stimulated with Alexa-488-conjugated EGF (Invitrogen) at 2 μg/ml in Opti-MEM alone or together with affinity-purified mouse monoclonal antibody to CD4 for 30 min at 4 °C. Cells were washed twice with ice-cold PBS to remove free EGF and antibody and chased for 30 and 120 min at 37 °C. At each time point, cells were fixed by incubation for 15 min at room temperature with 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS) and processed for immunofluorescence analysis. For immunoblot analysis of EGFR degradation, starved cells were mock-incubated or incubated with 50 ng/ml unlabeled EGF (Invitrogen) for 3 h in Opti-MEM at 37 °C. After that, cells were washed twice with PBS, and proteins were solubilized with lysis buffer, separated in 7% SDS-PAGE, and immunoblotted using the indicated antibodies.
Bimolecular Fluorescence Complementation Analysis and Immunofluorescence Microscopy
Cells grown on glass coverslips were transfected with plasmids indicated in the figure legends. After 8 h of incubation, cells were fixed for 15 min at room temperature with 4% (w/v) paraformaldehyde in PBS at room temperature. Paraformaldehyde-fixed cells were permeabilized for 10 min with 0.1% (w/v) Triton X-100 in PBS and incubated for 30 min at 37 °C in blocking solution (0.2% (w/v) pork skin gelatin in PBS). Cells were incubated with primary and secondary antibodies in blocking solution. Coverslips were mounted on slides, and cells were imaged using a confocal laser scanning microscope. Images of EGF uptake experiments were acquired with an inverted Leica TCS SP5 laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany). For imaging of BiFC experiments, a Zeiss laser scanning 780 confocal microscope (Zeiss, Jena, Germany) was used. Post-acquisition image processing was performed with ImageJ 1.36 (48). Colocalization analyses by immunofluorescence were performed with sets of images of the same cells (Z-stack, with 0.3-μm intervals) for each marker. Quantification was performed with ImageJ and the plugin colocalization threshold to determine the Pearson's correlation coefficient and the Manders' coefficients (tM) to each channel. The Mander‘s coefficients vary from 0 to 1, corresponding to no colocalization and complete colocalization, respectively. Scores are calculated for pixels above an automatically determined threshold for both channels, according to the algorithm by Costes et al. (49). Pearson's correlation coefficient represents all nonzero-zero pixels that overlay the images of the channels, where 1 is total positive correlation and 0 is no correlation.
Statistical Analysis
Data were plotted and analyzed using GraphPad Prism 5.0 software. Statistical significance was determined by one-way analysis of variance, followed by Bonferroni post-test, and the p values are represented as follows: *, p < 0.01; **, p < 0.001; and ***, p < 0.0001. Differences were considered statistically significant if the p value was <0.05.
RESULTS
Bro1 and V Domain of Alix Mediate Interaction with Nef
Alix is composed of three distinct structural elements that individually interact with a number of cellular proteins and with retroviral Gag proteins (Fig. 1A). In addition, Alix physically interacts with Nef proteins from HIV-1 (32) and SIV (33). To determine the domain(s) of Alix that mediates interaction with Nef, we performed in vitro binding experiments using immobilized recombinant GST-Alix fusion proteins to pull down Nef from cell lysates. We were able to detect a robust interaction between full-length Alix (FL-Alix) and Nef (Fig. 1B). In addition, pulldown of Nef was observed when either the Bro1 domain or the VD of Alix was used as bait (Fig. 1B), although Alix VD yielded lower levels of Nef pulldown. There was no pulldown of Nef when Alix PRD or GST alone was used as bait. We performed similar in vitro binding analyses using C-terminal deletion mutants of Alix. Interestingly, truncated Bro1 comprising the first 167 amino acid residues was sufficient for Nef binding (Fig. 1C), but the entire Bro1-VD was required to pull down Nef at levels comparable with FL-Alix (Fig. 1C, see GST-Bro1VD(1–717)). Robust binding of Nef to FL-Alix was detected when FLAG- or V5-tagged Nef was used (Fig. 1, B and C, respectively). These results confirm that Alix interacts with Nef and indicate that both the Bro1 domain and the V domain mediate binding. As interactions detected using mammalian cell lysates may not be direct, we performed similar in vitro binding experiments using purified recombinant GST-Alix and His6-Nef proteins produced in E. coli. The results confirmed that Nef binds to Alix directly and that both the Bro1 and the V domains contribute to the interaction (Fig. 2A). Next, we tested the ability of different Nef alleles to bind Alix. We observed that HIV-1 Nef variants NA7 and DH12-3 and the SIV Nef variant SIVmac239, all of which were previously shown to down-regulate CD4 (19), interact with GST-Bro1VD(1–717) (Fig. 2B). We conclude that the ability to interact with Alix is a conserved feature of Nef proteins.
FIGURE 2.
Direct interaction of Alix with Nef from distinct HIV-1 and SIV variants. A, in vitro interaction analysis of recombinant Nef and truncated Alix recombinant proteins. His6-Nef, GST, GST-Alix FL, GST-Bro1, GST-VD, and GST-Bro1VD(1–717) (GST-Bro1-VD) were produced as described under “Experimental Procedures” and used in a GST pulldown assay followed by SDS-PAGE. Binding of recombinant His-Nef to GST fusion proteins was analyzed by immunoblotting with anti-His tag antibody (top panel). An aliquot of 10% input of recombinant proteins (Input) was resolved by SDS-PAGE and stained with Coomassie Blue (bottom panel). B, in vitro interaction analysis of Alix-Bro1VD(1–717) and Nef proteins from HIV-1 (NL4-3, NA7 and DH12-3) and SIVmac293. Recombinant HIV-1 and SIV Nef variants were produced in E. coli fused to N-terminal His6 tag and used for GST pulldown experiments with immobilized GST and GST-Bro1VD(1–717) proteins. Binding of Nef to GST proteins was analyzed by immunoblotting with anti-His tag antibody (top and middle panels). An aliquot of 10% input of recombinant proteins (Input) was resolved by SDS-PAGE and stained with Coomassie Blue (bottom panel). The molecular masses are indicated in kilodaltons.
Nef Interacts with Alix in Late Endosomes
To confirm the interaction between Nef and Alix within cells and to determine the subcellular localization of this interaction, we used the BiFC technique (50) and confocal microscopy. A similar approach was previously used to demonstrate dimerization of Nef (51) and dimerization of Alix (52) and, more recently, the mechanism controlling Alix association to endosomal membranes (53). We generated constructs encoding Nef and Alix fused to the N- or C-terminal halves of the Venus fluorescent protein, respectively (Nef-VNt and VCt-Alix), and we used these constructs to transfect HeLa cells. Transfected cells showed a punctate pattern of Venus fluorescence distribution mostly localized in the juxtanuclear region (Fig. 3, see A, D, and G). In contrast, no Venus fluorescence was detected when these constructs were expressed individually (result not shown). A great proportion (∼45%) of Venus-positive structures contained CD63, a marker for MVB/late endosomes (Fig. 3, A–C, and Table 1). As shown in Fig. 3, D–F, a smaller proportion of Venus fluorescence was associated with HRS, a subunit of the ESCRT-0 complex responsible for targeting cargo proteins to the MVB pathway. Sorting nexin 2 (SNX2), a component of the retromer complex that mediates retrograde transport from endosomes to the trans-Golgi network (54), was also rarely found in Venus loci (Fig. 3, G–I, and Table 1). These findings indicated that Nef interacts with Alix in specific endocytic compartments that are enriched in an MVB marker.
FIGURE 3.
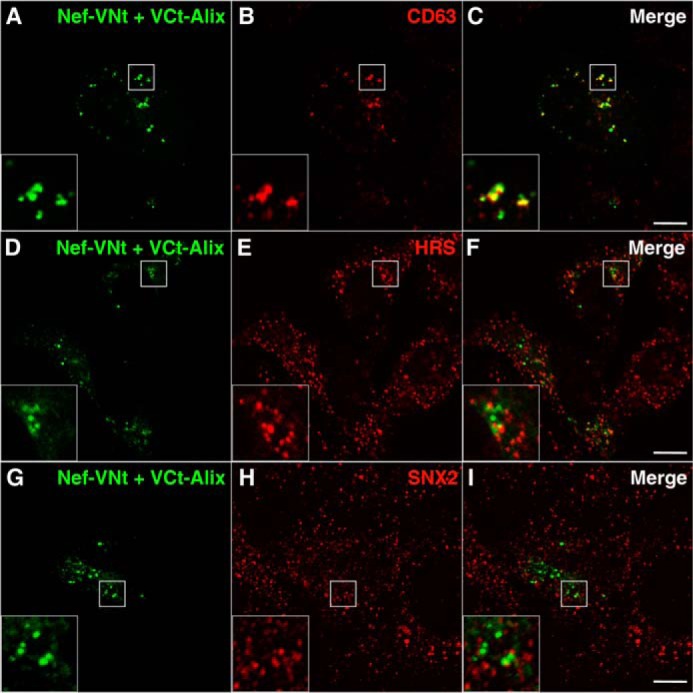
Nef interacts with Alix in late endosomes. A–I, HeLa cells grown on coverslips were transfected with plasmids encoding Nef fused to residues 1–158 of the Venus protein (Nef-VNt) and Alix fused to residues 159–239 of Venus (VCt-Alix). To achieve low expression levels and avoid mislocalization of proteins due to overexpression, cells were processed after 8 h of incubation. Cells were fixed, permeabilized, and stained with mouse monoclonal antibody to CD63 (A–C), mouse monoclonal antibody to HRS (D–F), or rabbit polyclonal antibody to SNX2 (G–I), followed by Alexa 594-conjugated donkey antibody to mouse or rabbit IgG (red channel). Cells were imaged by confocal laser scanning microscopy. Yellow in the merged images indicates colocalization. Bars, 10 μm. The insets represent the boxed areas at a magnification of ×3.
TABLE 1.
Quantitative analysis of colocalization between Nef/Alix BiFC signal and endosomal proteins
Quantification of colocalization was performed using the colocalization threshold plugin in the ImageJ software (48). Two independent parameters were obtained. The Pearson's correlation index represents all nonzero-zero pixels that overlie the images of the channels, where 1 is total positive correlation and 0 is no correlation. Also shown are the single-channel specific Manders' coefficients tM1 (the total intensity of pixels from channel 1 (green) for which the intensity in the channel 2 (red) is above zero, relative to total intensity in channel 1) and tM2 (the total intensity of pixels from channel 2 for which the intensity in channel 1 is above zero, relative to total intensity in channel 2). These coefficients were calculated using an automatically determined threshold value, for both channels, according to the algorithm of Costes et al. (49), where a value of 1 represents total colocalization. The table shows the mean ± S.E. of Pearson's and Manders' coefficients indicating the extent of overlap between Nef-VNt/VCt-Alix BiFC signal and the signal of specific endosomal markers (CD63, HRS, and SNX2) and CD4. Data for each pairwise comparison were obtained from series of Z-slices (with 0.3-μm intervals) of at least five cells from a total of three independent experiments.
| Proteins | Pearson's coefficient | Manders' coefficient (threshold) |
|
|---|---|---|---|
| tM1 | tM2 | ||
| Nef/Alix and CD63 | 0.43 ± 0.07 | 0.45 ± 0.08 | 0.31 ± 0.09 |
| Nef/Alix and HRS | 0.18 ± 0.03 | 0.24 ± 0.04 | 0.16 ± 0.04 |
| Nef/Alix and SNX2 | 0.14 ± 0.01 | 0.22 ± 0.04 | 0.14 ± 0.03 |
| Nef/Alix and CD4 | 0.48 ± 0.04 | 0.53 ± 0.03 | 0.46 ± 0.08 |
Overexpression of Alix V Domain Impairs Degradation of CD4 Induced by Nef
Down-regulation of CD4 by Nef involves two main steps as follows: induction of internalization and targeting to the MVB pathway that leads to degradation of CD4. Because Nef interacts with Alix in late endosomes, we next assessed whether interaction with Alix is required for Nef to direct CD4 to degradation. The V domain of Alix alone binds to Nef, but, differently from the Bro1 domain, it lacks the motifs that mediate interaction with subunits of the ESCRT complexes. Therefore, we hypothesized that truncated Alix-VD would act as a dominant-negative mutant by perturbing interaction of Nef with endogenous, functional Alix. Immunoblot analysis confirmed that expression of Nef decreased the total levels of CD4 in HeLa cells (Fig. 4A), whereas the Nef LL/AA mutant, with alanine substitutions in the endocytic dileucine motif, failed to decrease total levels of CD4, as observed previously (26). The decrease in CD4 expression mediated by Nef was largely prevented by coexpression of Alix VD (Fig. 4A) but not by coexpression of the Bro1 domain (Fig. 4B). Because Alix VD expression may compromise degradation of CD4 by blocking endocytosis, we next examined the effect of Alix VD expression on the cell surface levels of CD4 in cells expressing Nef. Fig. 4C shows that Alix VD expression did not prevent removal of CD4 from the cell surface. Taken together, the experiments using Alix VD expression strongly suggest that interaction of Nef with Alix in late endosomes is important for post-endocytic targeting of CD4 to a degradative pathway.
FIGURE 4.
Expression of Alix VD inhibits Nef-induced cellular depletion of CD4. A and B, HeLa cells were transfected with equal quantities of pCMV-CD4 and pCIneo-Nef LL/AA or pCIneo-Nef WT, in combination or not with pCINeo 3×HA-Alix VD (A) or pCINeo 3×HA-Alix Bro1 (B), as indicated above each lane. After 16 h, cell lysates were prepared, and equivalent amounts of proteins were analyzed by SDS-PAGE and immunoblotting with antibodies to CD4, Nef, and HA. Unspecific protein bands are indicated with an asterisk and serve as a control of sample loading. Bar graph showing signal intensity of CD4 relative to unspecific protein bands on Western blots. Data shown represent the mean ± S.E. of six independent experiments (A) or three independent experiments (B) and are expressed as percentage of the total amount of CD4 in the presence of Nef LL/AA. **, p < 0.001; ***, p < 0.0001; and ns, not significant. C, expression of Alix VD does not prevent cell surface removal of CD4 induced by Nef. HeLa cells were transfected with pCMV-CD4 together with pNef LL/AA.IRES.GFP or pNef wt.IRES.GFP alone or in combination with pCIneo 3×HA-Alix VD and analyzed for cell surface CD4 by flow cytometry, as described under “Experimental Procedures.” Bar graph represents surface CD4 levels (median values from FACS histogram plots) in cells transfected with pNef wt.IRES.GFP relative to levels in cells transfected with pNef LL/AA.IRES.GFP (100%). The data represent the mean ± S.E. (n = 3). ***, p < 0.0001; and ns, not significant.
Interaction with Alix Facilitates Targeting of CD4 to Lysosomes by Nef
We have previously shown that delivery of a GFP-tagged type I transmembrane protein to the lumen of a proteolytic compartment can be monitored by the detection of a soluble GFP degradation fragment in cell extracts, termed the GFP-core (55, 56). Therefore, to confirm that expression of Alix VD inhibits targeting of CD4 to lysosomes, we transfected a CD4-GFP plasmid in HeLa cells and monitored GFP-core formation by immunoblotting. Using an anti-GFP antibody, CD4-GFP expression was detected as two major bands as follows: an ∼80-kDa molecular mass precursor and a lower molecular mass (∼30 kDa) GFP-core fragment (Fig. 5A). Expression of Nef promotes GFP-core formation, and this can be reverted by treatment with bafilomycin, an inhibitor of acidification and protein degradation in lysosomes (Fig. 5A). Moreover, processing of CD4-GFP to the GFP-core fragment enhanced by Nef is impaired upon Alix VD coexpression (Fig. 5B). Together, the data shown in Fig. 5 indicate that Alix VD overexpression impairs targeting of CD4 to the lysosomal lumen, most likely by preventing delivery of CD4 to intraluminal vesicles of MVBs.
FIGURE 5.
Expression of Alix VD inhibits lysosomal processing of CD4. A, HeLa cells were cotransfected with equal amounts of pCMV-CD4-GFP and pCIneo-Nef WT as indicated above each lane, and 16 h later, the cells were incubated in the absence (−) or presence (+) of 1 μm bafilomycin A1 for the different periods indicated in the figure. Total cell extracts were analyzed by SDS-PAGE and immunoblotting with antibodies to GFP, Nef, and actin (loading control). Open arrowhead indicates a CD4-GFP degradation product termed GFP-core. Unspecific protein bands are indicated with an asterisk. Notice the increased level of GFP-core and the decrease of the full-length fusion protein in the presence of Nef and the reverse effect when cells were treated with bafilomycin A1. Gel image analysis from three independent experiments was conducted using ImageJ software. The signal from the GFP-core band was quantified in arbitrary units and calculated as a percentage of the total CD4-GFP FL plus GFP-core signal in each lane. The data represent the means ± S.E. (n = 3). **, p < 0.001; ***, p < 0.0001; and ns, not significant. B, HeLa cells were transfected with plasmids encoding CD4-GFP, Nef, and HA-Alix VD, as indicated at the right of each panel. After 16 h, cell lysates were prepared, equalized, and analyzed by SDS-PAGE and immunoblotting with antibodies to GFP, Nef, and HA. Unspecific protein bands are indicated with an asterisk and serve as loading control. The signal intensity for GFP-core was quantified using the ImageJ software and is expressed as percentage CD4-GFP FL plus GFP-core signals. The data represent the means ± S.E. of three independent experiments. *, p < 0.01; **, p < 0.001; and ns, not significant. The molecular masses are indicated in kilodaltons.
Sensitivity to Alix VD Distinguishes a Nef-mediated MVB Pathway
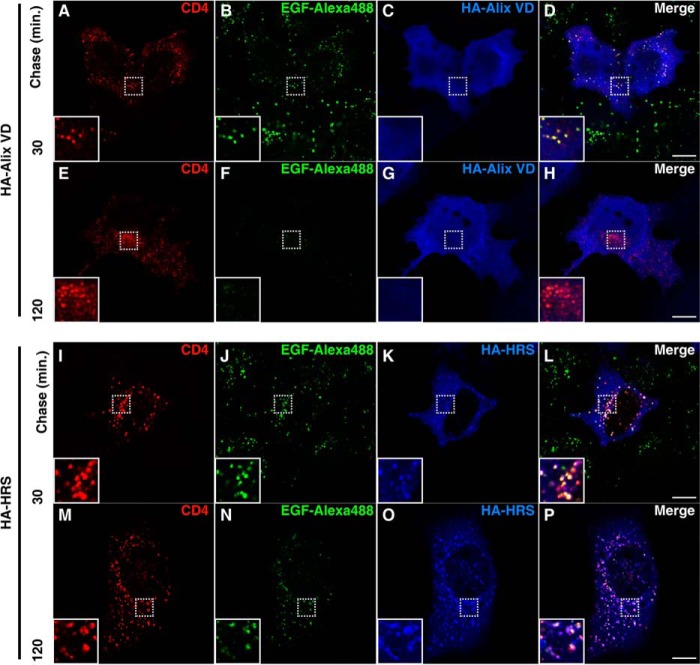
We have previously shown that Nef-mediated degradation of CD4 requires the ESCRT machinery (26). Thus, we asked whether Alix VD inhibits CD4 targeting to lysosomes by compromising the general functioning of the ESCRT-dependent MVB pathway. The EGFR has been extensively studied as a prototypical cargo of the MVB pathway. Following activation, ubiquitination, and endocytosis, EGFR-EGF complexes are targeted to intraluminal vesicles of MVBs and degraded in lysosomes. Thus, we examined the fate of internalized EGF over time. Control and Alix-VD-expressing cells were incubated with Alexa 488-EGF for 30 min at 4 °C before chasing for 30 or 120 min at 37 °C. In both control cells (Fig. 6, A and B) and Alix-VD-expressing cells (Fig. 6, C and D), disappearance of internalized EGF was essentially complete after 2 h. In contrast, overexpression of HRS, previously shown to have a dominant-negative effect on the ESCRT machinery (57), clearly compromised degradation of internalized EGF (Fig. 6, E and F). Next, we compared the subcellular distribution of CD4 that is internalized by Nef and EGFR-EGF complexes en route to lysosomes. To this end, cells expressing CD4 and Nef together with either HA-Alix VD or HA-HRS were incubated with both Alexa 488-EGF and mouse monoclonal antibody to CD4 for 30 min at 4 °C. In cells coexpressing Alix-VD, after 30 min of chase, internalized CD4 and EGF sorted to the same cytoplasmic vesicles (Fig. 7, A–D). After 2 h of chase, Alexa 488-EGF was barely detected, whereas internalized CD4 could still be seen in endosomes in the juxtanuclear region (Fig. 7, E–H). In HRS-overexpressing cells, internalized CD4 and EGF largely colocalized with each other after 30 min of chase (Fig. 7, I–L) and remained colocalized in endosomes decorated with HA-HRS after 2 h of incubation (Fig. 7, M–P). Some of these endosomes appeared enlarged, a phenotype characteristic of ESCRT machinery perturbation (58). Taken together, these results indicate that expression of Alix VD does not negatively perturb canonical ESCRT-dependent delivery of cargo to intraluminal vesicles of MVBs. Therefore, sensitivity to Alix VD expression, together with cargo ubiquitination independence (26), distinguishes Nef-mediated targeting of CD4 to degradation from the canonical MVB pathway.
FIGURE 6.
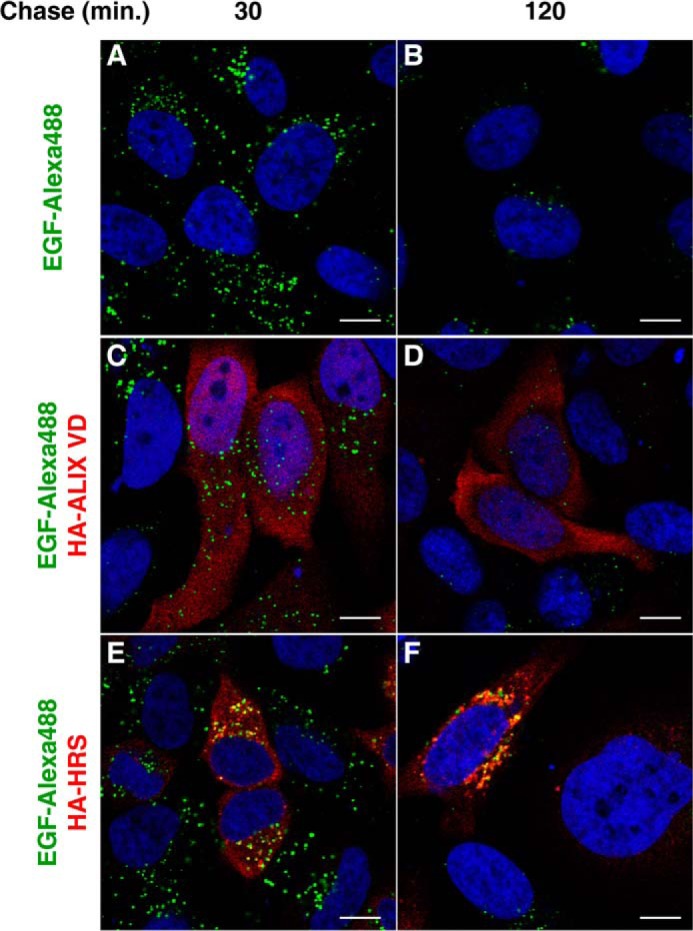
Alix VD expression does not block the EGF degradation pathway. A–F, HeLa cells were grown on coverslips and transfected with control plasmid (empty pCIneo) (A and B), pCIneo 3×-HA-Alix VD (C and D), or pHA-HRS (E and F). The cells were incubated in Opti-MEM media for 1 h followed by incubation with the same media supplemented with Alexa 488-EGF (2 μg/ml) for 30 min at 4 °C. Cells were then incubated at 37 °C for the times indicated above the panels. After incubation, cells were fixed, permeabilized, and stained with mouse monoclonal antibody to HA, followed by Alexa 594-conjugated donkey antibody to mouse IgG (red channel). Nuclei were labeled with DAPI (blue channel). Cells were imaged by confocal laser scanning microscopy. Bars, 10 μm.
FIGURE 7.
Internalized CD4 and EGF are sorted to the same endosomal population in cells expressing Nef. A–P, HeLa cells were grown on coverslips and transfected with pCMV-CD4, pCIneo-Nef WT, and pCIneo 3×HA-Alix VD (A–H) or pHA-HRS (I–P). After 20 h, cells were washed and incubated in Opti-MEM for 1 h followed by incubation with Alexa 488-EGF (2 μg/ml) and mouse monoclonal antibody to CD4 in Opti-MEM media for 30 min at 4 °C. Cells were washed with ice-cold PBS and incubated at 37 °C for the times indicated on the left of the panels. After incubation, cells were fixed, permeabilized, and stained with rabbit antibody to HA, followed by Alexa 594-conjugated donkey antibody to mouse IgG (red channel) and Alexa 647-conjugated donkey antibody to rabbit IgG (blue channel). Cells were imaged by confocal laser scanning microscopy. Bars, 10 μm. The insets represent the boxed areas at a magnification of ×2.5.
Requirement of Alix for Nef-induced Degradation of CD4
Our results indicate a functional interaction between Nef and Alix to promote down-regulation of CD4. To directly test the requirement of Alix for Nef-induced targeting of CD4 to lysosomes, we depleted Alix expression in HeLa cells and assessed processing of CD4-GFP. An approximate 90% reduction in Alix expression was achieved. Although an overall reduction in transfection efficiency was noticed in Alix-depleted cells, GFP-core generation promoted by Nef was clearly impaired by Alix depletion (Fig. 8A). It was previously shown that Alix is not essential for ubiquitin-dependent targeting of the EGFR to the MVB pathway leading to lysosomal degradation (59, 60). To confirm the specific effect of Alix depletion in the Nef-mediated sorting of cargo to the MVB pathway, we assayed for EGF-induced degradation of EGFR. Control HeLa cells and cells depleted of Alix by RNAi were incubated with EGF for 3 h and lysed, and the levels of EGFR were verified by immunoblotting. We confirmed that Alix depletion does not impair EGFR down-regulation under our experimental conditions (Fig. 8B), leading us to conclude that Alix plays a more direct role in Nef-mediated sorting of cargo to the MVB pathway.
FIGURE 8.
Depletion of Alix inhibits Nef-mediated targeting of CD4 to lysosomes. A, cells were either mock-transfected or transfected with siRNA for Alix and incubated for 48 h. Cells were transfected with pCMV-CD4-GFP and pCIneo-Nef WT, as indicated, and after 16 h equivalent amounts of cell lysates were subjected to SDS-PAGE and immunoblotting with antibodies to GFP, Nef, and Alix. Nonspecific protein bands are indicated with an asterisk and serve as loading control. The GFP-core signal intensity for each condition shown in the top panel was determined using the ImageJ software and is expressed as percentage CD4-GFP FL plus GFP-core signals. Bars represent the means ± S.E. of five independent experiments. **, p < 0.001; ns, not significant. B, cells were mock-transfected or transfected with siRNA for Alix as in A, and after 48 h cells were incubated without (−) or with (+) EGF (50 ng/ml) for 3 h at 37 °C. Total cell extracts were analyzed by SDS-PAGE and immunoblotting with antibodies to EGFR, Alix, and actin (loading control). Nonspecific protein bands are indicated with an asterisk. Gel image analysis from three independent experiments was conducted using ImageJ software. The signal from the EGFR bands was quantified in arbitrary units and expressed as percentage of signal from EGFR in control samples (cells incubated without EGF). The data represent the means ± S.E. (n = 3). ***, p < 0.0001.
Nef and Alix Associate in Endosomes Containing CD4
Nef has been previously shown to localize to clathrin-coated pits at the plasma membrane (16, 17), through which CD4 becomes rapidly internalized. Our findings indicate that Nef also acts on endosomes to target CD4 to the MVB pathway. Indeed, fluorescence microscopy of transfected HeLa cells showed that Nef-GFP localized not only to the plasma membrane but also to a population of cytoplasmic vesicles that contained down-regulated CD4 (26). To investigate whether Nef associates with Alix during endosomal targeting of CD4, we used BiFC analyses in HeLa cells. Immunofluorescence microscopy showed that Nef expression causes a dramatic redistribution of CD4 from the plasma membrane (Fig. 9A) to cytoplasmic vesicles (Fig. 9B). A similar redistribution of CD4 is seen when Nef-VNt and VCt-Alix are coexpressed (Fig. 9, C–E). Strikingly, the majority (∼53%, Table 1) of the CD4-containing vesicles displayed Nef-VNt/VCt-Alix fluorescence. We propose that CD4 molecules internalized by Nef are directed to the MVB pathway via the association of Nef with the ESCRT machinery through interaction with Alix.
FIGURE 9.
Nef-Alix interaction colocalizes with internalized CD4. HeLa cells grown on coverslips were transfected with pCMV-CD4 (A), pCMV-CD4 and pCIneo Nef (B), or pCMV-CD4, pNef-VNt and pVCt-Alix (C–E). After 8 h, cells were fixed, permeabilized, and stained with mouse monoclonal antibody to CD4, followed by Alexa 594-conjugated donkey antibody to mouse IgG (red channel). Cells were imaged by confocal laser scanning microscopy. Yellow in the merged images indicates colocalization. Bar, 10 μm. The insets represent the boxed areas at a magnification of ×3.
DISCUSSION
The results of our study provide strong evidence that Nef uses Alix as an adaptor to direct internalized CD4 into the MVB pathway leading to lysosomal degradation. We show that Nef interacts with Alix through both the Bro1 and V domains. Nef and Alix interact in endosomal structures that contain MVB proteins and are enriched with internalized CD4. Overexpression of Alix VD impairs lysosomal degradation of CD4 induced by Nef. This effect is not due to a general disturbance of the MVB pathway but most likely because overexpressed Alix VD competes with endogenous Alix for binding to Nef. We also show that delivery of CD4 to lysosomes is reduced by depletion of Alix. Thus, interaction with Alix appears to be an important step in the pathway mediated by Nef to reduce the levels of CD4 in the endosomal system.
Alix Links Nef to the ESCRT Machinery
Alix has several interacting partners in endosomes (Fig. 1A) and is thought to play multiple roles during MVB biogenesis (61). Here, we show that Nef interacts with both the Bro1 and the V domains of Alix (Figs. 1 and 2). Alix directly binds to ESCRT proteins TSG101 (ESCRT-I) (34, 35) and CHMP4 (ESCRT-III) (34–37) that are respectively involved in cargo sorting and ILV formation/scission in MVBs. Interaction with TSG101 is mediated by the Alix PRD, whereas the Bro1 domain mediates interaction with CHMP4 and the recruitment of Alix to late endosomes (38, 53). An exposed hydrophobic patch on Bro1, in which residues Leu-216 and Phe-199 are crucially involved, provides an interaction site for CHMP4 (38, 39, 62, 63). Our finding that Bro1(1–167) is sufficient for Nef binding (Fig. 1) suggests that Nef and CHMP4 bind to Alix via distinct regions of the Bro1 domain; thus, interaction of Alix with Nef and CHMP4 might not be mutually exclusive events. Studies on retroviral budding revealed that Alix functions as an adaptor in the ESCRT system. Alix mediates HIV-1 budding at the plasma membrane, a process that requires ESCRT III and is topologically equivalent to ILV formation (39, 62). This is achieved through a direct interaction between Alix VD and Gag p6 late assembly domains (YPXnL, where n = 1–3). Consistently, HIV-1 budding is strongly impaired by overexpression of an Alix VD (residues 364–716) fragment (64). As demonstrated here, overexpression of Alix VD partially inhibits CD4 targeting to lysosomes (Figs. 4 and 5), without preventing the reduction of surface CD4 induced by Nef (Fig. 4). Importantly, Alix VD overexpression does not seem to affect the activity of the ESCRT machinery or general targeting of ubiquitinated cargo to the MVB pathway (Figs. 6 and 7). We believe that this dominant-negative construct may specifically hinder association of Nef with endogenous Alix molecules, preventing Nef from hijacking the ESCRT machinery to target CD4 to lysosomes. The inhibitory effect of Alix depletion further supports that it may function as an adaptor for Nef-dependent targeting of CD4 to the MVB pathway (Fig. 8). Nef plays a positive role in MVB biogenesis (32, 65), an activity that is related to Nef's ability to interact with Alix (32). It remains to be determined whether the roles of the Nef-Alix interaction in CD4 down-regulation and in MVB proliferation are linked. It would be of interest to test whether overexpression of truncated Alix VD can inhibit MVB proliferation induced by Nef.
Targeting of CD4 to Lysosomes by Nef
Consistent with our data, it has been proposed that Nef modifies intracellular trafficking of transmembrane proteins, such as CD4, by linking their cytosolic tail to components of the protein sorting machinery (66). Down-regulation of CD4 starts at the plasma membrane, where Nef accelerates CD4 endocytosis mediated by clathrin-coated vesicles (16, 17). Nef binds to the clathrin-associated AP-2 complex through interaction with the α-σ2 hemicomplex (18–20). This process requires a dileucine motif and a diacidic motif within the conserved 27-amino acid C-terminal loop of Nef (14, 19, 22, 23). Nef also interacts with the cytosolic tail of CD4 directly, albeit with low affinity (67). Chaudhuri et al. (18) demonstrated that binding to the α-σ2 hemicomplex increases the avidity of Nef for the cytosolic tail of CD4. Although the CD4 tail is also thought to interact with AP-2 directly (68), binding of Nef to AP-2 may cooperatively enhance these interactions and promote CD4 incorporation into nascent clathrin-coated vesicles destined for early endosomes. CD4 molecules that have been internalized by Nef do not efficiently enter a retrieval pathway from early endosomes back to the plasma membrane and are delivered to lysosomes instead. We and others have shown that Nef targets CD4 to intraluminal vesicles of MVBs en route to lysosomes (26, 69).
As for most MVB cargoes, targeting of CD4 to the MVB/lysosomal pathway by Nef depends on the ESCRT machinery (26). However, MVB targeting of CD4 by Nef appears to circumvent the requirement for ubiquitination, as ubiquitination-deficient CD4 is also targeted to MVBs and efficiently degraded (26). Therefore, how Nef can ultimately target CD4 to the MVB pathway remains unknown. Here, we provide evidence that Nef also acts on endosomes by directly interacting with Alix in these organelles. We use the BiFC technique to show that Nef associates with Alix in endosomal structures, most of which contain the MVB marker CD63 and internalized CD4 (Figs. 3 and 9). We propose that in endosomes, Alix functions as an adaptor that connects Nef-bound CD4 molecules to the ESCRT machinery leading to sorting of CD4 into nascent ILVs. Indeed, Alix is known to play a direct role in ubiquitin-independent sorting of G protein-coupled receptor PAR1 into MVBs (41, 42). This is possible because the V domain of Alix binds to a YPXnL motif in the cytosolic tail of PAR1 and links this receptor to the ESCRT-III protein CHMP4b (41). Alix may link Nef-CD4 complexes to ESCRT-III directly or via ESCRT-I, which then engages ESCRT-III. Blockage of CD4 targeting to the MVB pathway by perturbing the ESCRT machinery activity (26) or by Alix VD overexpression (Figs. 4 and 5) leads to a partial recovery of total CD4, without restoring CD4 levels at the cell surface (Fig. 4C) (26). This suggests that in addition to targeting CD4 to the MVB pathway, Nef also prevents CD4 recycling to the plasma membrane by retaining it in endosomes. In fact, Nef has been shown to reduce the recycling rate of internalized CD4 (70).
Ubiquitin-dependent sorting of ILV cargo requires retention of cargo in specific clathrin-coated subdomains of early endosomes before recruitment of ESCRT III for vesicle formation. This is mediated by HRS, a component of ESCRT-0 that binds ubiquitin and prevents retrieval of MVB cargo to the plasma membrane (71). How would Nef-CD4 complexes be retained in early endosomes prior to Alix/ESCRTIII engagement? It has been proposed that AP-3 plays a cargo concentration role in early endosomes and is required for Alix-mediated sorting of PAR-1 (42). Interestingly, Nef also binds AP-3 (19, 20, 72, 73) and stabilizes association of AP-3 to endosomal membranes, where it colocalizes with Nef (73, 74). However, a role for AP-3 in CD4 down-regulation is unknown and requires further study. Alternatively, Nef-CD4 concentration in maturing early endosomes could be mediated by β-COP, a component of the COPI coat that interacts with Nef and has been implicated in targeting of CD4 to lysosomes (69, 75, 76). However, β-COP is not known to bind ESCRT proteins, and a role for COPI in CD4 down-regulation by Nef has been debated (77). Our data provide molecular insight into the mechanism by which Nef targets CD4 to MVBs and the lumen of lysosomes for degradation. Nef also targets MHC-I to MVBs (69, 78) and down-regulates a number of cell surface transmembrane proteins, such as CD8, CD28, CXCR4, CCR5, and CD80/CD86 (79, 80 and references therein) through mechanisms that are mostly unknown. It would be of interest to test whether Nef targets these proteins to the MVBs and whether this mechanism is ESCRT-dependent and requires Alix function.
Acknowledgments
We thank C. Haft, R. Hegde, J. Muller, K. Strebel, and the National Institutes of Health AIDS Research and Reference Reagent Program for the kind gifts of reagents; R. N. Silva for use of important laboratory equipment; A. N. de Carvalho and M. H. S. Martins for expert technical assistance; and R. R. C. Rosales and E. Rosa (Ribeirão Preto Medical School Bioimaging Facility) for help with confocal microscopy and colocalization analyses.
This work was supported, in whole or in part, by National Institutes of Health Grant ZIA HD001607-22 Intramural Program, NICHD (to J. S. B.). This work was also supported by São Paulo Research Foundation (FAPESP) Young Investigators Grant 2009/50650-6, the Brazilian National Council for Scientific and Technological Development (CNPq) Grant 470707/2009-7, and the Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas FMRP-USP (FAEPA).
- SIV
- simian immunodeficiency virus
- ILV
- intraluminal vesicle
- MVB
- multivesicular body
- PRD
- proline-rich domain
- BiFC
- bimolecular fluorescent complementation
- EGFR
- epidermal growth factor receptor
- VD
- V domain
- FL
- full length
- HRS
- hepatocyte growth factor-regulated tyrosine kinase substrate.
REFERENCES
- 1. Kirchhoff F. (2010) Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe 8, 55–67 [DOI] [PubMed] [Google Scholar]
- 2. Malim M. H., Emerman M. (2008) HIV-1 accessory proteins–ensuring viral survival in a hostile environment. Cell Host Microbe 3, 388–398 [DOI] [PubMed] [Google Scholar]
- 3. Deacon N. J., Tsykin A., Solomon A., Smith K., Ludford-Menting M., Hooker D. J., McPhee D. A., Greenway A. L., Ellett A., Chatfield C., Lawson V. A., Crowe S., Maerz A., Sonza S., Learmont J., Sullivan J. S., Cunningham A., Dwyer D., Dowton D., Mills J. (1995) Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270, 988–991 [DOI] [PubMed] [Google Scholar]
- 4. Kestler H. W., 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. (1991) Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65, 651–662 [DOI] [PubMed] [Google Scholar]
- 5. Kirchhoff F., Greenough T. C., Brettler D. B., Sullivan J. L., Desrosiers R. C. (1995) Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332, 228–232 [DOI] [PubMed] [Google Scholar]
- 6. Mariani R., Kirchhoff F., Greenough T. C., Sullivan J. L., Desrosiers R. C., Skowronski J. (1996) High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70, 7752–7764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salvi R., Garbuglia A. R., Di Caro A., Pulciani S., Montella F., Benedetto A. (1998) Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J. Virol. 72, 3646–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foster J. L., Garcia J. V. (2008) HIV-1 Nef: at the crossroads. Retrovirology 5, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bowers K., Pitcher C., Marsh M. (1997) CD4: a co-receptor in the immune response and HIV infection. Int. J. Biochem. Cell Biol. 29, 871–875 [DOI] [PubMed] [Google Scholar]
- 10. Lama J., Mangasarian A., Trono D. (1999) Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9, 622–631 [DOI] [PubMed] [Google Scholar]
- 11. Michel N., Allespach I., Venzke S., Fackler O. T., Keppler O. T. (2005) The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr. Biol. 15, 714–723 [DOI] [PubMed] [Google Scholar]
- 12. Ross T. M., Oran A. E., Cullen B. R. (1999) Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 9, 613–621 [DOI] [PubMed] [Google Scholar]
- 13. Anderson S. J., Lenburg M., Landau N. R., Garcia J. V. (1994) The cytoplasmic domain of CD4 is sufficient for its down-regulation from the cell surface by human immunodeficiency virus type 1 Nef. J. Virol. 68, 3092–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bresnahan P. A., Yonemoto W., Ferrell S., Williams-Herman D., Geleziunas R., Greene W. C. (1998) A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 8, 1235–1238 [DOI] [PubMed] [Google Scholar]
- 15. Rhee S. S., Marsh J. W. (1994) Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J. Virol. 68, 5156–5163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burtey A., Rappoport J. Z., Bouchet J., Basmaciogullari S., Guatelli J., Simon S. M., Benichou S., Benmerah A. (2007) Dynamic interaction of HIV-1 Nef with the clathrin-mediated endocytic pathway at the plasma membrane. Traffic 8, 61–76 [DOI] [PubMed] [Google Scholar]
- 17. Greenberg M. E., Bronson S., Lock M., Neumann M., Pavlakis G. N., Skowronski J. (1997) Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16, 6964–6976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaudhuri R., Mattera R., Lindwasser O. W., Robinson M. S., Bonifacino J. S. (2009) A basic patch on α-adaptin is required for binding of human immunodeficiency virus type 1 Nef and cooperative assembly of a CD4-Nef-AP-2 complex. J. Virol. 83, 2518–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chaudhuri R., Lindwasser O. W., Smith W. J., Hurley J. H., Bonifacino J. S. (2007) Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J. Virol. 81, 3877–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindwasser O. W., Smith W. J., Chaudhuri R., Yang P., Hurley J. H., Bonifacino J. S. (2008) A diacidic motif in human immunodeficiency virus type 1 Nef is a novel determinant of binding to AP-2. J. Virol. 82, 1166–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ren X., Park S. Y., Bonifacino J. S., Hurley J. H. (2014) How HIV-1 Nef hijacks the AP-2 clathrin adaptor to downregulate CD4. Elife 3, e01754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greenberg M., DeTulleo L., Rapoport I., Skowronski J., Kirchhausen T. (1998) A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 8, 1239–1242 [DOI] [PubMed] [Google Scholar]
- 23. Aiken C., Krause L., Chen Y. L., Trono D. (1996) Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology 217, 293–300 [DOI] [PubMed] [Google Scholar]
- 24. Sanfridson A., Cullen B. R., Doyle C. (1994) The simian immunodeficiency virus Nef protein promotes degradation of CD4 in human T cells. J. Biol. Chem. 269, 3917–3920 [PubMed] [Google Scholar]
- 25. Raiborg C., Stenmark H. (2009) The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445–452 [DOI] [PubMed] [Google Scholar]
- 26. daSilva L. L., Sougrat R., Burgos P. V., Janvier K., Mattera R., Bonifacino J. S. (2009) Human immunodeficiency virus type 1 Nef protein targets CD4 to the multivesicular body pathway. J. Virol. 83, 6578–6590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hislop J. N., Marley A., Von Zastrow M. (2004) Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes. J. Biol. Chem. 279, 22522–22531 [DOI] [PubMed] [Google Scholar]
- 28. Yamashita Y., Kojima K., Tsukahara T., Agawa H., Yamada K., Amano Y., Kurotori N., Tanaka N., Sugamura K., Takeshita T. (2008) Ubiquitin-independent binding of Hrs mediates endosomal sorting of the interleukin-2 receptor β-chain. J. Cell Sci. 121, 1727–1738 [DOI] [PubMed] [Google Scholar]
- 29. Henry A. G., White I. J., Marsh M., von Zastrow M., Hislop J. N. (2011) The role of ubiquitination in lysosomal trafficking of δ-opioid receptors. Traffic 12, 170–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolfe B. L., Marchese A., Trejo J. (2007) Ubiquitination differentially regulates clathrin-dependent internalization of protease-activated receptor-1. J. Cell Biol. 177, 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Henne W. M., Buchkovich N. J., Emr S. D. (2011) The ESCRT pathway. Dev. Cell 21, 77–91 [DOI] [PubMed] [Google Scholar]
- 32. Costa L. J., Chen N., Lopes A., Aguiar R. S., Tanuri A., Plemenitas A., Peterlin B. M. (2006) Interactions between Nef and AIP1 proliferate multivesicular bodies and facilitate egress of HIV-1. Retrovirology 3, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jesus da Costa L., Lopes Dos Santos A., Mandic R., Shaw K., Santana de Aguiar R., Tanuri A., Luciw P. A., Peterlin B. M. (2009) Interactions between SIVNef, SIVGagPol and Alix correlate with viral replication and progression to AIDS in rhesus macaques. Virology 394, 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strack B., Calistri A., Craig S., Popova E., Göttlinger H. G. (2003) AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114, 689–699 [DOI] [PubMed] [Google Scholar]
- 35. von Schwedler U. K., Stuchell M., Müller B., Ward D. M., Chung H. Y., Morita E., Wang H. E., Davis T., He G. P., Cimbora D. M., Scott A., Kräusslich H. G., Kaplan J., Morham S. G., Sundquist W. I. (2003) The protein network of HIV budding. Cell 114, 701–713 [DOI] [PubMed] [Google Scholar]
- 36. Katoh K., Shibata H., Hatta K., Maki M. (2004) CHMP4b is a major binding partner of the ALG-2-interacting protein Alix among the three CHMP4 isoforms. Arch. Biochem. Biophys. 421, 159–165 [DOI] [PubMed] [Google Scholar]
- 37. Katoh K., Shibata H., Suzuki H., Nara A., Ishidoh K., Kominami E., Yoshimori T., Maki M. (2003) The ALG-2-interacting protein Alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. J. Biol. Chem. 278, 39104–39113 [DOI] [PubMed] [Google Scholar]
- 38. Kim J., Sitaraman S., Hierro A., Beach B. M., Odorizzi G., Hurley J. H. (2005) Structural basis for endosomal targeting by the Bro1 domain. Dev. Cell 8, 937–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fisher R. D., Chung H. Y., Zhai Q., Robinson H., Sundquist W. I., Hill C. P. (2007) Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell 128, 841–852 [DOI] [PubMed] [Google Scholar]
- 40. Pashkova N., Gakhar L., Winistorfer S. C., Sunshine A. B., Rich M., Dunham M. J., Yu L., Piper R. C. (2013) The yeast Alix homolog Bro1 functions as a ubiquitin receptor for protein sorting into multivesicular endosomes. Dev. Cell 25, 520–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dores M. R., Chen B., Lin H., Soh U. J., Paing M. M., Montagne W. A., Meerloo T., Trejo J. (2012) ALIX binds a YPX(3)L motif of the GPCR PAR1 and mediates ubiquitin-independent ESCRT-III/MVB sorting. J. Cell Biol. 197, 407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dores M. R., Paing M. M., Lin H., Montagne W. A., Marchese A., Trejo J. (2012) AP-3 regulates PAR1 ubiquitin-independent MVB/lysosomal sorting via an ALIX-mediated pathway. Mol. Biol. Cell 23, 3612–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baietti M. F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A., Ivarsson Y., Depoortere F., Coomans C., Vermeiren E., Zimmermann P., David G. (2012) Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14, 677–685 [DOI] [PubMed] [Google Scholar]
- 44. Matsuo H., Chevallier J., Mayran N., Le Blanc I., Ferguson C., Fauré J., Blanc N. S., Matile S., Dubochet J., Sadoul R., Parton R. G., Vilbois F., Gruenberg J. (2004) Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303, 531–534 [DOI] [PubMed] [Google Scholar]
- 45. Falguières T., Luyet P. P., Bissig C., Scott C. C., Velluz M. C., Gruenberg J. (2008) In vitro budding of intralumenal vesicles into late endosomes is regulated by Alix and Tsg101. Mol. Biol. Cell 19, 4942–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. MacDonald M. L., Lamerdin J., Owens S., Keon B. H., Bilter G. K., Shang Z., Huang Z., Yu H., Dias J., Minami T., Michnick S. W., Westwick J. K. (2006) Identifying off-target effects and hidden phenotypes of drugs in human cells. Nat. Chem. Biol. 2, 329–337 [DOI] [PubMed] [Google Scholar]
- 47. Bardens A., Döring T., Stieler J., Prange R. (2011) Alix regulates egress of hepatitis B virus naked capsid particles in an ESCRT-independent manner. Cell. Microbiol. 13, 602–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rasband W. (1997) ImageJ Version 1.47. National Institutes of Health, Bethesda, MD [Google Scholar]
- 49. Costes S. V., Daelemans D., Cho E. H., Dobbin Z., Pavlakis G., Lockett S. (2004) Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys. J. 86, 3993–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kerppola T. K. (2008) Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu. Rev. Biophys. 37, 465–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Poe J. A., Smithgall T. E. (2009) HIV-1 Nef dimerization is required for Nef-mediated receptor downregulation and viral replication. J. Mol. Biol. 394, 329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pires R., Hartlieb B., Signor L., Schoehn G., Lata S., Roessle M., Moriscot C., Popov S., Hinz A., Jamin M., Boyer V., Sadoul R., Forest E., Svergun D. I., Göttlinger H. G., Weissenhorn W. (2009) A crescent-shaped ALIX dimer targets ESCRT-III CHMP4 filaments. Structure 17, 843–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bissig C., Lenoir M., Velluz M. C., Kufareva I., Abagyan R., Overduin M., Gruenberg J. (2013) Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Dev. Cell 25, 364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bonifacino J. S., Hurley J. H. (2008) Retromer. Curr. Opin. Cell Biol. 20, 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. daSilva L. L., Foresti O., Denecke J. (2006) Targeting of the plant vacuolar sorting receptor BP80 is dependent on multiple sorting signals in the cytosolic tail. Plant Cell 18, 1477–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. daSilva L. L., Taylor J. P., Hadlington J. L., Hanton S. L., Snowden C. J., Fox S. J., Foresti O., Brandizzi F., Denecke J. (2005) Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17, 132–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bishop N., Horman A., Woodman P. (2002) Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J. Cell Biol. 157, 91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Katzmann D. J., Odorizzi G., Emr S. D. (2002) Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 3, 893–905 [DOI] [PubMed] [Google Scholar]
- 59. Schmidt M. H., Hoeller D., Yu J., Furnari F. B., Cavenee W. K., Dikic I., Bögler O. (2004) Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex. Mol. Cell. Biol. 24, 8981–8993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cabezas A., Bache K. G., Brech A., Stenmark H. (2005) Alix regulates cortical actin and the spatial distribution of endosomes. J. Cell Sci. 118, 2625–2635 [DOI] [PubMed] [Google Scholar]
- 61. Bissig C., Gruenberg J. (2014) ALIX and the multivesicular endosome: ALIX in Wonderland. Trends Cell Biol. 24, 19–25 [DOI] [PubMed] [Google Scholar]
- 62. Usami Y., Popov S., Göttlinger H. G. (2007) Potent rescue of human immunodeficiency virus type 1 late domain mutants by ALIX/AIP1 depends on its CHMP4 binding site. J. Virol. 81, 6614–6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McCullough J., Fisher R. D., Whitby F. G., Sundquist W. I., Hill C. P. (2008) ALIX-CHMP4 interactions in the human ESCRT pathway. Proc. Natl. Acad. Sci. U.S.A. 105, 7687–7691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Munshi U. M., Kim J., Nagashima K., Hurley J. H., Freed E. O. (2007) An Alix fragment potently inhibits HIV-1 budding: characterization of binding to retroviral YPXL late domains. J. Biol. Chem. 282, 3847–3855 [DOI] [PubMed] [Google Scholar]
- 65. Stumptner-Cuvelette P., Jouve M., Helft J., Dugast M., Glouzman A. S., Jooss K., Raposo G., Benaroch P. (2003) Human immunodeficiency virus-1 Nef expression induces intracellular accumulation of multivesicular bodies and major histocompatibility complex class II complexes: potential role of phosphatidylinositol 3-kinase. Mol. Biol. Cell 14, 4857–4870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roeth J. F., Collins K. L. (2006) Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol. Mol. Biol. Rev. 70, 548–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Grzesiek S., Stahl S. J., Wingfield P. T., Bax A. (1996) The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry 35, 10256–10261 [DOI] [PubMed] [Google Scholar]
- 68. Kelly B. T., McCoy A. J., Späte K., Miller S. E., Evans P. R., Höning S., Owen D. J. (2008) A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature 456, 976–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schaefer M. R., Wonderlich E. R., Roeth J. F., Leonard J. A., Collins K. L. (2008) HIV-1 Nef targets MHC-I and CD4 for degradation via a final common β-COP-dependent pathway in T cells. PLoS Pathog. 4, e1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rose J. J., Janvier K., Chandrasekhar S., Sekaly R. P., Bonifacino J. S., Venkatesan S. (2005) CD4 down-regulation by HIV-1 and simian immunodeficiency virus (SIV) Nef proteins involves both internalization and intracellular retention mechanisms. J. Biol. Chem. 280, 7413–7426 [DOI] [PubMed] [Google Scholar]
- 71. Raiborg C., Bache K. G., Gillooly D. J., Madshus I. H., Stang E., Stenmark H. (2002) Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 4, 394–398 [DOI] [PubMed] [Google Scholar]
- 72. Janvier K., Kato Y., Boehm M., Rose J. R., Martina J. A., Kim B. Y., Venkatesan S., Bonifacino J. S. (2003) Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 g-s1 and AP-3 d-s3 hemicomplexes. J. Cell Biol. 163, 1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Coleman S. H., Van Damme N., Day J. R., Noviello C. M., Hitchin D., Madrid R., Benichou S., Guatelli J. C. (2005) Leucine-specific, functional interactions between human immunodeficiency virus type 1 Nef and adaptor protein complexes. J. Virol. 79, 2066–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Janvier K., Craig H., Hitchin D., Madrid R., Sol-Foulon N., Renault L., Cherfils J., Cassel D., Benichou S., Guatelli J. (2003) HIV-1 Nef stabilizes the association of adaptor protein complexes with membranes. J. Biol. Chem. 278, 8725–8732 [DOI] [PubMed] [Google Scholar]
- 75. Benichou S., Bomsel M., Bodéus M., Durand H., Douté M., Letourneur F., Camonis J., Benarous R. (1994) Physical interaction of the HIV-1 Nef protein with β-COP, a component of non-clathrin-coated vesicles essential for membrane traffic. J. Biol. Chem. 269, 30073–30076 [PubMed] [Google Scholar]
- 76. Piguet V., Gu F., Foti M., Demaurex N., Gruenberg J., Carpentier J. L., Trono D. (1999) Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell 97, 63–73 [DOI] [PubMed] [Google Scholar]
- 77. Janvier K., Craig H., Le Gall S., Benarous R., Guatelli J., Schwartz O., Benichou S. (2001) Nef-induced CD4 downregulation: a diacidic sequence in human immunodeficiency virus type 1 Nef does not function as a protein sorting motif through direct binding to β-COP. J. Virol. 75, 3971–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lubben N. B., Sahlender D. A., Motley A. M., Lehner P. J., Benaroch P., Robinson M. S. (2007) HIV-1 Nef-induced down-regulation of MHC class I requires AP-1 and clathrin but not PACS-1 and is impeded by AP-2. Mol. Biol. Cell 18, 3351–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Leonard J. A., Filzen T., Carter C. C., Schaefer M., Collins K. L. (2011) HIV-1 Nef disrupts intracellular trafficking of major histocompatibility complex class I, CD4, CD8, and CD28 by distinct pathways that share common elements. J. Virol. 85, 6867–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Landi A., Iannucci V., Nuffel A. V., Meuwissen P., Verhasselt B. (2011) One protein to rule them all: modulation of cell surface receptors and molecules by HIV Nef. Curr. HIV Res. 9, 496–504 [DOI] [PMC free article] [PubMed] [Google Scholar]